Targeted Theranostic Nanoparticles for Brain Tumor Treatment
Abstract
:1. Introduction
2. Glioblastoma
2.1. Barriers and Transport Pathways for the Treatment of Glioblastoma
2.1.1. Blood–Brain Barrier
Adsorptive-Mediated Transport
Carrier-Mediated Transcytosis
Receptor-Mediated Transcytosis
2.1.2. Blood–Brain–Tumor Barrier
2.1.3. Tumor Microenvironment
2.1.4. Glioma Stem Cells
3. Strategies to Enhance the Permeability of the Blood–Brain Barrier for Treatment of Glioblastoma
3.1. Nanoparticles for GB Treatment
3.2. Passive Targeting
3.3. Active Targeting
3.4. Stimuli-Responsive Strategies
4. Theranostics
4.1. Personalized Medicine
4.2. Preclinical Phase and Clinical Trials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Preusser, M.; De Ribaupierre, S.; Wöhrer, A.; Erridge, S.C.; Hegi, M.; Weller, M.; Stupp, R. Current concepts and management of glioblastoma. Ann. Neurol. 2011, 70, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Jendrossek, V.; Belka, C.; Bamberg, M. Novel chemotherapeutic agents for the treatment of glioblastoma multiforme. Expert Opin. Investig. Drugs 2003, 12, 1899–1924. [Google Scholar] [CrossRef] [PubMed]
- Chandana, S.R.; Movva, S.; Arora, M.; Singh, T. Primary brain tumors in adults. Am. Fam. Physician 2008, 77, 1423–1430. [Google Scholar] [PubMed]
- Young, R.M.; Jamshidi, A.; Davis, G.; Sherman, J.H. Current trends in the surgical management and treatment of adult glioblastoma. Ann. Transl. Med. 2015, 3, 121. [Google Scholar] [PubMed]
- Pourgholi, F.; hajivalili, M.; Farhad, J.N.; Kafil, H.S.; Yousefi, M. Nanoparticles: Novel vehicles in treatment of Glioblastoma. Biomed. Pharmacother. 2016, 77, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Jie, X.; Hua, L.; Jiang, W.; Feng, F.; Feng, G.; Hua, Z. Clinical application of a dendritic cell vaccine raised against heat-shocked glioblastoma. Cell Biochem. Biophys. 2012, 62, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Pellegatta, S.; Poliani, P.L.; Corno, D.; Menghi, F.; Ghielmetti, F.; Suarez-Merino, B.; Caldera, V.; Nava, S.; Ravanini, M.; Facchetti, F. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res. 2006, 66, 10247–10252. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, G.; Yuan, X.; Xu, M.; Wang, H.; Ji, J.; Konda, B.; Black, K.L.; Yu, J.S. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells 2009, 27, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Polivka, J.; Holubec, L.; Kubikova, T.; Priban, V.; Hes, O.; Pivovarcikova, K.; Treskova, I. Advances in experimental targeted therapy and immunotherapy for patients with glioblastoma multiforme. Anticancer Res. 2017, 37, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Chekhonin, I.V.; Chekhonin, V.P. The EGFR variant III mutant as a target for immunotherapy of glioblastoma multiforme. Eur. J. Pharmacol. 2017, 810, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, A.; Nandhu, M.S.; Behera, P.; Chiocca, E.A.; Viapiano, M.S. Strategies in gene therapy for glioblastoma. Cancers (Basel) 2013, 5, 1271–1305. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.V.C.; Costanzi-Strauss, E.; Strauss, B.E. A bicistronic adenoviral vector for glioblastoma gene therapy. In Proceedings of the AACR International Conference Held in Cooperation with the Latin American Cooperative Oncology Group (LACOG) on Translational Cancer Medicine, São Paulo, Brazil, 4–6 May 2017. [Google Scholar]
- Yu, D.; Khan, O.F.; Suvà, M.L.; Dong, B.; Panek, W.K.; Xiao, T.; Wu, M.; Han, Y.; Ahmed, A.U.; Balyasnikova, I.V.; et al. Multiplexed RNAi therapy against brain tumor-initiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression. Proc. Natl. Acad. Sci. USA 2017, 114, E6147–E6156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okura, H.; Smith, C.A.; Rutka, J.T. Gene therapy for malignant glioma. Mol. Cell. Ther. 2014, 2, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serlin, Y.; Shelef, I.; Knyazer, B.; Friedman, A. Anatomy and physiology of the blood–brain barrier. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 38, pp. 2–6. [Google Scholar]
- Saunders, N.R.; Habgood, M.D.; Møllgård, K.; Dziegielewska, K.M. The biological significance of brain barrier mechanisms: Help or hindrance in drug delivery to the central nervous system? F1000Reaserch 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Haseloff, R.F.; Dithmer, S.; Winkler, L.; Wolburg, H.; Blasig, I.E. Transmembrane proteins of the tight junctions at the blood–brain barrier: Structural and functional aspects. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 38, pp. 16–25. [Google Scholar]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and dysfunction of the blood-brain barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS delivery via adsorptive transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Thuenauer, R.; Müller, S.K.; Römer, W. Pathways of protein and lipid receptor-mediated transcytosis in drug delivery. Expert Opin. Drug Deliv. 2017, 14, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, X.; Ying, M.; Lu, W. Brain tumor-targeted drug delivery strategies. Acta Pharm. Sin. B 2014, 4, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Parikh, T.; Bommana, M.M.; Squillante, E., III. Efficacy of surface charge in targeting pegylated nanoparticles of sulpiride to the brain. Eur. J. Pharm. Biopharm. 2010, 74, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wan, J.; Zhang, Q.; She, Z.; Jiang, X. Aclarubicin-loaded cationic albumin-conjugated pegylated nanoparticle for glioma chemotherapy in rats. Int. J. Cancer 2007, 120, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Thöle, M.; Nobmann, S.; Huwyler, J.; Bartmann, A.; Fricker, G. Uptake of cationized albumin coupled liposomes by cultured porcine brain microvessel endothelial cells and intact brain capillaries. J. Drug Target. 2002, 10, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Tan, Y.-Z.; Hu, K.-L.; Jiang, X.-G. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood–brain barrier. Int. J. Pharm. 2005, 295, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kamal, A.; Abdinejad, M.; Mahajan, R.K.; Kraatz, H.-B. Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, M.; Rafiee, A.; Chen, D.W.; Foldvari, M. A flow cytometric approach to study the mechanism of gene delivery to cells by gemini-lipid nanoparticles: An implication for cell membrane nanoporation. J. Nanobiotechnol. 2015, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.A.S.; Marques, E.F.; Jurado, A.S.; Pais, A.C.C. The effect of cationic gemini surfactants upon lipid membranes. An experimental and molecular dynamics simulation study. Phys. Chem. Chem. Phys. 2010, 12, 14462–14476. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.Q.; Morais, C.M.; Cardoso, A.M.; Silva, S.G.; Vale, M.L.; Marques, E.F.; de Lima, M.C.P.; Jurado, A.S. Enhancing glioblastoma cell sensitivity to chemotherapeutics: A strategy involving survivin gene silencing mediated by gemini surfactant-based complexes. Eur. J. Pharm. Biopharm. 2016, 104, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.V. Nanomedicine: Principles, properties and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar]
- Forest, V.; Pourchez, J. Preferential binding of positive nanoparticles on cell membranes is due to electrostatic interactions: A too simplistic explanation that does not take into account the nanoparticle protein corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-X.; Zuo, Z.-Q.; Du, J.-Z.; Wang, Y.-C.; Sun, R.; Cao, Z.-T.; Ye, X.-D.; Wang, J.-L.; Leong, K.W.; Wang, J. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nanotoday 2016, 11, 133–144. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Kurtz-Chalot, A.; Villiers, C.; Pourchez, J.; Boudard, D.; Martini, M.; Marche, P.N.; Cottier, M.; Forest, V. Impact of silica nanoparticle surface chemistry on protein corona formation and consequential interactions with biological cells. Mater. Sci. Eng. C 2017, 75, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barar, J.; Rafi, M.A.; Pourseif, M.M.; Omidi, Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts 2016, 6, 225–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, R.A.; O’kane, R.L.; Simpson, I.A.; Vina, J.R. Structure of the blood-brain barrier and its role in the transport of amino acids. J. Nutr. 2006, 136, 218S–226S. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Y.; Kuang, Y.; An, S.; Ma, H.; Jiang, C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials 2013, 34, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Iwao, B.; Yara, M.; Hara, N.; Kawai, Y.; Yamanaka, T.; Nishihara, H.; Inoue, T.; Inazu, M. Functional expression of choline transporter like-protein 1 (CTL1) and CTL2 in human brain microvascular endothelial cells. Neurochem. Int. 2016, 93, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Chang, N.; Sun, S.; Li, M.; Yu, H.; Liu, M.; Liu, X.; Wang, G.; Li, H.; Liu, X. The role of glucose transporters in the distribution of p-aminophenyl-α-d-mannopyranoside modified liposomes within mice brain. J. Control. Release 2014, 182, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wen, H.E.; Lu, W.-L.; Du, J.; Guo, J.; Tian, W.; Men, Y.; Zhang, Y.; Li, R.-J.; Yang, T.-Y. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Control. Release 2010, 141, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escuredo, J.; Van Hée, V.F.; Sboarina, M.; Falces, J.; Payen, V.L.; Pellerin, L.; Sonveaux, P. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2481–2497. [Google Scholar] [CrossRef] [PubMed]
- Nawashiro, H.; Otani, N.; Uozumi, Y.; Ooigawa, H.; Toyooka, T.; Suzuki, T.; Katoh, H.; Tsuzuki, N.; Ohnuki, A.; Shima, K. High expression of l-type amino acid transporter 1 in infiltrating glioma cells. Brain Tumor Pathol. 2005, 22, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Ohnishi, A.; Promsuk, J.; Shimizu, S.; Kanai, Y.; Shiokawa, Y.; Nagane, M. Enhanced tumor growth elicited by L-type amino acid transporter 1 in human malignant glioma cells. Neurosurgery 2008, 62, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Geier, E.G.; Schlessinger, A.; Fan, H.; Gable, J.E.; Irwin, J.J.; Sali, A.; Giacomini, K.M. Structure-based ligand discovery for the Large-neutral Amino Acid Transporter 1, LAT-1. Proc. Natl. Acad. Sci. USA 2013, 110, 5480–5485. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Smith, Q.R. Transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr. 2000, 130, 1016S–1022S. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Koczwara, J.B.; Gallelli, C.A.; Vergara, D.; Di Bonaventura, M.V.M.; Gaetani, S.; Giudetti, A.M. Fats for thoughts: An update on brain fatty acid metabolism. Int. J. Biochem. Cell Biol. 2017, 84, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. Kellen Brunaldi A model for fatty acid transport into the brain. J. Mol. Neurosci. 2007, 33, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.; Stoll, E.A. Metabolic reprogramming in glioma. Front. Cell Dev. Biol. 2017, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Bell, E.H.; Chakravarti, A. Lipid metabolism emerges as a promising target for malignant glioma therapy. CNS Oncol. 2013, 2, 289–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Arcas, M.; Casado, F.J.; Pastor-Anglada, M. Nucleoside transporter proteins. Curr. Vasc. Pharmacol. 2009, 7, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Lui, P.C.W.; Li, J.Y. Receptor-mediated therapeutic transport across the blood-brain barrier. Immunotherapy 2009, 1, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zou, D.; Wang, W.; Yin, Y.; Yin, T.; Hao, S.; Wang, B.; Wang, G.; Wang, Y. Non-invasive approaches for drug delivery to the brain based on the receptor mediated transport. Mater. Sci. Eng. C 2017, 76, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin-and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Jallouli, Y.; Kroubi, M.; Yuan, X.; Feng, W.; Kang, C.; Pu, P.; Betbeder, D. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood-brain barrier. Int. J. Pharm. 2009, 379, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, Y.; Tosi, G.; Ruozi, B.; Belletti, D.; Vilella, A.; Zoli, M.; Vandelli, M.A.; Forni, F.; López, B.L.; Sierra, L. PEG-g-chitosan nanoparticles functionalized with the monoclonal antibody OX26 for brain drug targeting. Nanomedicine 2015, 10, 1735–1750. