Ophthalmic Drug Delivery Systems for Antibiotherapy—A Review
Abstract
1. Introduction
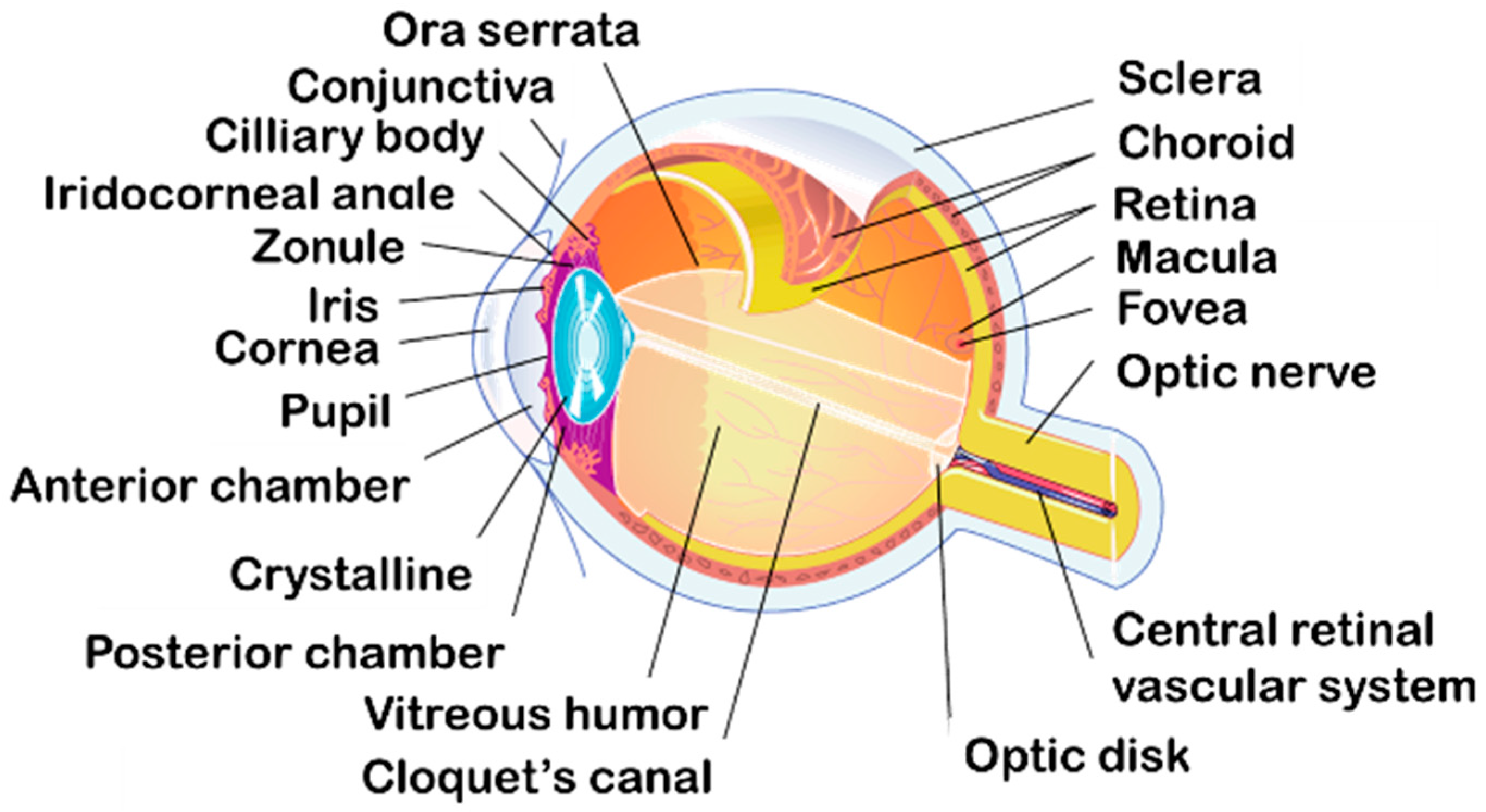
2. Anatomy and Physiology of the Eye for Ocular Drug Delivery
2.1. Anatomy and Physiology of the Eye
2.1.1. Three Different Layers
2.1.2. Inside the Globe
2.1.3. Ocular Annexes
2.2. Blood-Ocular Barriers
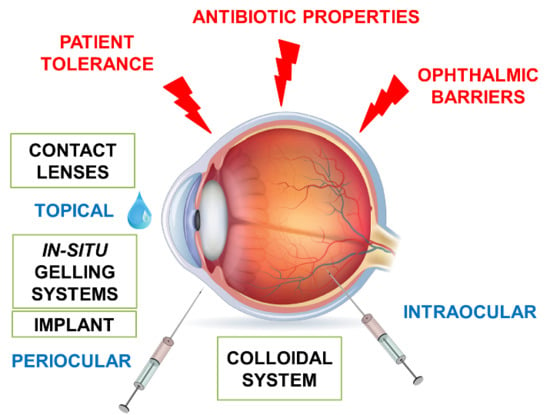
3. Ophthalmic Forms
3.1. Eye Drops
3.2. Ointments
3.3. Hydrogels
3.4. Emulsions
3.5. Ophthalmic Insert
3.6. Contact Lenses
3.7. Intraocular Injections
3.8. Innovative Forms
4. Recent Advances for Ocular Antibiotics Administration
4.1. Antibiotics and Ophthalmic Delivery
4.2. Recent Advances in Ocular Delivery of Antibiotics
4.2.1. Improvement of Drug Dissolution and Stability Using Cyclodextrins
4.2.2. Contact Lens for Antibiotic Delivery
4.2.3. Ocular Inserts for Antibiotic Delivery
4.2.4. In Situ Gelling Systems for Antibiotic Delivery
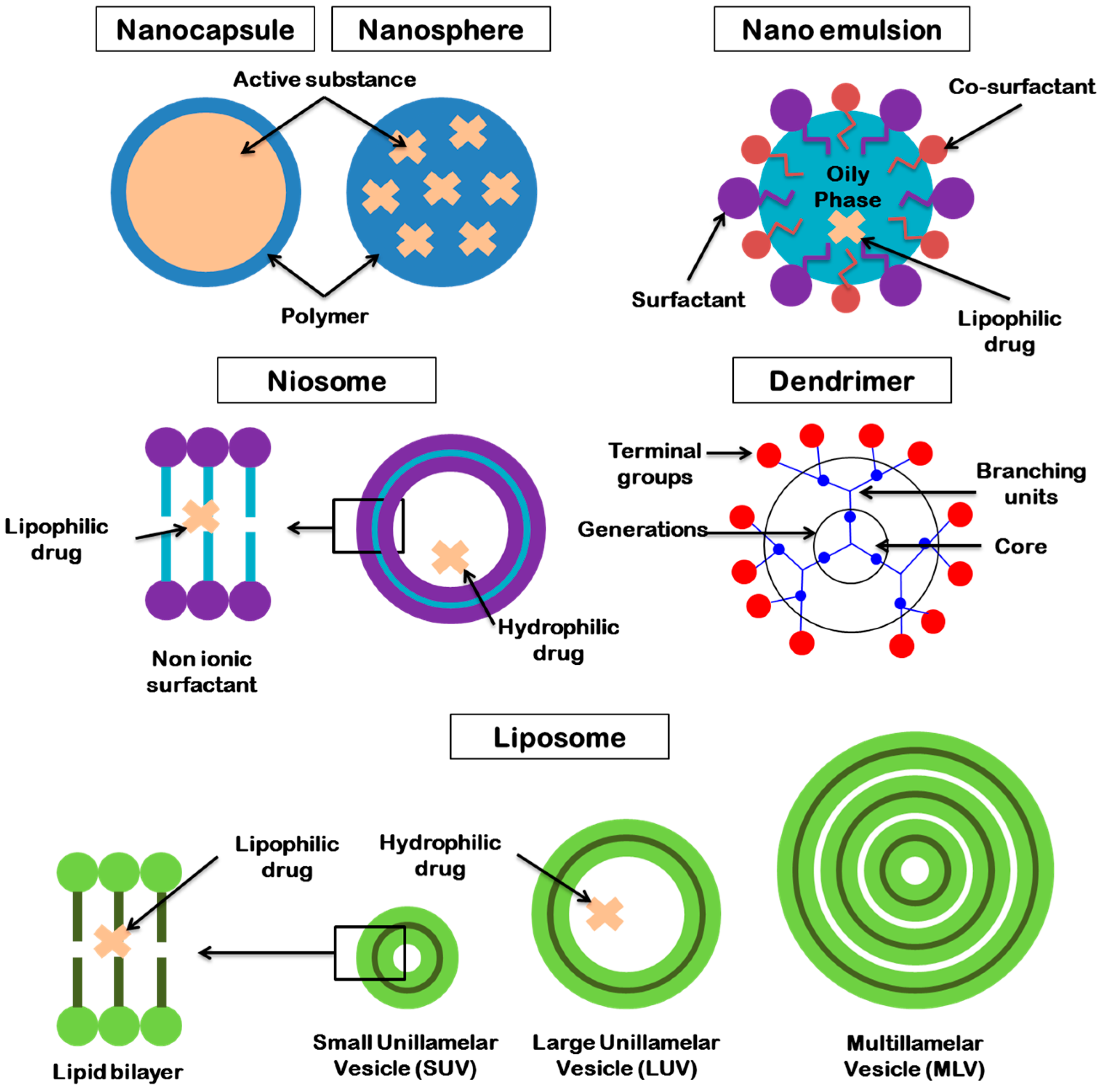
4.2.5. Colloidal Systems for Antibiotic Delivery
Microemulsions for Antibiotic Delivery
Nanoemulsions for Antibiotic Delivery
Nanoparticles and Microparticles for Antibiotic Delivery
Liposomes for Antibiotic Delivery
Niosomes for Antibiotic Delivery
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gan, L.; Wang, J.; Jiang, M.; Bartlett, H.; Ouyang, D.; Eperjesi, F.; Liu, J.; Gan, Y. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov. Today 2013, 18, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Le Bourlais, C.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems—Recent advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- Yellepeddi, V.K.; Palakurthi, S. Recent advances in topical ocular drug delivery. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2016, 32, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Antibiotic resistance in ocular bacterial pathogens. Indian J. Med. Microbiol. 2011, 29, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Remington, L.A. Clinical anatomy and physiology of the visual system, 3rd ed.; Elsevier/Butterworth Heinemann: St. Louis, MO, USA, 2012; ISBN 978-1-4377-1926-0. [Google Scholar]
- Rathbone, M.J.; Hadgraft, J.; Roberts, M.S.; Lane, M.E. Modified-release drug delivery technology (Drugs and the pharmaceutical sciences), 2nd ed.; Informa Healthcare: New York, NY, USA, 2008; Volume 2, ISBN 978-1-4200-4435-5. [Google Scholar]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cholkar, K.; Dasari, S.R.; Pal, D.; Mitra, A.K. Eye: anatomy, physiology and barriers to drug delivery. In Ocular Transporters and Receptors; Mitra, A.K., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 1–36. ISBN 978-1-907568-86-2. [Google Scholar]
