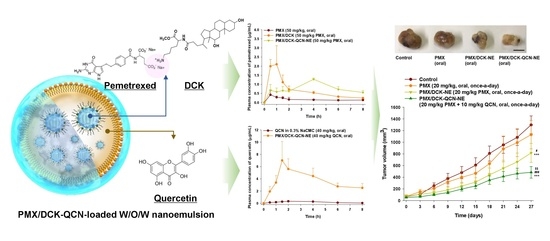
Preparation, Characterization, and In Vivo Evaluation of an Oral Multiple Nanoemulsive System for Co-Delivery of Pemetrexed and Quercetin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
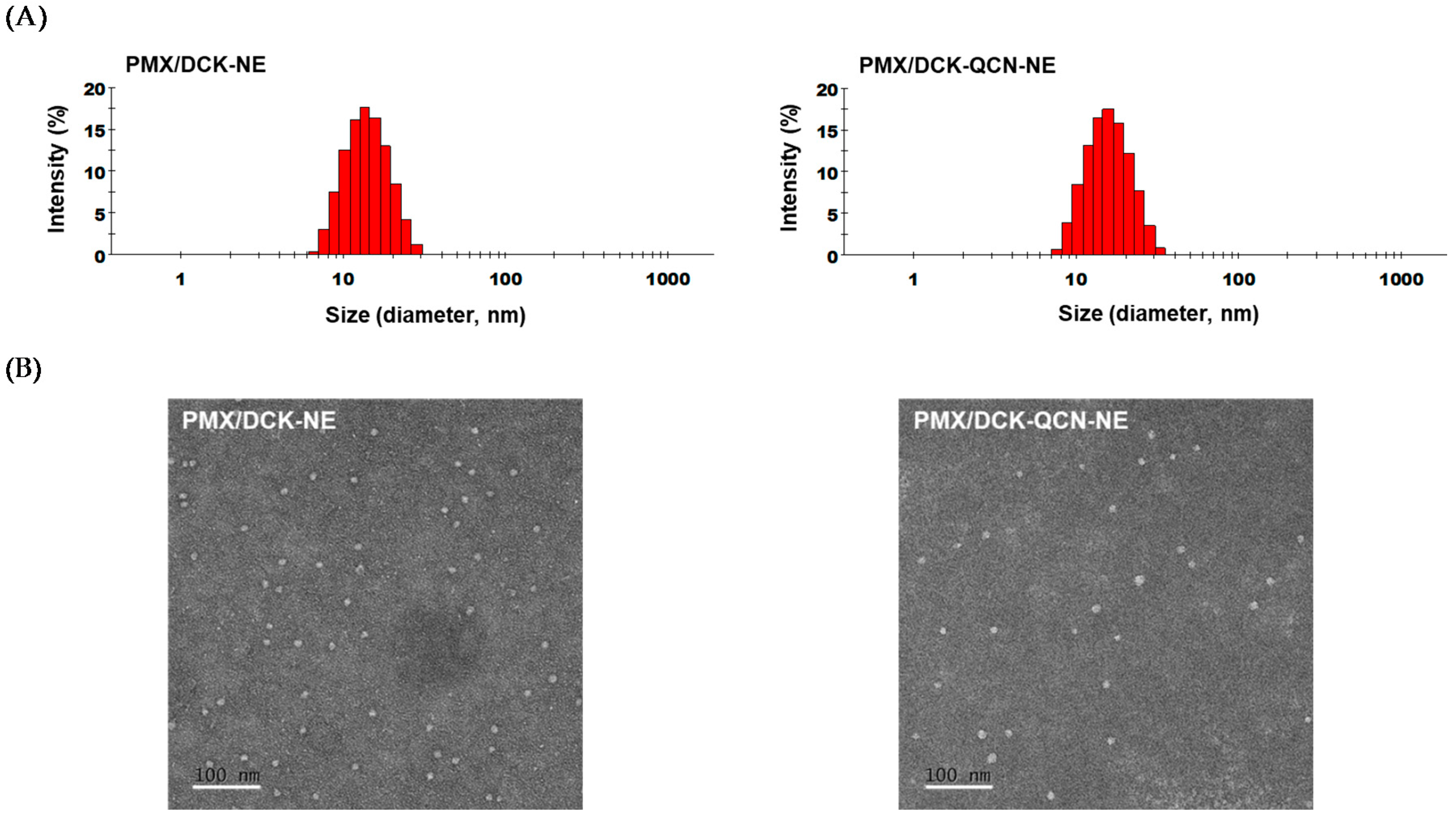
2.3. Preparation and Characterization of PMX/DCK-QCN-NE
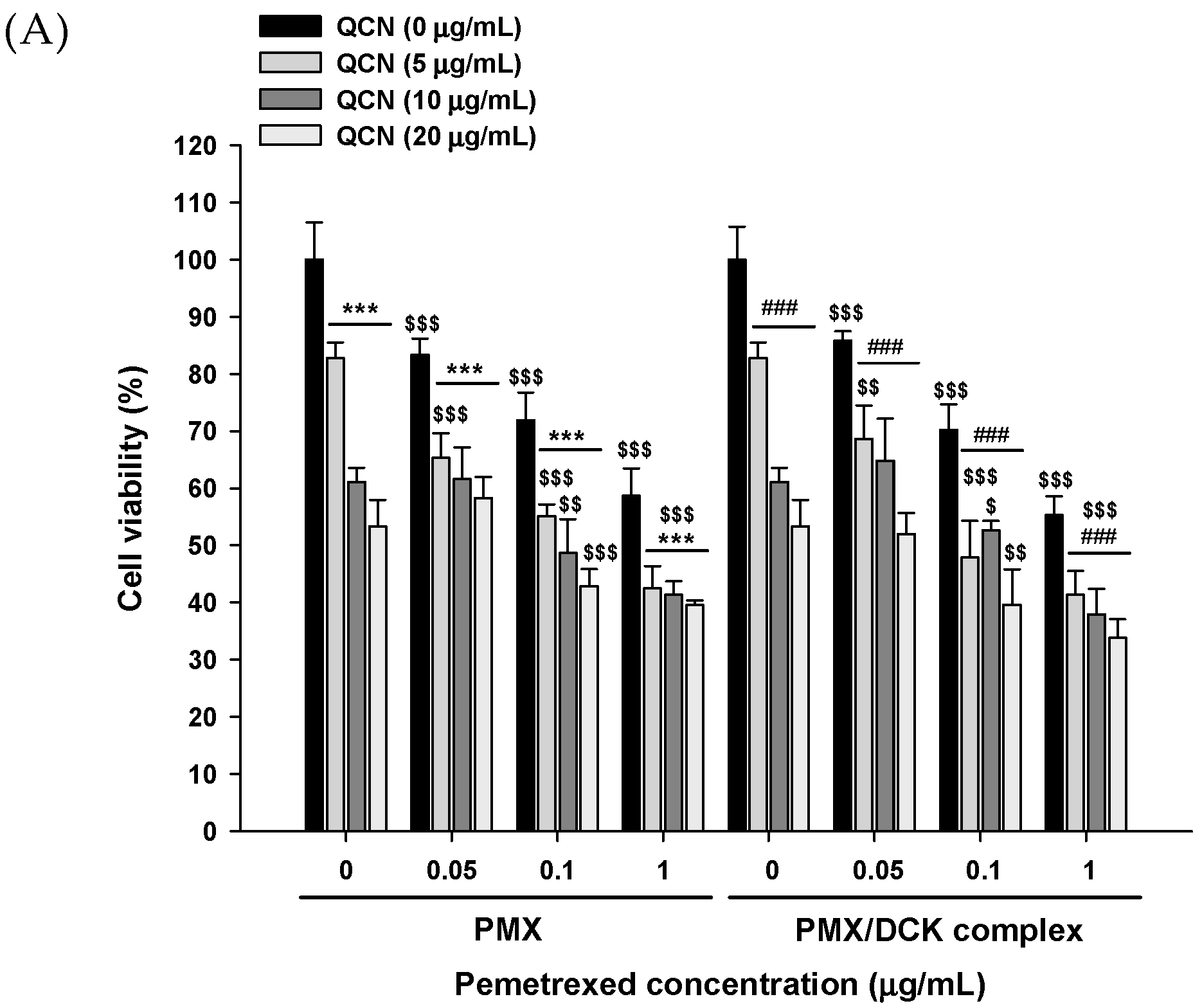
2.4. In Vitro Cytotoxicity
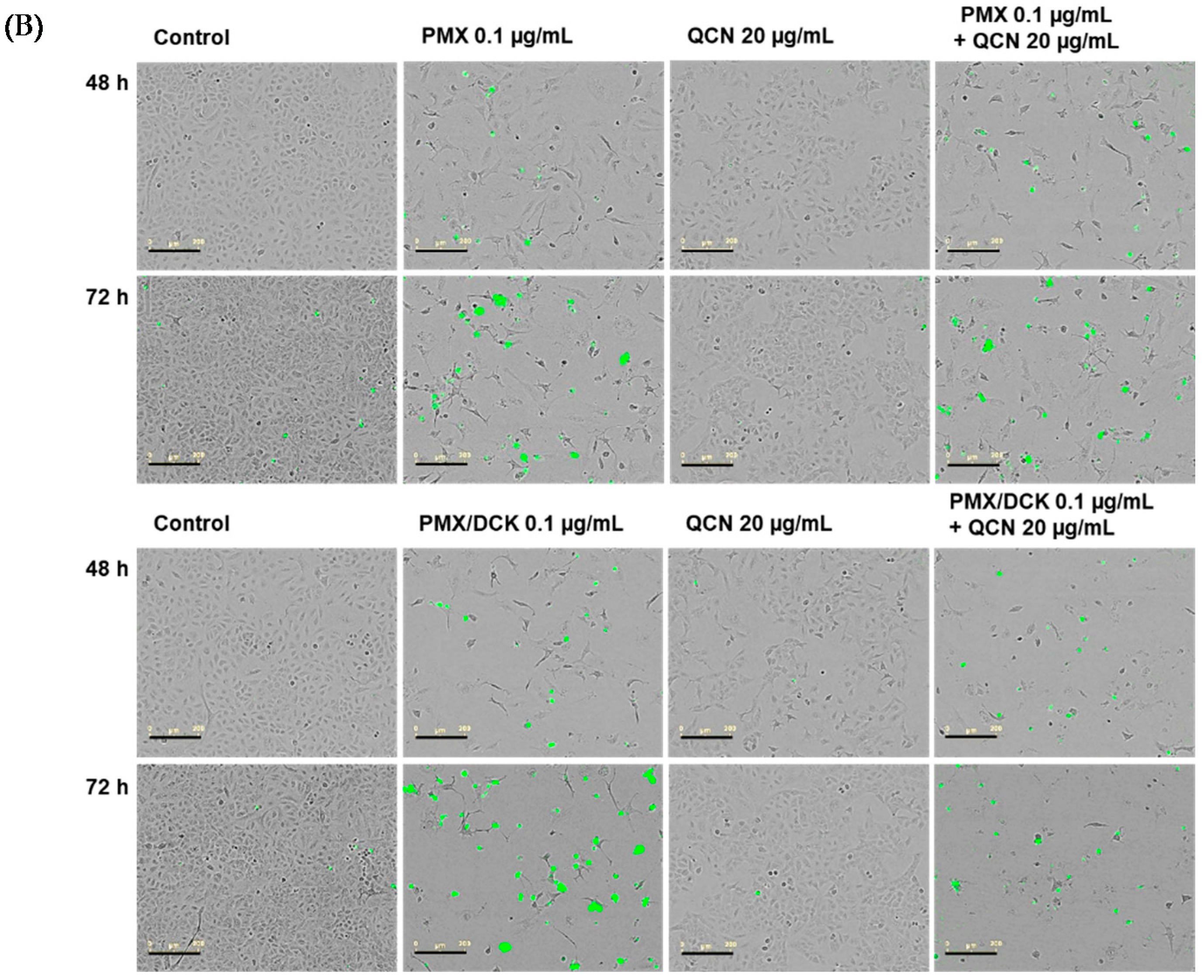
2.5. In Vitro Apoptosis Assay
2.6. In Vitro Inhibitory Effect on Cancer Cell Proliferation and Migration
2.7. In Vitro Permeability Across an Artificial Intestinal Membrane and Caco-2 Cell Monolayer
2.8. Cellular Uptake by Caco-2 and ASBT-Transfected MDCK Cells
2.9. Oral Absorption in Rats
2.10. In Vivo Antitumor Efficacy in Mice
2.11. Pharmacokinetic and Statistical Analyses
3. Results and Discussion
3.1. Preparation and Characterization of PMX/DCK-QCN-NE
3.2. In Vitro Cytotoxic Effects of PMX, PMX/DCK, and QCN
3.3. In Vitro Inhibition of Cancer Cell Proliferation and Migration
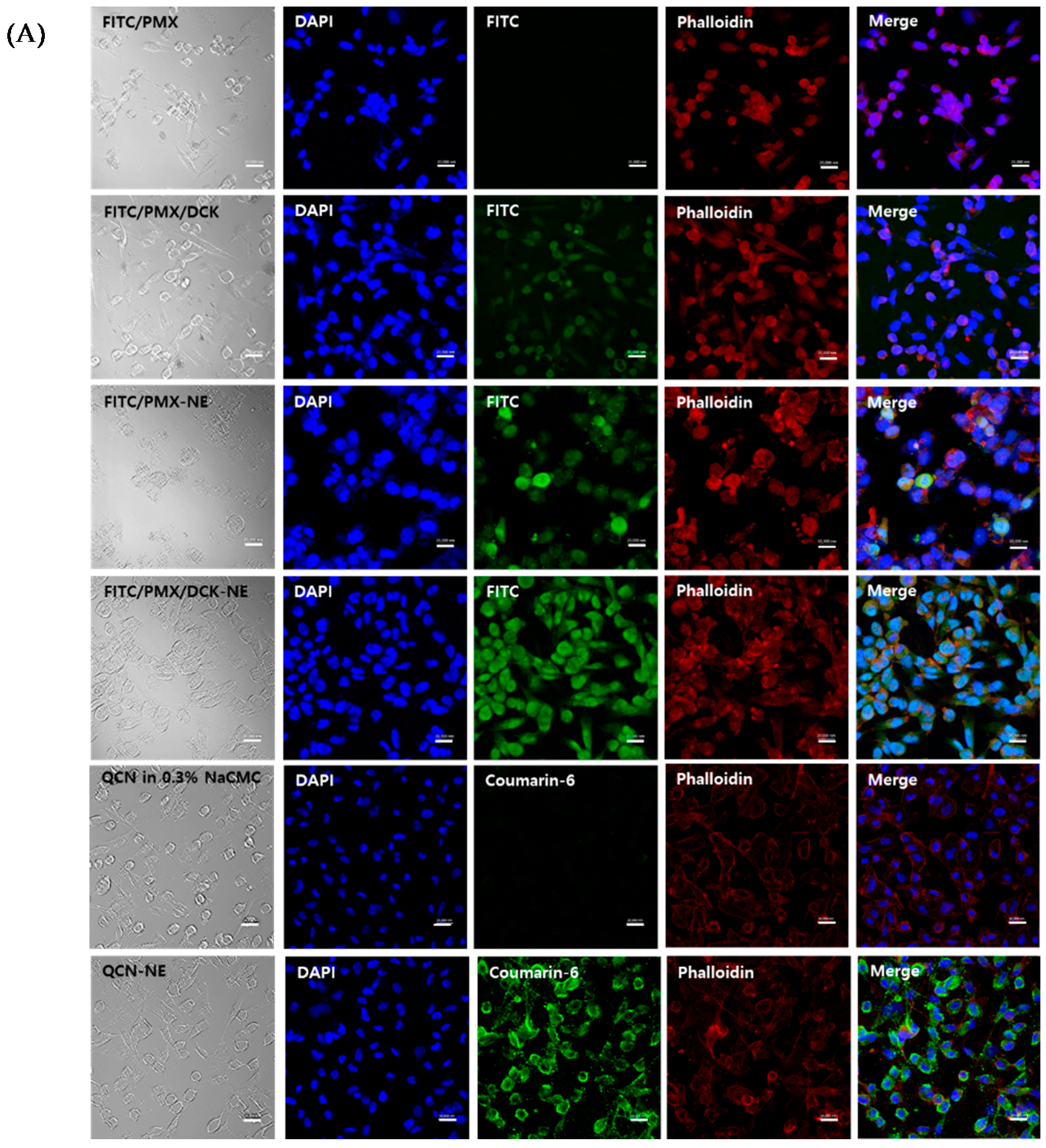
3.4. In Vitro Membrane Permeability
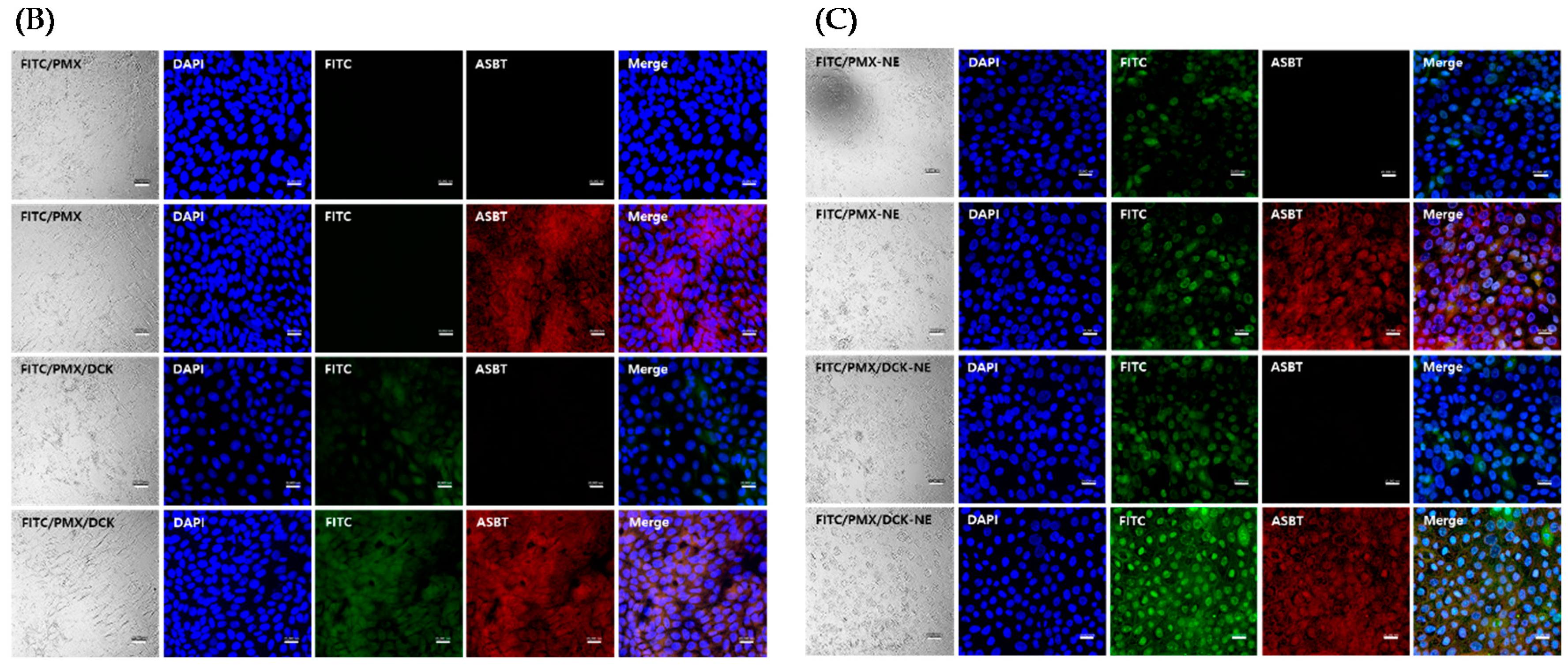
3.5. Uptake into Caco-2 and ASBT-Expressing MDCK Cells
3.6. Oral Absorption in Rats
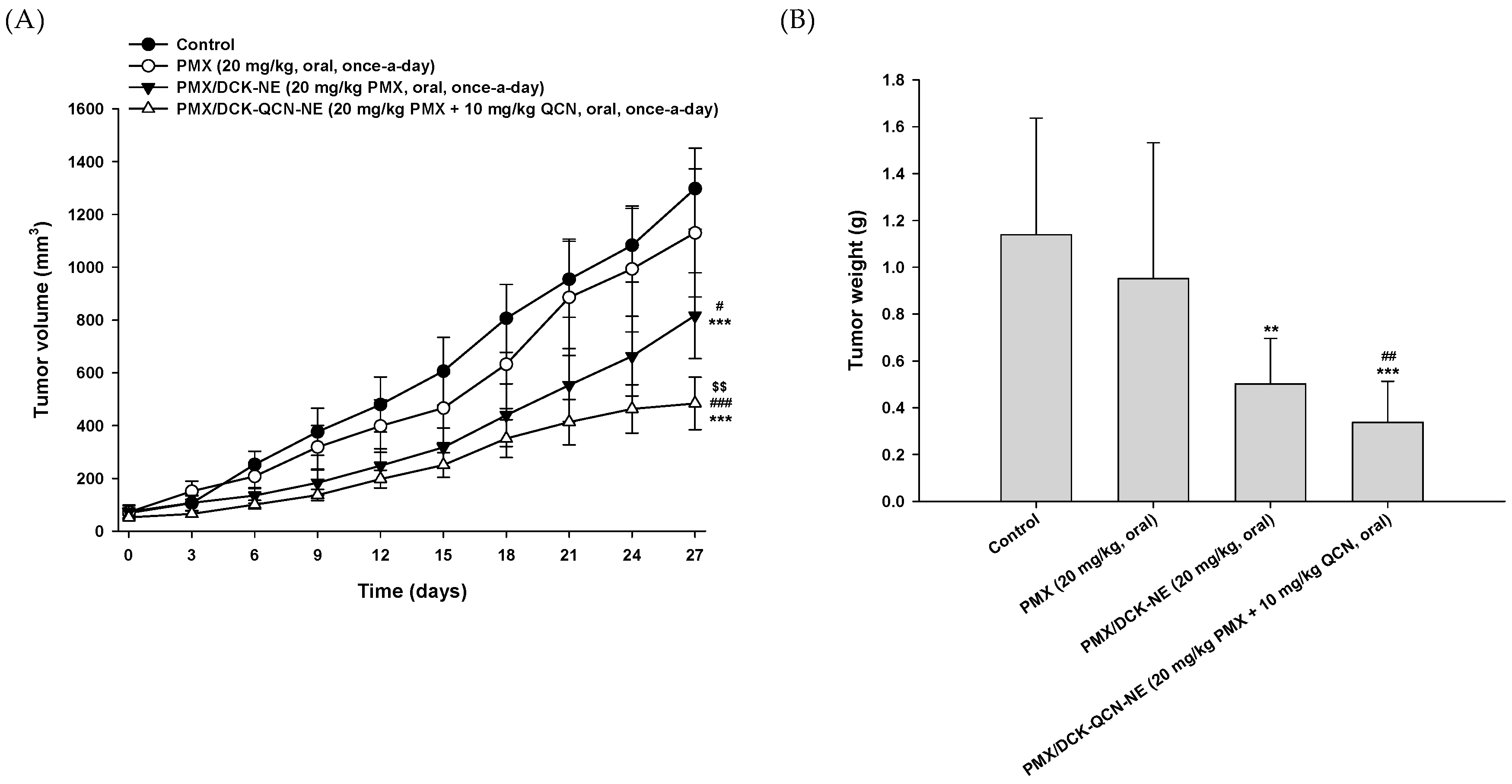
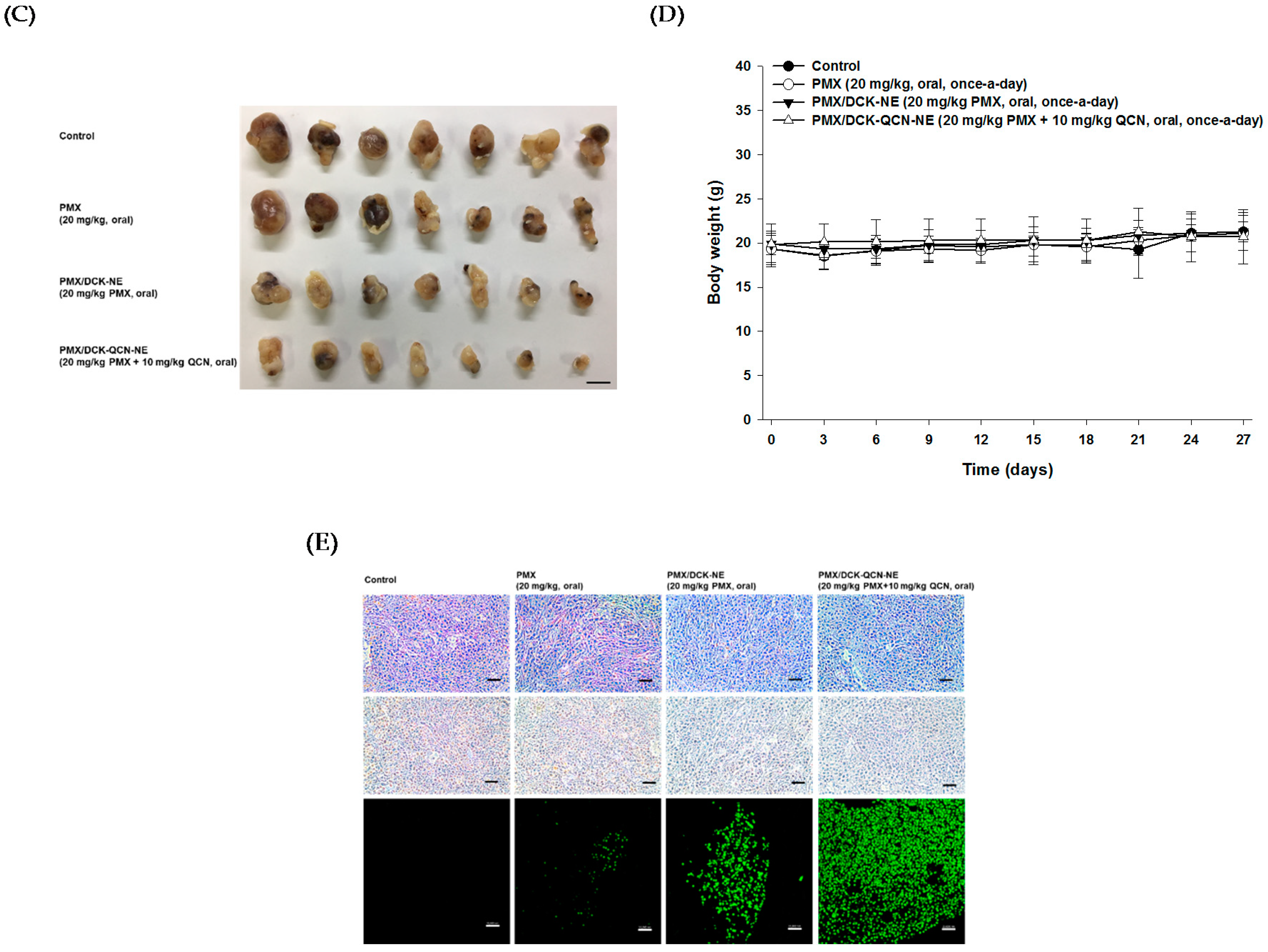
3.7. In Vivo Tumor Growth Inhibition Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, K.; Song, X.; Yang, L.; Li, L.; Wan, Z.; Sun, X.; Gong, T.; Lin, Q.; Zhang, Z. Enhanced antitumor and anti-metastasis efficacy against aggressive breast cancer with a fibronectin-targeting liposomal doxorubicin. J. Control. Release 2018, 271, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, J.; Hao, Y.; Zhao, Y.; Qiu, Y.; Jiang, J.; Yu, T.; Ji, P.; Liu, Y. Application of a lipid-coated hollow calcium phosphate nanoparticle in synergistic co-delivery of doxorubicin and paclitaxel for the treatment of human lung cancer A549 cells. Int. J. Nanomed. 2017, 12, 7979–7992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Fai Chan, H.; Xie, W.; Chen, S.; He, C.; Wang, Y.; Chen, M. Co-delivery of paclitaxel and tetrandrine via iRGD peptide conjugated lipid-polymer hybrid nanoparticles overcome multidrug resistance in cancer cells. