Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview
Abstract
1. Introduction
2. Bioadhesion and Mucoadhesion
2.1. Theories and Mechanism
2.2. Mucoadhesive Polymers
3. Poloxamers
3.1. General Characteristics and Proprieties
3.2. Poloxamer 407
3.3. Preparation of Thermo-Reversible Hydrogels
3.4. Measurement of Sol–Gel Transition Temperature and Gelation Time
4. Poloxamer 407-Based Mucoadhesive Formulations
4.1. Rectal Formulations
4.2. Vaginal Formulations
4.3. Ophthalmic Formulations
4.4. Nasal Formulations
4.5. Buccal Formulations
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Agrawal, A.K.; Das, M.; Jain, S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert. Opin. Drug Deliv. 2012, 9, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariepy, E.; Leroux, J.C. In situ-forming hydrogels–review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Agnello, S.; Gasperini, L.; Mano, J.F.; Pitarresi, G.; Palumbo, F.S.; Reis, R.L.; Giammona, G. Synthesis, mechanical and thermal rheological properties of new gellan gum derivatives. Int. J. Biol. Macromol. 2017, 98, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, I.R. Physical basis for poloxamer interactions. Ann. N. Y. Acad. Sci. 1994, 720, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Mayol, L.; Biondi, M.; Quaglia, F.; Fusco, S.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. Injectable thermally responsive mucoadhesive gel for sustained protein delivery. Biomacromolecules 2011, 12, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Peppas, N.A.; Buri, P.A. Surface interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release 1985, 2, 257–275. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Nicolini, M.; Morales, J.O. Overview and Future Potential of Buccal Mucoadhesive Films as Drug Delivery Systems for Biologics. AAPS PharmSciTech 2017, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F. Mucoadhesive therapeutic compositions: A patent review (2011–2014). Expert Opin. Ther. Pat. 2016, 26, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; Gremião, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 1–17. [Google Scholar] [CrossRef]
- Shaikh, R.; Raj Singh, T.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied. Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Dodou, D.; Breedveld, P.; Wieringa, P.A. Mucoadhesives in the gastrointestinal tract: Revisiting the literature for novel applications. Eur. J. Pharm. Biopharm. 2005, 60, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Hägerström, H.; Edsman, K.; Strømme, M. Low-frequency dielectric spectroscopy as a tool for studying the compatibility between pharmaceutical gels and mucus tissue. J. Pharm. Sci. 2003, 92, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Sahlin, J.J. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials 1996, 17, 1553–1561. [Google Scholar] [CrossRef]
- Sankar, R.; Jain, S.K. Development and characterization of gastroretentive sustained-release formulation by combination of swelling and mucoadhesive approach: A mechanistic study. Drug Des. Dev. Ther. 2013, 7, 1455–1469. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wu, S. Polymer Interface and Adhesion, 1st ed.; Marcel Dekker Inc.: New York, NY, USA, 1982; pp. 359–447. ISBN 0-8247-1533-0. [Google Scholar]
- Rossi, S.; Vigani, B.; Bonferoni, M.C.; Sandri, G.; Caramella, C.; Ferrari, F. Rheological analysis and mucoadhesion: A 30 year-old and still active combination. J. Pharm. Biomed. Anal. 2018, 156, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Hombach, J.; Bernkop-Schnürch, A. Mucoadhesive drug delivery systems. Handb. Exp. Pharmacol. 2010, 197, 251–266. [Google Scholar] [CrossRef]
- Mathiowitz, E.; Chickering, D.E. Definitions, mechanisms and theories of bioadhesion. In Bioadhesive Drug Delivery Systems: Fundamentals, Novel Approaches and Development, 1st ed.; Mathiowitz, E., Chickering, D.E., Lehr, C.-M., Eds.; Marcel Decker Inc.: New York, NY, USA, 1999; Volume 98, pp. 1–10. ISBN 0-8247-1995-6. [Google Scholar]
- Smart, J.D. The role of water movement and polymer hydration in mucoadhesion. In Bioadhesive Drug Delivery Systems: Fundamentals, Novel Approaches and Development, 1st ed.; Mathiowitz, E., Chickering, D.E., Lehr, C.-M., Eds.; Marcel Decker Inc.: New York, NY, USA, 1999; Volume 98, pp. 11–23. ISBN 0-8247-1995-6. [Google Scholar]
- Agarwal, S.; Aggarwal, S. Mucoadhesive polymeric platform for drug delivery; A comprehensive review. Curr. Drug Deliv. 2015, 12, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Serra, L.; Doménech, J.; Peppas, N.A. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur. J. Pharm. Biopharm. 2009, 71, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef]
- Huang, Y.; Leobandung, W.; Foss, A.; Peppas, N.A. Molecular aspects of mucoadhesion and bioadhesion: Tethered structures and site specific surfaces. J. Control. Release 2000, 65, 63–71. [Google Scholar] [CrossRef]
- Tiwari, D.; Goldman, D.; Sause, R.; Madan, P.L. Evaluation of polyoxyethylene homopolymers for buccal bioadhesive drug delivery device formulations. AAPS PharmSci 1999, 1, E13. [Google Scholar] [CrossRef] [PubMed]