- Occhiutto, M.L.; Freitas, F.R.; Maranhao, R.C.; Costa, V.P. Breakdown of the Blood-Ocular Barrier as a Strategy for the Systemic Use of Nanosystems. Pharmaceutics 2012, 4, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J. The blood-ocular barriers. Surv. Ophthalmol. 1979, 23, 279–296. [Google Scholar] [CrossRef]
- Chen, M.-S.; Hou, P.-K.; Tai, T.-Y.; Lin, B.J. Blood-ocular barriers. Tzu Chi Med. J. 2008, 20, 25–34. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Patel, A. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Pisella, P.J.; Fillacier, K.; Elena, P.P.; Debbasch, C.; Baudouin, C. Comparison of the effects of preserved and unpreserved formulations of timolol on the ocular surface of albino rabbits. Ophthalmic Res. 2000, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Van der Bijl, P.; van Eyk, A.D.; Meyer, D. Effects of three penetration enhancers on transcorneal permeation of cyclosporine. Cornea 2001, 20, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Jaanus, S.D. Clinical ocular pharmacology; Butterworth-Heinemann: Oxford, United Kingdom, 1989; ISBN 0-409-90058-3. [Google Scholar]
- Bakkour, Y.; Vermeersch, G.; Morcellet, M.; Boschin, F.; Martel, B.; Azaroual, N. Formation of cyclodextrin inclusion complexes with doxycyclin-hyclate: NMR investigation of their characterisation and stability. J. Incl. Phenom. Macrocycl. Chem. 2006, 54, 109–114. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Stefánsson, E.; Gudmundsdóttir, E.; Eysteinsson, T.; Thorsteinsdóttir, M.; Loftsson, T. Cyclodextrin formulation of dorzolamide and its distribution in the eye after topical administration. J. Controlled Release 2005, 102, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Järvinen, T. Cyclodextrins in ophthalmic drug delivery. Adv. Drug Deliv. Rev. 1999, 36, 59–79. [Google Scholar] [CrossRef]
- Al-Ghabeish, M.; Xu, X.; Krishnaiah, Y.S.R.; Rahman, Z.; Yang, Y.; Khan, M.A. Influence of drug loading and type of ointment base on the in vitro performance of acyclovir ophthalmic ointment. Int. J. Pharm. 2015, 495, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Khare, A.; Grover, K.; Pawar, P.; Singh, I. Mucoadhesive polymers for enhancing retention in ocular drug delivery: A critical review. Rev. Adhes. Adhes. 2014, 2, 467–502. [Google Scholar] [CrossRef]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in mucoadhesive drug-delivery systems: A brief note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef]