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska-Wisniewska, A.; Halas-Wisniewska, M.; Izdebska, M.; Gagat, M.; Grzanka, A.; Grzanka, D. Antiproliferative and antimetastatic action of quercetin on A549 non-small cell lung cancer cells through its effect on the cytoskeleton. Acta Histochem. 2017, 119, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Cooray, H.C.; Janvilisri, T.; van Veen, H.W.; Hladky, S.B.; Barrand, M.A. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem. Biophys. Res. Commun. 2004, 317, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, J.; Ji, C. Quercetin: A potential drug to reverse multidrug resistance. Life Sci. 2010, 87, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Chemotherapy and dietary phytochemical agents. Chemother. Res. Pract. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, X.; Yang, L.; Mao, Y.; Wei, Y.; Chen, L. Co-delivery of doxorubicin and plasmid by a novel FGFR-mediated cationic liposome. Int. J. Pharm. 2010, 393, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Batlle, J.F.; Arranz, E.E.; De Castro Carpeño, J.; Sáez, E.C.; Auñón, P.Z.; Sánchez, A.R.; Barón, M.G. Oral chemotherapy: Potential benefits and limitations. Revista de Oncología 2004, 6, 335–340. [Google Scholar] [CrossRef]
- Cardoso, F.; Colleoni, M.; Di Leo, A.; Francia, G.; Gennari, A.; Gligorov, J.; Llombart, A. Oral chemotherapy in advanced breast cancer: Expert perspectives on its role in clinical practice. Cancer Treat. Commun. 2016, 6, S1–S10. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, D.; Wang, D.; Wang, Y.; Fu, Q.; Fallon, J.K.; Yang, X.; He, Z.; Liu, F. Combinational delivery of hydrophobic and hydrophilic anticancer drugs in single nanoemulsions to treat MDR in cancer. Mol. Pharm. 2014, 11, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Kuppens, I.E.; Bosch, T.M.; van Maanen, M.J.; Rosing, H.; Fitzpatrick, A.; Beijnen, J.H.; Schellens, J.H. Oral bioavailability of docetaxel in combination with OC144-093 (ONT-093). Cancer Chemother. Pharmacol. 