- Salamat-Miller, N.; Chittchang, M.; Johnston, T.P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1666–1691. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-castellanos, M.R.; Zia, H.; Rhodes, C.T. Mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 1993, 19, 143–194. [Google Scholar] [CrossRef]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in mucoadhesive drug-delivery systems: A brief note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Vauthier, C.; Farabollini, A.; Palmieri, G.F.; Ponchel, G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutiylcyanoacrylate) core-shell nanoparticles. Biomaterials 2007, 28, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnurch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Delivery Rev. 2005, 57, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev. Ind. Pharm. 2004, 30, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Portero, A.; Teijeiro, D.; Alonso, M.; Remuñán-López, C. Development of chitosan sponges for buccal administration of insulin. Carbohydr. Polym. 2007, 68, 617–625. [Google Scholar] [CrossRef]
- Wittaya-areekul, S.; Kruenate, J.; Prahsarn, C. Preparation and in vitro evaluation of mucoadhesive properties of alginate/chitosan microparticles containing prednisolone. Int. J. Pharm. 2006, 312, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnurch, A.; Schwarz, V.; Steininger, S. Polymers with thiol groups: A new generation of mucoadhesive polymers? Pharm. Res. 1999, 16, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Lehr, C.M. Lectin-mediated drug delivery: The second generation of bioadhesives. J. Control. Release 2000, 65, 19–29. [Google Scholar] [CrossRef]
- Duggan, S.; Cummins, W.; O’Donovan, O.; Hughes, H.; Owens, E. Thiolated polymers as mucoadhesive drug delivery systems. Eur. J. Pharm. Sci. 2017, 100, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Grabovac, V.; Guggi, D.; Bernkop-Schnurch, A. Comparison of the mucoadhesive properties of various polymers. Adv. Drug Deliv. Rev. 2005, 57, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Ikada, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef]
- Nowak, J.; Laffleur, F.; Bernkop-Schnurch, A. Preactivated hyaluronic acid: A potential mucoadhesive polymer for vaginal delivery. Int. J. Pharm. 2014, 478, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Shahnaz, G.; Islambulchilar, Z.; Bernkop-Schnurch, A. Design and in vitro evaluation of a novel polymeric excipient for buccal applications. Future Med. Chem. 2013, 5, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)–poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Federico, C.; Maiuolo, J.; Bulotta, S.; Molinaro, R.; Paolino, D.; Tassone, P.; Fresta, M. Physicochemical features and transfection properties of chitosan/poloxamer 188/poly(d,l-lactide-co-glycolide) nanoplexes. Int. J. Nanomed. 2014, 9, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Paolino, D.; Iannone, M.; Palma, E.; Fresta, M.; Cosco, D. Sodium deoxycholate-decorated zein nanoparticles for a stable colloidal drug delivery system. Int. J. Nanomed. 2018, 13, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Kabanov, A.; Nazarova, I.; Astafieva, I.; Batrakova, E.; Alakhov, V.; Yaroslavov, A.; Kabanov, V. Micelle formation and solubilization of fluorescent probes in poly(oxyethylene-boxypropilene-b-oxyethylene) solutions. Macromolecules 1995, 28, 2303–2314. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarthf, J.F.; Hatton, T.A. Micellization of Poly(ethy1ene oxide)-Poly(propy1ene oxide)-Poly(ethy1ene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Bonacucina, G.; Spina, M.; Misici-Falzi, M.; Cespi, M.; Pucciarelli, S.; Angeletti, M.; Palmieri, G.F. Effect of hydroxypropyl beta-cyclodextrin on the self-assembling and thermogelation properties of Poloxamer 407. Eur. J. Pharm. Sci. 2007, 32, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Bedrov, D.; Ayyagari, C.; Smith, G.D. Multiscale Modeling of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymer Micelles in Aqueous Solution. J. Chem. Theory Comput. 2006, 2, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.; Rehman, K. Recent progress in biomedical applications of pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Cilurzo, F.; Maiuolo, J.; Federico, C.; Di Martino, M.T.; Cristiano, M.C.; Tassone, P.; Fresta, M.; Paolino, D. Delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci. Rep. 2015, 5, 17579. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.E.; Maggisano, V.; Celano, M.; Cosco, D.; Mignogna, C.; Baldan, F.; Lepore, S.M.; Allegri, L.; Moretti, S.; Durante, C.; et al. Anti-hTERT siRNA-Loaded Nanoparticles Block the Growth of Anaplastic Thyroid Cancer Xenograft. Mol. Cancer Ther. 2018, 6, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chavez, J.J.; Lopez-Cervantes, M.; Naik, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic f-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar] [PubMed]