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, Y.; Yemisci, M.; Andrieux, K.; Gürsoy, R.N.; Alonso, M.J.; Fernandez-Megia, E.; Novoa-Carballal, R.; Quiñoá, E.; Riguera, R.; Sargon, M.F. Development and brain delivery of chitosan-PEG nanoparticles functionalized with the monoclonal antibody OX26. Bioconjug. Chem. 2005, 16, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, H.; Shu, L.; Zhang, Y.; Okeke, C.; Zhang, L.; Li, J.; Li, N. Preparation and evaluation of Baicalin-loaded cationic solid lipid nanoparticles conjugated with OX26 for improved delivery across the BBB. Drug Dev. Ind. Pharm. 2015, 41, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Nikanjam, M.; Blakely, E.A.; Bjornstad, K.A.; Shu, X.; Budinger, T.F.; Forte, T.M. Synthetic nano-low density lipoprotein as targeted drug delivery vehicle for glioblastoma multiforme. Int. J. Pharm. 2007, 328, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Li, Y.; Lee, J.; Schwartz, A.L.; Bu, G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 2009, 69, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Tao, J.; Hua, H.; Sun, P.; Zhao, Y. Low-density lipoprotein peptide-combined DNA nanocomplex as an efficient anticancer drug delivery vehicle. Eur. J. Pharm. Biopharm. 2015, 94, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.A.; Fares, K. Alanazi Targeting cancer using cholesterol conjugates. Saudi Pharm. J. 2014, 22, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Lima, S.A.C.; Reis, S. Apo E-Functionalization of Solid Lipid Nanoparticles Enhances Brain Drug Delivery: Uptake Mechanism and Transport Pathways. Bioconjug. Chem. 2017, 28, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Rajora, M.; Ding, L.; Valic, M.; Jiang, W.; Overchuk, M.; Chen, J.; Zheng, G. Tailored theranostic apolipoprotein E3 porphyrin-lipid nanoparticles target glioblastoma tumours. Chem. Sci. 2017, 8, 5371–5384. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, M.; Khan, R.A.; Maghrabi, I.A.; Ahmed, B. Polysorbate-80-coated, polymeric curcumin nanoparticles for in vivo anti-depressant activity across BBB and envisaged biomolecular mechanism of action through a proposed pharmacophore model. J. Microencapsul. 2016, 33, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Huang, R.; Li, J.; Han, L.; Ye, L.; Lou, J.; Jiang, C. Angiopep-2 modified PE-PEG based polymeric micelles for amphotericin B delivery targeted to the brain. J. Control. Release 2010, 147, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Yin, G.; Pu, X.; Chen, X.; Liao, X.; Huang, Z. Glioma targeted delivery strategy of doxorubicin-loaded liposomes by dual-ligand modification. J. Biomater. Sci. Polym. Ed. 2017, 28, 1695–1712. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Shin, D.H.; Kim, J.-S. Dual-targeting immunoliposomes using angiopep-2 and Cd133 antibody for glioblastoma stem cells. J. Control. Release 2018, 269, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, S.; Tu, T.; Qi, X.; Xiong, Y.; Du, S.; Shen, Y.; Tu, J.; Sun, C. Paclitaxel-loaded cholesterol-conjugated polyoxyethylene sorbitol oleate polymeric micelles for glioblastoma therapy across the blood-brain barrier. Polym. Chem. 2015, 6, 2740–2751. [Google Scholar] [CrossRef]
- Li, C.; Sun, C.; Li, S.; Han, P.; Sun, H.; Ouahab, A.; Shen, Y.; Xu, Y.; Xiong, Y.; Tu, J. Novel designed polyoxyethylene nonionic surfactant with improved safety and efficiency for anticancer drug delivery. Int. J. Nanomed. 2014, 9, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardridge, W.M.; Kang, Y.-S.; Buciak, J.L.; Yang, J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood–brain barrier in vivo in the primate. Pharm. Res. 1995, 12, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the insulin receptor: Nanoparticles for drug delivery across the blood–brain barrier (BBB). J. Drug Target. 2011, 19, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Li, B.; Hu, L.; Wei, X.; Feng, L.; Fu, W.; Lu, W. Micelle-based brain-targeted drug delivery enabled by a nicotine acetylcholine receptor ligand. Angew. Chem. Int. Ed. 2011, 50, 5482–5485. [Google Scholar] [CrossRef] [PubMed]
- Rama, A.R.; Alvarez, P.J.; Madeddu, R.; Aranega, A. ABC transporters as differentiation markers in glioblastoma cells. Mol. Biol. Rep. 2014, 41, 4847–4851. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S. Regulation of ABC Transporters Blood-Brain Barrier. The Good, the Bad, and the Ugly, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 125. [Google Scholar]
- Dean, M. ABC transporters, drug resistance, and cancer stem cells. J. Mammary Gland Biol. Neoplasia 2009, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ganugula, R.; Arora, M.; Guada, M.; Saini, P.; Kumar, M.N.V.R. Noncompetitive Active Transport Exploiting Intestinal Transferrin Receptors for Oral Delivery of Proteins by Tunable Nanoplatform. ACS Macro Lett. 2017, 6, 161–164. [Google Scholar] [CrossRef]
- Ussar, S.; Haering, M.-F.; Fujisaka, S.; Lutter, D.; Lee, K.Y.; Li, N.; Gerber, G.K.; Bry, L.; Kahn, C.R. Regulation of glucose uptake and enteroendocrine function by the intestinal epithelial insulin receptor. Diabetes 2017. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.D.; Ding, Q.; Chorazyczewski, J.; Gros, R. GPER Regulation of LDL Receptor Expression in Human Hepatocytes. Atheroscler. Suppl. 2018, 32, 58. [Google Scholar] [CrossRef]
- Suhorutsenko, J.; Oskolkov, N.; Arukuusk, P.; Kurrikoff, K.; Eriste, E.; Copolovici, D.-M.; Langel, U. Cell-penetrating peptides, PepFects, show no evidence of toxicity and immunogenicity in vitro and in vivo. Bioconjug. Chem. 2011, 22, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Yum, S.Y.; Jang, G.; Ahn, D.-R. Discovery of a non-cationic cell penetrating peptide derived from membrane-interacting human proteins and its potential as a protein delivery carrier. Sci. Rep. 2015, 5, 11719. [Google Scholar] [Green Version]
- Liu, R.; Li, X.; Xiao, W.; Lam, K.S. Tumor-targeting peptides from combinatorial libraries. Adv. Drug Deliv. Rev. 2017, 110, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, S.; Denny, W.A.; Gamage, S.; Sarojini, V. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J. Control. Release 2017, 250, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Milletti, F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, R. pH-Responsive, Lysine-based, hyperbranched polymers mimicking endosomolytic cell-penetrating peptides for efficient intracellular delivery. Chem. Mater. 2017. [Google Scholar] [CrossRef]
- Hu, X.; Tan, J.J.; Ye, S. Reversible Activation of pH-Responsive Cell-Penetrating Peptides in Model Cell Membrane Relies on the Nature of Lipid. J. Phys. Chem. C 2017, 121, 15181–15187. [Google Scholar] [CrossRef]
- Yoo, J.; Rejinold, N.S.; Lee, D.; Jon, S.; Kim, Y.-C. Protease-activatable cell-penetrating peptide possessing ROS-triggered phase transition for enhanced cancer therapy. J. Control. Release 2017, 264, 89–101. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sun, L.; Ye, J.; Liu, E.; Chen, S.; Liang, Q.; Shin, M.C.; Yang, V.C. Enzyme-triggered, cell penetrating peptide-mediated delivery of anti-tumor agents. J. Control. Release 2016, 240, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Noh, I.; Yoo, J.; Rejinold, N.S.; Kim, Y.-C. pH-controllable cell-penetrating polypeptide that exhibits cancer targeting. Acta Biomater. 2017, 57, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; Xie, X.; Cai, X.; Mei, X. Preparation and characterization of photo-responsive cell-penetrating peptide-mediated nanostructured lipid carrier. J. Drug Target. 2014, 22, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mei, L.; Yu, Q.; Xu, C.; Qiu, Y.; Yang, Y.; Shi, K.; Zhang, Q.; Gao, H.; Zhang, Z. Multifunctional tandem peptide modified paclitaxel-loaded liposomes for the treatment of vasculogenic mimicry and cancer stem cells in malignant glioma. ACS Appl. Mater. Interfaces 2015, 7, 16792–16801. [Google Scholar] [CrossRef] [PubMed]
- Higa, M.; Katagiri, C.; Shimizu-Okabe, C.; Tsumuraya, T.; Sunagawa, M.; Nakamura, M.; Ishiuchi, S.; Takayama, C.; Kondo, E.; Matsushita, M. Identification of a novel cell-penetrating peptide targeting human glioblastoma cell lines as a cancer-homing transporter. Biochem. Biophys. Res. Commun. 2015, 457, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Opačak-Bernardi, T.; Ryu, J.S.; Raucher, D. Effects of cell penetrating Notch inhibitory peptide conjugated to elastin-like polypeptide on glioblastoma cells. J. Drug Target. 2017, 25, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Long, Y.; Xu, C.; Wang, Y.; Qiu, Y.; Yu, Q.; Liu, Y.; Zhang, Q.; Gao, H.; Zhang, Z. Liposomes combined an integrin αvβ3-specific vector with pH-responsible cell-penetrating property for highly effective antiglioma therapy through the blood–brain barrier. ACS Appl. Mater. Interfaces 2015, 7, 21442–21454. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, M.; Yu, J.; Wang, X.; Zhang, Q.; Yang, X.; Li, J.; Zhao, C.; Feng, B. Glioma targeting peptide in combination with the P53 C terminus inhibits glioma cell proliferation in vitro. Cytotechnology 2017, 70, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Eriste, E.; Kurrikoff, K.; Suhorutšenko, J.; Oskolkov, N.; Copolovici, D.M.; Jones, S.; Laakkonen, P.; Howl, J.; Langel, U. Peptide-based glioma-targeted drug delivery vector gHoPe2. Bioconjug. Chem. 2013, 24, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Z.; Mei, L.; Yu, Q.; Tai, X.; Wang, Y.; Shi, K.; Zhang, Z.; He, Q. Tandem peptide based on structural modification of poly-arginine for enhancing tumor targeting efficiency and therapeutic effect. ACS Appl. Mater. Interfaces 2017, 9, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, H.; Yuan, W.; Kuai, R.; Zhang, Q.; Xie, F.; Zhang, L.; Zhang, Z.; Liu, J.; He, Q. Liposome formulated with TAT-modified cholesterol for enhancing the brain delivery. Int. J. Pharm. 2011, 419, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Modgil, A.; Layek, B.; Arora, K.; Sun, C.; Law, B.; Singh, J. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: Biodistribution and transfection. J. Control. Release 2013, 167, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Cooke, J.R.N.; Ellis, J.A.; Emala, C.W.; Bruce, J.N. Targeting brain tumors by intra-arterial delivery of cell-penetrating peptides: A novel approach for primary and metastatic brain malignancy. J. Neurooncol. 2017, 135, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.; Mei, L.; Gao, H.; Cai, W.; Zhu, P.; Shi, K.; Chen, J.; Wang, Y.; Gao, F.; He, Q. Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals. Mol. Pharm. 2014, 11, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mei, L.; Yu, Q.; Zhang, Q.; Gao, H.; Zhang, Z.; He, Q. Integrin αvβ3 targeting activity study of different retro-inverso sequences of RGD and their potentiality in the designing of tumor targeting peptides. Amino Acids 2015, 47, 2533–2539. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Pang, Z.; Yang, X.; Jiang, X. Glioma-homing peptide with a cell-penetrating effect for targeting delivery with enhanced glioma localization, penetration and suppression of glioma growth. J. Control. Release 2013, 172, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, K.; Wang, Y.; Wang, S.; Li, J.; Lou, J.; Ye, L.; Yan, X.; Lu, W.; Huang, R. Enhanced blood-brain barrier penetration and glioma therapy mediated by a new peptide modified gene delivery system. Biomaterials 2015, 37, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Niwa, M.; Yang, H.; Yoshida, T. Nucleolar localization signals of LIM kinase 2 function as a cell-penetrating peptide. Protein Pept. Lett. 2010, 17, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Shao, K.; Huang, R.; Ye, L.; Lou, J.; Jiang, C. A leptin derived 30-amino-acid peptide modified pegylated poly-l-lysine dendrigraft for brain targeted gene delivery. Biomaterials 2010, 31, 5246–5257. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mann, A.P.; Mölder, T.; Kotamraju, V.R.; Mattrey, R.; Teesalu, T.; Ruoslahti, E. Vascular changes in tumors resistant to a vascular disrupting nanoparticle treatment. J. Control. Release 2017, 268, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Barchanski, A.; Taylor, U.; Klein, S.; Rath, D.; Barcikowski, S. Penetratin-Conjugated Gold Nanoparticles-Design of Cell-Penetrating Nanomarkers by Femtosecond Laser Ablation. J. Phys. Chem. C 2010, 115, 5152–5159. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Xie, X.; Cai, X.; Zhang, H.; Gong, W.; Wang, Z.; Mei, X. PEGylated liposomes with NGR ligand and heat-activable cell-penetrating peptide–doxorubicin conjugate for tumor-specific therapy. Biomaterials 2014, 35, 4368–4381. [Google Scholar] [CrossRef] [PubMed]