2005, 55, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Garbuzenko, O.B.; Minko, T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine 2008, 3, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Zhang, M.; Wei, D.; Stueber, D.; Taratula, O.; Minko, T.; He, H. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small 2009, 5, 2673–2677. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thanki, K.; Jain, S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: Implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol. Pharm. 2013, 10, 3459–3474. [Google Scholar] [CrossRef] [PubMed]
- Adjei, A.A. Pharmacology and mechanism of action of pemetrexed. Clin. Lung Cancer 2004, 5, S51–S55. [Google Scholar] [CrossRef] [PubMed]
- Hanauske, A.R.; Chen, V.; Paoletti, P.; Niyikiza, C. Pemetrexed disodium: A novel antifolate clinically active against multiple solid tumors. Oncologist 2001, 6, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.; Ma, Y.; Yu, A.; Cai, F.; Shao, W.; Zhai, G. Formulation optimization and in situ absorption in rat intestinal tract of quercetin-loaded microemulsion. Colloids Surf. B Biointerfaces 2009, 71, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Sharma, A.R.; Nguyen, L.T.; Chakraborty, C.; Sharma, G.; Lee, S.S. Application of bioactive quercetin in oncotherapy: From nutrition to nanomedicine. Molecules 2016, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Daker, M.; Ahmad, M.; Khoo, A.S. Quercetin-induced inhibition and synergistic activity with cisplatin—A chemotherapeutic strategy for nasopharyngeal carcinoma cells. Cancer Cell Int. 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Demiroglu-Zergeroglu, A.; Ergene, E.; Ayvali, N.; Kuete, V.; Sivas, H. Quercetin and cisplatin combined treatment altered cell cycle and mitogen activated protein kinase expressions in malignant mesotelioma cells. BMC Complement. Altern. Med. 2016, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, W.; Yang, T.; Wen, B.; Ding, D.; Keidar, M.; Tang, J.; Zhang, W. Paclitaxel and quercetin nanoparticles co-loaded in microspheres to prolong retention time for pulmonary drug delivery. Int. J. Nanomed. 2017, 12, 8239–8255. [Google Scholar] [CrossRef] [PubMed]
