Effects of Formulation and Process Variables on Gastroretentive Floating Tablets with A High-Dose Soluble Drug and Experimental Design Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Screening of Hydrophilic Polymers
2.3. Box-Behnken Experimental Design
2.4. Preparation of Floating Tablets
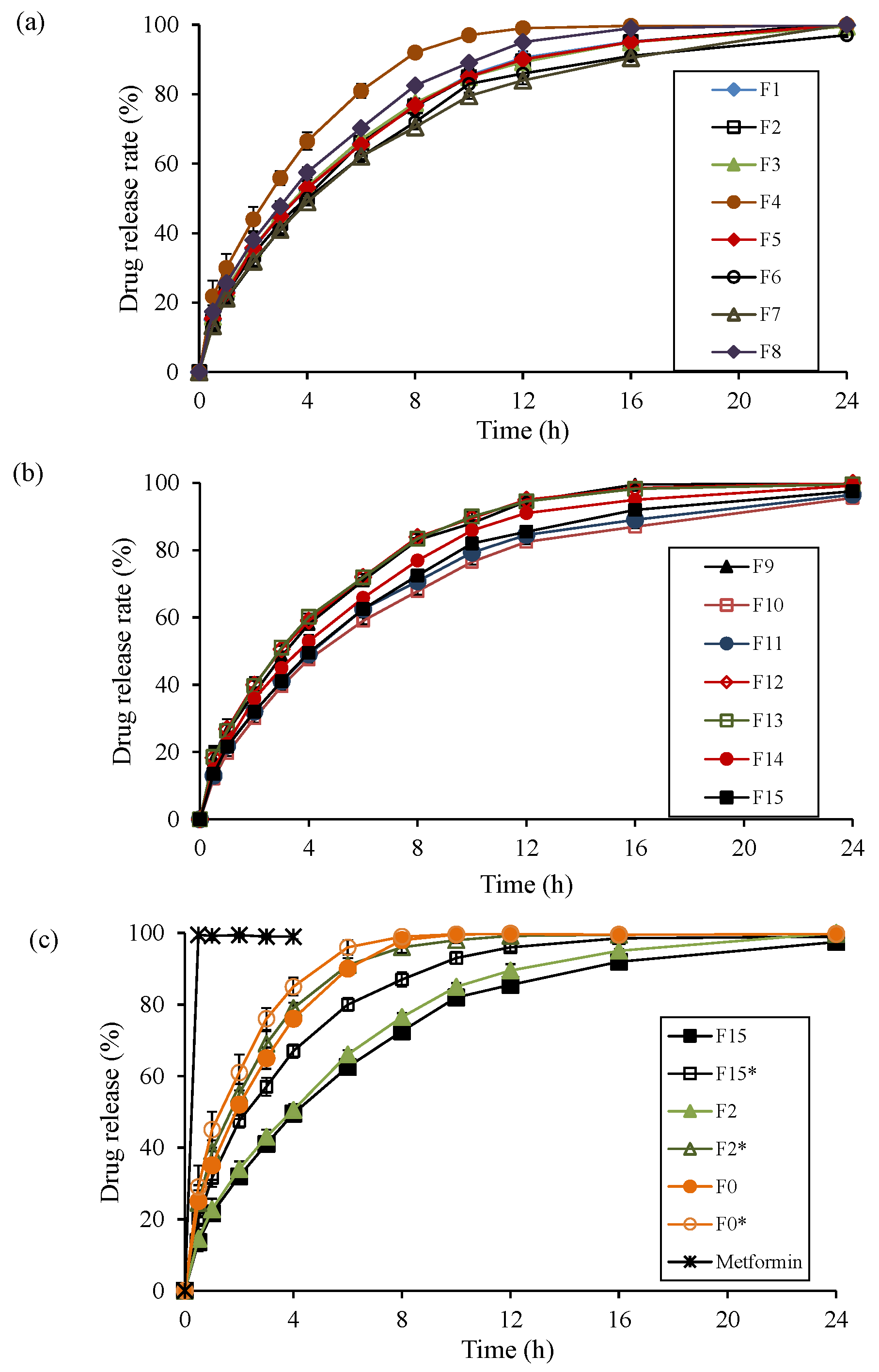
2.5. In vitro Drug Release Studies
2.6. In Vitro Floating Behavior
2.7. Medium Uptake and Mass Loss of Tablets
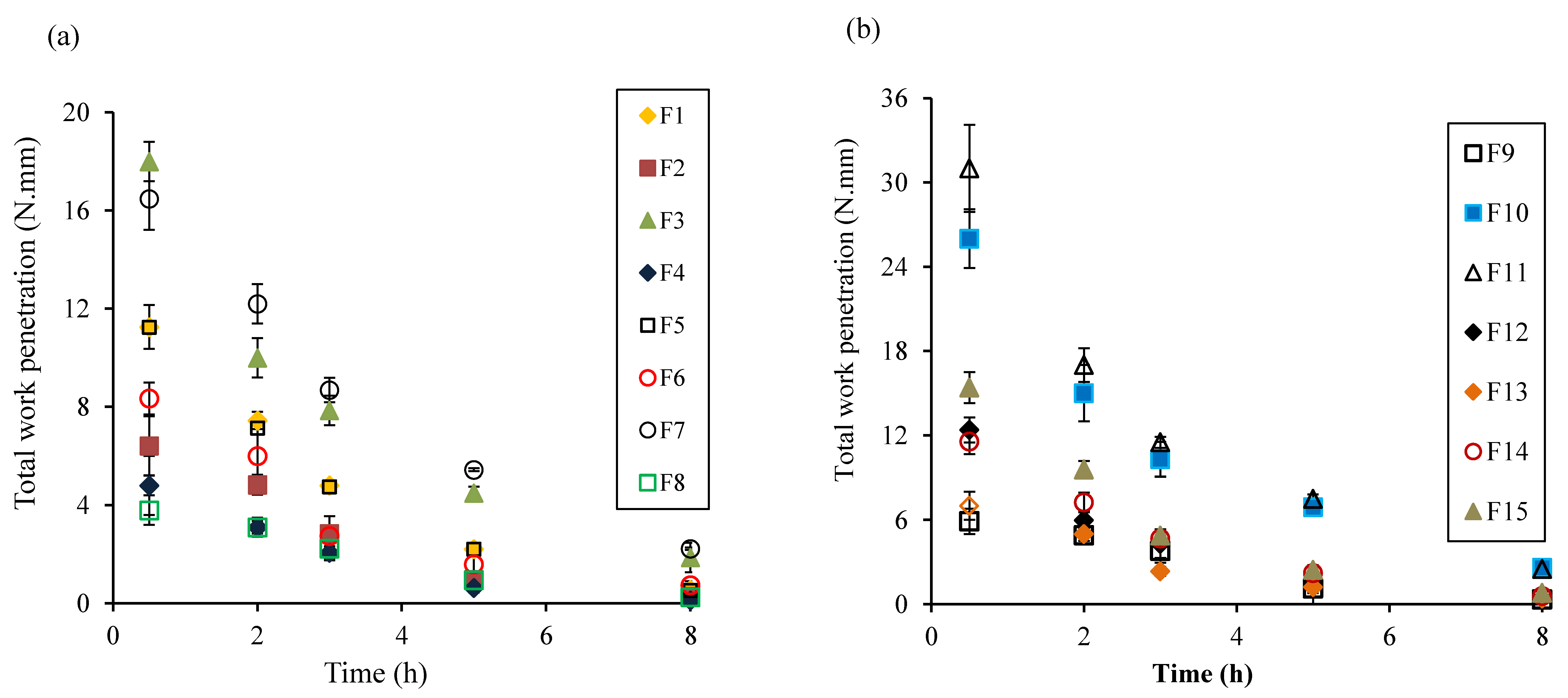
2.8. Total Fwork of Penetration
2.9. Tablet Ejection Force
2.10. Tablet Tensile Strength and Porosity
3. Results and Discussion
3.1. Risk Assessment
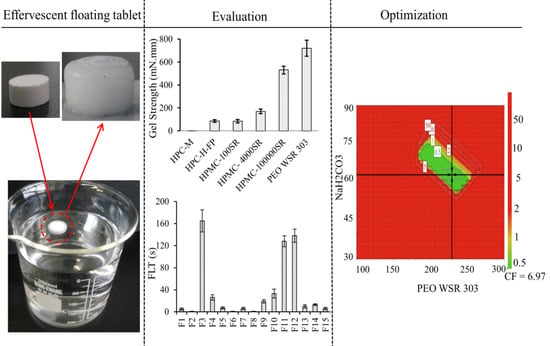
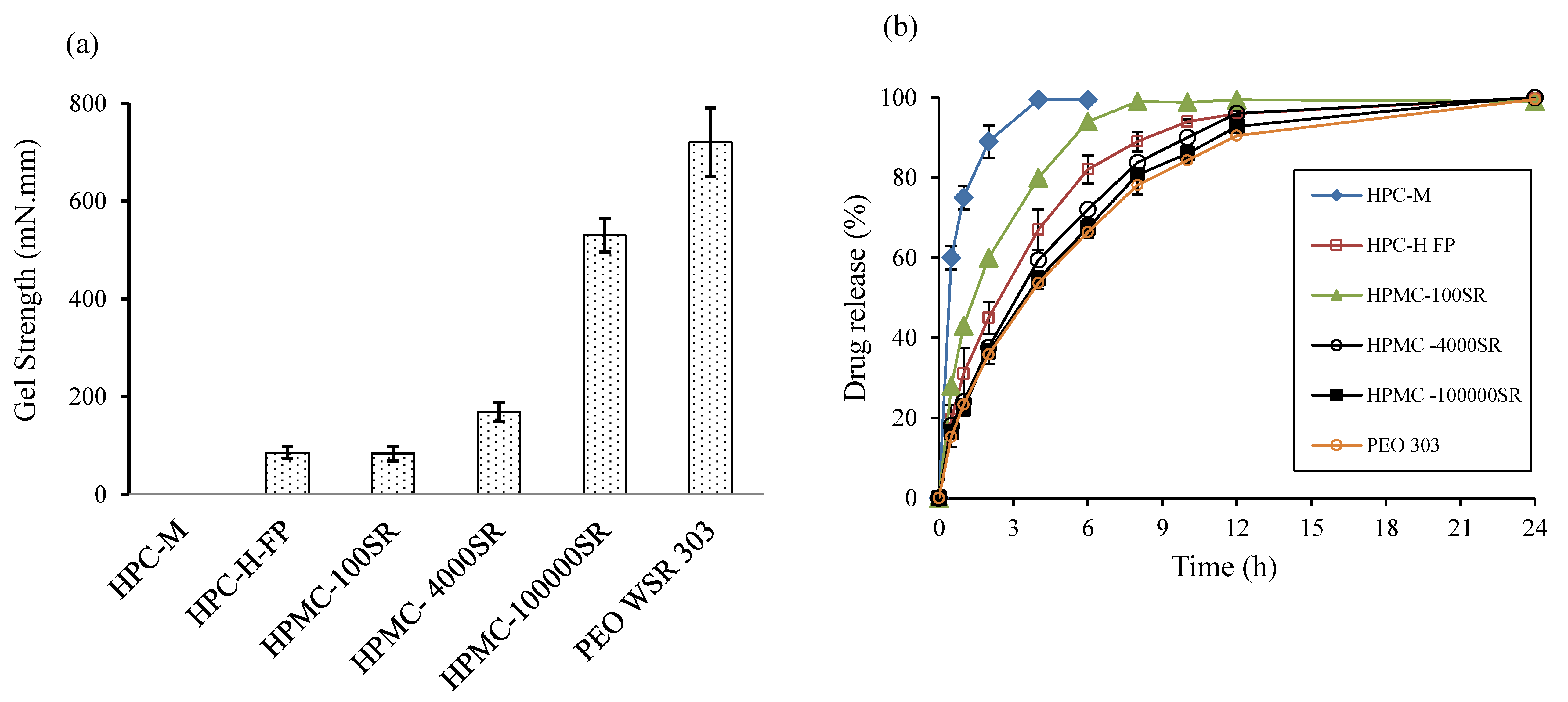
3.2. Polymer Screening
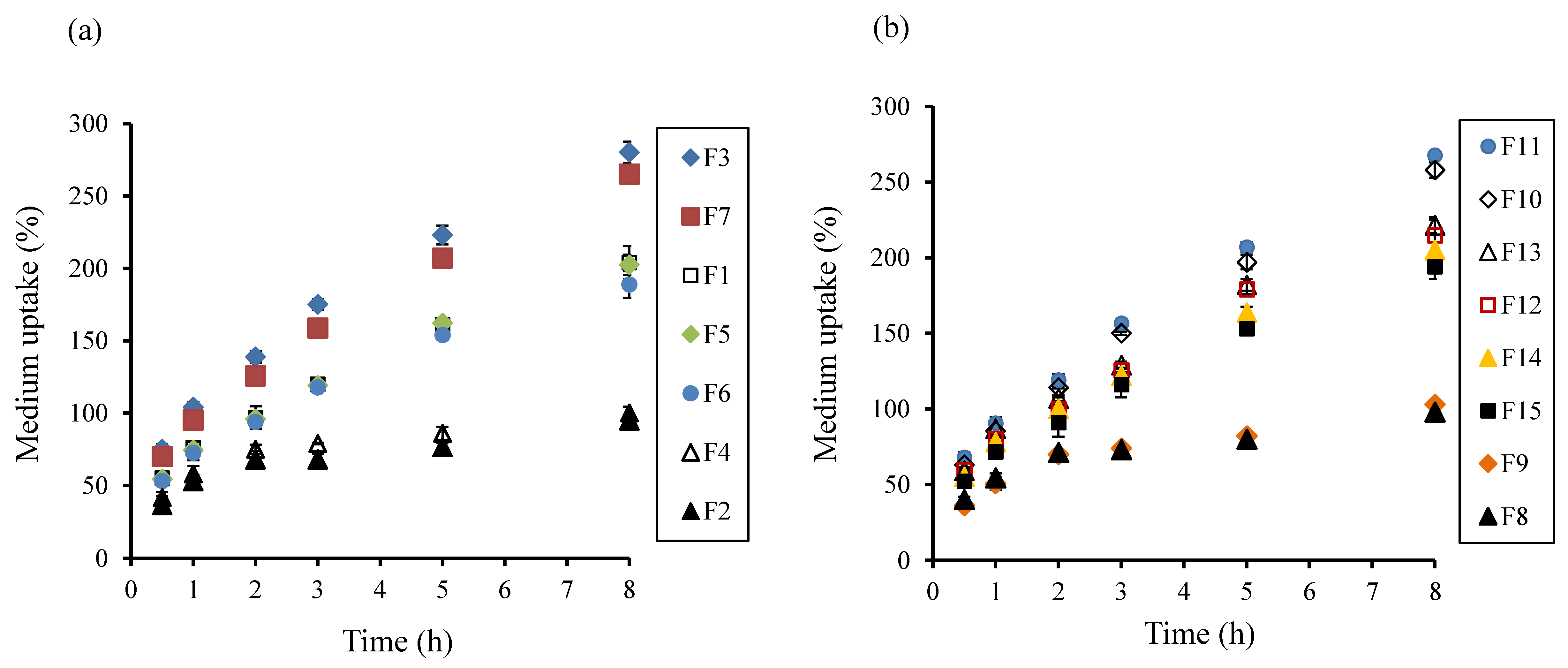
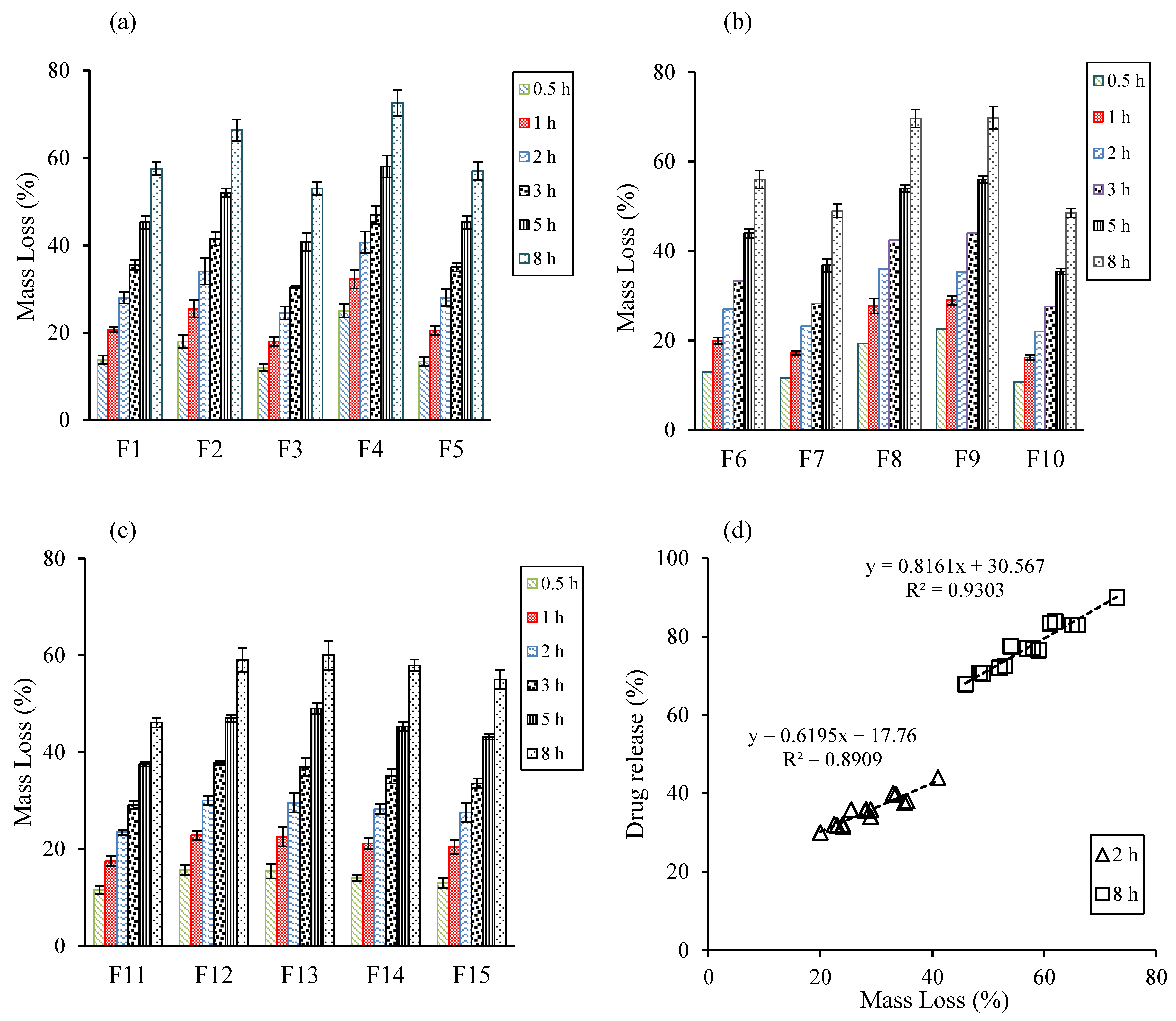
3.3. Evaluation of Medium Uptake, Mass Loss, and Gel Strength
3.4. Evaluation of Drug Release Kinetics
3.5. Statistical Analysis and Summary of Fit
3.6. Effect of Control Factors on Drug Release Profiles
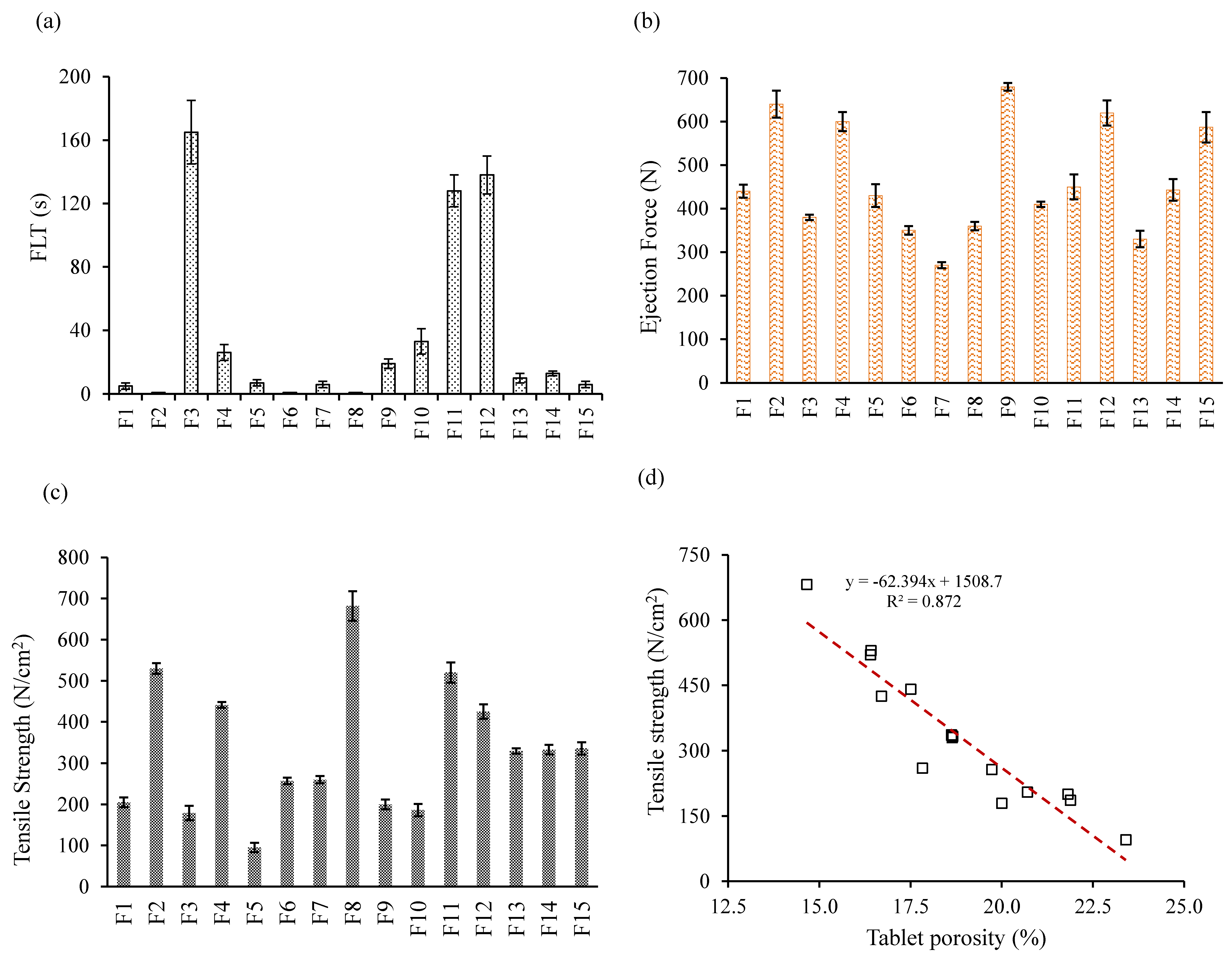
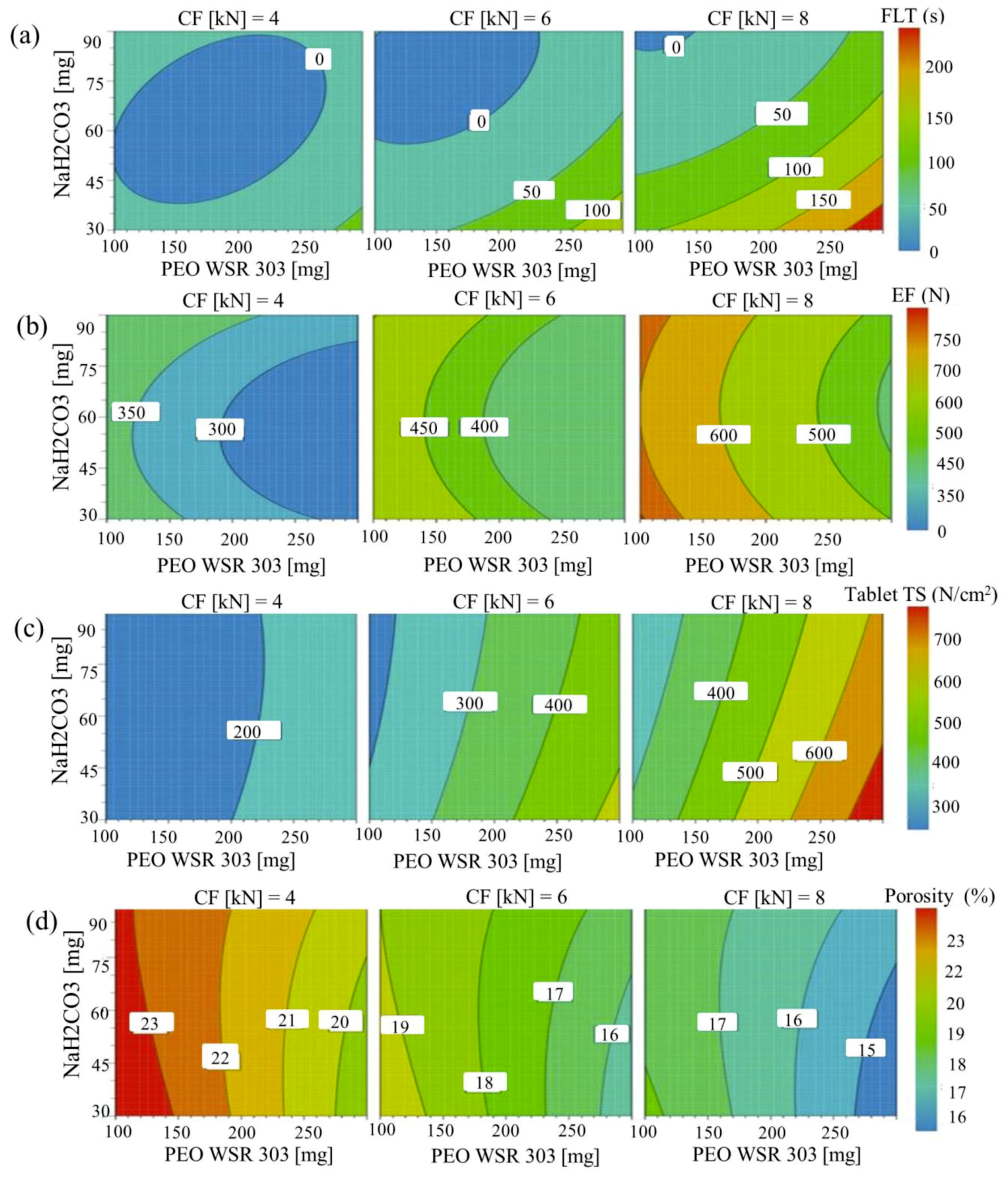
3.7. Effect of Control Factors on Floating Lag Time (FLT)
3.8. Effect of Control Factors on Tablet Properties
3.8.1. Effects of Control Factors on Tablet Ejection Force
3.8.2. Effect of Control Factors on Tablet Tensile Strength
3.8.3. Effect of Control Factors on Tablet Porosity
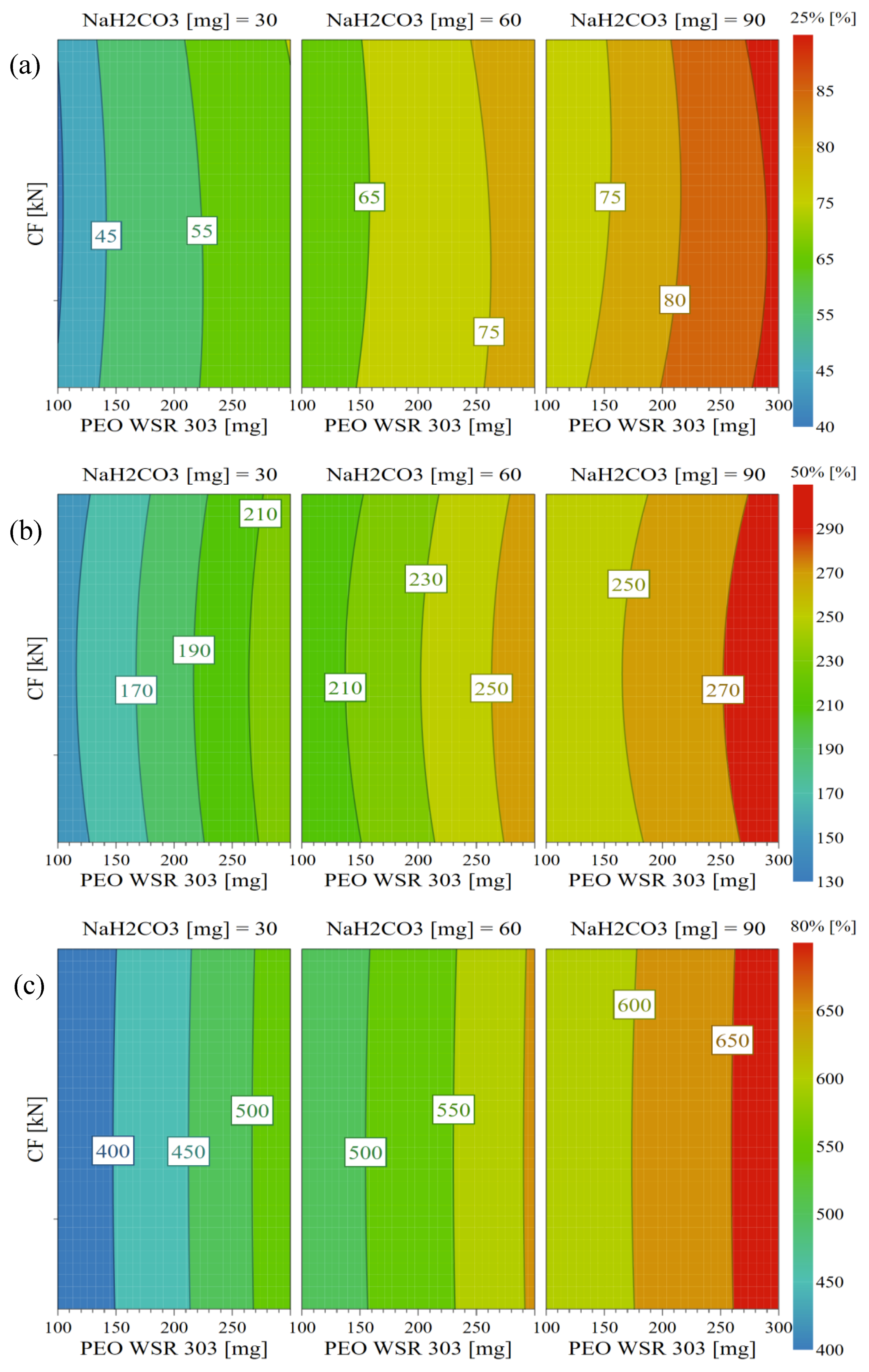
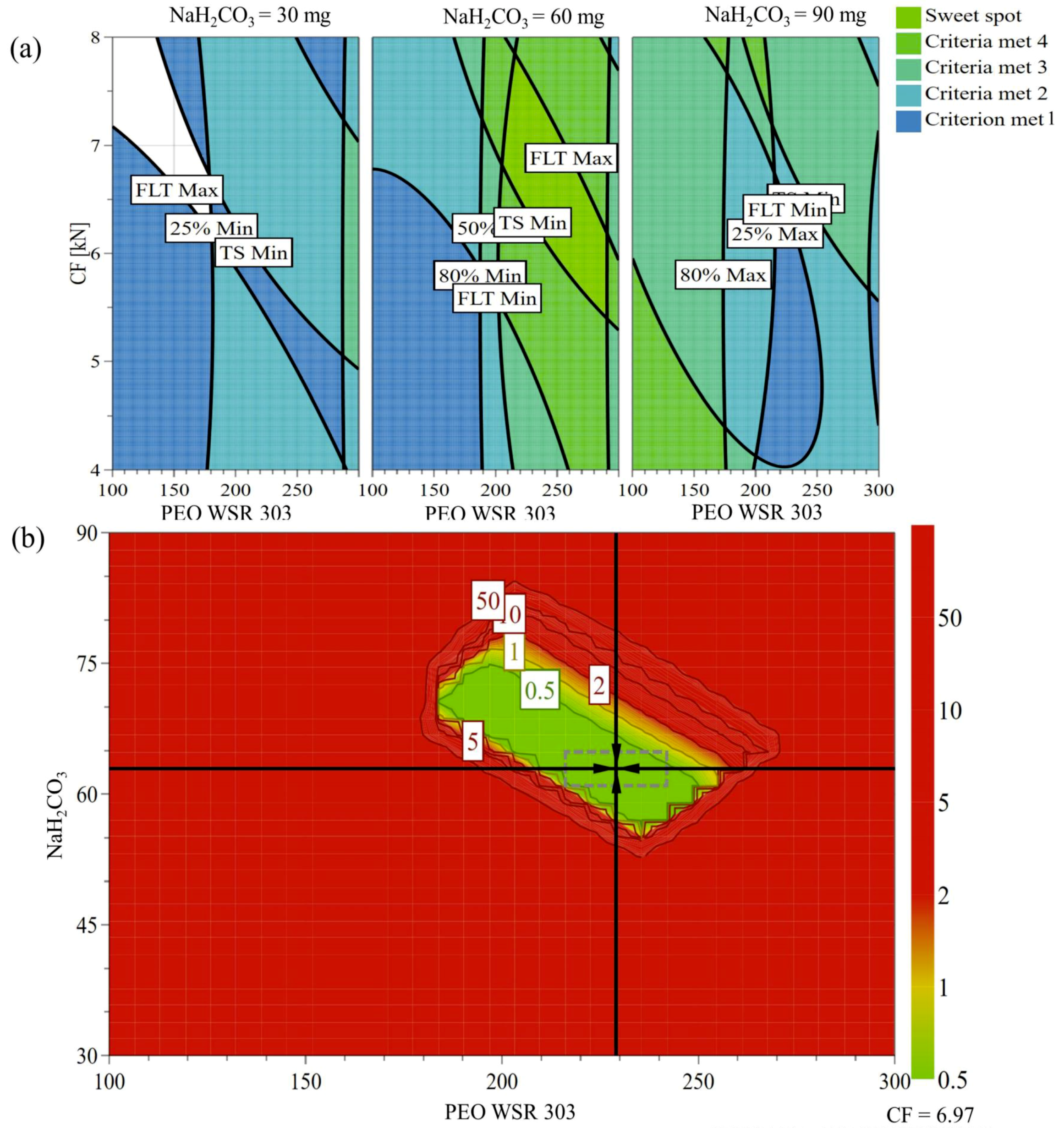
3.9. Design Space and Optimization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoffman, A. Pharmacodynamic aspects of sustained release preparations. Adv. Drug Deliv. Rev. 1998, 33, 185–199. [Google Scholar] [CrossRef]
- Nayak, A.K.; Malakar, J.; Sen, K.K. Gastroretentive drug delivery technologies: Current approaches and future potential. J. Pharm. Edu. Res. 2010, 1, 1. [Google Scholar]