- Bora, M.; Mundargi, R.C.; Chee, Y.; Wong, T.T.L.; Venkatraman, S.S. 5-Flurouracil microencapsulation and impregnation in hyaluronic acid hydrogel as composite drug delivery system for ocular fibrosis. Cogent Med. 2016, 3. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Ma, D.H.-K.; Cheng, H.-Y.; Sun, C.-C.; Huang, S.-J.; Li, Y.-T.; Hsiue, G.-H. Ocular biocompatibility of Carbodiimide cross-linked hyaluronic acid hydrogels for cell sheet delivery carriers. J. Biomater. Sci. Polym. Ed. 2010, 21, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, L.K.; Bora, M.; Chan, P.N.P.H.; Lipik, V.; Wong, T.T.L.; Venkatraman, S.S. Hyaluronic acid-based nanocomposite hydrogels for ocular drug delivery applications: Ha-based nanocomposite hydrogels. J. Biomed. Mater. Res. A 2014, 102, 3056–3065. [Google Scholar] [CrossRef] [PubMed]
- Rajoria, G.; Gupta, A. In situ Gelling System: A novel approach for ocular drug delivery. Am. J. PharmTech Res. 2012, 2, 25–53. [Google Scholar]
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L.; Ping, Q. Poly(N-isopropylacrylamide)–chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Controlled Release 2007, 120, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Mane, K.; Dhole, S. In situ gelling system - A novel approach for ocular drug delivery. World J. Pharm. Pharm. Sci. 2014, 3, 317–333. [Google Scholar]
- Gonjari, I.D.; Karmarkar, A.B.; Khade, T.S.; Hosmani, A.H.; Navale, R.B. Use of factorial design in formulation and evaluation of ophthalmic gels of gatifloxacin: Comparison of different mucoadhesive polymers. Drug Discov. Ther. 2010, 4, 423–434. [Google Scholar] [PubMed]
- Buchan, B.; Kay, G.; Heneghan, A.; Matthews, K.H.; Cairns, D. Gel formulations for treatment of the ophthalmic complications in cystinosis. Int. J. Pharm. 2010, 392, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. Applications of poloxamers in ophthalmic pharmaceutical formulations: An overview. Expert Opin. Drug Deliv. 2013, 10, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, Z.; Zhang, H.; Pan, X.; Su, W.; Liang, D.; Wu, C. Doxycycline and hydroxypropyl-β-cyclodextrin complex in poloxamer thermal sensitive hydrogel for ophthalmic delivery. Acta Pharm. Sin. B 2011, 1, 254–260. [Google Scholar] [CrossRef]
- Cho, K. Release of ciprofloxacin from poloxamer-graft-hyaluronic acid hydrogels in vitro. Int. J. Pharm. 2003, 260, 83–91. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; Rotonda, M. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Shastri, D.H.; Prajapati, S.T.; Patel, L.D. Studies on poloxamer based mucoadhesive insitu ophthalmic hydrogel of moxifloxacin HCL. Curr. Drug Deliv. 2010, 3, 238–243. [Google Scholar] [CrossRef]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F.V. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Srividya, B.; Cardoza, R.M.; Amin, P. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J. Controlled Release 2001, 73, 205–211. [Google Scholar] [CrossRef]
