Enhanced Bioavailability of Tadalafil after Intranasal Administration in Beagle Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Study
2.3. Determination of Tadalafil Concentration in Plasma Using LC-MS/MS
2.4. Noncompartmental Pharmacokinetic Analysis
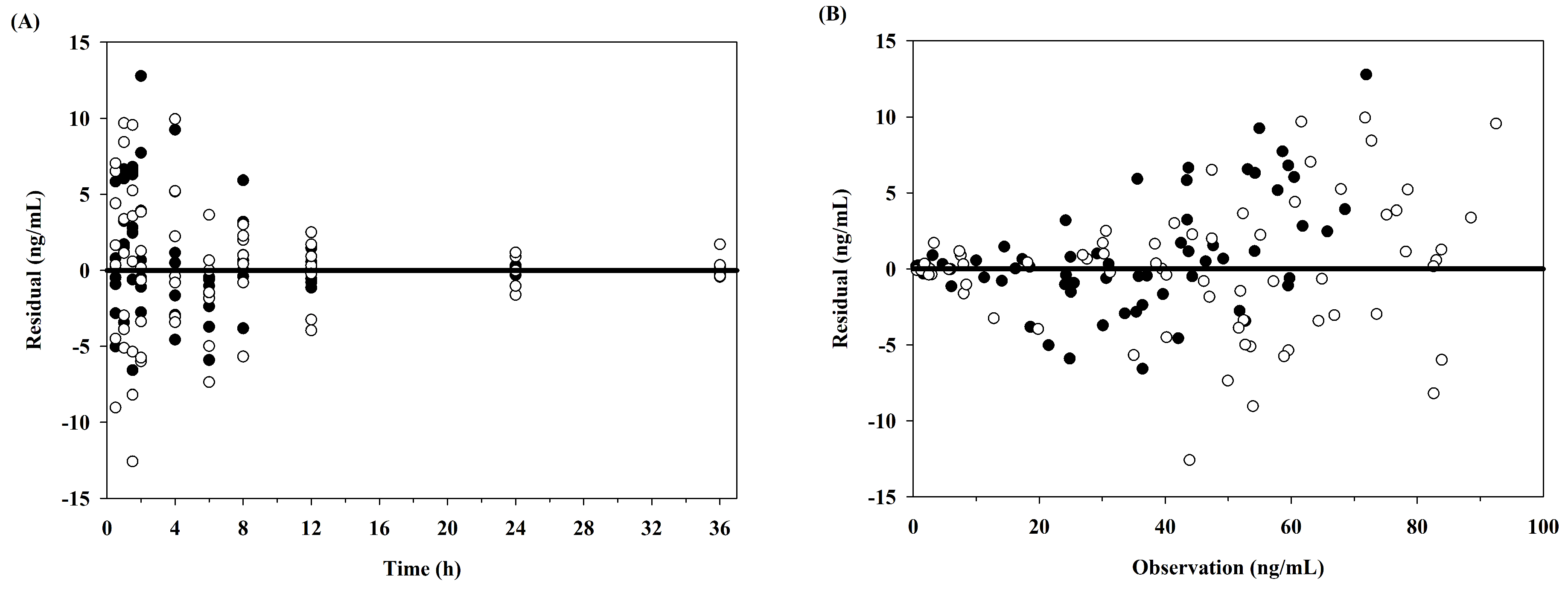
2.5. Pharmacokinetic Modeling Analysis
2.6. Statistical Analysis
3. Results
3.1. Noncompartmental Pharmacokinetic Analysis
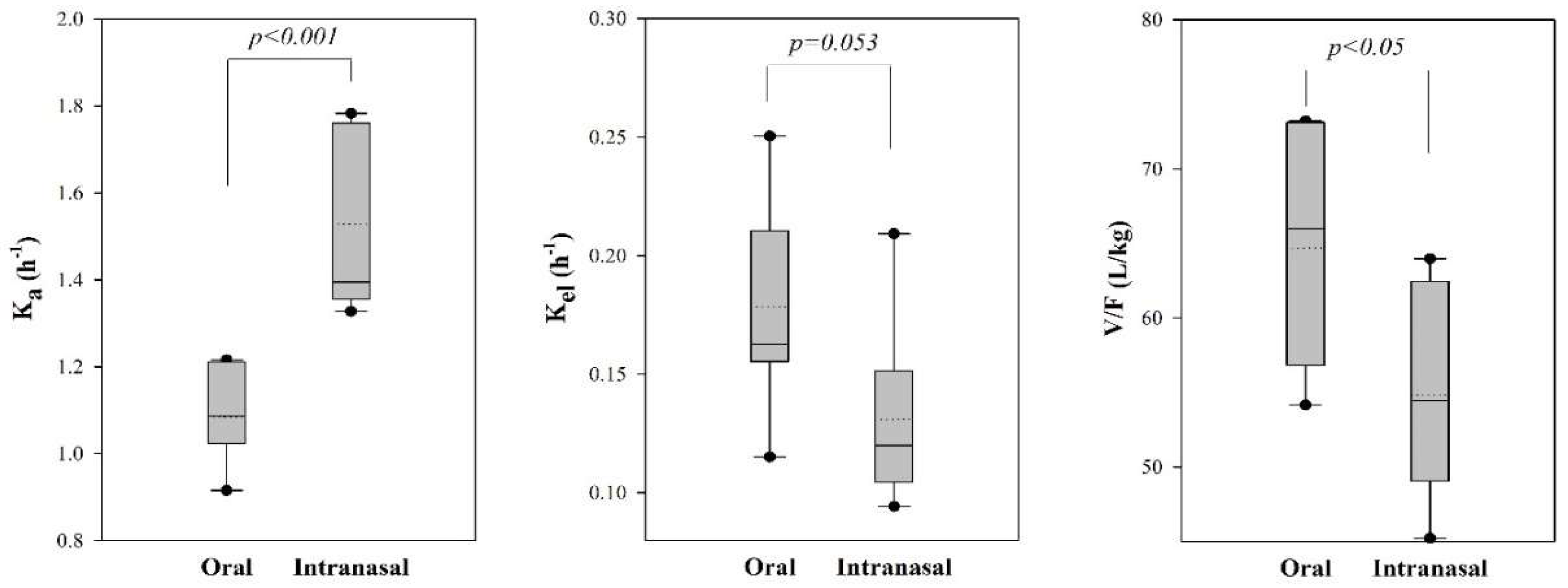
3.2. Pharmacokinetic Model Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brock, G.B.; McMahon, C.G.; Chen, K.; Costigan, T.; Shen, W.; Watkins, V.; Anglin, G.; Whitaker, S. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: Results of integrated analyses. J. Urol. 2002, 168, 1332–1336. [Google Scholar] [CrossRef]
- Padma-Nathan, H. Efficacy and tolerability of tadalafil, a novel phosphodiesterase 5 inhibitor, in treatment of erectile dysfunction. Am. J. Cardiol. 2003, 92, 19–25. [Google Scholar] [CrossRef]
- Bischoff, E. Potency, selectivity, and consequences of nonselectivity of pde inhibition. Int. J. Impot. Res. 2004, 16, S11. [Google Scholar] [CrossRef] [PubMed]
- Washington, S.L., III; Shindel, A.W. A once-daily dose of tadalafil for erectile dysfunction: Compliance and efficacy. Drug Des. Dev. Ther. 2010, 4, 159. [Google Scholar]
- Park, S.-I.; Heo, S.-H.; Kim, G.; Chang, S.; Song, K.-H.; Kim, M.-G.; Jin, E.-H.; Kim, J.; Lee, S.; Hong, J.H. Comparison of tadalafil pharmacokinetics after administration of a new orodispersible film versus a film-coated tablet. Drug Des. Dev. Ther. 2018, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Swindle, R.W.; Cameron, A.E.; Lockhart, D.C.; Rosen, R.C. The psychological and interpersonal relationship scales: Assessing psychological and relationship outcomes associated with erectile dysfunction and its treatment. Arch. Sex. Behav. 2004, 33, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Panchal, M.; Shah, S.; Vadalia, K. Formulation and evaluation of transdermal gel of sildenafil citrate. Int. J. Pharm. Res. Allied. Sci. 2012, 1, 103–118. [Google Scholar]