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.Q.; Feng, X.; Pimparade, M.; Ye, X.; Kim, D.W.; Martin, S.T.; Repka, M.A. Dual-mechanism gastroretentive drug delivery system loaded with an amorphous solid dispersion prepared by hot-melt extrusion. Eur. J. Pharm. Sci. 2017, 102, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ngwuluka, N.C.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Pillay, V. An optimized gastroretentive nanosystem for the delivery of levodopa. Int. J. Pharm. 2015, 494, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006, 6, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Kim, K.H. Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention. J. Control. Release 2000, 63, 235–259. [Google Scholar] [CrossRef]
- Bardonnet, P.; Faivre, V.; Pugh, W.; Piffaretti, J.; Falson, F. Gastroretentive dosage forms: Overview and special case of Helicobacter pylori. J. Control. Release 2006, 111, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Gupta, G.D. Preparation and Evaluation of Gastroretentive Floating Tablets of Silymarin. Chem. Pharm. Bull. 2009, 57, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.K.; Verma, A. Development and statistical optimization of chitosan and eudragit based gastroretentive controlled release multiparticulate system for bioavailability enhancement of metformin HCl. J. Pharm. Investig. 2016, 46, 239–252. [Google Scholar] [CrossRef]
- Xu, X.; Sun, M.; Zhi, F.; Hu, Y. Floating matrix dosage form for phenoporlamine hydrochloride based on gas forming agent: In vitro and in vivo evaluation in healthy volunteers. Int. J. Pharm. 2006, 310, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Patra, S.; Pani, N.R. Optimization of HPMC and carbopol concentrations in non-effervescent floating tablet through factorial design. Carbohydr. Polym. 2014, 102, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hwang, K.-M.; Park, Y.S.; Nguyen, T.-T.; Park, E.-S. Preparation and evaluation of non-effervescent gastroretentive tablets containing pregabalin for once-daily administration and dose proportional pharmacokinetics. Int. J. Pharm. 