- Patil, S.; Kadam, A.; Bandgar, S.; Patil, S. Formulation and evaluation of an in situ gel for ocular drug delivery of anticonjunctival drug. Cellulose Chem. Technol. 2015, 49, 35–40. [Google Scholar]
- Makwana, S.B.; Patel, V.A.; Parmar, S.J. Development and characterization of in situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Prabhushankar, G.; Thimmasetty, M.; Geetha, M. Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. Int. J. Pharm. Investig. 2012, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Carlfors, J.; Edsman, K.; Petersson, R.; Jörnving, K. Rheological evaluation of Gelrite® in situ gels for ophthalmic use. Eur. J. Pharm. Sci. 1998, 6, 113–119. [Google Scholar] [CrossRef]
- Sultana, Y.; Aqil, M.; Ali, A. Ion-Activated, Gelrite®-Based in Situ Ophthalmic Gels of Pefloxacin Mesylate: Comparison with Conventional Eye Drops. Drug Deliv. 2006, 13, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Ding, S.; Himmelstein, K.J. Reversible Gelation Compositions and Methods of Use. U.S. Patent 5252318 A, 15 June 1990. [Google Scholar]
- Liu, Y.; Liu, J.; Zhang, X.; Zhang, R.; Huang, Y.; Wu, C. In situ gelling gelrite/alginate formulations as vehicles for ophthalmic drug delivery. AAPS PharmSciTech 2010, 11, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Fanun, M. Microemulsions as delivery systems. Curr. Opin. Colloid Interface Sci. 2012, 17, 306–313. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Murthy, R.S.R. Microemulsions: A potential drug delivery system. Curr. Drug Deliv. 2006, 3, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, T.F. Microemulsions as ocular drug delivery systems: Recent developments and future challenges. Prog. Retin. Eye Res. 2002, 21, 15–34. [Google Scholar] [CrossRef]
- Tamilvanan, S.; Benita, S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur. J. Pharm. Biopharm. 2004, 58, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S. Successfully improving ocular drug delivery using the cationic nanoemulsion, Novasorb. J. Drug Deliv. 2012, 2012, 604204. [Google Scholar] [CrossRef] [PubMed]
- Van, A. Eye irritation: studies relating to responses in man and laboratory animals. J. Soc. Cosmet. Chem. Jpn. 1973, 24, 685–692. [Google Scholar]
- Yin, J.; Xiang, C.; Lu, G. Cationic lipid emulsions as potential bioadhesive carriers for ophthalmic delivery of palmatine. J. Microencapsul. 2016, 33, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Ocular Insert. U.S. Patent 3,630,200 A, 28 December 1971. [Google Scholar]