- Balzeau, J.; Pinier, M.; Berges, R.; Saulnier, P.; Benoit, J.-P.; Eyer, J. The effect of functionalizing lipid nanocapsules with NFL-TBS. 40-63 peptide on their uptake by glioblastoma cells. Biomaterials 2013, 34, 3381–3389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, L.; Perkins, E.; Kratz, F.; Raucher, D. Cell penetrating peptides fused to a thermally targeted biopolymer drug carrier improve the delivery and antitumor efficacy of an acid-sensitive doxorubicin derivative. Int. J. Pharm. 2012, 436, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunkin, C.M.; Pokorny, A.; Almeida, P.F.; Lee, H.-S. Molecular dynamics studies of transportan 10 (Tp10) interacting with a POPC lipid bilayer. J. Phys. Chem. B 2010, 115, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Aroui, S.; Dardevet, L.; Ajmia, W.; de Boisvilliers, M.; Perrin, F.; Laajimi, A.; Boumendjel, A.; Kenani, A.; Muller, J.M.; De Waard, M. A Novel Platinum–Maurocalcine Conjugate Induces Apoptosis of Human Glioblastoma Cells by Acting through the ROS-ERK/AKT-p53 Pathway. Mol. Pharm. 2015, 12, 4336–4348. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Kar, R.K.; Mondal, S.; Pahan, K.; Bhunia, A. Structural Elucidation of the Cell-Penetrating Penetratin Peptide in Model Membranes at the Atomic Level: Probing Hydrophobic Interactions in the Blood–Brain Barrier. Biochemistry 2016, 55, 4982–4996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, Y.; Tai, L.; Jiang, K.; Xie, C.; Li, Z.; Lin, Y.-Z.; Wei, G.; Lu, W.; Pan, W. Functionalized cell nucleus-penetrating peptide combined with doxorubicin for synergistic treatment of glioma. Acta Biomater. 2016, 42, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, S.; Cao, S.; Yang, Z.; Pang, Z.; Jiang, X. Angiopep-2 and activatable cell-penetrating peptide dual-functionalized nanoparticles for systemic glioma-targeting delivery. Mol. Pharm. 2014, 11, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Zha, Z.; Mukerabigwi, J.F.; Chen, W.; Wang, Y.; He, C.; Ge, Z. Matrix metalloproteinase-responsive multifunctional peptide-linked amphiphilic block copolymers for intelligent systemic anticancer drug delivery. Bioconjug. Chem. 2017, 28, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.J.; von Maltzahn, G.; Lord, M.E.; Park, J.; Agrawal, A.; Min, D.; Sailor, M.J.; Bhatia, S.N. Protease-Triggered Unveiling of Bioactive Nanoparticles. Small 2008, 4, 1307–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.-X.; Zhao, Y.; Huang, Y.; Luo, L.-M.; Song, P.; Wang, X.; Chen, S.; Yu, K.-F.; Zhang, X.; Zhang, Q. The efficiency of tumor-specific pH-responsive peptide-modified polymeric micelles containing paclitaxel. Biomaterials 2012, 33, 2508–2520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.-Q.; Zhang, X. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ma, Y.; Zhang, W.; Li, L.; Zhang, Y.; Zhang, L.; Liu, H.; Ni, J.; Wang, R. Design of new acid-activated cell-penetrating peptides for tumor drug delivery. PeerJ 2017, 5, e3429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, K.; Zhang, L.; Hu, G.; Wan, J.; Tang, J.; Yin, S.; Duan, J.; Qin, M.; Wang, N. Significantly enhanced tumor cellular and lysosomal hydroxychloroquine delivery by smart liposomes for optimal autophagy inhibition and improved antitumor efficiency with liposomal doxorubicin. Autophagy 2016, 12, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, Z.; Zhang, Y.; Lv, H.; Zhou, J.; Li, C.; Hou, L.; Zhang, Q. Dual-functional liposomes based on pH-responsive cell-penetrating peptide and hyaluronic acid for tumor-targeted anticancer drug delivery. Biomaterials 2012, 33, 9246–9258. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Preusser, M. Role of the Blood–Brain Barrier in Metastatic Disease of the Central Nervous System, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 149. [Google Scholar]
- Karim, R.; Palazzo, C.; Evrard, B.; Piel, G. Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art. J. Control. Release 2016, 227, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Angara, K.; Rashid, M.H.; Shankar, A.; Ara, R.; Iskander, A.; Borin, T.F.; Jain, M.; Achyut, B.R.; Arbab, A.S. Vascular mimicry in glioblastoma following anti-angiogenic and anti-20-HETE therapies. Histol. Histopathol. 2017, 32, 917–928. [Google Scholar] [PubMed]
- Arbab, A.S.; Rashid, M.H.; Angara, K.; Borin, T.F.; Lin, P.-C.; Jain, M.; Achyut, B.R. Major challenges and potential microenvironment-targeted therapies in glioblastoma. Int. J. Mol. Sci. 2017, 18, 2732. [Google Scholar] [CrossRef] [PubMed]
- Alifieris, C.; Dimitrios, T. Trafalis. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Kanapathipillai, M.; Brock, A.; Ingber, D.E. Nanoparticle targeting of anti-cancer drugs that alter intracellular signaling or influence the tumor microenvironment. Adv. Drug Deliv. Rev. 2014, 79, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Winquist, R.J.; Boucher, D.M.; Wood, M.; Furey, B.F. Targeting cancer stem cells for more effective therapies: Taking out cancer’s locomotive engine. Biochem. Pharmacol. 2009, 78, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Finger, E.C.; Giaccia, A.J. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010, 29, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, L.A.; Anelli, V.; Guarnaccia, L.; Navone, S.; Beretta, M.; Moccia, F.; Tringali, C.; Urechie, V.; Campanella, R.; Marfia, G. A bidirectional crosstalk between glioblastoma and brain endothelial cells potentiates the angiogenic and proliferative signaling of sphingosine-1-phosphate in the glioblastoma microenvironment. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Q.; Feng, L.; Liu, Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today 2018, 21, 55–73. [Google Scholar] [CrossRef]
- Shimizu, T.; Kurozumi, K.; Ishida, J.; Ichikawa, T.; Date, I. Adhesion molecules and the extracellular matrix as drug targets for glioma. Brain Tumor Pathol. 2016, 33, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Faissner, A.; Roll, L.; Theocharidis, U. Tenascin-C in the matrisome of neural stem and progenitor cells. Mol. Cell. Neurosci. 2017, 81, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Malric, L.; Monferran, S.; Gilhodes, J.; Boyrie, S.; Dahan, P.; Skuli, N.; Sesen, J.; Filleron, T.; Kowalski-Chauvel, A.; Moyal, E.C.-J. Interest of integrins targeting in glioblastoma according to tumor heterogeneity and cancer stem cell paradigm: An update. Oncotarget 2017, 8, 86947–86968. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Duan, Z.; Yuan, Y.; Li, R.; Pang, L.; Liang, J.; Xu, X.; Wang, J. Peptide-22 and cyclic RGD functionalized liposomes for glioma targeting drug delivery overcoming BBB and BBTB. ACS Appl. Mater. Interfaces 2017, 9, 5864–5873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Hua, H.; Wang, A.; Liu, W.; Li, Y.; Fu, F.; Shi, Y.; Sun, K. Cyclic hexapeptide-conjugated nanoparticles enhance curcumin delivery to glioma tumor cells and tissue. Int. J. Nanomed. 2017, 12, 5717–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Jiang, Y.; Zou, Y.; Meng, F.; Zhang, J.; Deng, C.; Sun, H.; Zhong, Z. Targeted glioma chemotherapy by cyclic RGD peptide-functionalized reversibly core-crosslinked multifunctional poly(ethylene glycol)-b-poly(ε-caprolactone) micelles. Acta Biomater. 2017, 50, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Mao, G.; Du, J.; Zhu, X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016, 23, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xiong, Y.; Zhang, S.; Yang, Z.; Cao, S.; Jiang, X. RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol. Pharm. 2014, 11, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, M.; Serra, M.; Schinelli, S. Integrins in glioblastoma: Still an attractive target? Pharmacol. Res. 2016, 113, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Friedmann-Morvinski, D.; Narasimamurthy, R.; Xia, Y.; Myskiw, C.; Soda, Y.; Verma, I.M. Targeting NF-κB in glioblastoma: A therapeutic approach. Sci. Adv. 2016, 2, e1501292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannappan, V.; Wang, Z.; Liu, P.; Brown, S.; Butcher, M.K.; Azar, M.K.; Bian, X.; Armesilla, A.; Darling, J.; Wang, W. PP54. Nano-encapsulated disulfiram targets glioblastoma stem cells in vitro and in vivo by modulation of hypoxia-nf-kb pathway. Neuro. Oncol. 2017, 19, i15. [Google Scholar] [CrossRef]
- Park, H.-Y.; Lee, K.-J.; Lee, S.-J.; Yoon, M.-Y. Screening of peptides bound to breast cancer stem cell specific surface marker CD44 by phage display. Mol. Biotechnol. 2012, 51, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.-M.; Lee, K.E.; Jin, B.E.; Aujla, P.S.; Gholamin, S.; Li, G. Immune evasion strategies of glioblastoma. Front. Surg. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining tumor niches. Trends Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Morgante, L.; Coccè, V.; Bonomi, A.; Buccarelli, M.; Pascucci, L.; Alessandri, G.; Pessina, A. Human mesenchymal stromal cells inhibit tumor growth in orthotopic glioblastoma xenografts. Stem Cell Res. Ther. 2017, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Perez, R.M.; Voytik-Harbin, S.L.; Sarkaria, J.N.; Pollok, K.E.; Fishel, M.L.; Rickus, J.L. Presence of stromal cells in a bioengineered tumor microenvironment alters glioblastoma migration and response to STAT3 inhibition. PLoS ONE 2018, 13, e0194183. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Ngambenjawong, C.; Heather, H.G.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.H.; Cannarile, M.A.; Hoves, S.; Benz, J.; Wartha, K.; Runza, V.; Rey-Giraud, F.; Pradel, L.P.; Feuerhake, F.; Klaman, I. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014, 25, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.E.; Kirkpatrick, N.D.; Huang, Y.; Farrar, C.T.; Marijt, K.A.; Kloepper, J.; Datta, M.; Amoozgar, Z.; Seano, G.; Jung, K. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl. Acad. Sci. USA 2016, 113, 4470–4475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.; Pawar, V.K.; Meher, J.G.; Raval, K.; Kumar, A.; Shrivastava, R.; Bhadauria, S.; Chourasia, M.K. Targeting tumor associated macrophages (TAMs) via nanocarriers. J. Control. Release 2017, 254, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, L.; Ajmone-Cat, M.A.; Cecchetti, S.; Ricci, A.; Bozzuto, G.; Molinari, A.; Manni, I.; Pollo, B.; Scala, S.; Carpinelli, G. Targeting CXCR4 by a selective peptide antagonist modulates tumor microenvironment and microglia reactivity in a human glioblastoma model. J. Exp. Clin. Cancer Res. 2016, 35, 55. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Zheng, Y.-R.; Gadde, S.; Pfirschke, C.; Zope, H.; Engblom, C.; Kohler, R.H.; Iwamoto, Y.; Yang, K.S.; Askevold, B. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt (IV) pro-drug. Nat. Commun. 2015, 6, 8692. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.L. Exploiting the cancer niche: Tumor-associated macrophages and hypoxia as promising synergistic targets for nano-based therapy. J. Control. Release 2017, 253, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Goswami, K.K.; Ghosh, T.; Ghosh, S.; Sarkar, M.; Bose, A.; Baral, R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell. Immunol. 2017, 316, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.; Warren, G.; Wei, X. Macrophages associated with tumors as potential targets and therapeutic intermediates. Nanomedicine 2014, 9, 695–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, R.L.; Joyce, J.A. Therapeutic targeting of tumor-associated macrophages and microglia in glioblastoma. Immunotherapy 2014, 6, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Feng, X.; Herting, C.J.; Garcia, V.A.; Nie, K.; Pong, W.W.; Rasmussen, R.; Dwivedi, B.; Seby, S.; Wolf, S.A. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Donthireddy, L.; Marvel, D.; Condamine, T.; Wang, F.; Lavilla-Alonso, S.; Hashimoto, A.; Vonteddu, P.; Behera, R.; Goins, M.A. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 2017, 32, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Talks, K.L.; Turley, H.; Gatter, K.C.; Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J.; Harris, A.L. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 2000, 157, 411–421. [Google Scholar] [CrossRef]
- Vaupel, P.; Kelleher, D.K.; Höckel, M. Oxygenation status of malignant tumors: Pathogenesis of hypoxia and significance for tumor therapy. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 28, pp. 29–35. [Google Scholar]