- Fakhar Ud, D.; Khan, G.M. Development and characterisation of levosulpiride-loaded suppositories with improved bioavailability in vivo. Pharm. Dev. Technol. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Burgalassi, S.; Rossato, M.S.; Albertini, B.; Passerini, N.; Rodriguez, L.; Chetoni, P. Poloxamer 407 microspheres for orotransmucosal drug delivery. Part II: In vitro/in vivo evaluation. Int. J. Pharm. 2010, 400, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobao, P.; Lobo, J.M. Pluronic(r) f-127 and pluronic lecithin organogel (plo): Main features and their applications in topical and transdermal administration of drugs. J. Pharm. Pharm. Sci. 2012, 15, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C.; Cho, C.W. Physicochemical characterizations of piroxicam–poloxamer solid dispersion. Pharm. Dev. Technol. 1997, 2, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Chutimaworapan, S.; Ritthidej, G.C.; Yonemochi, E.; Oguchi, T.; Yamamoto, K. Effect of water-soluble carriers on dissolution characteristics of nifedipine solid dispersions. Drug Dev. Ind. Pharm. 2000, 26, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.J.; Bentley, M.V.; Farah, M.; Bretas, R.E.; Marchetti, J.M. Rheological characterization of Poloxamer 407 lidocaine hydrochloride gels. Eur. J. Pharm. Sci. 2002, 17, 161–167. [Google Scholar] [CrossRef]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.J.; Balakrishnan, P.; Oh, D.H.; Yeo, W.H.; Park, S.M.; Yong, C.S.; Choi, H.G. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int. J. Pharm. 2010, 395, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Mittal, N.; Yadav, S.K.; Khan, G.; Gupta, P.; Mishra, B.; Nath, G. Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: Preparation, in-vitro characterization and antimicrobial study. J. Oral Biol. Craniofac. Res. 2018, 8, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, X.; Lin, X.; Yao, C.; Shen, L.; Feng, Y. Poloxamer-based in situ hydrogels for controlled delivery of hydrophilic macromolecules after intramuscular injection in rats. Drug Deliv. 2015, 22, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.; Rehman, K.; Li, N.; Gao, J.Q.; Sun, H.; Chen, S. Sustained delivery of IL-1Ra from pluronic F127-based thermosensitive gel prolongs its therapeutic potentials. Pharm. Res. 2012, 29, 3475–3485. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. Applications of poloxamers in ophthalmic pharmaceutical formulations: An overview. Expert Opin. Drug Deliv. 2013, 10, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Schwartz, J.; Larrañeta, E.; Nguewa, P.A.; Sanmartín, C.; Agüeros, M.; Irache, J.M.; Espuelas, S. Thermosensitive hydrogels of poly(methyl vinyl ether-co-maleic anhydride)–Pluronic(®) F127 copolymers for controlled protein release. Int. J. Pharm. 2014, 459, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ur-Rehman, T.; Tavelin, S.; Grobner, G. Chitosan in situ gelation for improved drug loading and retention in poloxamer 407 gels. Int. J. Pharm. 2011, 409, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Koffi, A.A.; Agnely, F.; Ponchel, G.; Grossiord, J.L. Modulation of the rheological and mucoadhesive properties of thermosensitive poloxamer-based hydrogels intended for the rectal administration of quinine. Eur. J. Pharm. Sci. 2006, 27, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, M.; Kim, M.; Kim, C. Effect of additives on the physicochemical properties of liquid suppository bases. Int. J. Pharm. 1999, 190, 13–19. [Google Scholar] [CrossRef]
- Schmolka, I.R. Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.G.; Dimer, F.A.; Guterres, S.S.; Kechinski, C.P.; Granada, J.E.; Cardozo, N.S.M. Formulation and characterization of poloxamer 407: Thermoreversible gel containing polymeric microparticles and hyaluronic acid. Quim. Nova 2013, 36, 1121–1125. [Google Scholar] [CrossRef]
- Choi, H.G.; Jung, J.H.; Ryu, J.M.; Yoon, S.J.; Oh, Y.K.; Kim, C.K. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int. J. Pharm. 1998, 165, 33–44. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.P.; Guo, Y.S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.H.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef] [PubMed]
- De Souza Ferreira, S.B.; Moço, T.D.; Borghi-Pangoni, F.B.; Junqueira, M.V.; Bruschi, M.L. Rheological, mucoadhesive and textural properties of thermoresponsive polymer blends for biomedical applications. J. Mech. Behav. Biomed. Mater. 2015, 55, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassas, R.; Wen, J.; Cheng, A.E.; Kim, A.M.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wong, Y.C.; Zuo, Z. Development, characterization and application of in situ gel systems for intranasal delivery of tacrine. Int. J. Pharm. 2014, 468, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Shaker, D.S.; Shaker, M.A.; Klingner, A.; Hanafy, M.S. In situ thermosensitive Tamoxifen citrate loaded hydrogels: An effective tool in breast cancer loco-regional therapy. J. Drug Deliv. Sci. Technol. 2016, 35, 155–164. [Google Scholar] [CrossRef]
- Dimitrova, E.; Bogdanova, S.; Mitcheva, M.; Tanev, I.; Minkov, E. Development of model aqueous ophthalmic solution of indomethacin. Drug Dev. Ind. Pharm. 2000, 26, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Asasutjarit, R.; Thanasanchokpibull, S.; Fuongfuchat, A.; Veeranondha, S. Optimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gels. Int. J. Pharm. 2011, 411, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Veyries, M.L.; Couarraze, G.; Geiger, S.; Agnely, F.; Massias, L.; Kunzli, B.; Faurisson, F.; Rouveix, B. Controlled release of vancomycin from poloxamer 407 gels. Int. J. Pharm. 1999, 192, 183–193. [Google Scholar] [CrossRef]