- Kumari, A.; Sharma, P.; Garg, V.; Garg, G. Ocular inserts—Advancement in therapy of eye diseases. J. Adv. Pharm. Technol. Res. 2010, 1, 291. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Sharma, S.; Bhat, S.A.; Bhandari, A.; Deva, A.S.; Rathore, M.S.; Khan, R.A.; Bhatia, N. Preparation and evaluation of ocular inserts of diclofenac sodium for controlled drug delivery. Int. J. Sci. Res. Publ. 2015, 5, 93–99. [Google Scholar]
- Shukr, M. Formulation, in vitro and in vivo evaluation of lidocaine HCl ocular inserts for topical ocular anesthesia. Arch. Pharm. Res. 2014, 37, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Sampath Kumar, K.P.; Bhowmik, D.; Harish, G.; Duraivel, S.; Pragathi Kumar, B. Ocular inserts: A novel controlled drug delivery system. The Pharm. Innov. 2012, 1, 1–16. [Google Scholar]
- Gurtler, F.; Gurny, R. Patent literature review of ophthalmic inserts. Drug Dev. Ind. Pharm. 1995, 21, 1–18. [Google Scholar] [CrossRef]
- Baranowski, P.; Karolewicz, B.; Gajda, M.; Pluta, J. Ophthalmic drug dosage forms: Characterisation and research methods. Sci. World J. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Aranguez, A.; Colligris, B.; Pintor, J. Contact lenses: promising devices for ocular drug delivery. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2013, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Stretton, S.; Papas, E.; Skotnitsky, C.; Sweeney, D.F. Silicone hydrogel contact lenses and the ocular surface. Ocul. Surf. 2006, 4, 24–43. [Google Scholar] [CrossRef]
- Van der Worp, E.; Bornman, D.; Ferreira, D.L.; Faria-Ribeiro, M.; Garcia-Porta, N.; González-Meijome, J.M. Modern scleral contact lenses: A review. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2014, 37, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Harthan, J.S. Therapeutic use of mini-scleral lenses in a patient with Graves’ ophthalmopathy. J. Optom. 2014, 7, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Rathi, V.M.; Dumpati, S.; Mandathara, P.S.; Taneja, M.M.; Sangwan, V.S. Scleral contact lenses in the management of pellucid marginal degeneration. Contact Lens Anterior Eye 2016, 39, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Severinsky, B.; Behrman, S.; Frucht-Pery, J.; Solomon, A. Scleral contact lenses for visual rehabilitation after penetrating keratoplasty: Long term outcomes. Contact Lens Anterior Eye 2014, 37, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Romero-Rangel, T.; Stavrou, P.; Cotter, J.; Rosenthal, P.; Baltatzis, S.; Foster, C.S. Gas-permeable scleral contact lens therapy in ocular surface disease. Am. J. Ophthalmol. 2000, 130, 25–32. [Google Scholar] [CrossRef]