- Nessa, M.U.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Synergism from combinations of cisplatin and oxaliplatin with quercetin and thymoquinone in human ovarian tumour models. Anticancer Res. 2011, 31, 3789–3797. [Google Scholar] [PubMed]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Kavuru, P.; Wojtas, L.; Zaworotko, M.J.; Shytle, R.D. Cocrystals of quercetin with improved solubility and oral bioavailability. Mol. Pharm. 2011, 8, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Caibiao, H.; Airui, Q.; Tian, L.; Hong, Z.; Yali, Z.; Qiang, X. Enhanced oral bioavailability of quercetin by a new non-aqueous self-double-emulsifying drug delivery system. Eur. J. Lipid Sci. Technol. 2017, 119, 1–12. [Google Scholar]
- Pangeni, R.; Kang, S.-W.; Oak, M.; Park, E.Y.; Park, J.W. Oral delivery of quercetin in oil-in-water nanoemulsion: In vitro characterization and in vivo anti-obesity efficacy in mice. J. Funct. Foods 2017, 38, 571–581. [Google Scholar] [CrossRef]
- Wang, J.; Shi, A.; Agyei, D.; Wang, Q. Formulation of water-in-oil-in-water (w/o/w) emulsions containing trans-resveratrol. RSC Adv. 2017, 7, 35917–35927. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Wu, Y.; Hu, Y.L.; Nan, K.; Nie, G.; Chen, H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials 2011, 32, 8281–8290. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.C.; Seo, D.H.; Kim, H.S.; Byun, Y.; Park, J.W. Oral delivery of zoledronic acid by non-covalent conjugation with lysine-deoxycholic acid: In vitro characterization and in vivo anti-osteoporotic efficacy in ovariectomized rats. Eur. J. Pharm. Sci. 2016, 82, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, F.; Jeon, O.C.; Al-Hilal, T.A.; Kweon, S.; Yang, V.C.; Lee, D.S.; Byun, Y. Absorption mechanism of a physical complex of monomeric insulin and deoxycholyl-l-lysyl-methylester in the small intestine. Mol. Pharm. 