- Vaupel, P.; Mayer, A.; Hockel, M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004, 23, 335–354. [Google Scholar]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Tafani, M.; Di Vito, M.; Frati, A.; Pellegrini, L.; De Santis, E.; Sette, G.; Eramo, A.; Sale, P.; Mari, E.; Santoro, A. Pro-inflammatory gene expression in solid glioblastoma microenvironment and in hypoxic stem cells from human glioblastoma. J. Neuroinflamm. 2011, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Wigerup, C.; Sven, P.; Bexell, D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol. Ther. 2016, 164, 152–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, L.J.; Niemans, R.; van Kuijk, S.J.A.; Panth, K.M.; Parvathaneni, N.-K.; Peeters, S.G.J.A.; Zegers, C.M.L.; Rekers, N.H.; van Gisbergen, M.W.; Biemans, R. New ways to image and target tumour hypoxia and its molecular responses. Radiother. Oncol. 2015, 116, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Cheng, Z.; Liu, J.; Torre-Healy, L.; Lathia, J.D.; Nakano, I.; Guo, Y.; Thompson, R.C.; Freeman, M.L.; Wang, J. Inhibition of Farnesyltransferase Potentiates NOTCH-Targeted Therapy against Glioblastoma Stem Cells. Stem Cell Rep. 2017, 9, 1948–1960. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, H.; Xu, H.; Xiong, H.; Chu, Q.; Yu, S.; Wu, G.S.; Wu, K. Notch signaling: An emerging therapeutic target for cancer treatment. Cancer Lett. 2015, 369, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Canalis, E. Notch signaling and the skeleton. Endocr. Rev. 2016, 37, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Natsumeda, M.; Maitani, K.; Liu, Y.; Miyahara, H.; Kaur, H.; Chu, Q.; Zhang, H.; Kahlert, U.D.; Eberhart, C.G. Targeting notch signaling and autophagy increases cytotoxicity in glioblastoma neurospheres. Brain Pathol. 2016, 26, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.K.; McFarland, B.C.; Nozell, S.E.; Benveniste, E.N. NF-κB and STAT3 in glioblastoma: Therapeutic targets coming of age. Expert Rev. Neurother. 2014, 14, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Xu, X.-Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: From basic research to clinical application. Am. J. Cancer Res. 2015, 5, 1602. [Google Scholar] [PubMed]
- Liebelt, B.D.; Shingu, T.; Zhou, X.; Ren, J.; Shin, S.A.; Hu, J. Glioma stem cells: Signaling, microenvironment, and therapy. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kesari, S.; Rooney, C.; Strack, P.R.; Chen, J.; Shen, H.; Wu, L.; Griffin, J.D. Inhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer 2010, 1, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Kim, A.-R.; Kim, S.-H.; Lee, S.-J.; Chung, H.; Yoon, M.-Y. Development of a novel imaging agent using peptide-coated gold nanoparticles toward brain glioma stem cell marker CD133. Acta Biomater. 2017, 47, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.P.; Arce, M.; Yameen, B.; Vilos, C. Targeted brain delivery nanoparticles for malignant gliomas. Nanomedicine 2017, 12, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Sakurai, Y.; Kajimoto, K.; Nakamura, T.; Yamada, Y.; Akita, H.; Harashima, H. Innovative Technologies in Nanomedicines: From Passive Targeting to Active Targeting/From Controlled Pharmacokinetics to Controlled Intracellular Pharmacokinetics. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Wadajkar, A.S.; Dancy, J.G.; Hersh, D.S.; Anastasiadis, P.; Tran, N.L.; Woodworth, G.F.; Winkles, J.A.; Kim, A.J. Tumor-targeted nanotherapeutics: Overcoming treatment barriers for glioblastoma. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef]
- Lee, G.Y.; Qian, W.P.; Wang, L.; Wang, Y.A.; Staley, C.A.; Satpathy, M.; Nie, S.; Mao, H.; Yang, L. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano 2013, 7, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, S.Y.; Green, J.J. Therapeutic nanomedicine for brain cancer. Ther. Deliv. 2013, 4, 687–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Lee, J.-Y.; Kim, H.-S. Selective fluorescence sensing of 3, 5-dinitrosalicylic acid based on pyrenesulfonamide-functionalized inorganic/organic hybrid nanoparticles. J. Ind. Eng. Chem. 2016, 44, 82–89. [Google Scholar] [CrossRef]
- Di Martino, A.; Guselnikova, O.A.; Trusova, M.E.; Postnikov, P.S.; Sedlarik, V. Organic-inorganic hybrid nanoparticles controlled delivery system for anticancer drugs. Int. J. Pharm. 2017, 526, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-Q.; Lv, Q.; Li, L.-M.; Tang, X.-J.; Li, F.-Z.; Hu, Y.-L.; Han, M. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials 2013, 34, 5628–5639. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Madhankumar, A.B.; Miller, P.A.; Duck, K.A.; Hafenstein, S.; Rizk, E.; Slagle-Webb, B.; Sheehan, J.M.; Connor, J.R.; Yang, Q.X. MRI contrast agent for targeting glioma: Interleukin-13 labeled liposome encapsulating gadolinium-DTPA. Neuro Oncol. 2015, 18, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, Z.; Zhan, C.; Ying, M.; Wei, X.; Xie, C.; Yan, Z.; Lu, W. Multifunctional targeted liposomal drug delivery for efficient glioblastoma treatment. Oncotarget 2017, 8, 66889–66900. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Hu, X.; Wei, X.; Zhan, C.; Lu, L.; Jiang, K.; Su, B.; Ruan, H.; Ran, D.; Fang, R.H. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J. Control. Release 2017, 264, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Messaoudi, K.; Lemaire, L.; Benoit, J.-P.; Lagarce, F. Combined anti-Galectin-1 and anti-EGFR siRNA-loaded chitosan-lipid nanocapsules decrease temozolomide resistance in glioblastoma: In vivo evaluation. Int. J. Pharm. 2015, 481, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Lai, X.; Song, S.; Zhu, X.; Zhu, J. Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy. Drug Deliv. 2016, 23, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Fan, Y.; Lv, S.; Xiao, B.; Ye, M.; Zhu, X. Vincristine and temozolomide combined chemotherapy for the treatment of glioma: A comparison of solid lipid nanoparticles and nanostructured lipid carriers for dual drugs delivery. Drug Deliv. 2016, 23, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, L.; Chen, Z.; Mao, G.; Gao, Z.; Qu, J.; Zhang, L.; Chen, Z.; Mao, G.; Gao, Z.; et al. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: Which kind of drug delivery system is better for glioblastoma chemotherapy? Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles. Drug Deliv. 2016, 23, 3048–3416. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Cheng, S.-J. Brain targeted delivery of carmustine using solid lipid nanoparticles modified with tamoxifen and lactoferrin for antitumor proliferation. Int. J. Pharm. 2016, 499, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Lee, C.-H. Inhibition Against Growth of Glioblastoma Multiforme In Vitro Using Etoposide-Loaded Solid Lipid Nanoparticles with p-Aminophenyl-α-d-Manno-Pyranoside and Folic Acid. J. Pharm. Sci. 2015, 104, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Graverini, G.; Piazzini, V.; Landucci, E.; Pantano, D.; Nardiello, P.; Casamenti, F.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Solid lipid nanoparticles for delivery of andrographolide across the blood-brain barrier: In vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2018, 161, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Won, N.; Bang, J.; Jin, H.; Park, J.; Jung, S.; Jung, S.; Park, Y.; Kim, S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.; Xu, Y.; Lovas, K.; Qin, Y.; Bao, Y. Surface functionalization of dopamine coated iron oxide nanoparticles for various surface functionalities. J. Magn. Magn. Mater. 2017, 427, 220–224. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, S.H.; Seong, H. Multifunctional ultrasmall superparamagnetic iron oxide nanoparticles as a theranostic agent. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 892–902. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Baalousha, M.; Manciulea, A.; Cumberland, S.; Kendall, K.; Lead, J.R. Aggregation and surface properties of iron oxide nanoparticles: Influence of pH and natural organic matter. Environ. Toxicol. Chem. 2008, 27, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Gulati, N.; Nagaich, U.; Pal, A. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.-W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4, 2041731413503357. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tan, Y.; Xu, K.; Zhang, L.; Qiu, B.; Guo, L.; Lin, Z.; Chen, G. Stimulus-response mesoporous silica nanoparticle-based chemiluminescence biosensor for cocaine determination. Biosens. Bioelectron. 2016, 75, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-J.; Liu, L.-H.; Li, Z.-Y.; Zhuo, R.-X.; Zhang, X.-Z. MMP-responsive theranostic nanoplatform based on mesoporous silica nanoparticles for tumor imaging and targeted drug delivery. J. Mater. Chem. B 2016, 4, 1932–1940. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, T.; Kim, J.; Hyeon, T. Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, S.; Benito-Peña, E.; Navarro-Villoslada, F.; Langer, J.; Sanz-Ortiz, M.N.; Reguera, J.; Liz-Marzán, L.M.; Moreno-Bondi, M.C. Multibranched gold–mesoporous silica nanoparticles coated with a molecularly imprinted polymer for label-free antibiotic surface-enhanced raman scattering analysis. Chem. Mater. 2016, 28, 7947–7954. [Google Scholar] [CrossRef]
- Dhamecha, D.; Jalalpure, S.; Jadhav, K.; Jagwani, S.; Chavan, R. Doxorubicin loaded gold nanoparticles: Implication of passive targeting on anticancer efficacy. Pharmacol. Res. 2016, 113, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Amoli-Diva, M.; Sadighi-Bonabi, R.; Pourghazi, K. Switchable on/off drug release from gold nanoparticles-grafted dual light-and temperature-responsive hydrogel for controlled drug delivery. Mater. Sci. Eng. C 2017, 76, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Lim, I.G.; Oh, J. Paclitaxel-loaded chitosan oligosaccharide-stabilized gold nanoparticles as novel agents for drug delivery and photoacoustic imaging of cancer cells. Int. J. Pharm. 2016, 511, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, T.; Pandey, A.; Talpur, F.N.; Kaushik, A.; Niazi, J.H. Gold nanoparticles based sensor for in vitro analysis of drug-drug interactions using imipramine and isoniazid drugs: A proof of concept approach. Sens. Actuators B Chem. 2017, 252, 1055–1062. [Google Scholar] [CrossRef]
- Bishop, C.J.; Stephany, Y.; Green, J.J. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater. 2015, 11, 393–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perche, F.; Yi, Y.; Hespel, L.; Mi, P.; Dirisala, A.; Cabral, H.; Miyata, K.; Kataoka, K. Hydroxychloroquine-conjugated gold nanoparticles for improved siRNA activity. Biomaterials 2016, 90, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Khodadadei, F.; Safarian, S.; Ghanbari, N. Methotrexate-loaded nitrogen-doped graphene quantum dots nanocarriers as an efficient anticancer drug delivery system. Mater. Sci. Eng. C 2017, 79, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.; Thakur, N.; Katyal, S.C.; Barman, P.B.; Sharma, V.; Sharma, P. Recent developments on the synthesis, structural and optical properties of chalcogenide quantum dots. Sol. Energy Mater. Sol. Cells 2017, 168, 183–200. [Google Scholar] [CrossRef]
- Svitkova, V.; Blaskovicova, J.; Tekelova, M.; Kallai, B.M.; Ignat, T.; Horackova, V.; Skladal, P.; Kopel, P.; Adam, V.; Farkasova, D. Assessment of CdS quantum dots effect on UV damage to DNA using a DNA/quantum dots structured electrochemical biosensor and DNA biosensing in solution. Sens. Actuators B Chem. 2017, 243, 435–444. [Google Scholar] [CrossRef]