2018, 550, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, K.H.; Park, J.S.; Jeong, S.H.; Park, K. Evaluation of drug delivery profiles in geometric three-layered tablets with various mechanical properties, in vitro–in vivo drug release, and Raman imaging. J. Control. Release 2013, 172, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Beg, S.; Garg, B.; Asthana, A.; Asthana, G.S.; Singh, B. QbD-oriented development and characterization of effervescent floating-bioadhesive tablets of cefuroxime axetil. AAPS PharmSciTech 2016, 17, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, I.; Quirino-Barreda, T.; Villafuerte-Robles, L. Sustained delivery of captopril from floating matrix tablets. Int. J. Pharm. 2008, 362, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Bukhari, N.I.; Karim, S.; Hafiz, M.A.; Hayat, U. Floating tablets of minocycline hydrochloride: Formulation, in-vitro evaluation and optimization. Futu. J. Pharm. Sci. 2017, 3, 131–139. [Google Scholar] [CrossRef]
- Rahim, S.A.; Carter, P.; Elkordy, A.A. Influence of calcium carbonate and sodium carbonate gassing agents on pentoxifylline floating tablets properties. Powder Technol. 2017, 322, 65–74. [Google Scholar] [CrossRef]
- Arza, R.A.K.; Gonugunta, C.S.R.; Veerareddy, P.R. Formulation and evaluation of swellable and floating gastroretentive ciprofloxacin hydrochloride tablets. AAPS PharmSciTech 2009, 10, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Tadros, M.I. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: Development, optimization and in vitro–in vivo evaluation in healthy human volunteers. Eur. J. Pharm. Biopharm. 2010, 74, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Chen, H.; Rui, Y.; Yang, F.; Ma, N.; Wu, Z. Floating tablets for controlled release of ofloxacin via compression coating of hydroxypropyl cellulose combined with effervescent agent. Int. J. Pharm. 2015, 489, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Vaingankar, P.; Amin, P. Continuous melt granulation to develop high drug loaded sustained release tablet of Metformin HCl. Asian. J. Pharm. Sci. 2017, 12, 37–50. [Google Scholar] [CrossRef]