- Chang, J.Y.; Oh, Y.K.; Choi, H.G.; Kim, Y.B.; Kim, C.K. Rheological evaluation of thermosensitive and mucoadhesive vaginals gels in physiological conditions. Int. J. Pharm. 2002, 241, 155–163. [Google Scholar] [CrossRef]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Guneri, T. Rheological and mechanical properties of poloxamer mixtures as a mucoadhesive gel base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Zheng, W.S.; Chen, S.H.; Fang, X.Q. Development of in situ gelling and bio adhesive 5-Fluorouracil enema. PLoS ONE. 2013, 8, e71037. [Google Scholar] [CrossRef] [PubMed]
- Bonacucina, G.; Martelli, S.; Palmieri, G.F. Rheological, mucoadhesive and release properties of Carbopol gels in hydrophilic cosolvents. Int. J. Pharm. 2004, 282, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Edsman, K.; Carlfors, J.; Petersson, R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur. J. Pharm. Sci. 1998, 6, 105–112. [Google Scholar] [CrossRef]
- Kim, C.K.; Lee, S.W.; Choi, H.G.; Lee, M.K.; Gao, Z.G.; Kim, I.S. Trials of in-situ gelling and mucoadhesive acetaminophen liquid suppository in human subjects. Int. J. Pharm. 1998, 174, 201–207. [Google Scholar] [CrossRef]
- Ryu, J.M.; Chung, S.J.; Lee, M.H.; Kim, C.K.; Shim, C.K. Increased bioavailability of propranolol in rats by retaining thermally gelling liquid suppositories in the rectum. J. Control. Release 1999, 59, 163–172. [Google Scholar] [CrossRef]
- Park, Y.J.; Yong, C.S.; Kim, H.M.; Rhee, J.D.; Oh, Y.K.; Kim, C.K.; Choi, H.G. Effect of sodium chloride on the release, absorption and safety of diclofenac sodium delivered by poloxamer gel. Int. J. Pharm. 2003, 263, 105–111. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Liu, Y.; Di, X. Thermosensitive in situ gel based on solid dispersion for rectal delivery of ibuprofen. AAPS PharmSciTech 2018, 19, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Moawad, F.A.; Ali, A.A.; Salem, H.F. Nanotransfersomes-loaded thermosensitive in situ gel as a rectal delivery system of tizanidine HCl: Preparation, in vitro and in vivo performance. Drug Deliv. 2017, 24, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.H.; Ramasamy, T.; Kim, D.W.; Cho, H.J.; Kim, Y.I.; Cho, K.H.; Yong, C.S.; Kim, J.O.; Choi, H.G. Docetaxel-loaded thermosensitive liquid suppository: Optimization of rheological properties. Arch Pharm. Res. 2013, 36, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Shastri, D.H.; Prajapati, S.T.; Patel, L.D. Thermoreversible mucoadhesive ophthalmic in situ hydrogel: Design and optimization using a combination of polymers. Acta Pharm. 2010, 60, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.R.; Sung, K.C.; Vong, W.J. In situ gelling of alginate/pluronic solutions for ophthalmic delivery of pilocarpine. Biomacromolecules 2004, 5, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.; Sarkar, G.; Bhowmik, M.; Das, B.; Chattoapadhyay, A.K.; Rana, D.; Chattopadhyay, D. Effect of gellan gum on the thermogelation property and drug release profile of poloxamer 407 based ophthalmic formulation. Int. J. Biol. Macromol. 2017, 102, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Warade, S.; Singhavi, D.J. Improvement in ocular bioavailability and prolonged delivery of tobramycin sulfate following topical ophthalmic administration of drug-loaded mucoadhesive microparticles incorporated in thermosensitive in situ gel. J. Ocul. Pharmacol. Ther. 2018, 34, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Balakrishnan, P.; Park, E.K.; Song, K.W.; Hong, S.S.; Jang, T.Y.; Kim, K.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Poloxamer/cyclodextrin/chitosan-based thermoreversible gel for intranasal delivery of fexofenadine hydrochloride. J. Pharm. Sci. 2011, 100, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Mennini, N.; Nativi, C.; Richichi, B. In situ mucoadhesive-thermosensitive liposomal gel as a novel vehicle for nasal extended delivery of opiorphin. Eur. J. Pharm. Biopharm. 2018, 122, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, E.; Yener, G. Formulation and Evaluation of Thermoreversible In Situ Nasal Gels Containing Mometasone Furoate for Allergic Rhinitis. AAPS PharmSciTech 2017, 18, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Wairkar, S.; Gaud, R.; Bajaj, A.; Meshram, P. Brain targeted delivery of mucoadhesive thermosensitive nasal gel of selegiline hydrochloride for treatment of parkinson’s disease. J. Drug Target. 2018, 26, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Panda, A.K.; Asif, S.; Verma, D.; Talegaonkar, S.; Manzoor, N.; Khan, A.; Ahmed, F.J.; Dudeja, M.; Iqbal, Z. A vaginal drug delivery model. Drug Deliv. 2016, 23, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Rençber, S.; Karavana, S.Y.; Şenyiğit, Z.A.; Eraç, B.; Limoncu, M.H.; Baloğlu, E. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: Formulation, preparation, and in vitro/in vivo evaluation. Pharm. Dev. Technol. 2017, 22, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.M.; Fetih, G.; Abbas, A.M. Thermosensitive bioadhesive gels for the vaginal delivery of sildenafil citrate: In vitro characterization and clinical evaluation in women using clomiphene citrate for induction of ovulation. Drug. Dev. Ind. Pharm. 2017, 43, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.S.; Robinson, J.R. Polymer structure features contributing to mucoadhesion. II. J. Control. Release 1990, 12, 187–194. [Google Scholar] [CrossRef]