- Roh, H.; Son, H.; Lee, D.; Yeon, K.J.; Kim, H.S.; Kim, H.; Park, K. Pharmacokinetic comparison of an orally disintegrating film formulation with a film-coated tablet formulation of sildenafil in healthy korean subjects: A randomized, open-label, single-dose, 2-period crossover study. Clin. Ther. 2013, 35, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, F.M.; Gittelman, M.; Sperling, H.; Börner, M.; Beneke, M. Time to onset of action of vardenafil: A retrospective analysis of the pivotal trials for the orodispersible and film-coated tablet formulations. J. Sex. Med. 2011, 8, 2912–2923. [Google Scholar] [CrossRef] [PubMed]
- Radicioni, M.; Castiglioni, C.; Giori, A.; Cupone, I.; Frangione, V.; Rovati, S. Bioequivalence study of a new sildenafil 100 mg orodispersible film compared to the conventional film-coated 100 mg tablet administered to healthy male volunteers. Drug Des. Dev. Ther. 2017, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, K.; Kim, M.; Choi, D.H.; Jeong, S.H. Orally disintegrating films focusing on formulation, manufacturing process, and characterization. J. Pharm. Investig. 2017, 47, 183–201. [Google Scholar] [CrossRef]
- Elshafeey, A.H.; Bendas, E.R.; Mohamed, O.H. Intranasal microemulsion of sildenafil citrate: In vitro evaluation and in vivo pharmacokinetic study in rabbits. AAPS PharmSciTech 2009, 10, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Ku, W.-S.; Cho, H.-J.; Yoon, I.-S.; Kim, J.H.; Cha, B.-J.; Kim, J.S.; Kim, K.-M.; Kang, S.-K.; Chung, S.-J.; Shim, C.-K. Rapid and sensitive determination of udenafil in plasma by lc-ms/ms for intranasal pharmacokinetic study in rats. Chem. Pharm. Bull. 2011, 59, 1083–1088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romeo, V.; DeMeireles, J.; Sileno, A.; Pimplaskar, H.; Behl, C. Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 89. [Google Scholar] [PubMed]
- Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007, 337, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Kumar, A.; Sharma, S. Nanoemulsions loaded carbopol® 934 based gel for intranasal delivery of neuroprotective centella asiatica extract: In–vitro and ex–vivo permeation study. J. Pharm. Investig. 2016, 46, 79–89. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research (CDER). Guidance for Industry: Bioanalytical Method Validation. In US Food and Drug Administration. Available online: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm070107.pdf (accessed on 3 May 2018).
- WinNonlin, T. User’s guide (ver. 5.2); Pharsight Corporation: Mountain View, CA, USA, 1999. [Google Scholar]
- D’Argenio, D.Z.; Schumitzky, A.; Wang, X. ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic system analysis software; Biomedical Simulations Resource: Los Angeles, CA, USA, 2009. [Google Scholar]
- Kletting, P.; Glatting, G. Model selection for time-activity curves: The corrected akaike information criterion and the f-test. Z. Med. Phys. 2009, 19, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Ku, W.-S.; Termsarasab, U.; Yoon, I.; Chung, C.-W.; Moon, H.T.; Kim, D.-D. Development of udenafil-loaded microemulsions for intranasal delivery: In vitro and in vivo evaluations. Int. J. Pharm. 2012, 423, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Baek, I.-H.; Lee, B.-Y.; Kang, W.; Kwon, K.-I. Pharmacokinetic analysis of two different doses of duloxetine following oral administration in dogs. Drug Res. 2013, 63, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Forgue, S.T.; Patterson, B.E.; Bedding, A.W.; Payne, C.D.; Phillips, D.L.; Wrishko, R.E.; Mitchell, M.I. Tadalafil pharmacokinetics in healthy subjects. Br. J. Clin. Pharmacol. 2006, 61, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research (CDER). Cialis® Pharmacological Review. In US Food Drug Administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-368_Cialis_Pharmr.pdf (accessed on 2 October 2018).
- Court, M.H. Canine cytochrome p450 (cyp) pharmacogenetics. Vet. Clin. N. Am. Small. Anim. Pract. 2013, 43, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kleiber, M. Body size and metabolic rate. Physiol. Rev. 1947, 27, 511–541. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Lee, S.; Wilson, C. Intranasal drug delivery by spray and drops. J. Pharm. Pharmacol. 