- Colombo, P.; Bettini, R.; Peppas, N.A. Observation of swelling process and diffusion front position during swelling in hydroxypropyl methyl cellulose (HPMC) matrices containing a soluble drug. J. Control. Release 1999, 61, 83–91. [Google Scholar] [CrossRef]
- Bettini, R.; Catellani, P.L.; Santi, P.; Massimo, G.; Peppas, N.A.; Colombo, P. Translocation of drug particles in HPMC matrix gel layer: Effect of drug solubility and influence on release rate. J. Control. Release 2001, 70, 383–391. [Google Scholar] [CrossRef]
- Qin, C.; Wu, M.; Xu, S.; Wang, X.; Shi, W.; Dong, Y.; Yang, L.; He, W.; Han, X.; Yin, L. Design and optimization of gastro-floating sustained-release tablet of pregabalin: In vitro and in vivo evaluation. Int. J. Pharm. 2018, 545, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Vanhoorne, V.; Janssens, L.; Vercruysse, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation of controlled release formulations with various HPMC grades. Int. J. Pharm. 2016, 511, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Saeidipour, F.; Mansourpour, Z.; Mortazavian, E.; Rafiee-Tehrani, N.; Rafiee-Tehrani, M. New comprehensive mathematical model for HPMC-MCC based matrices to design oral controlled release systems. Eur. J. Pharm. Biopharm. 2017, 121, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Furubayashi, T.; Tomisaki, M.; Kawakami, M.; Kimura, S.; Inoue, D.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Nasal drug absorption from powder formulations: The effect of three types of hydroxypropyl cellulose (HPC). Eur. J. Pharm. Sci. 2017, 96, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Lim, J.Y.; Shin, S.; Choi, W.J.; Jeong, S.H.; Lee, S. A Novel Experimental Design Method to Optimize Hydrophilic Matrix Formulations with Drug Release Profiles and Mechanical Properties. J. Pharm. Sci. 2014, 103, 3083–3094. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Thapa, R.; Choi, D.H.; Jeong, S.H. Effects of pharmaceutical processes on the quality of ethylcellulose coated pellets: Quality by design approach. Powder Technol. 2018, 339, 25–38. [Google Scholar] [CrossRef]