2015, 12, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hwang, S.R.; Jeon, O.C.; Moon, H.T.; Byun, Y. Enhanced oral absorption of ibandronate via complex formation with bile acid derivative. J. Pharm. Sci. 2013, 102, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Haq, N.; Al-Dhfyan, A.; Alanazi, F.K.; Alsarra, I.A. Double w/o/w nanoemulsion of 5-fluorouracil for self-nanoemulsifying drug delivery system. J. Mol. Liq. 2014, 200, 183–190. [Google Scholar] [CrossRef]
- Saxena, A.; Valicherla, G.R.; Joshi, P.; Saxena, R.; Cheruvu, S.H.; Bhunia, S.S.; Jain, G.K.; Siddiqui, H.H.; Saxena, A.K.; Gayen, J.R. Pharmacokinetics, dose proportionality and permeability of S002-333 and its enantiomers, a potent antithrombotic agent, in rabbits. Xenobiotica 2015, 45, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Spinks, C.B.; Zidan, A.S.; Khan, M.A.; Habib, M.J.; Faustino, P.J. Pharmaceutical characterization of novel tenofovir liposomal formulations for enhanced oral drug delivery: In vitro pharmaceutics and Caco-2 permeability investigations. Clin. Pharmacol. 2017, 9, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Bobin-Dubigeon, C.; Amiand, M.B.; Herrenknecht, C.; Bard, J.M. Development and validation of an improved liquid chromatography-mass spectrometry method for the determination of pemetrexed in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Bali, V.; Ali, M.; Ali, J. Study of surfactant combinations and development of a novel nanoemulsion for minimising variations in bioavailability of ezetimibe. Colloids Surf. B Biointerfaces 2010, 76, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Solans, C.; Morales, D.; Homs, M. Spontaneous emulsification. Curr. Opin. Colloid Interface Sci. 2016, 22, 88–93. [Google Scholar] [CrossRef]
- Seo, Y.G.; Kim, D.H.; Ramasamy, T.; Kim, J.H.; Marasini, N.; Oh, Y.K.; Kim, D.W.; Kim, J.K.; Yong, C.S.; Kim, J.O.; et al. Development of docetaxel-loaded solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced chemotherapeutic effect. Int. J. Pharm. 2013, 452, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.Y.; Wu, Y.C.; Chung, J.G.; Yang, J.S.; Lu, H.F.; Tsou, M.F.; Wood, W.G.; Kuo, S.J.; Chen, D.R. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp. Toxicol. 2009, 28, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Khuda-Bukhsh, A.R. Quercetin down-regulates IL-6/STAT-3 signals to induce mitochondrial-mediated apoptosis in a nonsmall- cell lung-cancer cell line, A549. J. Pharmacopunct. 2015, 18, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosisinducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.E.; Kim, Y.S.; Hwang, Y.R.; Kwon, S.J.; Park, D.S.; Cha, B.K.; Kim, B.R.; Yoon, K.H.; Jeong, E.T.; Kim, H.R. Enhanced apoptosis by pemetrexed and simvastatin in malignant mesothelioma and lung cancer cells by reactive oxygen species-dependent mitochondrial dysfunction and Bim induction. Int. J. Oncol. 2014, 45, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Bae, S.M.; Ahn, W.S. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch. Pharm. Res. 2008, 31, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Zhang, H.T.; Yang, W.F.; Li, Y.F.; Liu, C. Evaluation of METase-pemetrexed-loaded PEG-PLGA nanoparticles modified with anti-CD133-scFV for treatment of gastric carcinoma. Biosci. Rep. 2018, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fofaria, N.M.; Qhattal, H.S.; Liu, X.; Srivastava, S.K. Nanoemulsion formulations for anti-cancer agent piplartine–Characterization, toxicological, pharmacokinetics and efficacy studies. Int. J. Pharm. 2016, 498, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Monteforte, E.; Luceri, C.; Bigagli, E.; Bilia, A.R.; Bergonzi, M.C. Nanoemulsion for improving solubility and permeability of Vitex agnus-castus extract: Formulation and in vitro evaluation using PAMPA and Caco-2 approaches. Drug Deliv. 2017, 24, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.; Yan, G.; Wu, Y.; Li, J.; Fang, X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur. J. Pharm. Sci. 2005, 24, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Fu, L.; Zhu, Z.; Yang, Y.; Sun, B.; Shan, W.; Zhang, Z. Modified mixed nanomicelles with collagen peptides enhanced oral absorption of Cucurbitacin B: Preparation and evaluation. Drug Deliv. 