- Kramer, E.G.; Boshnick, E.L. Scleral lenses in the treatment of post-LASIK ectasia and superficial neovascularization of intrastromal corneal ring segments. Contact Lens Anterior Eye 2015, 38, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, Y.; Sun, Y.-C.; Flowers, M.E.D.; Carpenter, P.A.; Martin, P.J.; Li, P.; Wang, R.; Chai, X.; Storer, B.E.; Shen, T.T.; et al. Bandage soft contact lenses for ocular graft-versus-host disease. Biol. Blood Marrow Transplant. 2015, 21, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Glisoni, R.J.; García-Fernández, M.J.; Pino, M.; Gutkind, G.; Moglioni, A.G.; Alvarez-Lorenzo, C.; Concheiro, A.; Sosnik, A. β-Cyclodextrin hydrogels for the ocular release of antibacterial thiosemicarbazones. Carbohydr. Polym. 2013, 93, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Alonso, M.J.; Pinto, M.M.M.; Barbosa, C.M. Development and characterization of PLGA nanospheres and nanocapsules containing xanthone and 3-methoxyxanthone. Eur. J. Pharm. Biopharm. 2005, 59, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retin. Eye Res. 2010, 29, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Kambhampati, S.P.; Kannan, R.M. Nanotechnology approaches for ocular drug delivery. Middle East Afr. J. Ophthalmol. 2013, 20, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Yildirimer, L.; Thanh, N.T.K.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Duxfield, L.; Sultana, R.; Wang, R.; Englebretsen, V.; Deo, S.; Swift, S.; Rupenthal, I.; Al-Kassas, R. Development of gatifloxacin-loaded cationic polymeric nanoparticles for ocular drug delivery. Pharm. Dev. Technol. 2016, 21, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Mun, E.A.; Morrison, P.W.J.; Williams, A.C.; Khutoryanskiy, V.V. On the barrier properties of the cornea: A microscopy study of the penetration of fluorescently labeled nanoparticles, polymers, and sodium fluorescein. Mol. Pharm. 2014, 11, 3556–3564. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Meisner, D.; Mezei, M. Liposome ocular delivery systems. Adv. Drug Deliv. Rev. 1995, 16, 75–93. [Google Scholar] [CrossRef]
- Mishra, G.P.; Bagui, M.; Tamboli, V.; Mitra, A.K. Recent applications of liposomes in ophthalmic drug delivery. J. Drug Deliv. 2011, 2011, e863734. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Guinedi, A.S. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS PharmSciTech 2007, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Khanam, N.; Alam, M.I.; Sachan, A.K.; Sharma, R. Recent trends in drug delivery by niosomes: A review. Research Gate 2013, 1, 115–122. [Google Scholar]