- Thapa, P.; Lee, A.R.; Choi, D.H.; Jeong, S.H. Effects of moisture content and compression pressure of various deforming granules on the physical properties of tablets. Powder Technol. 2017, 310, 92–102. [Google Scholar] [CrossRef]
- Turkoglu, M.; Sakr, A. Mathematical modelling and optimization of a rotary fluidized-bed coating process. Int. J. Pharm. 1992, 88, 75–87. [Google Scholar] [CrossRef]
- Liu, H.; Wang, K.; Schlindwein, W.; Li, M. Using the Box–Behnken experimental design to optimise operating parameters in pulsed spray fluidised bed granulation. Int. J. Pharm. 2013, 448, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, T.R.; Young, S.; Dunstan, D.E.; Alany, R.G. Texture and rheological characterization of kappa and iota carrageenan in the presence of counter ions. Carbohydr. Polym. 2010, 82, 69–77. [Google Scholar] [CrossRef]
- Bono, A.; Anisuzzaman, S.M.; Ding, O.W. Effect of process conditions on the gel viscosity and gel strength of semi-refined carrageenan (SRC) produced from seaweed (Kappaphycus alvarezii). J. King Saud University-Eng. Sci. 2014, 26, 3–9. [Google Scholar] [CrossRef]
- Wichianphong, N.; Charoenchaitrakool, M. Application of Box–Behnken design for processing of mefenamic acid–paracetamol cocrystals using gas anti-solvent (GAS) process. J. CO2 Util. 2018, 26, 212–220. [Google Scholar] [CrossRef]
- Jamzad, S.; Tutunji, L.; Fassihi, R. Analysis of macromolecular changes and drug release from hydrophilic matrix systems. Int. J. Pharm. 2005, 292, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Lim, D.G.; Son, H.M.; Jeong, S.H. Comprehensive evaluation of layer separation tendency of novel three-layered tablets with geometric and mechanical properties. Int. J. Pharm. 2014, 465, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Cascone, S.; Cafaro, M.M.; Titomanlio, G.; d’Amore, M.; Barba, A.A. Measurements of water content in hydroxypropyl-methyl-cellulose based hydrogels via texture analysis. Carbohydr. Polym. 