- Li, Z.; Xu, W.; Wang, Y.; Shah, B.R.; Zhang, C.; Chen, Y.; Li, Y.; Li, B. Quantum dots loaded nanogels for low cytotoxicity, pH-sensitive fluorescence, cell imaging and drug delivery. Carbohydr. Polym. 2015, 121, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.B.; Mrowczynski, O.; Patel, S.; Weston, C.; Zacharia, B.; Glantz, M.; Siedlecki, C.; Xu, L.; Connor, J.R. Interleukin-13 conjugated quantum dots for identification of glioma initiating cells and their extracellular vesicles. Acta Biomater. 2017, 58, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ding, Y.; Zhou, L.; Shi, D.; Sun, L.; Webster, T.J.; Shen, Y. Noninvasive nanoparticle strategies for brain tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2605–2621. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Blanco, J.; Torres-Suárez, A.-I. Towards tailored management of malignant brain tumors with nanotheranostics. Acta Biomater. 2018, 73, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Blanco-Prieto, M.J.; Sousa, J.; Pais, A.; Vitorino, C. Breaching barriers in glioblastoma. Part II: Targeted drug delivery and lipid nanoparticles. Int. J. Pharm. 2017, 531, 389–410. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Morshed, R.A.; Auffinger, B.; Tobias, A.L.; Lesniak, M.S. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv. Drug Deliv. Rev. 2014, 66, 42–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orunoğlu, M.; Kaffashi, A.; Pehlivan, S.B.; Şahin, S.; Söylemezoğlu, F.; Karlı-Oğuz, K.; Mut, M. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater. Sci. Eng. C 2017, 78, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhao, M.; Fu, C.; Yu, Y.; Fu, A. Targeted therapy of intracranial glioma model mice with curcumin nanoliposomes. Int. J. Nanomed. 2018, 13, 1601–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.-M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, S.; Miller, K.; Zhu, Y.; McKinnon, E.; Novak, T.; Kenney, M.E.; Broome, A.-M. Dual receptor-targeted theranostic nanoparticles for localized delivery and activation of photodynamic therapy drug in glioblastomas. Mol. Pharm. 2015, 12, 3250–3260. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, Z.; Ying, M.; Cao, X.; Hu, X.; Zhan, C.; Wei, X.; Gao, J.; Wang, X.; Yan, Z.; Lu, W. Design of Y-shaped targeting material for liposome-based multifunctional glioblastoma-targeted drug delivery. J. Control. Release 2017, 255, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.L.; Wilson, C.L.; Kidambi, S. Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing glioblastoma cells. Oncotarget 2016, 7, 34158–34171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Kievit, F.M.; Jeon, M.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Nanoparticle-mediated target delivery of trail as gene therapy for glioblastoma. Adv. Healthc. Mater. 2015, 4, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Rabanel, J.M.; Latreille, P.L.; Lalloz, A.; Hildgen, P.; Banquy, X. Chapter 4-Nanostructured Nanoparticles for Improved Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Bregoli, L.; Movia, D.; Gavigan-Imedio, J.D.; Lysaght, J.; Reynolds, J.; Prina-Mello, A. Nanomedicine applied to translational oncology: A future perspective on cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, M.; Sousa, J.; Pais, A.; Vitorino, C. Clinical applications of nanostructured drug delivery systems: From basic research to translational medicine. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 43–116. [Google Scholar]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; Jiang, X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int. J. Pharm. 2006, 310, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Fan, T.M.; Borst, L.B.; Cheng, J. Synthesis and biological response of size-specific, monodisperse drug–silica nanoconjugates. ACS Nano 2012, 6, 3954–3966. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.H.; Kim, K.-W.; Kim, M.H.; Yu, Y.S. Intravenously administered gold nanoparticles pass through the blood–retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology 2009, 20, 505101. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. In Nanoscience And Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 239–250. [Google Scholar]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood-brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar] [PubMed]
- Mikitsh, J.L.; Chacko, A.-M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Med. Chem. 2014, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Amiji, M.M. Therapeutic targeting strategies using endogenous cells and proteins. J. Control. Release 2017, 258, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Schlich, M.; Cryan, J.F.; O’Driscoll, C.M. Targeted drug delivery via folate receptors for the treatment of brain cancer: Can the promise deliver? J. Pharm. Sci. 2017, 106, 3413–3420. [Google Scholar] [CrossRef] [PubMed]
- Razpotnik, R.; Novak, N.; Čurin Šerbec, V.; Rajcevic, U. Targeting Malignant Brain Tumors with Antibodies. Front. Immunol. 2017, 8, 1181. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Li, F.; Baik, S.; Shao, W.; Ling, D.; Hyeon, T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017, 136, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Gu, L.; Ren, W.; Liu, Y. Stimuli-responsive polymers for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 45, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C 2017, 71, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Huang, P.; Fan, W.; Wang, Z.; Liu, Y.; Wang, S.; Zhang, G.; Hu, J.; Liu, W.; Niu, G. Tri-stimuli-responsive biodegradable theranostics for mild hyperthermia enhanced chemotherapy. Biomaterials 2017, 126, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gerweck, L.E.; Seetharaman, K. Cellular pH Gradient in Tumor versus Normal Tissue: Potential Exploitation for the Treatment of Cancer Advances in Brief Cellular. Cancer Res. 1996, 56, 1194–1198. [Google Scholar] [PubMed]
- Karimi, M.; Eslami, M.; Sahandi-Zangabad, P.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 696–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Li, D.; He, B.; Xu, X.; Sheng, M.; Lai, Y.; Wang, G.; Gu, Z. Anti-tumor drug delivery of pH-sensitive poly (ethylene glycol)-poly (l-histidine-)-poly (l-lactide) nanoparticles. J. Control. Release 2011, 152, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, Z.S.; Al-Jamal, W.; Bossche, J.V.; Bui, T.T.; Drake, A.F.; Mason, A.J.; Kostarelos, K. Lipid–peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano 2012, 6, 9335–9346. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.-Q.; Gao, W.; Xiang, B.; Qi, X.-R. Enhancing cellular uptake of activable cell-penetrating peptide–doxorubicin conjugate by enzymatic cleavage. Int. J. Nanomed. 2012, 7, 1613–1621. [Google Scholar]
- Liu, Z.; Xiong, M.; Gong, J.; Zhang, Y.; Bai, N.; Luo, Y.; Li, L.; Wei, Y.; Liu, Y.; Tan, X. Legumain protease-activated TAT-liposome cargo for targeting tumours and their microenvironment. Nat. Commun. 2014, 5, 4280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, G.; Xia, H.; Hu, Q.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Tu, Y.; Pang, Z.; Song, Q. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials 2013, 34, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, F.; Hu, R.G.; Becker, D.L.; Xu, C. Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.M.; Kim, H.; Park, D.; Kim, J.; Kim, W.J. Phenylboronic acid-sugar grafted polymer architecture as a dual stimuli-responsive gene carrier for targeted anti-angiogenic tumor therapy. Biomaterials 2016, 75, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Wang, I.-H. Using catanionic solid lipid nanoparticles with wheat germ agglutinin and lactoferrin for targeted delivery of etoposide to glioblastoma multiforme. J. Taiwan Inst. Chem. Eng. 2017, 77, 73–82. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, N.; Wang, H.; Gu, W.; Liu, K.; Ai, P.; Yan, C.; Ye, L. Dual-targeting superparamagnetic iron oxide nanoprobes with high and low target density for brain glioma imaging. J. Colloid Interface Sci. 2016, 469, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yao, J.; Gao, X.; Jing, Y.; Kang, T.; Jiang, D.; Jiang, T.; Feng, J.; Zhu, Q.; Jiang, X. Multi-targeting peptide-functionalized nanoparticles recognized vasculogenic mimicry, tumor neovasculature, and glioma cells for enhanced anti-glioma therapy. ACS Appl. Mater. Interfaces 2015, 7, 27885–27899. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Qian, J.; Cao, S.; Yang, Z.; Pang, Z.; Pan, S.; Fan, L.; Xi, Z.; Jiang, X.; Zhang, Q. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials 2012, 33, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Agemy, L.; Kotamraju, V.R.; Braun, G.; Teesalu, T.; Sugahara, K.N.; Hamzah, J.; Ruoslahti, E. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene 2012, 31, 3754–3763. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-M.; Huang, Y.; Zhao, B.-X.; Zhao, X.; Duan, Y.; Du, R.; Yu, K.-F.; Song, P.; Zhao, Y.; Zhang, X. Anti-tumor and anti-angiogenic effect of metronomic cyclic NGR-modified liposomes containing paclitaxel. Biomaterials 2013, 34, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Shen, Q.; Zhan, C.; Wei, X.; Gao, J.; Xie, C.; Yao, B.; Lu, W. A stabilized peptide ligand for multifunctional glioma targeted drug delivery. J. Control. Release 2016, 243, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Koivunen, E.; Kain, R.; Lahdenranta, J.; Sakamoto, M.; Stryhn, A.; Ashmun, R.A.; Shapiro, L.H.; Arap, W.; Ruoslahti, E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000, 60, 722–727. [Google Scholar] [PubMed]
- Jiang, X.; Xin, H.; Ren, Q.; Gu, J.; Zhu, L.; Du, F.; Feng, C.; Xie, Y.; Sha, X.; Fang, X. Nanoparticles of 2-deoxy-D-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials 2014, 35, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H.J.; Lee, S.; Min, S.Y.; Lee, E.S.; Shin, B.S.; Choi, H.-G.; Youn, Y.S. Doxorubicin-loaded nanoparticles consisted of cationic-and mannose-modified-albumins for dual-targeting in brain tumors. J. Control. Release 2016, 225, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.; Mei, L.; Gao, H.; Shi, K.; Chen, J.; Wang, Y.; Zhang, Q.; Yang, Y.; He, Q. Enhanced glioma targeting and penetration by dual-targeting liposome co-modified with T7 and TAT. J. Pharm. Sci. 2014, 103, 3891–3901. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Guo, X.Y.; Yang, T.; Yu, M.Z.; Chen, D.W.; Wang, J.C. Brain tumor-targeted therapy by systemic delivery of siRNA with Transferrin receptor-mediated core-shell nanoparticles. Int. J. Pharm. 2016, 510, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Hsu, C.-C. Anti-melanotransferrin and apolipoprotein E on doxorubicin-loaded cationic solid lipid nanoparticles for pharmacotherapy of glioblastoma multiforme. J. Taiwan Inst. Chem. Eng. 2017, 77, 10–20. [Google Scholar] [CrossRef]
- Wang, Z.; He, Q.; Zhao, W.; Luo, J.; Gao, W. Tumor-homing, pH-and ultrasound-responsive polypeptide-doxorubicin nanoconjugates overcome doxorubicin resistance in cancer therapy. J. Control. Release 2017, 264, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Jin, H.; Li, D.; Xu, F.; Wu, A.; Wang, J.; Huang, Y. Glioma dual-targeting nanohybrid protein toxin constructed by intein-mediated site-specific ligation for multistage booster delivery. Theranostics 2017, 7, 3489–3503. [Google Scholar] [CrossRef] [PubMed]
- Zamykal, M.; Martens, T.; Matschke, J.; Günther, H.S.; Kathagen, A.; Schulte, A.; Peters, R.; Westphal, M.; Lamszus, K. Inhibition of intracerebral glioblastoma growth by targeting the insulin-like growth factor 1 receptor involves different context-dependent mechanisms. Neuro. Oncol. 2014, 17, 1076–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iaboni, M.; Fontanella, R.; Rienzo, A.; Capuozzo, M.; Nuzzo, S.; Santamaria, G.; Catuogno, S.; Condorelli, G.; De Franciscis, V.; Esposito, C.L. Targeting insulin receptor with a novel internalizing aptamer. Mol. Ther. Acids 2016, 5, e365. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Kulkarni, S.A.; Xiong, J.; Feng, S.-S. Vitamin E TPGS coated liposomes enhanced cellular uptake and cytotoxicity of docetaxel in brain cancer cells. Int. J. Pharm. 2011, 421, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Singh, R.P.; Kumari, L.; Sharma, G.; Koch, B.; Rajesh, C.V.; Mehata, A.K.; Singh, S.; Pandey, B.L.; Muthu, M.S. TPGS-chitosan cross-linked targeted nanoparticles for effective brain cancer therapy. Mater. Sci. Eng. C 2017, 74, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Yao, Q.; Zhu, L. MMP2-sensitive peg–lipid copolymers: A new type of tumor-targeted p-glycoprotein inhibitor. ACS Appl. Mater. Interfaces 2016, 8, 12661–12673. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Grassi, E.S.; Pantazopoulou, V.; Tong, B.; Lindgren, D.; Berg, T.J.; Pietras, E.J.; Axelson, H.; Pietras, A. CD44 interacts with HIF-2α to modulate the hypoxic phenotype of perinecrotic and perivascular glioma cells. Cell Rep. 2017, 20, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Onnis, B.; Rapisarda, A.; Melillo, G. Development of HIF-1 inhibitors for cancer therapy. J. Cell. Mol. Med. 2009, 13, 2780–2786. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cabello, J.C.; Piña, M.J.; Ibáñez-Fonseca, A.; Fernández-Colino, A.; Arias, F.J. Nanotechnological approaches to therapeutic delivery using elastin-like recombinamers. Bioconjug. Chem. 2015, 26, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.S.; Raucher, D. Anti-tumor efficacy of a therapeutic peptide based on thermo-responsive elastin-like polypeptide in combination with gemcitabine. Cancer Lett. 2014, 348, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shen, S.; Liao, Z.; Shi, W.; Wang, Y.; Zhao, J.; Hu, Y.; Yang, J.; Chen, J.; Mei, H. Targeting fibronectins of glioma extracellular matrix by CLT1 peptide-conjugated nanoparticles. Biomaterials 2014, 35, 4088–4098. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2016, 110, 3–12. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Cen, B.; Liao, L.; Wang, Z.; Qin, Y.; Wu, Z.; Liao, W.; Zhang, Z.; Ji, A. A tumor-targeting cRGD-EGFR siRNA conjugate and its anti-tumor effect on glioblastoma in vitro and in vivo. Drug Deliv. 2017, 24, 471–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quader, S.; Liu, X.; Chen, Y.; Mi, P.; Chida, T.; Ishii, T.; Miura, Y.; Nishiyama, N.; Cabral, H.; Kataoka, K. cRGD peptide-installed epirubicin-loaded polymeric micelles for effective targeted therapy against brain tumors. J. Control. Release 2017, 258, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kulhari, H.; Telukutla, S.R.; Pooja, D.; Shukla, R.; Sistla, R.; Bansal, V.; Adams, D.J. Peptide grafted and self-assembled poly(γ-glutamic acid)-phenylalanine nanoparticles targeting camptothecin to glioma. Nanomedicine 2017, 12, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Diao, Y.; Li, W.; Yang, Z.; Zhang, L.; Chen, Z.; Wu, Y. RGD peptide conjugation results in enhanced antitumor activity of PD0325901 against glioblastoma by both tumor-targeting delivery and combination therapy. Int. J. Pharm. 2016, 505, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Thysiadis, S.; Katsamakas, S.; Dalezis, P.; Chatzisideri, T.; Trafalis, D.; Sarli, V. Novel c (RGDyK)-based conjugates of POPAM and 5-fluorouracil for integrin-targeted cancer therapy. Future Med. Chem. 2017, 9, 2181–2196. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Ding, Y.; Mujeeb, A.; Zhao, Y.; Nie, G. Tumor microenvironment targeting and responsive peptide-based nanoformulations for improved tumor therapy. Mol. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, J.; Gao, L.; Sun, L.; Peng, T.; Wang, J.; Zhu, J.; Lu, W.; Zhang, L.; Yan, Z. iNGR-modified liposomes for tumor vascular targeting and tumor tissue penetrating delivery in the treatment of glioblastoma. Mol. Pharm. 2017, 14, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Cheng, S.; Zhang, X.; Tian, Q.; Pi, J.; Tang, J.; Huang, Q.; Wang, F.; Chen, J.; Xie, Z. Efficacy of NGR peptide-modified PEGylated quantum dots for crossing the blood-brain barrier and targeted fluorescence imaging of glioma and tumor vasculature. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Li, Y.; Pang, H.; Zheng, Y.; Zhao, Y. Pep-1 peptide-functionalized liposome to enhance the anticancer efficacy of cilengitide in glioma treatment. Colloids Surf. B Biointerfaces 2017, 158, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lv, L.; Wang, Z.; Jiang, Y.; Lv, W.; Liu, X.; Wang, Z.; Zhao, Y.; Xin, H.; Xu, Q. Improved anti-glioblastoma efficacy by IL-13Rα2 mediated copolymer nanoparticles loaded with paclitaxel. Sci. Rep. 2015, 5, 16589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicks, M.J.; Chiuchiolo, M.J.; Ballon, D.; Dyke, J.P.; Aronowitz, E.; Funato, K.; Tabar, V.; Havlicek, D.; Fan, F.; Sondhi, D. Anti-Epidermal Growth Factor Receptor Gene Therapy for Glioblastoma. PLoS ONE 2016, 11, e0162978. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Wang, H.; Gao, Y.; Li, L.; Hwang, S.H.; Ji, X.; Hammock, B.D. COX-2/sEH dual inhibitor PTUPB suppresses glioblastoma growth by targeting epidermal growth factor receptor and hyaluronan mediated motility receptor. Oncotarget 2017, 8, 87353. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.T.; Furnari, B.F.; Cavenee, W.K. Targeting EGFR for treatment of glioblastoma: Molecular basis to overcome resistance. Curr. Cancer Drug Targets 2012, 12, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Eller, J.L.; Longo, S.L.; Hicklin, D.J.; Canute, G.W. Activity of anti-epidermal growth factor receptor monoclonal antibody C225 against glioblastoma multiforme. Neurosurgery 2002, 51, 1005–1014. [Google Scholar] [PubMed]
- Wei, J.; Cui, J.; Zhou, X.; Fang, C.; Tan, Y.; Chen, L.; Yang, C.; Liu, M.; Kang, C. F25P preproinsulin abrogates the secretion of pro-growth factors from EGFRvIII cells and suppresses tumor growth in an EGFRvIII/wt heterogenic model. Cancer Lett. 2016, 380, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chu, J.; Chan, W.K.; Zhang, J.; Wang, Y.; Cohen, J.B.; Victor, A.; Meisen, W.H.; Kim, S.; Grandi, P. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci. Rep. 2015, 5, 11483. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Cecile, L.M.; Lamszus, K. EGFR as a target for glioblastoma treatment: An unfulfilled promise. CNS Drugs 2017, 31, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Archer, G.E.; Mitchell, D.A.; Heimberger, A.B.; McLendon, R.E.; Bigner, D.D.; Sampson, J.H. EGFRvIII-Targeted Vaccination Therapy of Malignant Glioma. Brain Pathol. 2009, 19, 713–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, A.; Jain, M.; Lim, M.J.; Angara, K.; Zeng, P.; Arbab, S.A.; Iskander, A.S.M.; Ara, R.; Arbab, A.S.; Achyut, B.R. Anti-VEGFR2 driven nuclear translocation of VEGFR2 and acquired malignant hallmarks are mutation dependent in glioblastoma. J. Cancer Sci. Ther. 2016, 8, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Momeny, M.; Moghaddaskho, F.; Gortany, N.K.; Yousefi, H.; Sabourinejad, Z.; Zarrinrad, G.; Mirshahvaladi, S.; Eyvani, H.; Barghi, F.; Ahmadinia, L. Blockade of vascular endothelial growth factor receptors by tivozanib has potential anti-tumour effects on human glioblastoma cells. Sci. Rep. 2017, 7, 44075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biel, N.M.; Dietmar, W. Siemann Targeting the Angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference. Cancer Lett. 2016, 380, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Modulation of MMP-2 and MMP-9 secretion by cytokines, inducers and inhibitors in human glioblastoma T-98G cells. Oncol. Rep. 2017, 37, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Aroui, S.; Aouey, B.; Chtourou, Y.; Meunier, A.-C.; Fetoui, H.; Kenani, A. Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MMP-9) via the inhibition of ERK-P38-JNK signaling pathway in human glioblastoma. Chem. Biol. Interact. 2016, 244, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Piromkraipak, P.; Sangpairoj, K.; Tirakotai, W.; Chaithirayanon, K.; Unchern, S.; Supavilai, P.; Power, C.; Vivithanaporn, P. Cysteinyl leukotriene receptor antagonists inhibit migration, invasion, and expression of mmp-2/9 in human glioblastoma. Cell. Mol. Neurobiol. 2017, 38, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Zaaroor, M. Glioblastoma multiforme targeted therapy: The Chlorotoxin story. J. Clin. Neurosci. 2016, 33, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Cheng, Y.; Morshed, R.; Nord, K.; Han, Y.; Wegscheid, M.L.; Auffinger, B.; Wainwright, D.A.; Lesniak, M.S.; Tirrell, M. V Fibrin-binding, peptide amphiphile micelles for targeting glioblastoma. Biomaterials 2014, 35, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, B.; Shen, S.; Chen, J.; Zhang, Q.; Jiang, X.; Pang, Z. CREKA peptide-conjugated dendrimer nanoparticles for glioblastoma multiforme delivery. J. Colloid Interface Sci. 2015, 450, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, O.R.; Choi, Y.S.; Lee, H.; Park, K.; Lee, C.-T.; Kang, K.W.; Jeong, S. Selection and characterization of tenascin C targeting peptide. Mol. Cells 2012, 33, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.E. Dual Targeting of Angiopoietin-2 and VEGF Signaling for the Treatment of Glioblastoma; Harvard University: Cambridge, MA, USA, 2015. [Google Scholar]
- Sato, A.; Mizobuchi, Y.; Nakajima, K.; Shono, K.; Fujihara, T.; Kageji, T. Blocking COX-2 induces apoptosis and inhibits cell proliferation via the Akt/survivin- and Akt/ID3 pathway in low-grade-glioma. J. Neurooncol. 2017, 132, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Z.; Wang, C.; Cheng, W.; Tian, X.; Huo, X.; Wang, Y.; Sun, C.; Feng, L.; Xing, J. Alantolactone, a natural sesquiterpene lactone, has potent antitumor activity against glioblastoma by targeting IKKβ kinase activity and interrupting NF-κB/COX-2-mediated signaling cascades. J. Exp. Clin. Cancer Res. 2017, 36, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Qin, J.; Li, Y.; Li, G.; Wang, Y.; Zhang, N.; Chen, P.; Li, C. Combination therapy of PKCζ and COX-2 inhibitors synergistically suppress melanoma metastasis. J. Exp. Clin. Cancer Res. 2017, 36, 115. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, L.; Wang, J.; Zhou, H. Baicalein induces the apoptosis of U251 glioblastoma cell lines via the NF-kB-p65-mediated mechanism. Animal Cells Syst. (Seoul) 2016, 20, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.; Duan, Y.; Wang, P.; Gao, R.; Chen, X.; Ou, Y.; Liang, M.; Wang, Z.; Yuan, Y.; Wang, L. DYT-40, a novel synthetic 2-styryl-5-nitroimidazole derivative, blocks malignant glioblastoma growth and invasion by inhibiting AEG-1 and NF-κB signaling pathways. Sci. Rep. 2016, 6, 27331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Ren, X.; Cheng, Y.; Liu, X.; Allen, J.E.; Zhang, Y.; Yuan, Y.; Huang, S.-Y.; Yang, W.; Berg, A. The NFκB inhibitor, SN50, induces differentiation of glioma stem cells and suppresses their oncogenic phenotype. Cancer Biol. Ther. 2014, 15, 602–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, K.E.; Morshed, R.A.; Yamini, B. Nuclear factor-κB in glioblastoma: Insights into regulators and targeted therapy. Neuro. Oncol. 2015, 18, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, W.W.; Lam, A.K.Y.; Wang, Y.; Hu, H.-F.; Guan, X.Y.; Qin, Y.R.; Saremi, N.; Tsao, S.W.; He, Q.-Y. Significance of PI3K/AKT signaling pathway in metastasis of esophageal squamous cell carcinoma and its potential as a target for anti-metastasis therapy. Oncotarget 2017, 8, 38755. [Google Scholar] [CrossRef] [PubMed]
- Bumpers, H.; Huang, M.-B.; Katkoori, V.; Manne, U.; Bond, V. Nef-M1, a CXCR4 peptide antagonist, enhances apoptosis and inhibits primary tumor growth and metastasis in Breast Cancer. J. Cancer Ther. 2013, 4, 898. [Google Scholar] [CrossRef] [PubMed]
- Katkoori, V.R.; Basson, M.D.; Bond, V.C.; Manne, U.; Bumpers, H.L. Nef-M1, a peptide antagonist of CXCR4, inhibits tumor angiogenesis and epithelial-to-mesenchymal transition in colon and breast cancers. Oncotarget 2015, 6, 27763. [Google Scholar] [CrossRef] [PubMed]
- Kapur, A.; Beres, T.; Rathi, K.; Nayak, A.P.; Czarnecki, A.; Felder, M.; Gillette, A.; Ericksen, S.S.; Sampene, E.; Skala, M.C. Oxidative stress via inhibition of the mitochondrial electron transport and Nrf-2-mediated anti-oxidative response regulate the cytotoxic activity of plumbagin. Sci. Rep. 2018, 8, 1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scodeller, P.; Simón-Gracia, L.; Kopanchuk, S.; Tobi, A.; Kilk, K.; Säälik, P.; Kurm, K.; Squadrito, M.L.; Kotamraju, V.R.; Rinken, A. Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide. Sci. Rep. 2017, 7, 14655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannarile, M.A.; Weisser, M.; Jacob, W.; Jegg, A.-M.; Ries, C.H.; Rüttinger, D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer 2017, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Cieslewicz, M.; Tang, J.; Jonathan, L.Y.; Cao, H.; Zavaljevski, M.; Motoyama, K.; Lieber, A.; Raines, E.W.; Pun, S.H. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc. Natl. Acad. Sci. USA 2013, 110, 15919–15924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segers, F.M.E.; Yu, H.; Molenaar, T.J.M.; Prince, P.; Tanaka, T.; van Berkel, T.J.C.; Biessen, E.A.L. Design and validation of a specific scavenger receptor class AI binding peptide for targeting the inflammatory atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Hierro, C.; Rodon, J.; Tabernero, J. Fibroblast growth factor (FGF) receptor/FGF inhibitors: Novel targets and strategies for optimization of response of solid tumors. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 42, pp. 801–819. [Google Scholar]