2018, 25, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.-G.; Dong, L.C.; Song, Y. Enhanced bioavailability of poorly absorbed hydrophilic compounds through drug complex/in situ gelling formulation. Int. J. Pharm. 2013, 457, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Polli, J.E. Apical sodium dependent bile acid transporter (ASBT, SLC10A2): A potential prodrug target. Mol. Pharm. 2006, 3, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kim, S.K.; Al-Hilal, T.A.; Jeon, O.C.; Moon, H.T.; Byun, Y. Strategies for oral delivery of macromolecule drugs. Biotechnol. Bioprocess Eng. 2010, 15, 66–75. [Google Scholar] [CrossRef]
- Forsgard, R.A.; Korpela, R.; Stenman, L.K.; Osterlund, P.; Holma, R. Deoxycholic acid induced changes in electrophysiological parameters and macromolecular permeability in murine small intestine with and without functional enteric nervous system plexuses. Neurogastroenterol. Motil. 2014, 26, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Santoro, P.; Barone, M.V.; Pappacoda, S.; Barretta, M.L.; Nanayakkara, M.; Apicella, C.; Capasso, L.; Paludetto, R. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G906–G913. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Quercetin enhances intestinal barrier function through the assembly of zonula occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Sun, L.; Ding, Y.; Liu, X.; Zhang, Y.; Webster, T.J.; Zheng, C. A solid self-nanoemulsifying system of the BCS class IIb drug dabigatran etexilate to improve oral bioavailability. Nanomedicine 2016, 11, 1801–1816. [Google Scholar] [CrossRef] [PubMed]


| Test Material | Effective Permeability Across an Artificial Membrane (Pe, ×10−6, cm/s) | Apparent Permeability Across a Caco-2 Cell Monolayer (Papp, ×10−6, cm/s) |
|---|---|---|
| PMX in water | 6.03 ± 1.41 | 1.57 ± 0.749 |
| PMX/DCK | 15.3 ± 3.65 *** | 11.9 ± 1.47 *** |
| PMX/DCK-NE | 33.1 ± 0.739 ***,### | 15.8 ± 3.53 ***,# |
| QCN in water | 0.000± 0.000 | 0.550 ± 0.032 |
| QCN in 0.3% NaCMC | 1.20 ± 0.527 ** | 2.21 ± 0.533 |