2013, 92, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G.; Titomanlio, G.; d’Amore, M.; Barba, A.A. Measurements of non-uniform water content in hydroxypropyl-methyl-cellulose based matrices via texture analysis. Carbohydr. Polym. 2014, 103, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Johnson, B.; Fassihi, R. Determination of continuous changes in the gel layer thickness of poly (ethylene oxide) and HPMC tablets undergoing hydration: A texture analysis study. Pharm. Res. 1998, 15, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gu, X. Correlation between drug dissolution and polymer hydration: A study using texture analysis. Int. J. Pharm. 2007, 342, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, S.; Nazzal, M.; El-Malah, Y. A novel texture-probe for the simultaneous and real-time measurement of swelling and erosion rates of matrix tablets. Int. J. Pharm. 2007, 330, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.; Newton, J. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Pavurala, N.; Manda, P.; Xu, X.; Cruz, C.N.; Krishnaiah, Y.S.R. Quality by Design approach for studying the impact of formulation and process variables on product quality of oral disintegrating films. Int. J. Pharm. 2017, 527, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Charoo, N.A.; Shamsher, A.A.A.; Zidan, A.S.; Rahman, Z. Quality by design approach for formulation development: A case study of dispersible tablets. Int. J. Pharm. 2012, 423, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Tomba, E.; Facco, P.; Bezzo, F.; Barolo, M. Latent variable modeling to assist the implementation of Quality-by-Design paradigms in pharmaceutical development and manufacturing: A review. Int. J. Pharm. 2013, 457, 283–297. [Google Scholar] [CrossRef] [PubMed]
- ICH Guidance for Industry Q8 (R2) Pharmaceutical Development. 2009. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm073507.pdf (accessed on 07.06.2018).