- Grassi, G.; Crevatin, A.; Farra, R.; Guarnieri, G.; Pascotto, A.; Rehimers, B.; Lapasin, R.; Grassi, M. Rheological properties of aqueous Pluronic-alginate systems containing liposomes. J. Colloid. Interface Sci. 2006, 301, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Failla, P.; Costa, N.; Pullano, S.; Fiorillo, A.; Mollace, V.; Fresta, M.; Paolino, D. Rutin-loaded chitosan microspheres: Characterization and evaluation of the anti-inflammatory activity. Carbohydr. Polym. 2016, 152, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Iannone, M.; Mare, R.; Paolino, D.; Gagliardi, A.; Froiio, F.; Cosco, D.; Fresta, M. Characterization and in vitro anticancer properties of chitosan-microencapsulated flavan-3-ols-rich grape seed extracts. Int. J. Biol. Macromol. 2017, 104, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Tsapis, N.; Nascimento, T.L.; Fresta, M.; Chapron, D.; Taverna, M.; Arpicco, S.; Fattal, E. Polysaccharide-coated liposomes by post-insertion of a hyaluronan-lipid conjugate. Colloids Surf. B Biointerfaces 2017, 158, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.A.; Kong, J.H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.L.; Hillaireau, H.; Noiray, M.; Bourgaux, C.; Arpicco, S.; Pehau-Arnaudet, G.; Taverna, M.; Cosco, D.; Tsapis, N.; Fattal, E. Supramolecular Organization and siRNA Binding of Hyaluronic Acid-Coated Lipoplexes for Targeted Delivery to the CD44 Receptor. Langmuir 2015, 31, 11186–11194. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Bentley, M.; Marchetti, J.M.; Nágila, R.; Ali-Abi, Z.; Collet, J.H. Influence of lecithin on some physical chemical properties of poloxamer gels: Rheological, microscopic and in vitro permeation studies. Int. J. Pharm. 1999, 193, 49–55. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Cilurzo, F.; Fresta, M. Innovative drug delivery systems for the administration of natural compounds. Curr. Bioact. Compd. 2007, 3, 262–277. [Google Scholar] [CrossRef]
- Jannin, V.; Lemagnen, G.; Gueroult, P.; Larrouture, D.; Tuleu, C. Rectal route in the 21st Century to treat children. Adv. Drug Deliv. Rev. 2014, 73, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Cheng, Y.M.; Lai, P.H.; Wu, J.F.; Hsu, Y.C. In vitro biocompatibility of thermally gelling liquid mucoadhesive loaded curcuminoids in colorectal cancer chemoprevention. Int. J. Colorectal Dis. 2012, 27, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Özgüney, I.; Kardhiqi, A.; Yıldız, G.; Ertan, G. In vitro-in vivo evaluation of in situ gelling and thermosensitive ketoprofen liquid suppositories. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.S.; Sah, H.; Jahng, Y.; Chang, H.W.; Son, J.K.; Lee, S.H.; Jeong, T.C.; Rhee, J.D.; Baek, S.H.; Kim, C.K.; et al. Physicochemical characterization of diclofenac sodium-loaded poloxamer gel as a rectal delivery system with fast absorption. Drug Dev. Ind. Pharm. 2003, 29, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Agnely, F.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010, 76, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Faccendini, A.; Puccio, A.; Caramella, C. Comparison of poloxamer- and chitosan-based thermally sensitive gels for the treatment of vaginal mucositis. Drug Dev. Ind. Pharm. 2014, 40, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, F.; Feng, L.; Yang, L.; Chen, L.; Wei, G.; Lu, W. In vivo retention of poloxamer-based in situ hydrogels for vaginal application in mouse and rat models. Acta Pharm. Sin. B 2017, 7, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Timur, S.S.; Sahin, A.; Aytekin, E.; Ozturk, N.; Polat, K.H.; Tezel, N.; Gursoy, R.N.; Calis, S. Design and in vitro evaluation of tenofovir-loaded vaginal gels for the prevention of HIV infections. Pharm. Dev. Technol. 2018, 23, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.S.; Dismukes, W.E. Azole antifungal agents: Emphasis on new triazoles. Antimicrob. Agents Chemother. 1988, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.M.; Dehghani, F.; Foster, N.R. Increasing the dissolution rate of itraconazole processed by gas antisolvent techniques using polyethylene glycol as a carrier. Pharm. Res. 2008, 25, 1274–1289. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; McGinity, J.W. Bioadhesive properties of hydroxypropylcellulose topical films produced by hot-melt extrusion. J. Control. Release 2001, 70, 341–351. [Google Scholar] [CrossRef]
- Homburg, R. Clomiphene citrate-end of an era? A mini-review. Hum. Reprod. 2005, 20, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Sher, G.; Fisch, J.D. Vaginal sildenafil (Viagra): A preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum. Reprod. 2000, 15, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.M.; Robinson, J.R. Relationship of chemical structure to corneal penetration and influence of low viscosity solution on ocular bioavailability. J. Pharm. Sci. 1984, 73, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Haglund, B.O.; Himmelstein, K.J. In situ-forming gels for ophthalmic drug delivery. J. Ocul. Pharmacol. 1994, 10, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bourlais, C.L.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems--recent advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Fathalla, Z.M.A.