| QCN-NE | 16.6 ± 0.621 ***,$$$ | 8.46 ± 2.21 ***,$$$ |
| Test Material | PMX | QCN | |||
|---|---|---|---|---|---|
| PMX in Water | PMX/DCK in Water | PMX/DCK-QCN-NE | QCN in 0.3% NaCMC | PMX/DCK-QCN-NE | |
| Administration | Oral | Oral | Oral | Oral | Oral |
| Dose of PMX or QCN (mg/kg) | 50 | 50 | 50 | 40 | 40 |
| Tmax (h) | 0.833 ± 0.577 | 0.667 ± 0.289 | 4.00 ± 0.000 ***,### | 2.00 ± 0.000 | 1.67 ± 0.289 |
| T1/2 (h) | 6.06 ± 1.83 | 4.10 ± 1.40 | 3.34 ± 0.589 | 4.83 ± 2.68 | 4.76 ± 0.551 |
| Cmax (μg/mL) | 0.452 ± 0.221 | 2.28 ± 1.05 ** | 1.28 ± 0.072 | 0.336 ± 0.122 | 7.38 ± 3.03 $$ |
| AUClast (μg∙h/mL) | 1.39 ± 0.395 | 5.49 ± 1.01 *** | 6.29 ± 0.677 *** | 1.26 ± 0.665 | 30.1 ± 7.57 $$$ |
| AUCinf (μg∙h/mL) | 2.56 ± 0.755 | 6.99 ± 1.63 ** | 9.02 ± 1.38 *** | 2.60 ± 1.03 | 47.6 ± 12.9 $$$ |
| Relative bioavailability | 1.00 | 3.94 ± 0.727 *** | 4.51 ± 0.486 *** | 1.00 | 23.9 ± 6.00 $$$ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pangeni, R.; Panthi, V.K.; Yoon, I.-S.; Park, J.W. Preparation, Characterization, and In Vivo Evaluation of an Oral Multiple Nanoemulsive System for Co-Delivery of Pemetrexed and Quercetin. Pharmaceutics 2018, 10, 158. https://doi.org/10.3390/pharmaceutics10030158
Pangeni R, Panthi VK, Yoon I-S, Park JW. Preparation, Characterization, and In Vivo Evaluation of an Oral Multiple Nanoemulsive System for Co-Delivery of Pemetrexed and Quercetin. Pharmaceutics. 2018; 10(3):158. https://doi.org/10.3390/pharmaceutics10030158
Chicago/Turabian StylePangeni, Rudra, Vijay Kumar Panthi, In-Soo Yoon, and Jin Woo Park. 2018. "Preparation, Characterization, and In Vivo Evaluation of an Oral Multiple Nanoemulsive System for Co-Delivery of Pemetrexed and Quercetin" Pharmaceutics 10, no. 3: 158. https://doi.org/10.3390/pharmaceutics10030158
APA StylePangeni, R., Panthi, V. K., Yoon, I.-S., & Park, J. W. (2018). Preparation, Characterization, and In Vivo Evaluation of an Oral Multiple Nanoemulsive System for Co-Delivery of Pemetrexed and Quercetin. Pharmaceutics, 10(3), 158. https://doi.org/10.3390/pharmaceutics10030158