- Bertram, U.; Bodmeier, R. In situ gelling, bioadhesive nasal inserts for extended drug delivery: In vitro characterization of a new nasal dosage form. Eur. J. Pharm. Sci. 2006, 27, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control. Release 2011, 154, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Controlled drug release from hydrogel-based matrices: Experiments and modeling. Int. J. Pharm. 2015, 486, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kaunisto, E.; Tajarobi, F.; Abrahmsen-Alami, S.; Larsson, A.; Nilsson, B.; Axelsson, A. Mechanistic modelling of drug release from a polymer matrix using magnetic resonance microimaging. Eur. J. Pharm. Sci. 2013, 48, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Podual, K.; Sriwongjanya, M.; Peppas, N.A.; Bodmeier, R. A New Model Describing the Swelling and Drug Release Kinetics from Hydroxypropyl Methylcellulose Tablets. J. Pharm. Sci. 1999, 88, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Segale, L.; Torre, M.L.; Ochoa Machiste, E.; Conte, U. Dissolution behaviour of hydrophilic matrix tablets containing two different polyethylene oxides (PEOs) for the controlled release of a water-soluble drug. Dimensionality study. Biomaterials 2002, 23, 1113–1119. [Google Scholar] [CrossRef]
- Siepmann, F.; Karrout, Y.; Gehrke, M.; Penz, F.K.; Siepmann, J. Limited drug solubility can be decisive even for freely soluble drugs in highly swollen matrix tablets. Int. J. Pharm. 2017, 526, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Meng, F.; Lubach, J.; Koleng, J.; Watson, N.A. Properties and mechanisms of drug release from matrix tablets containing poly(ethylene oxide) and poly(acrylic acid) as release retardants. Eur. J. Pharm. Biopharm. 2016, 105, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.S.; Kumar, A.; Pathak, K. Osmotically regulated floating asymmetric membrane capsule for controlled site-specific delivery of ranitidine hydrochloride: Optimization by central composite design. AAPS PharmSciTech 2012, 13, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, Å.; Nyström, C. Tablet strength, porosity, elasticity and solid state structure of tablets compressed at high loads. Int. J. Pharm. 1996, 132, 95–106. [Google Scholar] [CrossRef]
- Sun, C.C. Dependence of ejection force on tableting speed—A compaction simulation study. Powder Technol. 2015, 279, 123–126. [Google Scholar] [CrossRef]
- Wang, J.; Wen, H.; Desai, D. Lubrication in tablet formulations. Eur. J. Pharm. Biopharm. 2010, 75, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Sun, C.C. Dependence of friability on tablet mechanical properties and a predictive approach for binary mixtures. Pharm. Res. 2017, 34, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Uzunović, A.; Vranić, E. Effect of magnesium stearate concentration on dissolution properties of ranitidine hydrochloride coated tablets. Bosn. J. Basic Med. Sci. 2007, 7, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Hilden, J.; Litster, J.D. Compaction mechanics of plastically deformable dry granules. Powder Technol. 2016, 291, 328–336. [Google Scholar] [CrossRef]
- Taipale-Kovalainen, K.; Karttunen, A.-P.; Ketolainen, J.; Korhonen, O. Lubricant based determination of design space for continuously manufactured high dose paracetamol tablets. Eur. J. Pharm. Sci. 2018, 115, 1–10. [Google Scholar] [CrossRef] [PubMed]
| Run | Independent Variables | Dependent Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | Y7 | |
| PEO WSR (mg) | Sodium Bicarbonate (mg) | Compression Force (kN) | T25% (min) | T50% (min) | T80% (min) | FLT (s) | Ejection Force (N) | Tensile Strength (N/cm2) | Tablet Porosity (%) | |
| 1 | 200 | 60 | 6 | 70 | 229 | 528 | 5 | 445 | 342 | 18.7 |
| 2 | 100 | 90 | 6 | 72 | 238 | 516 | 1 | 625 | 179 | 20.0 |
| 3 | 300 | 30 | 6 | 61 | 224 | 529 | 165 | 390 | 524 | 16.4 |
| 4 | 100 | 30 | 6 | 44 | 143 | 391 | 26 | 600 | 207 | 20.7 |
| 5 | 200 | 60 | 6 | 69 | 228 | 531 | 7 | 430 | 330 | 18.6 |
| 6 | 200 | 90 | 4 | 79 | 254 | 580 | 1 | 350 | 188 | 21.9 |
| 7 | 300 | 60 | 4 | 80 | 260 | 612 | 6 | 280 | 271 | 19.7 |
| 8 | 100 | 60 | 4 | 59 | 193 | 461 | 1 | 360 | 90 | 23.4 |
| 9 | 100 | 60 | 8 | 57 | 194 | 459 | 19 | 680 | 260 | 17.8 |
| 10 | 300 | 90 | 6 | 87 | 281 | 655 | 33 | 410 | 441 | 17.5 |
| 11 | 300 | 60 | 8 | 81 | 259 | 610 | 128 | 450 | 690 | 14.7 |
| 12 | 200 | 30 | 8 | 55 | 183 | 444 | 138 | 620 | 503 | 16.4 |
| 13 | 200 | 30 | 4 | 53 | 181 | 450 | 10 | 330 | 200 | 21.8 |
| 14 | 200 | 60 | 6 | 69 | 228 | 525 | 13 | 440 | 336 | 18.6 |
| 15 | 200 | 90 | 8 | 79 | 252 | 575 | 6 | 587 | 430 | 16.7 |
| QTPP | Target | Justification | |
|---|---|---|---|
| Dosage form | Controlled release effervescent floating tablet | Metformin is well absorbed from the upper part of the small intestine Short half-life and highly water-soluble | |
| Route of administration | Oral | Designed for oral administration | |
| Dose strength | 500 mg/Tablet | Commonly accepted strength | |
| Product quality attributes | Tablet TS | 500 N/cm2 | Softer tablet could chip or break during packaging and/or shipping, unacceptable for controlled release formulations. High tablet hardness can increase FLT. |