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M. In situ gelling systems: A strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Fatouros, D.G. Smart materials: In situ gel-forming systems for nasal delivery. Drug Discov. Today 2016, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.M.; Kumar, A.; Pathak, K. Mucoadhesive in situ nasal gelling drug delivery systems for modulated drug delivery. Expert. Opin. Drug Deliv. 2013, 10, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Salib, R.J.; Howarth, P.H. Safety and tolerability profiles of intranasal antihistamines and intranasal corticosteroids in the treatment of allergic rhinitis. Drug Saf. 2003, 26, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O.; Munafo, D.A.; Chung, W.; Gopalan, G.; Varghese, S.T. Intranasal mometasone furoate therapy for allergic rhinitis symptoms and rhinitis-disturbed sleep. Ann. Allergy Asthma Immunol. 2010, 105, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.L.; Ren, X.W.; Zhang, Q.Z.; Chen, E.; Xu, F.; Chen, J.; Liu, L.C.; Jiang, X.G. In situ gel based on gellan gum as new carrier for nasal administration of mometasone furoate. Int. J. Pharm. 2009, 365, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery–recent developments and future prospects. J. Control. Release 2012, 161, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, Y.; Güngör, S. Nasal route: An alternative approach for antiemetic drug delivery. Expert Opin. Drug Deliv. 2011, 8, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Wisner, A.; Dufour, E.; Messaoudi, M.; Nejdi, A.; Marcel, A.; Ungeheuer, M.N.; Rougeot, C. Human Opiorphin, a natural antinociceptive modulator of opioid-dependent pathways. Proc. Natl. Acad. Sci. USA 2006, 103, 17979–17984. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Z.; Chen, J.; Xiong, W.; He, T.; Chen, Q. Effects and underlying mechanisms of human opiorphin on colonic motility and nociception in mice. Peptides 2009, 30, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Mennini, N.; Mura, P.; Nativi, C.; Richichi, B.; Di Cesare Mannelli, L.; Ghelardini, C. Injectable liposomal formulations of opiorphin as a new therapeutic strategy in pain management. Future Sci. 2015, 1, FSO2. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. In situ-based gels for nose to brain delivery for the treatment of neurological diseases. Pharmaceutics 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Di Marzio, L.; Marianecci, C.; Trapasso, E.; Paolino, D.; Celia, C.; Carafa, M.; Fresta, M. Colloidal supramolecular aggregates for therapeutic application in neuromedicine. Curr. Med. Chem. 2014, 21, 4132–4153. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lohan, S.; Murthy, R.S. Formulation and characterization of intranasal mucoadhesive nanoparticulates and thermo-reversible gel of levodopa for brain delivery. Drug Dev. Ind. Pharm. 2014, 40, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, S.; Wang, H.; Bie, H. A mucoadhesive, thermoreversible in situ nasal gel of geniposide for neurodegenerative diseases. PLoS ONE 2017, 12, e0189478. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Jelvehgari, M. Thermosensitive in situ nanocomposite of rivastigmine hydrogen tartrate as an intranasal delivery system: Development, characterization, ex vivo permeation and cellular studies. Colloids Surf. B 2017, 159, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Abouhussein, D.M.; Khattab, A.; Bayoumi, N.A.; Mahmoud, A.F.; Sakr, T.M. Brain targeted rivastigmine mucoadhesive thermosensitive In situ gel: Optimization, in vitro evaluation, radiolabeling, in vivo pharmacokinetics and biodistribution. J. Drug Deliv. Sci. Technol. 2018, 43, 129–140. [Google Scholar] [CrossRef]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Agomelatine-based in situ gels for brain targeting via the nasal route: Statistical optimization, in vitro, and in vivo evaluation. Drug Deliv. 2017, 24, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Shahi, S.; Jadhav, K.; Dhamecha, D.; Tiwari, R.; Patil, H. Thermoreversible nanoethosomal gel for the intranasal delivery of eletriptan hydrobromide. J. Mater. Sci. Mater. Med. 2016, 27, 103. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D. Poloxamer 407-based intranasal thermoreversible gel of zolmitriptan-loaded nanoethosomes: Formulation, optimization, evaluation and permeation studies. J. Liposome Res. 2016, 26, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Junginger, H.E.; Hoogstraate, J.A.; Verhoef, J.C. Recent advances in buccal drug delivery and absorption–in vitro and in vivo studies. J. Control. Release 1999, 62, 149–159. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of polymeric dosage forms in buccal drug delivery: State of art, design of formulations and their in vivo performance evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Sheshala, R.; Quah, S.Y.; Tan, G.C.; Meka, V.S.; Jnanendrappa, N.; Sahu, P.S. Investigation on solution-to-gel characteristic of thermosensitive and mucoadhesive biopolymers for the development of moxifloxacin-loaded sustained release periodontal in situ gels. Drug Deliv. Transl. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nasra, M.M.A.; Khiri, H.M.; Hazzah, H.A.; Abdallah, O.Y. Formulation, in-vitro characterization and clinical evaluation of curcumin in-situ gel for treatment of periodontitis. Drug Deliv. 2017, 24, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Patil, D.; Hegde, S.; Potdar, R.; Metgud, R.; Jalalpure, S.; Roy, S.; Jadhav, K.; et al. Formulation of thermoreversible gel of cranberry juice concentrate: Evaluation, biocompatibility studies and its antimicrobial activity against periodontal pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1506–1514. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