- Babina, I.S.; Nicholas, C. Turner. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 2017, 17, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Ader, I.; Delmas, C.; Skuli, N.; Bonnet, J.; Schaeffer, P.; Bono, F.; Cohen-Jonathan-Moyal, E.; Toulas, C. Preclinical evidence that SSR128129E–A novel small-molecule multi-fibroblast growth factor receptor blocker–Radiosensitises human glioblastoma. Eur. J. Cancer 2014, 50, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Ding, Y.; Zhao, Y.; Wang, J.; Qin, H.; Liu, X.; Lang, J.; Zhao, R.; Zhang, Y.; Shi, J. Peptide assembly integration of fibroblast-targeting and cell-penetration features for enhanced antitumor drug delivery. Adv. Mater. 2015, 27, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.G.; Tentori, L.; Ruffini, F.; Ceci, C.; Lisi, L.; Bonanno, E.; Scimeca, M.; Eskilsson, E.; Daubon, T.; Miletic, H. The anti-vascular endothelial growth factor receptor-1 monoclonal antibody D16F7 inhibits invasiveness of human glioblastoma and glioblastoma stem cells. J. Exp. Clin. Cancer Res. 2017, 36, 106. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wang, A.; Yang, Z.; Liu, Y.; Qi, X. Enhance cancer cell recognition and overcome drug resistance using hyaluronic acid and α-tocopheryl succinate based multifunctional nanoparticles. Mol. Pharm. 2015, 12, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Burattini, L.; Nabissi, M.; Beatrice Morelli, M.; Berardi, R.; Santoni, G.; Cascinu, S. Essential role of Gli proteins in glioblastoma multiforme. Curr. Protein Pept. Sci. 2013, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cenciarelli, C.; Marei, H.E.S.; Zonfrillo, M.; Pierimarchi, P.; Paldino, E.; Casalbore, P.; Felsani, A.; Vescovi, A.L.; Maira, G.; Mangiola, A. PDGF receptor alpha inhibition induces apoptosis in glioblastoma cancer stem cells refractory to anti-Notch and anti-EGFR treatment. Mol. Cancer 2014, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, F.; Evangelisti, C.; McCubrey, J.A.; Martelli, A.M. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol. Sci. 2015, 36, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Luwor, R.B.; Kaye, A.H.; Zhu, H.-J. Transforming growth factor-beta (TGF-β) and brain tumours. J. Clin. Neurosci. 2008, 15, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Bierie, B.; Moses, H.L. Transforming growth factor beta (TGF-β) and inflammation in cancer. Cytokine Growth Factor Rev. 2010, 21, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, J.R.D.; Regad, T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct. Target. Ther. 2017, 2, 17040. [Google Scholar] [CrossRef] [PubMed]
- Phuphanich, S.; Raizer, J.; Chamberlain, M.; Canelos, P.; Narwal, R.; Hong, S.; Miday, R.; Nade, M.; Laubscher, K. Phase II study of MEDI-575, an anti-platelet-derived growth factor-α antibody, in patients with recurrent glioblastoma. J. Neurooncol. 2017, 131, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Gerstner, E.R.; Ye, X.; Desideri, S.; Duda, D.G.; Peereboom, D.; Lesser, G.J.; Chowdhary, S.; Wen, P.Y.; Grossman, S. Feasibility, phase I, and phase II studies of tandutinib, an oral platelet-derived growth factor receptor-β tyrosine kinase inhibitor, in patients with recurrent glioblastoma. Neuro. Oncol. 2017, 19, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Joglekar-Javadekar, M.; Van Laere, S.; Bourne, M.; Moalwi, M.; Finetti, P.; Vermeulen, P.B.; Birnbaum, D.; Dirix, L.Y.; Ueno, N.; Carter, M. Characterization and targeting of platelet-derived growth factor receptor alpha (PDGFRA) in inflammatory breast cancer (IBC). Neoplasia 2017, 19, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Altaba, A.R. Hedgehog signaling and the Gli code in stem cells, cancer, and metastases. Sci. Signal. 2011, 4, pt9. [Google Scholar]
- Yu, S.; Ping, Y.; Yi, L.; Zhou, Z.; Chen, J.; Yao, X.; Gao, L.; Wang, J.M.; Bian, X. Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett. 2008, 265, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Hyvönen, M.; Enbäck, J.; Huhtala, T.; Lammi, J.; Sihto, H.; Weisell, J.; Joensuu, H.; Rosenthal-Aizman, K.; El-Andaloussi, S.; Langel, U. Novel target for peptide-based imaging and treatment of brain tumors. Mol. Cancer Ther. 2014, 13, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Sailor, M.J.; Park, J. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012, 24, 3779–3802. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, A.H.; Caruthers, S.D.; Zhang, H.; Williams, T.A.; Robertson, J.D.; Wickline, S.A.; Lanza, G.M. Three-dimensional MR mapping of angiogenesis with α5β1 (ανβ3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model. FASEB J. 2008, 22, 4179–4189. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, V.J.; Gambhir, S.S. Molecular imaging with theranostic nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y.; Liang, X.-J. Theranostic nanoparticles engineered for clinic and pharmaceutics. Acc. Chem. Res. 2011, 44, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Hsu, C.-H.; Li, Z.; Kim, T.; Hwang, L.-P.; Lin, Y.-C.; Lin, Y.-Y. Magnetic resonance nano-theranostics for glioblastoma multiforme. Curr. Pharm. Des. 2015, 21, 5256–5266. [Google Scholar] [CrossRef] [PubMed]
- Bhojani, M.S.; Van Dort, M.; Rehemtulla, A.; Ross, B.D. Targeted imaging and therapy of brain cancer using theranostic nanoparticles. Mol. Pharm. 2010, 7, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Colombeau, L.; Gries, M.; Peterlini, T.; Mathieu, C.; Thomas, N.; Boura, C.; Frochot, C.; Vanderesse, R.; Lux, F. Ultrasmall AGuIX theranostic nanoparticles for vascular-targeted interstitial photodynamic therapy of glioblastoma. Int. J. Nanomed. 2017, 12, 7075–7088. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Chen, Z.; Li, K.; Morais, G.R.; Klockow, J.; Yerneni, K.; Pisani, L.; Chin, F.T.; Mitra, S.; Cheshier, S. A novel theranostic strategy for MMP-14 expressing glioblastomas impacts survival. Mol. Cancer Ther. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Joh, D.Y.; Al-Zaki, A.; Stangl, M.; Murty, S.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Kao, G.D.; Tsourkas, A. Theranostic application of mixed gold and superparamagnetic iron oxide nanoparticle micelles in glioblastoma multiforme. J. Biomed. Nanotechnol. 2016, 12, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E. Rethinking brain cancer therapy: Tumor enzyme activatable theranostic nanoparticles. Mol. Imaging 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y.; Dobrodumov, A.V.; Zhakhov, A.V.; Mikhrina, A.L.; Pitkin, E.; Parr, M.A.; Rolich, V.I.; Simbircev, A.S. Recombinant interleukin-1 receptor antagonist conjugated to superparamagnetic iron oxide nanoparticles for theranostic targeting of experimental glioblastoma. Neoplasia 2015, 17, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.R.; Kievit, F.M.; Veiseh, O.; Chiarelli, P.A.; Fang, C.; Wang, K.; Hatzinger, S.J.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M. Redox-responsive magnetic nanoparticle for targeted convection-enhanced delivery of O 6-benzylguanine to brain tumors. ACS Nano 2014, 8, 10383–10395. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Ifediba, M.A.; Ghosh, S.; Medarova, Z.; Moore, A. Combination treatment with theranostic nanoparticles for glioblastoma sensitization to TMZ. Mol. Imaging Biol. 2014, 16, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Mosafer, J.; Teymouri, M.; Abnous, K.; Tafaghodi, M.; Ramezani, M. Study and evaluation of nucleolin-targeted delivery of magnetic PLGA-PEG nanospheres loaded with doxorubicin to C6 glioma cells compared with low nucleolin-expressing L929 cells. Mater. Sci. Eng. C 2017, 72, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Tafaghodi, M.; Abbasi, B.; Daroudi, M.; Kazemi Oskuee, R. Preparation of superparamagnetic iron oxide/doxorubicin loaded chitosan nanoparticles as a promising glioblastoma theranostic tool. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Melancon, M.P.; Xiong, C.; Huang, Q.; Elliott, A.; Song, S.; Zhang, R.; Flores, L.G.; Gelovani, J.G.; Wang, L.V. Effects of photoacoustic imaging and photothermal ablation therapy mediated by targeted hollow gold nanospheres in an orthotopic mouse xenograft model of glioma. Cancer Res. 2011, 71, 6116–6121. [Google Scholar] [CrossRef] [PubMed]
- Vant’s Veer, L.J.; Bernards, R. Personalized cancer medicine. In Encyclopedia of Cancer; Springer: New York, NY, USA, 2008; pp. 2291–2295. [Google Scholar]
- Bode, A.M.; Dong, Z. Precision oncology-the future of personalized cancer medicine? NPJ Precis. Oncol. 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Wistuba, I.I.; Gelovani, J.G.; Jacoby, J.J.; Davis, S.E.; Herbst, R.S. Methodological and practical challenges for personalized cancer therapies. Nat. Rev. Clin. Oncol. 2011, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.; Tatiparti, K.; Alsaab, H.O.; Kashaw, S.K.; Iyer, A.K. A tumor multicomponent targeting chemoimmune drug delivery system for reprograming the tumor microenvironment and personalized cancer therapy. Drug Discov. Today 2018, 23, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Stupp, R.; Hegi, M.; Wick, W. Individualized targeted therapy for glioblastoma: Fact or fiction? Cancer J. 2012, 18, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-J.; Wong, E. Personalized medicine for glioblastoma: Current challenges and future opportunities. Curr. Mol. Med. 2013, 13, 358–367. [Google Scholar] [PubMed]
- Ene, C.I.; Holland, E.C. Personalized medicine for gliomas. Surg. Neurol. Int. 2015, 6, S89. [Google Scholar] [PubMed]
- Muthu, M.S.; Kulkarni, S.A.; Raju, A.; Feng, S.-S. Theranostic liposomes of TPGS coating for targeted co-delivery of docetaxel and quantum dots. Biomaterials 2012, 33, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.-J.; Sung, C.T.; Aljuffali, I.A.; Huang, Y.-J.; Fang, J.-Y. Nanocomposite liposomes containing quantum dots and anticancer drugs for bioimaging and therapeutic delivery: A comparison of cationic, PEGylated and deformable liposomes. Nanotechnology 2013, 24, 325101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kulkarni, A.; Nagesha, D.K.; Sridhar, S. In vitro evaluation of theranostic polymeric micelles for imaging and drug delivery in cancer. Theranostics 2012, 2, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hong, H.; Chen, G.; Shi, S.; Nayak, T.R.; Theuer, C.P.; Barnhart, T.E.; Cai, W.; Gong, S. Theranostic unimolecular micelles based on brush-shaped amphiphilic block copolymers for tumor-targeted drug delivery and positron emission tomography imaging. ACS Appl. Mater. Interfaces 2014, 6, 21769–21779. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhang, K.; Cao, Y.; Chen, X.; Wang, K.; Liu, M.; Pei, R. Hydrophobic IR-780 dye encapsulated in CRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 12217–12226. [Google Scholar] [CrossRef] [PubMed]
- Sarvagalla, S.; Hsieh, H.P.; Coumar, M.S. Therapeutic polymeric nanoparticles and the methods of making and using thereof: A patent evaluation of WO2015036792. Expert Opin. Ther. Pat. 2016, 26, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, E.J.; Lee, Y.K.; Kim, K.; Kwon, I.C.; Lee, K.Y. Optical Imaging and Gene Therapy with Neuroblastoma-Targeting Polymeric Nanoparticles for Potential Theranostic Applications. Small 2016, 12, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.Y.; Achilli, E.; Grasselli, M. Radiation-induced preparation of core/shell gold/albumin nanoparticles. Radiat. Phys. Chem. 2017, 142, 60–62. [Google Scholar] [CrossRef]
- Croissant, J.G.; Zhang, D.; Alsaiari, S.; Lu, J.; Deng, L.; Tamanoi, F.; AlMalik, A.M.; Zink, J.I.; Khashab, N.M. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in-vivo tumor imaging. J. Control. Release 2016, 229, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
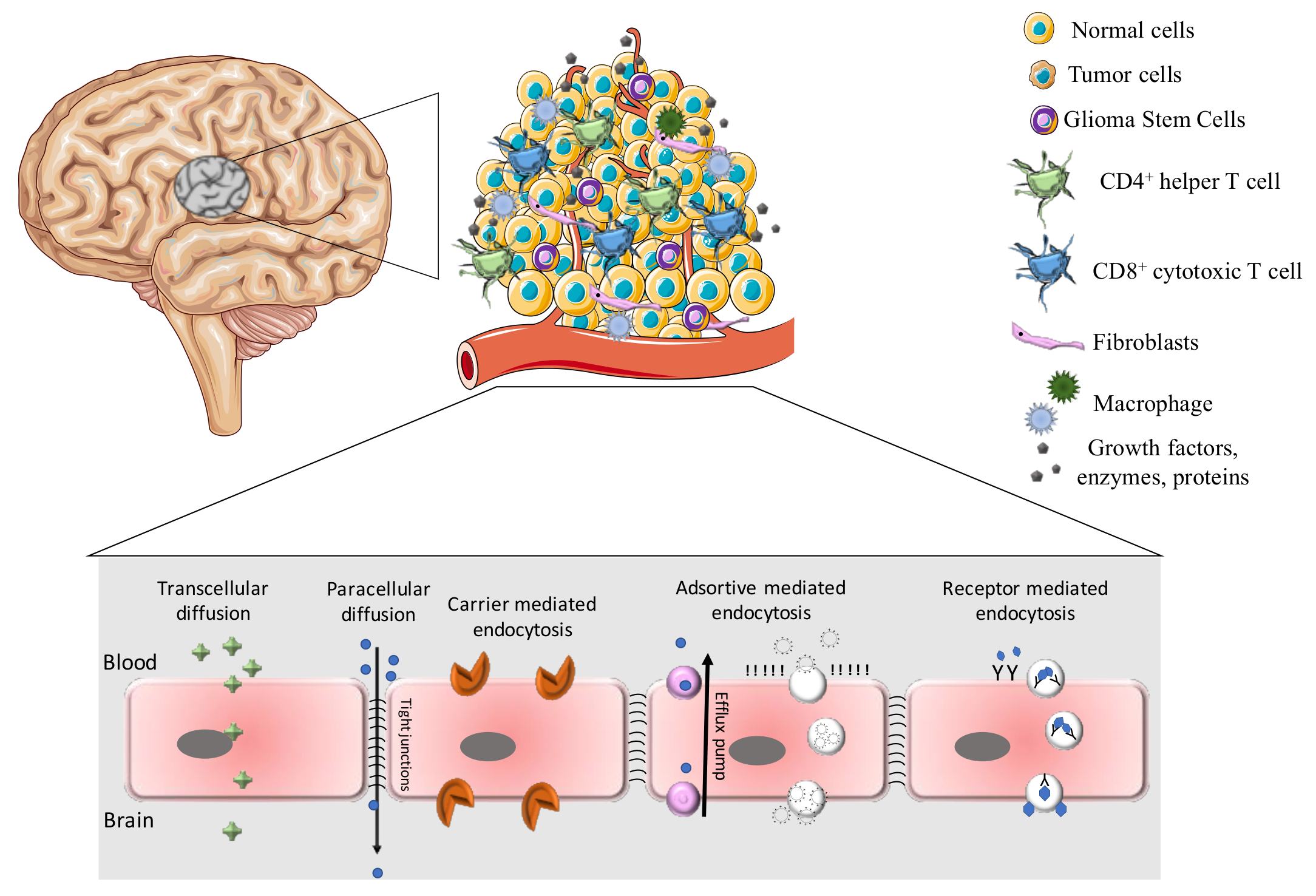
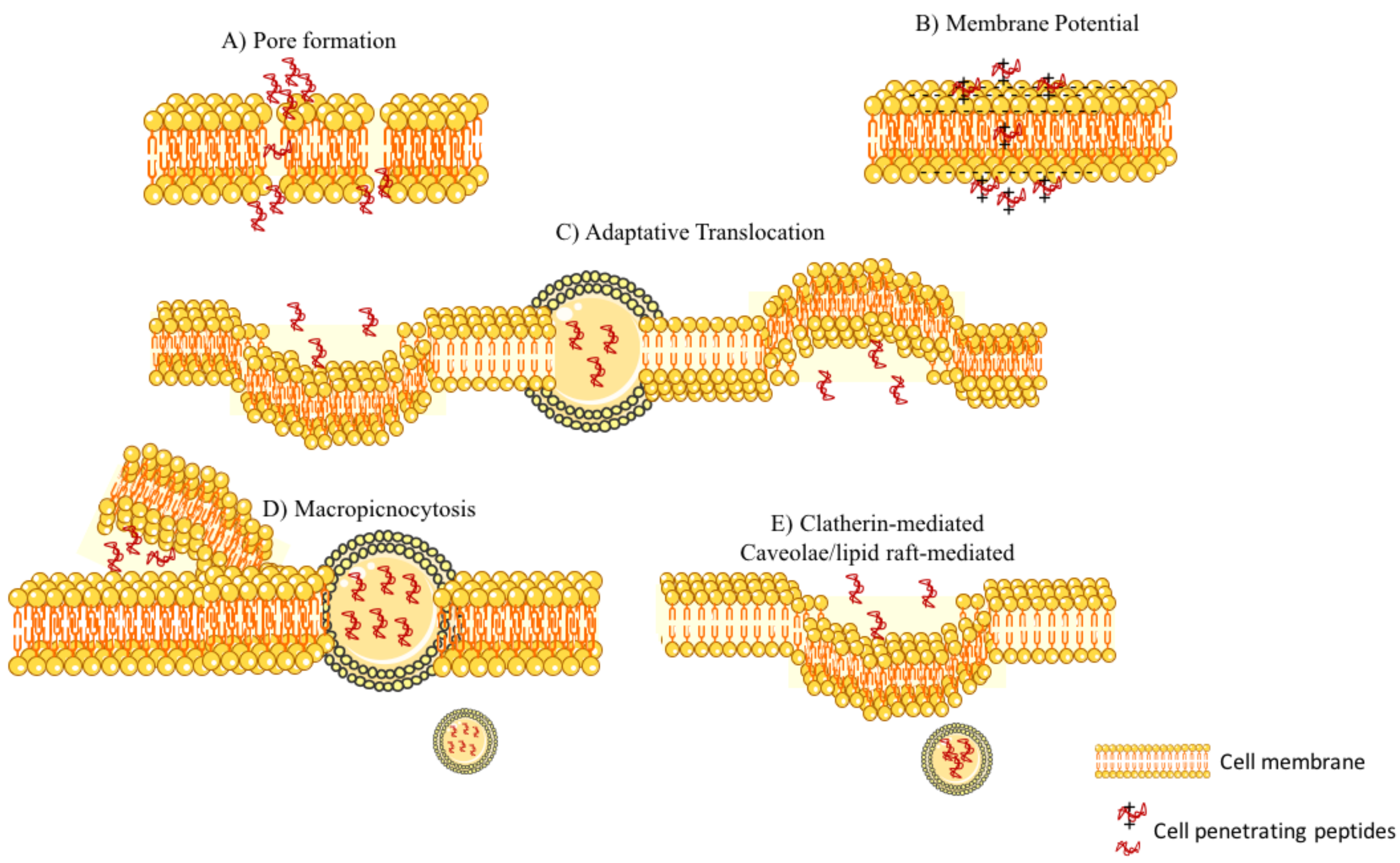


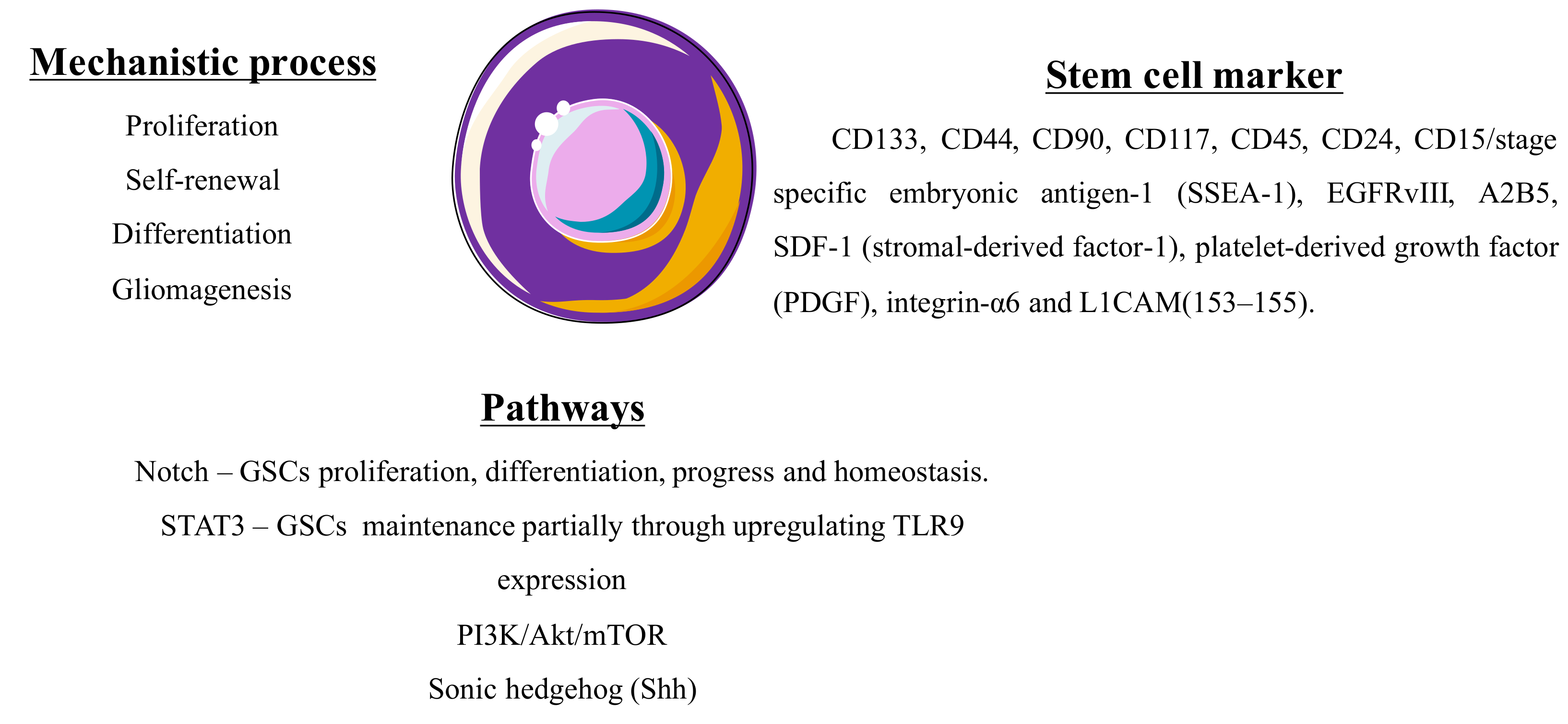
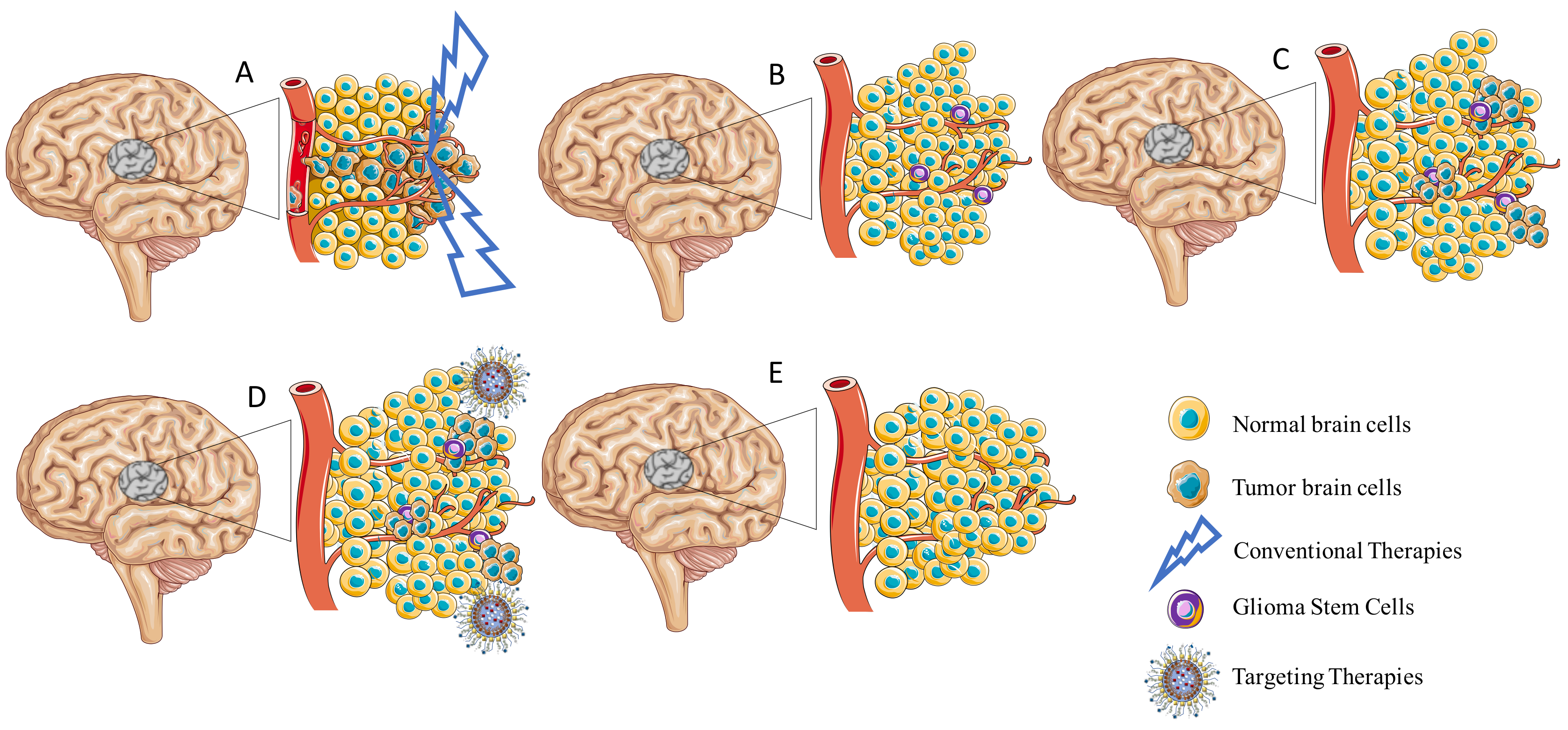
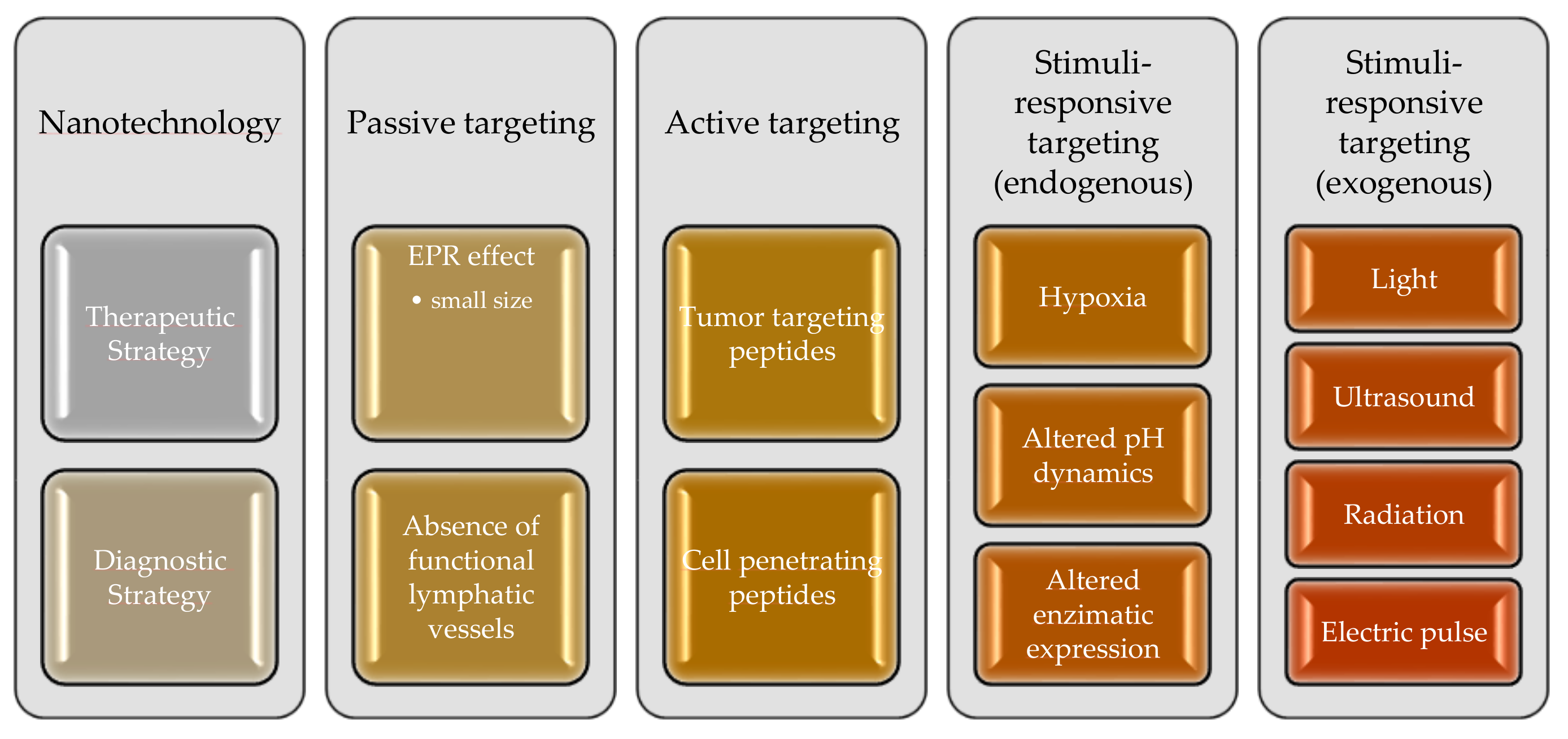
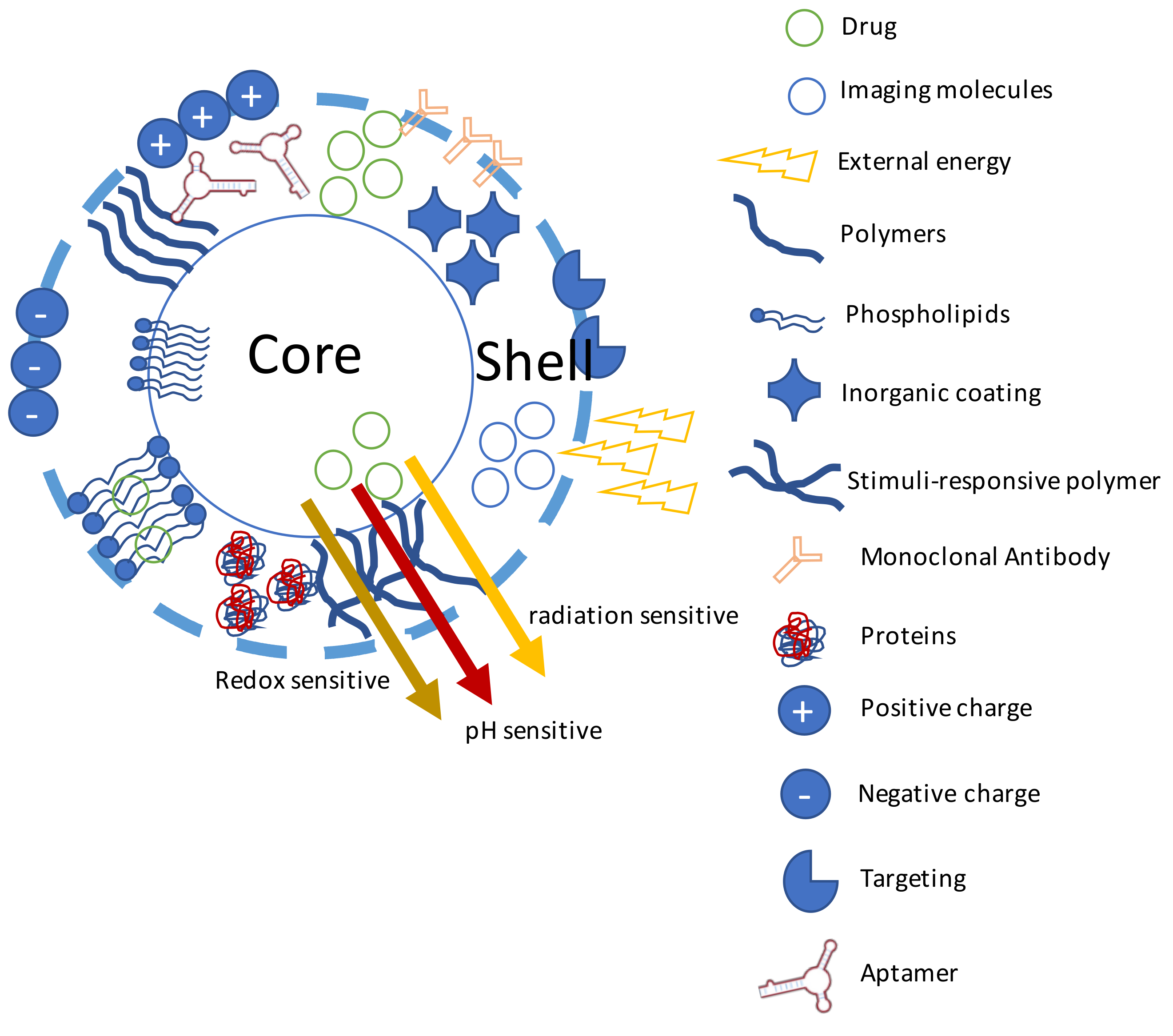
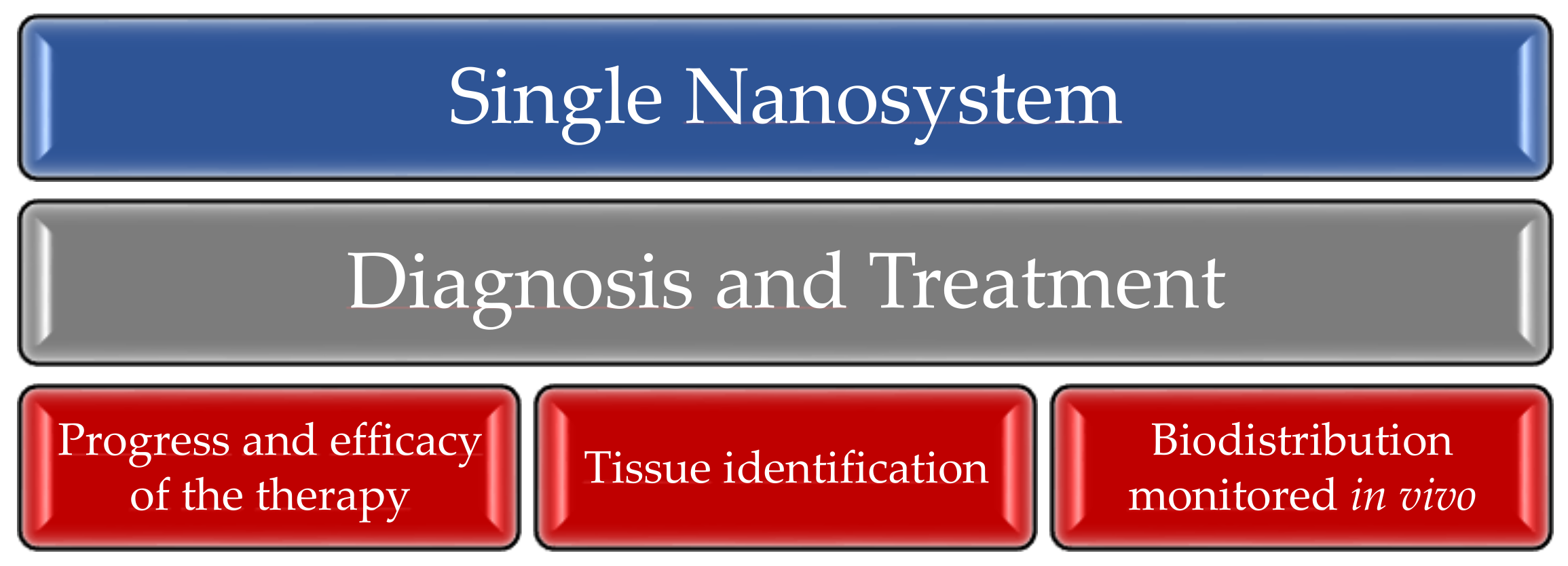
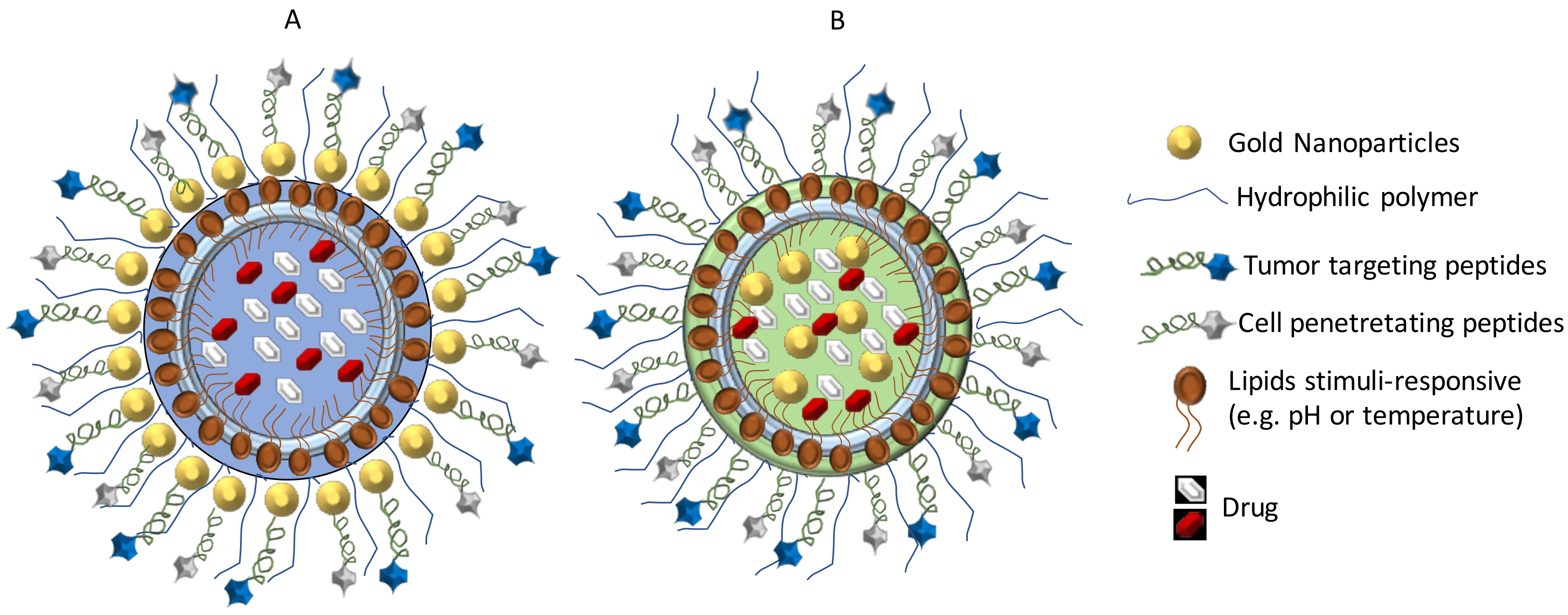

| Current Treatments | Pros | Cons | References |
|---|---|---|---|
| Surgical resection | Significant increase in survival rate. Possibility to apply radio-and chemotherapy, in order to remove residual tumor cells. | Damage of the surrounding cortex or brainstem structures, due to the diffuse nature of the tumor and inability to remove it. | [5] |
| Radiotherapy | Most frequent treatment. | Tumor response depends on its size. Acute side effects, such as damage of the epithelial surface, mouth, throat, gastrointestinal ulcers, swelling and infertility. Late effects, including fibrosis, hair loss, lymphedema and heart diseases. Effect only on the periphery of the tumor, with the core still being able to proliferate. | [6] |
| Chemotherapy | Cytotoxic and cytostatic agents act in tumor cells death through multiple mechanisms: angiogenesis, pro-differentiation, growth factor pathways and inhibition of tumor invasion. (e.g., temozolomide) | Several side effects including nerve damage, nausea, hair loss, vomiting, infertility, diarrhea, insomnia and skin rash. Effect only on the periphery of the tumor, with the core still being able to proliferate. | [6] |