| Assay | 100% w/w of label claim | Assay variability will affect safety and efficacy. Process variables could affect product assay. | |
| Dissolution | Sustained release up to 12 h | Failure to meet dissolution specifications can impact bioavailability. Amount of polymer and effervescence will impact drug release rate. | |
| FLT | As low as possible | Prevent gastric emptying | |
| Floating time | 12 h | Tablet should continuously float in medium. If not, gastric emptying of the tablet is likely to occur, which can be considered failure. | |
| Tablet ejection force | As low as possible | Minimize tablet defects and improve product quality and production output. | |
| Drug Product CQAs | Formulation Variables | Process Variables | |||||
|---|---|---|---|---|---|---|---|
| Type/Viscosity Grade of Polymer | PEO WSR 303 | Sodium Bicarbonate | MCC | Lactose Monohydrate | Compression Force | Dwell Time | |
| Drug release: T25%, T50% and T80% | High | High | High | Low | Low | Low | Low |
| Assay | Low | Low | Low | Low | Low | Low | Low |
| Floating lag time | Medium | Medium | High | Medium | Low | High | Medium |
| Tensile strength | High | High | Low | Medium | Low | High | Medium |
| Gel strength | High | High | Low | Low | Low | Medium | Low |
| Ejection force | High | High | Low | Medium | Medium | High | Low |
| Run | Zero-Order | Higuchi | Korsmeyer-Peppas | ||
|---|---|---|---|---|---|
| R2 | R2 | R2 | n | k | |
| F1 | 0.9355 | 0.9970 | 0.9999 | 0.575 | 0.375 |
| F2 | 0.9413 | 0.9959 | 0.9991 | 0.599 | 0.234 |
| F3 | 0.9229 | 0.9957 | 0.9996 | 0.567 | 0.376 |
| F4 | 0.8871 | 0.9979 | 0.9876 | 0.490 | 0.633 |
| F5 | 0.9401 | 0.9977 | 0.9995 | 0.584 | 0.370 |
| F6 | 0.9469 | 0.9944 | 0.9986 | 0.639 | 0.158 |
| F7 | 0.9408 | 0.9962 | 0.9990 | 0.612 | 0.210 |
| F8 | 0.9222 | 0.9961 | 0.9987 | 0.534 | 0.478 |
| F9 | 0.9238 | 0.9959 | 0.9969 | 0.535 | 0.488 |
| F10 | 0.9450 | 0.9940 | 0.9937 | 0.702 | 0.100 |
| F11 | 0.9415 | 0.9956 | 0.9987 | 0.632 | 0.210 |
| F12 | 0.9132 | 0.9945 | 0.9983 | 0.554 | 0.450 |
| F13 | 0.9167 | 0.9934 | 0.9984 | 0.541 | 0.452 |
| F14 | 0.9307 | 0.9969 | 0.9983 | 0.577 | 0.373 |
| F15 | 0.9475 | 0.9946 | 0.9945 | 0.641 | 0.160 |
| F0 | 0.7702 | 0.9936 | 0.9675 | 0.485 | 0.730 |
| F0* | 0.8333 | 0.9985 | 0.9825 | 0.474 | 0.792 |
| F2* | 0.8174 | 0.9947 | 0.9740 | 0.481 | 0.744 |
| F15* | 0.8787 | 0.9937 | 0.9838 | 0.512 | 0.600 |
| Terms | T25% | T50% | T80% | FLT | Tablet Ejection Force | Tablet Tensile Strength | Tablet Porosity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Y1) | (Y2) | (Y3) | (Y4) | (Y5) | (Y6) | (Y7) | ||||||||
| Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | |
| Constant | 67.667 | <0.0001 | 229.000 | <0.0001 | 527.077 | <0.0001 | 12.230 | 0.04712 | 440.714 | <0.0001 | 332.733 | <0.0001 | 18.633 | <0.0001 |
| X1 | 9.625 | <0.0001 | 32.000 | <0.0001 | 72.375 | <0.0001 | 35.625 | <0.0001 | −91.875 | <0.0001 | 148.750 | <0.0001 | −1.700 | <0.0001 |
| X2 | 13.000 | <0.0001 | 36.750 | <0.0001 | 64.000 | <0.0001 | −37.250 | <0.0001 | 4.0000 | 0.6248 | −24.500 | 0.0001 | 0.1000 | 0.001 |
| X3 | – | – | 0.0000 | 1.0000 | – | – | 34.125 | <0.0001 | 127.1200 | <0.0001 | 141.750 | <0.0001 | −2.650 | <0.0001 |
| X1*X2 | – | – | −9.500 | <0.0001 | – | – | 26.750 | 0.0019 | – | – | −13.750 | 0.0239 | −0.450 | <0.0001 |
| X2*X3 | – | – | – | – | – | – | −30.750 | 0.0010 | – | – | −15.250 | 0.0150 | 0.050 | 0.0408 |
| X1*X3 | – | – | – | – | – | – | 26.000 | 0.0022 | −37.5000 | 0.0140 | 62.250 | <0.0001 | 0.150 | 0.0004 |
| X12 | – | – | – | – | 9.115 | <0.0001 | 23.346 | 0.0045 | – | – | 9.5000 | 0.0010 | −0.141 | <0.0001 |
| X22 | – | – | −8.000 | <0.0001 | −14.134 | 0.0011 | 23.596 | 0.0043 | 48.2856 | 0.0043 | – | – | 0.158 | <0.0001 |
| X32 | – | – | −3.000 | 0.0180 | – | – | – | – | – | – | – | – | 0.408 | <0.0001 |
| R2 | 0.9702 | 0.9984 | 0.9980 | 0.9859 | 0.9747 | 0.9978 | 0.9999 | |||||||
| R2 (Adj) | 0.9653 | 0.9973 | 0.9972 | 0.9670 | 0.96.07 | 0.9962 | 0.9998 | |||||||
| R2 (Pred) | 0.9493 | 0.9910 | 0.9952 | 0.8668 | 0.9135 | 0.9896 | 0.9998 | |||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, P.; Jeong, S.H. Effects of Formulation and Process Variables on Gastroretentive Floating Tablets with A High-Dose Soluble Drug and Experimental Design Approach. Pharmaceutics 2018, 10, 161. https://doi.org/10.3390/pharmaceutics10030161
Thapa P, Jeong SH. Effects of Formulation and Process Variables on Gastroretentive Floating Tablets with A High-Dose Soluble Drug and Experimental Design Approach. Pharmaceutics. 2018; 10(3):161. https://doi.org/10.3390/pharmaceutics10030161
Chicago/Turabian StyleThapa, Prakash, and Seong Hoon Jeong. 2018. "Effects of Formulation and Process Variables on Gastroretentive Floating Tablets with A High-Dose Soluble Drug and Experimental Design Approach" Pharmaceutics 10, no. 3: 161. https://doi.org/10.3390/pharmaceutics10030161
APA StyleThapa, P., & Jeong, S. H. (2018). Effects of Formulation and Process Variables on Gastroretentive Floating Tablets with A High-Dose Soluble Drug and Experimental Design Approach. Pharmaceutics, 10(3), 161. https://doi.org/10.3390/pharmaceutics10030161