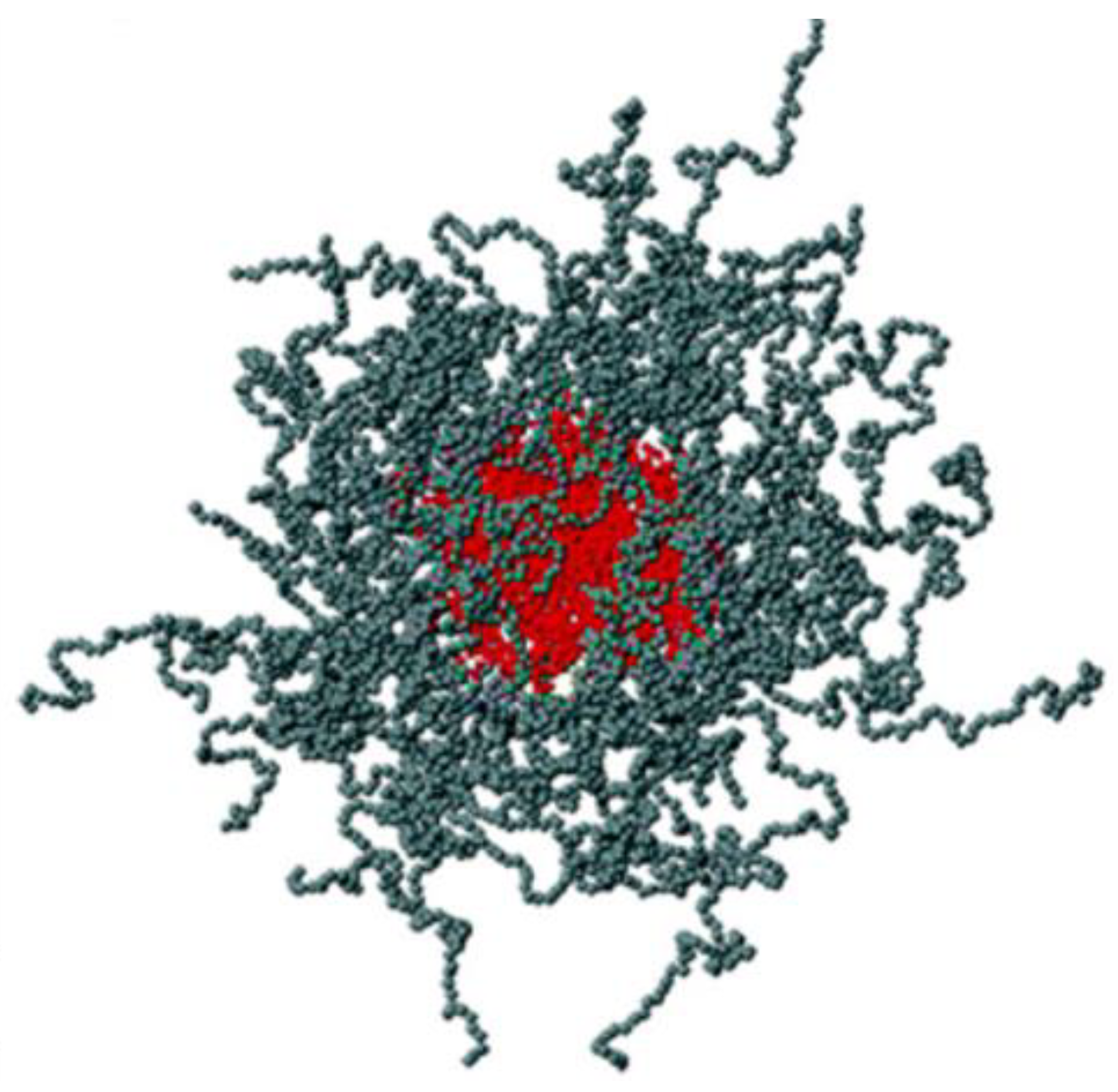
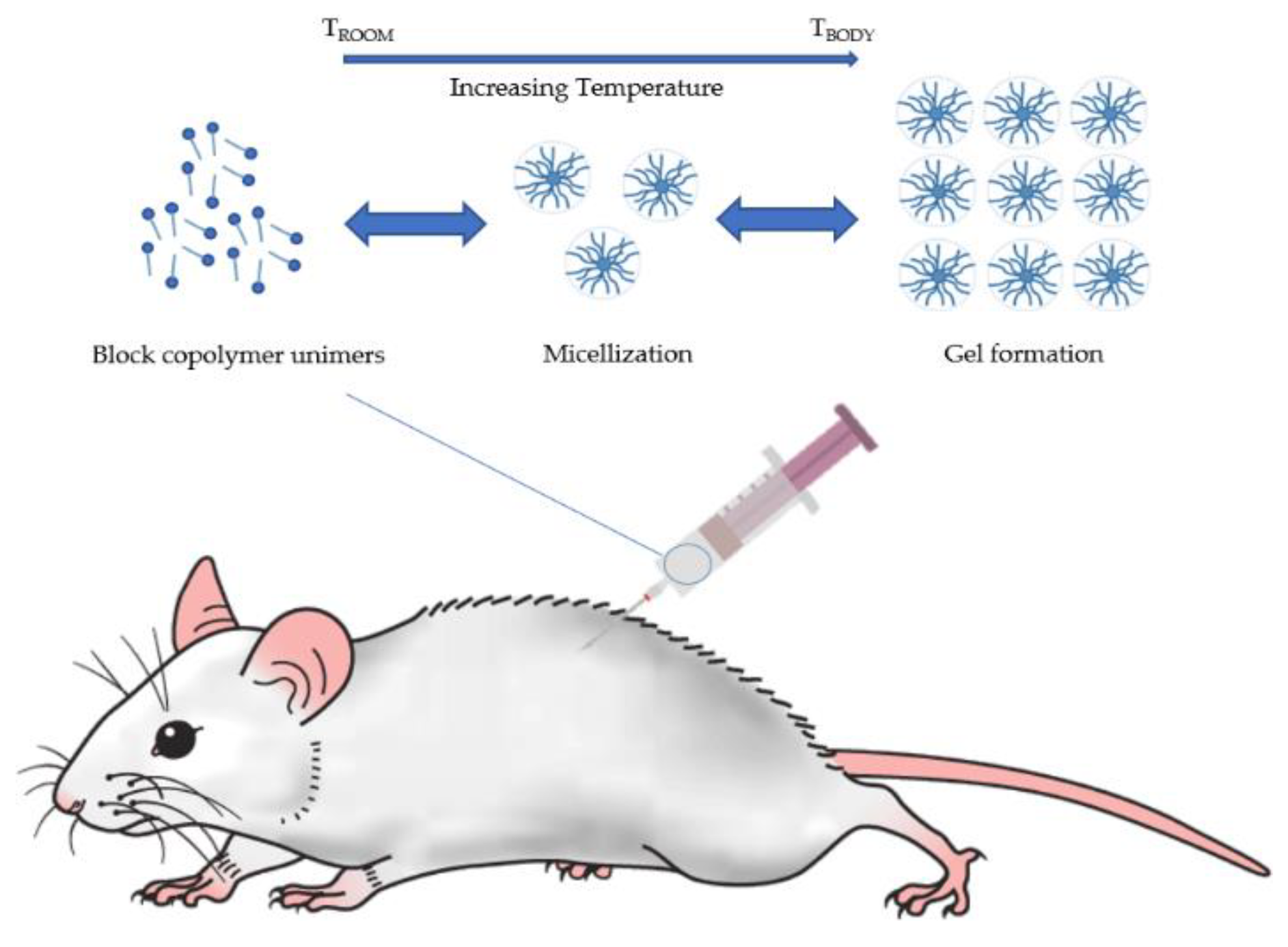
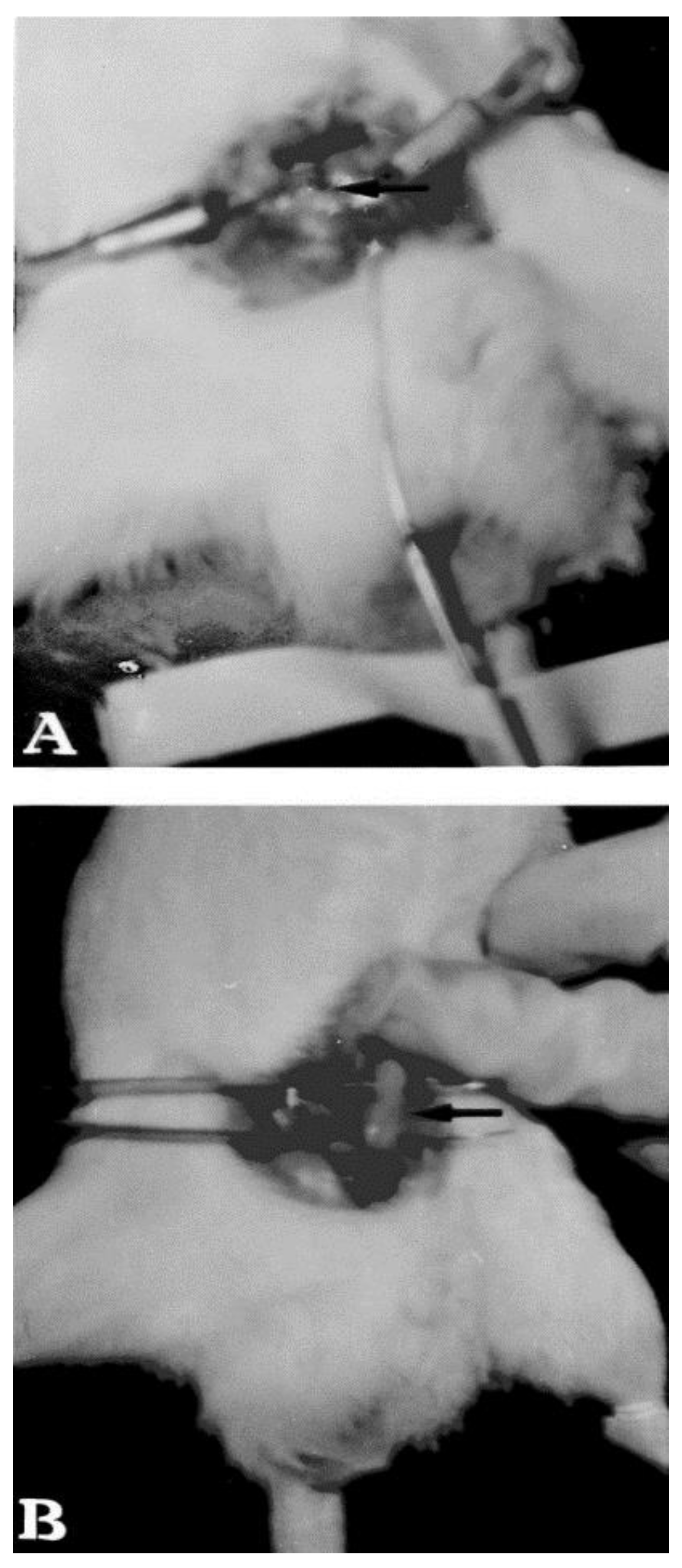
| Pharmaceutical Formulations | %P407 (/P188) 1 | Additives | Results | Reference |
|---|---|---|---|---|
| Rectal application | ||||
| Rectal administration of acetaminophen formulated as a liquid suppository. | 15/15 15/20 | PVP 2, HPMC 3, HPC 4, Carbopol 934P, Polycarbophil | PVP, HPMC, and HPC: non-affected Tsol-gel. Carbopol and polycabophil decreased Tsol-gel, and enhanced the gel strength and bioadhesive force. | [79] |
| P407/P188 liquid suppository bases | 15/15 | Ethanol, propylene glycol, glycerin, hydrochloric acid, sodium chloride, sodium monohydrogen phosphate, sodium dihydrogen phosphate | Sodium chloride, sodium monohydrogen phosphate, and sodium dihydrogen phosphate increased the gel strength and the bioadhesive force, with a decrease in gelation temperature. Glycerin slightly decreased the gelation temperature, and slightly increased the gel strength and bioadhesive force. | [76] |
| Propranolol mucoadhesive liquid suppositories. | 15/15 | HPC, PVP, Carbopol, sodium alginate, polycarbophil | Sodium alginate exhibited the greatest degree of mucoadhesion and caused no irritation of the rectal mucosal membrane. | [95] |
| Thermo-sensitive and mucoadhesive rectal in situ gel of nimesulide. | 18 | Sodium alginate, HPMC, polyethylene glycol (PEG 4000 and PEG 400) | The addition of PEG polymers increased the gelation temperature and the drug release rate. The P407/nimesulide/sodium alginate/PEG 4000 (18/2.0/0.5/1.2%) exhibited the appropriate gelation temperature, acceptable drug release rate, and rectal retention. | [81] |
| Thermo-sensitive gels based on poloxamer 407 and HPMC for the rectal delivery of quinine for the treatment of severe malaria in children. | 16, 17 | Propanediol-1,2, HPMC | 1,2-Propanediol limits HPMC precipitation in poloxamer 407 solution. Moreover, HPMC in the presence of propanediol-1,2 had a synergistic effect on the gelation of the poloxamer 407 solution. | [75] |
| Thermo-sensitive poloxamer gel containing diclofenac sodium in a rectal dosage form. | 15/17 | Sodium chloride | Rectal diclofenac sodium/P407/P188/sodium chloride gel could provide fast drug absorption, without damaging the rectum. | [96] |
| In situ gelling and mucoadhesive acetaminophen liquid suppository. | 15/19 | Sodium alginate | Acetaminophen liquid suppository allowed faster absorption of acetaminophen in human subjects than conventional suppositories, probably because of its greater dispersability and bioadhesive force. | [94] |
| Thermo-sensitive in situ gel based on solid dispersions for rectal delivery of ibuprofen. | 20 | HPMC, sodium alginate | HPMC and sodium alginate lowered Tsol-gel and increased gel strength. Liquid suppository showed better drug release performance than solid suppositories, and the drug absorption and bioavailability were both improved in rabbits. | [97] |
| Levosulpiride-loaded liquid suppositories with improved bioavailability. | 15/17 | Tween 80 | Tween 80 increased the mucoadhesive force and the gel strength. The system showed a suitable gelation temperature and exhibited an enhanced bioavailability with respect to the drug suspension in rats. | [61] |
| Nanotransfersome-loaded thermosensitve in situ gel as a rectal delivery system of tizanidine. | 21/3 | HPMC | An increase in the bioavailability and a sustained release of the drug were obtained by the synergic effect of a poloxamer gel and nanotransfersomes. | [98] |
| Docetaxel-loaded thermo-sensitive liquid suppository | 11/15 | Tween 80 | Tween 80 induced an increase in viscosity. | [99] |
| Ophthalmic application | ||||
| Thermo-reversible in situ gelling ophthalmic drug delivery system based on Pluronic F 127, containing moxifloxacin hydrochloride | 15 (w/v) | Gelrite® | Gelrite® showed a positive effect on the bioadhesive features. | [100] |
| P407/chitosan ophthalmic delivery system characterized by a prolonged retention time for the treatment of ocular diseases. | 14–20 | Chitosan | Chitosan improved the mechanical strength and textural properties of poloxamer formulations, characterized by a significant residence time in the eye. | [101] |
| Alginate and Pluronic-based in situ gelling system for ophthalmic delivery of pilocarpine. | 12–16 | Alginic acid | The rheological analysis as well as in vitro and in vivo studies demonstrated that the alginate/Pluronic mixture was used to retain pilocarpine in order to increase its ocular bioavailability. | [102] |
| Poloxamers/ hyaluronic acid (HA) gel for the ocular delivery of acyclovir | 15/10, 15 | Hyaluronic acid | The addition of HA caused a modulation in the rheological properties of the poloxamer. Mucoadhesion tests showed an increased interaction with mucin. In vitro analysis showed a controlled release of acyclovir. | [80] |