| Hyperthermia | Tumor cell eradication based on generation of heat at the target site. It induces physiological changes, which lead to their apoptosis. Temperature ranges from 41 °C to 46 °C, activating many intracellular and extracellular degradation mechanisms. | Late effects including problems associated with heart, blood vessels, and other major organs. | [6,7] |
| Immunotherapy | Promotion of an enhanced anti-tumor immune response with an adequate antigen presentation, and circumvention of immunosuppressive mechanisms. Immunotherapy may include:T-Cell based vaccine therapies: EGFRvIII vaccine, heat-shock protein (HSP) vaccine, dendritic cell (DC) vaccines, adoptive T-cell therapy. Immune Checkpoint Inhibition: Anti-PD1, anti-CTLA4. Adoptive T-Cell Therapy: chimeric antigen receptors (CARs) targeting proteins (IL-13 receptor, Her2, EphA2, and EGFRvIII. | Low response rates: only a relatively reduced fraction of patients obtain clinical benefit. Potential increase in the magnitude, frequency, and onset of side effects. Severe immunological reactions, including a systemic cytokine release syndrome (“cytokine storm”), cause a delayed and/or inappropriate response, and may contribute to tissue damage. | [6,8,9,10,11,12,13] |
| Gene Therapy | Direct inhibition of the expression of oncogenes and normalization of tumor suppressor gene expression. Gene therapy include: Suicide genes: HSV-TK, CDA, carboxypeptidase G2 and CYP450. Immunomodulatory genes: IFN-beta, IL-4, -12, -18, -23. Oncolytic virotherapy: Herpes simplex virus, CR adenovirus, measles virus.Tumor-suppressor genes: p53, p16, p27 and PTEN. | Deficiency of antigen presenting cells inside the brain. Inefficient distribution, resulting in a poor delivery of a gene to the tumor cells. | [14,15,16,17] |
| Type of Transport | Example Ligands | Biological Significance | Reference |
|---|---|---|---|
| Glucose receptors (GLUT) | Mannose; Glucose | GLT1 targeting occurs when the NPs are coated with mannose | [47,48] |
| Monocarboxylate transporter (MCT) | Lactate; Short-chain fatty acids; Biotin; Salicylic acid; Valproic acid; Phenylbutyrate; 3,5,3’-triiodo-l-thyronine | MCT inhibitors: MCT1 and MCT2 would play a role in tumor maintenance; MCT4 would increase tumor aggressiveness | [49] |
| Neutral amino acids transporter (NAAT) | Tyrosine; Thyroid hormones (e.g., triiodothyronine); Asparagine; Histidine; Isoleucine; Leucine; Methionine; Phenylalanine; Threonine; Tryptophan | Transportation of neutral amino acids and some drugs, such as L-dopa and anticonvulsant gabapentin | [50,51,52,53] |
| Cationic amino acids transporter (CAATs) | Arginine; Lysine | [43] | |
| Anionic amino acids transporters (AAATs) | l-glutamate; l-aspartate | [44,54] | |
| ßeta amino acid transporter (ßAATs) | Beta (β)-alanine | [44] | |
| Choline transporter (ChT) | Choline; Thiamine | Support of the neurological supplies of brain | [45,46] |
| Peptide transporters (PT) | Oligopeptide transporters (e.g., PepT1, PepT2); Polypeptide transport system (e.g., Oatp2, OAT-K1, OATP) | Covalently linked to a vehicle: chlorotoxin-based strategies | |
| Fatty acid transporters (FAT) | Fatty acids | Glioma cells use fatty acids as a substrate for energy production. Targeting lipid metabolism is a promising approach in treating malignant gliomas Energetic role: stimulate numerous neural functions, or performing potential pharmacological targeting | [55,56,57,58] |
| Nucleoside transporters (NTs) | Nucleoside transporters (ENT1, ENT2, ENT3, and ENT4); Concentration nucleoside transporters (CNT2) | NTs act as second messengers in many signal transduction pathways, in maintaining the homeostasis of the nucleosides within the CNS, such as adenosine, to keep it available to bind to receptors Important for recycling pathways for nucleosides transportation into CNS tissue that brain cannot synthesize | [59] |
| CPPs | Sequence | Mechanism of Action | Type of Interaction | TTP | References |
|---|---|---|---|---|---|
| Transactivating-transduction (TAT) | AYGRKKRRQRRR | Endocytosis Micropinocytosis Pore formation | RGD | [106,107,108,109] | |
| R8 (cell penetrating peptide octa-arginine) | RRRRRRRR | Affinity to neuropilin-1 (NRP-1) | RGD | [110] | |
| IL-13p (Interleukin 13 peptide) | TAMRAVDKLLLHLKKLFREGQFNRNFESIIICRDRT | Affinity to IL13Rα2 receptor | [111] | ||
| LIMK2 NoLS (nucleolar translocation signal (NoLS) sequence of the LIM Kinase 2 (LIMK2)) | KKRT LRKN DRKK RC | [112,113] | |||
| Leptin30 | C-terminal (YQQVLTSLPSQNVLQIANDLENLRDLLHLLC) | transcytosis across the BBBmediated endocytosis pathway. | [114] | ||
| peptide1-NS∆ | TCTWLKYH | (unknown) | [100] | ||
| D(KLAKLAK)2 | Disruption of the mitochondria membrane | NGR CGKRK | [115] | ||
| pVec | LLIILRRRIRKQAHAHSK-NH2 | non-endocytic pathway | gHoPe2 | [104] | |
| Penetratin | CKRRMKWKK | Direct penetration, endocytosis | [116,117] | ||
| NFL-TBS | Direct penetration, endocytosis | [118] | |||
| SynB1 SynB3 | RGGRLSYSRRRFSTSTGR RRLSYSRRRF | Direct penetration, endocytosis | Conjugated with elastin-like polypeptide | [101,119] | |
| Transportan 10 (TP10) | AGYLLGKINLKALAALAKKIL | Membrane Potential | - | [120] | |
| D-Maurocalcine (D-MCa) | Cys3−Cys17, Cys10−Cys21, and Cys16−Cys32 (Positive-charge) | Membrane Potential | - | [121] | |
| DK17 | DRQIKIWFQNRRMKWKK-NH2 | Membrane Potential | - | [122] | |
| CB5005 | KLKLALALALAVQRKRQKLMP | Membrane Potential | blocking agent of the NF-kB pathway | - | [123] |
| ACP | R8-EEEEEEEE (E8) | Membrane Potential (R8) | MMP-responsive (E8) | Angiopep-2 | [124] |
| GPLGVRGDG | MMP-responsive | RGD | [125] | ||
| polyarginine peptides | (NH2-RRRRGRRRRKGC) | MMP-responsive | [126] | ||
| (CKRRMKNvocWKNvo0cKNvoc) | Derived from CKRRMKWKK | cellular uptake after rapidly cleaving the photolabile-protective group. | photo-responsiveness | [98] | |
| LNP | KKRT LRKN DRKK RC | nucleolar translocation signal | pH-responsive | [112] | |
| H7K(R2)2 | RRK(HHHHHHH)RR | cross cell membranes in a seemingly energy-independent manner | pH-responsive | [127,128] | |
| TH | (AGYLLGHINLHHLAHL(Aib)HHIL-NH2 | histidine-rich TH peptide | pH-responsive | RGD | [102,129,130] |
| R6H4 | RRRRRRHHHH | Clathrin-mediated Caveolae/lipid raft-mediated | pH-responsive | HA | [131] |
| GB Phases | Morphology BBTB | Permeability BBTB | |
|---|---|---|---|
| First phase | Initial phase of malignant brain tumors | Brain capillaries provide enough nutrients for their growth. The capillaries are continuous and non-fenestrated. | BBTB integrity is not compromised |
| Second phase | Progression of the tumor | Cancer cells invade neighboring healthy cerebral tissues. High metabolic demands. Tumor volume increase. New capillaries have fenestrations (12 nm) | BBTB integrity is compromised, increasing the permeability and the molecules with size below 12 nm may pass through. |
| Third phase | Tumor growth | Inter-endothelial gaps are formed between cerebral endothelial cells Fenestration size increase from 48 nm to 1 µm. | BBTB damage and enhanced permeability and retention (EPR) effect favors NPs accumulation in the tumor tissues. |
| Targeting | Molecules | References |
|---|---|---|
| BBB | ||
| Ecs; P32; Neuropin (NRP-1/2); Aminopeptidase N (CD13) | Wheat germ agglutinin (WGA), Folic acid (FA), CVNHPAFACGYGHTMYYHHYQHHL-NH2, TNG; (TGNYKALHPHNG); LyP-1 (CGNKRTRGC); iNGR (CRNGRGPDC), L-peptide A7R (termed LA7R); NGR | [285,286,287,288,289,290,291,292] |
| Glucose receptors (GLUT) | 2-deoxy-D-glucose; Mannose | [293,294] |
| Transferrin receptor (Tfr) | T7 (HAIYPRH); Angiopep-2; Transferrin (Tfpep); Melanotransferrin (MT); OX26 and RI7217 | [64,65,66,251,295,296,297,298] |
| Low density lipoprotein receptor (LDLR) | Polypeptide LHRH-ELP-C8; Trichosanthin (TCS); Angiopep-2 (TFFYGGSRGKRNNFKTEEY); Lactoferrin (Lf); apolipoprotein E (ApoE); Polysorbate 80; polyoxyethylene sorbitol oleate (PSO) | [73,74,75,76,77,78,79,124,285,298,299,300] |
| Insulin receptor (IR) | Cixutumumab; GL56; 83-14 Mab; 29B4 Mab | [79,80,301,302] |
| ABC (ATP-binding cassette) | d-Alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS); Polysorbate 80; Polyoxyethylene sorbitol oleate (PSO) | [77,78,79,80,299,303,304,305] |
| TME | ||
| Hypoxic response | HIF-1α inhibitor; EZN-2968; Hsp90; CAIX inhibitor; LyP-1 (CGNKRTRGC) | [181,306,307] |
| Acid pH | TH peptide (AGYLLGHINLHHLAHL [Aib]HHIL-NH2); pHLIP (ACEQNPIYWARYADWLFTTPLLLLDLALLVDADET); R6H4; H7K(R2)2; LNP | [96,102,112,127,128,129,130,131,279] |
| Enzymatic alterations | R9; TAT; LMWP; ACP; GPLGVRGDG; polyarginine peptides | [124,125,126,280,281,282] |
| Temperature | L-zipper peptide; (VSSLESKVSSLESKVSKLESKKSKLESKVSKLESKVSSLESK); ELP (VPGXG) | [308,309,310] |
| BBTB | ||
| Integrins | RGD; c(RGDyK); NGR | [146,150,286,311,312,313,314,315,316,317,318,319] |
| 13 receptorα2 (IL-13Rα2) | Pep-1 (CGEMGWVRC) | [320,321] |
| EGFR | Cetuximab; Inhibitor of COX-2 (e.g., Celecoxib); 125I-mab 425; PKI166; Canertinib; Pelitinib Monoclonal antibody C225; D(KLAKLAK)2; Epidermal growth factor (egfpep) | [251,322,323,324,325] |
| EGFR variant III mutant (EGFRVII) | F25P preproinsulin; CAR-engineered NK cell lines such as NK-92; Rindopepimut; D2C7-IT; PEPvIII (H-Leu-Glu-Glu-Lys-Lys-Gln-Asn-Tyr-Val-Val-Thr-Asp-His-Cys-OH) | [326,327,328,329] |
| VEGF-1, -2 | Vatalanib; Bevacizumab; Tivozanib; L-peptide A7R (termed LA7R); K237 (HTMYYHHYQHHL-NH2) | [287,330,331,332] |
| ECM | ||
| Matrix metalloproteinases (MMPs) | inhibitor of matrix protease-3 (TIMP-3); histone deacetylase inhibitors; Chlorotoxin; LMWP | [253,333,334,335,336] |
| Fibrin | cysteineearginineeglutamic acidelysineealanine (CREKA) | [337,338] |
| Tenascin-C | FHK (FHKHKSPALSPV) | [143,339] |
| Angiopoietin 2 | MEDI3617; AMG780; Nesvacumab (REGN910); CVX-060 | [332,340] |
| Cox-2 | COX-2 inhibitors: celecoxib; Alantolactone; Plumbagin | [341,342,343] |
| NF-kB | Baicalein (BA); DYT-40; SN50; Natural compounds: Curcumin, Resveratrol, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO); Non-steroidal anti-inflammatory drugs: Irinotecan and celecoxib; Sulfasalazine, Disulfiram, Glutathione; Antibodies (bortezomib, lactacystin, and MG132) | [153,189,344,345,346,347] |
| TAMS | ||
| Cxcl12/cxcr4 | Plenrixafor (DY-[NMe]DOrn-R-2Nal-G); Peptide R (RACRFFC); NT21MP (LGASWHRPDKCCLGYQKRPLP); Nef-M1 (NAACAWLEAQ) | [167,348,349,350] |
| Nrp-1/2 | LyP-1 (CGNKRTRGC); TT1 (CKRGARSTC); Plumbagin | [351] |
| Multi-ligand endocytic receptor mannose receptor (CD206/MRC1) | CSPGAKVRC | [352] |
| Colony stimulating factor 1 (CSF1) | Emactuzumab (RG7155); AMG820; IMC-CS4 (LY3022855); PLX7486 | [353] |
| CD11b+ F4/80high M2-like tams | M2pep (YEQDPWGVKWWY) | [354,355] |
| CSF-1r | RG7155 | [164] |
| Tumor fibroblasts | ||
| Fibroblast growth factor (FGF) | brivanib, nindetanib, cediranib, lenvatinib, sulfatinib, dovitinib, ponatinib and lucitanib; SSR12819E; C2KG2R9 | [356,357,358,359] |
| Glioma Stem Cells, Invasion and Metastasis | ||
| VEGFR | D16F7 | [360] |
| CD44 | HA; Peptide 7 (FNLPLPSRPLLR) | [154,361] |
| CD133 | GL1 (LLADTTHHRPWT); CBP4 | [193,362] |
| PI3k/Akt/mTor | PP242, P30 and NVP-BEZ235; Temsirolimus; Sirolimus; Everolimus; XL765 (SAR245409) | [363,364] |
| Transforming growth factor-beta (TGF-β) | AP-12009; SD-208, SB-431542; PDX; LY2109761 and LY364947 (HTS466284) | [365,366,367] |
| Platelet-derived growth factor (PDGF) | MEDI-575; Tandutinib (MLN518); Crenolanib (CP-868-596) | [368,369,370] |
| Hedgehog | GL1 peptide (LLADTTHHRPWT); CVNHPAFAC-NH2; CK (CVNHPAFAC-HTMYYHHYQHHL) | [287,371,372] |
| Mammary-derived growth inhibitor (MDGI) | CooP (CGLSGLGVA) | [373] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, M.; Sousa, J.J.; Pais, A.; Vitorino, C. Targeted Theranostic Nanoparticles for Brain Tumor Treatment. Pharmaceutics 2018, 10, 181. https://doi.org/10.3390/pharmaceutics10040181
Mendes M, Sousa JJ, Pais A, Vitorino C. Targeted Theranostic Nanoparticles for Brain Tumor Treatment. Pharmaceutics. 2018; 10(4):181. https://doi.org/10.3390/pharmaceutics10040181
Chicago/Turabian StyleMendes, Maria, João José Sousa, Alberto Pais, and Carla Vitorino. 2018. "Targeted Theranostic Nanoparticles for Brain Tumor Treatment" Pharmaceutics 10, no. 4: 181. https://doi.org/10.3390/pharmaceutics10040181
APA StyleMendes, M., Sousa, J. J., Pais, A., & Vitorino, C. (2018). Targeted Theranostic Nanoparticles for Brain Tumor Treatment. Pharmaceutics, 10(4), 181. https://doi.org/10.3390/pharmaceutics10040181