1985, 37, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Musulin, S.; Mariani, C.; Papich, M. Diazepam pharmacokinetics after nasal drop and atomized nasal administration in dogs. J. Vet. Pharmacol. Ther. 2011, 34, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Kostis, J.B. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am. J. Cardiol. 2003, 92, 9–18. [Google Scholar] [CrossRef]
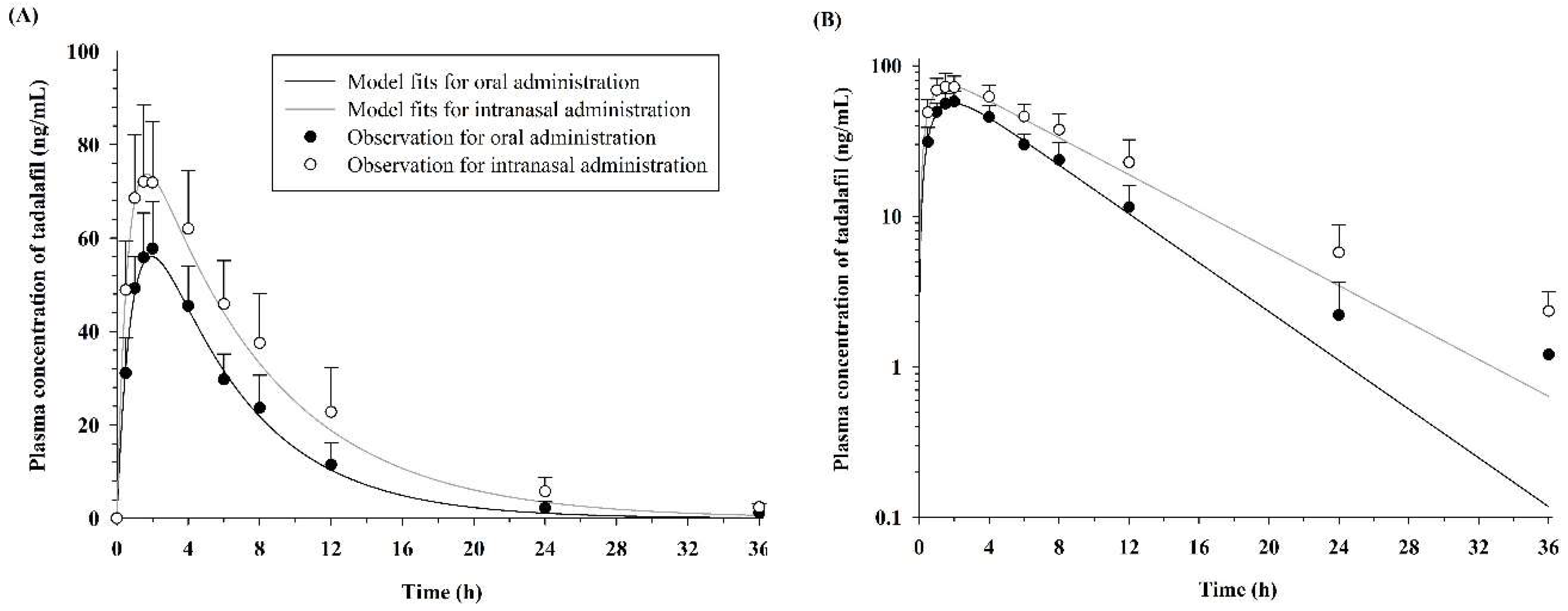
| Parameter (Units) | Oral (n = 7) | Intranasal (n = 7) | p Value |
|---|---|---|---|
| Tmax (h) | 1.71 ± 0.39 | 1.50 ± 0.41 | 0.337 |
| Cmax (ng/mL) | 59.49 ± 9.22 | 76.45 ± 12.07 | p < 0.05 |
| AUC36h (ng·h/mL) | 472.66 ± 102.70 | 771.10 ± 216.72 | p < 0.01 |
| AUCinf (ng·h/mL) | 479.33 ± 102.88 | 790.23 ± 224.91 | p < 0.01 |
| λz (h−1) | 0.17 ± 0.04 | 0.13 ± 0.04 | 0.06 |
| t1/2 (h) | 4.17 ± 1.01 | 5.79 ± 1.47 | p < 0.05 |
| Clt/F (L/h) | 10.87 ± 2.43 | 6.98 ± 2.77 | p < 0.05 |
| Vz/F (L) | 64.13 ± 14.80 | 53.63 ± 5.07 | 0.101 |
| Parameter (Units) | Oral (n = 7) | Intranasal (n = 7) | p Value |
|---|---|---|---|
| Ka (h−1) | 1.08 ± 0.11 | 1.53 ± 0.21 | p < 0.001 |
| Kel (h−1) | 0.18 ± 0.04 | 0.13 ± 0.04 | p = 0.053 |
| Vc/F (L) | 64.70 ± 8.41 | 54.83 ± 6.99 | p < 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Kim, M.-S.; Baek, I.-h. Enhanced Bioavailability of Tadalafil after Intranasal Administration in Beagle Dogs. Pharmaceutics 2018, 10, 187. https://doi.org/10.3390/pharmaceutics10040187
Kim J-S, Kim M-S, Baek I-h. Enhanced Bioavailability of Tadalafil after Intranasal Administration in Beagle Dogs. Pharmaceutics. 2018; 10(4):187. https://doi.org/10.3390/pharmaceutics10040187
Chicago/Turabian StyleKim, Jeong-Soo, Min-Soo Kim, and In-hwan Baek. 2018. "Enhanced Bioavailability of Tadalafil after Intranasal Administration in Beagle Dogs" Pharmaceutics 10, no. 4: 187. https://doi.org/10.3390/pharmaceutics10040187
APA StyleKim, J.-S., Kim, M.-S., & Baek, I.-h. (2018). Enhanced Bioavailability of Tadalafil after Intranasal Administration in Beagle Dogs. Pharmaceutics, 10(4), 187. https://doi.org/10.3390/pharmaceutics10040187