| A dual pH- and temperature-responsive poloxamer 407-hydrogel system containing carboxymethyl chitosan cross-linked by glutaraldehyde for ophthalmic drug delivery. | 1.5–20 (w/v) | Carboxymethyl chitosan | No toxicity on human corneal epithelial cells at a low concentration. The gelation temperature was 32–33 °C, suitable for ocular delivery, while the viscosity quickly increased after gelation, and a sustained release of the drug was observed. | [103] |
| Combined poloxamer 407/gellan gum in situ gel for the ocular delivery of pilocarpine hydrochloride | 18 | Gellan gum | Gellan gum caused a decrease in the gelation temperature and an increase of viscosity due to the formation of hydrogen bonds with the poloxamer. In addition, gellan gum largely decreased the gel dissolution rate, while in an vitro drug release study, it showed a better drug delivery time with respect to the poloxamer alone. | [104] |
| Tobramycin sulfate-loaded microparticles dispersed in poloxamer 407/chitosan thermosensitive gel for the treatment of ocular infections | 17 (w/v) | Chitosan | Addition of chitosan resulted in an increase in viscosity and in a greater mucoadhesive strength of the gel. It also evidenced a better in vitro permeability and a greater aqueous humor concentration of the drug, compared with commercial tobramycin eye drops with no signs of ocular irritation. | [105] |
| Nasal application | ||||
| Poloxamer/cyclodextrin/chitosan-based thermoreversible gel for the intranasal delivery of fexofenadine hydrochloride. | 17 (w/v) | Chitosan | Chitosan induced a slight increase in gelation temperature and viscosity, promoting a controlled release of the drug and a significant permeation through the nasal epithelium. | [106] |
| In situ mucoadhesive-thermosensitive liposomal gel as a novel formulation for the nasal delivery of opiorphin | 15–30 (w/v) | Carbopol 934P, HPMC, P188 | The formulation made up of poloxamer 407 (26.5% w/v) and carbopol 934P (1% w/v) showed the best properties in terms of proper gelation time, adequate mucoadhesive and gel strength, and mucoadhesion duration. This hydrogel had a prolonged, controlled delivery of the drug for more than 5 h, and the liposomes enhanced the permeability coefficient and the permeation rate of the peptide up to six times. | [107] |
| Thermo-reversible in situ nasal gels containing mometasone furoate for the treatment of allergic rhinitis | 18 | Carbopol 974P, PEG 400 | Carbopol 974P NF significantly decreased the Tsol-gel and increased the viscosity, while PEG 400 increased the Tsol-gel and decreased gel viscosity. Mucoadhesive strength was predominantly dependent on the Carbopol 974P NF. The release of the drug was prolonged, as demonstrated by in vitro experiments. | [108] |
| Mucoadhesive thermo-sensitive nasal gel of selegiline hydrochloride for the treatment of Parkinson’s disease. | 15–18 | Chitosan | The formulation showed desired characteristics such as sol–gel transition at nasal temperature, viscosity, pH, and mucoadhesive strength, and it improved the drug residence time in the nasal cavity. In vivo investigations confirmed that selegiline hydrochloride was more efficacious after encapsulation within the thermo-sensitive gel with respect to the nasal solution or oral tablets. | [109] |
| Vaginal application | ||||
| Thermo-sensitive and mucoadhesive vaginal gel containing clotrimazole | 15/15, 20 | Polycarbophil | The formulation had a useful gelation time and Tsol-gel values, as well as suitable rheological properties, even after dilution with simulated vaginal fluid. | [89] |
| Mucoadhesive and thermo-sensitive poloxamer 407-based gel for the topical delivery of itraconazole | 15, 18, 20 (w/v) | Carbopol CP 934 | The gel demonstrated an appreciable bioadhesion and non-toxicity. A remarkable decrease in the microbial count was observed, as compared to the marketed formulation. | [110] |
| Vaginal mucoadhesive in situ gel formulations of clotrimazole | 20/10 | HPMC E50, HPMC K100M | The rheological and texture analysis revealed a suitable gelation temperature and time, together with an appropriate consistency, high adhesiveness, cohesiveness, and mucoadhesiveness values. In vivo studies showed a long residence time in the vaginal compartment (up to 24 h). | [111] |
| In situ thermo-sensitive gels for the vaginal administration of sildenafil as a potential treatment of infertility in women. | 15–20/15, 20 | Sodium alginate, HEC 5 | P188 increased the Tsol-gel and mucoadhesive force. HEC and sodium alginate increased the viscosity and the mucoadhesion. All polymers showed a significant decrease of released sildenafil. Clinical results showed that the vaginal gel containing sildenafil significantly increased endometrial thickness and the uterine blood flow with no side effects. | [112] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. https://doi.org/10.3390/pharmaceutics10030159
Giuliano E, Paolino D, Fresta M, Cosco D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics. 2018; 10(3):159. https://doi.org/10.3390/pharmaceutics10030159
Chicago/Turabian StyleGiuliano, Elena, Donatella Paolino, Massimo Fresta, and Donato Cosco. 2018. "Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview" Pharmaceutics 10, no. 3: 159. https://doi.org/10.3390/pharmaceutics10030159
APA StyleGiuliano, E., Paolino, D., Fresta, M., & Cosco, D. (2018). Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics, 10(3), 159. https://doi.org/10.3390/pharmaceutics10030159