- Pham, T.T.; Jaafar-Maalej, C.; Charcosset, C.; Fessi, H. Liposome and niosome preparation using a membrane contactor for scale-up. Colloids Surf. B 2012, 94, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomedicine 2015, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Burçin, Y.; Bozdag Pehlivan, S.; Ünlü, S. Dendrimeric systems and their applications in ocular drug delivery. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Brobeck, L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Controlled Release 2005, 102, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzæ. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Gualerzi, C.O.; Brandi, L.; Fabbretti, A.; Pon, C.L. (Eds.) Antibiotics: Targets, Mechanisms and Resistance; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 978-3-527-65968-5. [Google Scholar]
- Kapoor, A.; Malhotra, R.; Grover, V.; Grover, D. Systemic antibiotic therapy in periodontics. Dent. Res. J. 2012, 9, 505–515. [Google Scholar] [CrossRef]
- Cornut, P.-L.; Chiquet, C. Intravitreal injection of antibiotics in endophthalmitis. J. Fr. Ophtalmol. 2008, 31, 815–823. [Google Scholar] [CrossRef]
- Barza, M. Factors affecting the intraocular penetration of antibiotics. The influence of route, inflammation, animal species and tissue pigmentation. Scand. J. Infect. Dis. Suppl. 1978, 151–159. [Google Scholar]
- Snyder, R.W.; Glasser, D.B. Antibiotic therapy for ocular infection. West. J. Med. 1994, 161, 579–584. [Google Scholar] [PubMed]
- Shimpi, S.; Chauhan, B.; Shimpi, P. Cyclodextrins: application in different routes of drug administration. Acta Pharm. Zagreb Croat. 2005, 55, 139–156. [Google Scholar]
- Tiwari, G.; Tiwari, R.; Rai, A.K. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied Sci. 2010, 2, 72. [Google Scholar] [CrossRef] [PubMed]
- Nijhawan, R.; Agarwal, S.P. Development of an ophthalmic formulation containing ciprofloxacin-hydroxypropyl-b-cyclodextrin complex. Boll. Chim. Farm. 2003, 142, 214–219. [Google Scholar] [PubMed]
- Bozkir, A.; Denli, Z.F.; Basaran, B. Effect of hydroxypropyl-beta-cyclodextrin on the solubility, stability and in-vitro release of ciprofloxacin for ocular drug delivery. Acta Pol. Pharm. 2012, 69, 719–724. [Google Scholar] [PubMed]
- Thatiparti, T.R.; von Recum, H.A. Cyclodextrin complexation for affinity-based antibiotic delivery. Macromol. Biosci. 2010, 10, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Pullum, K.W. The unique role of scleral lenses in contact lens practice. Contact Lens Anterior Eye 1999, 22, S26–S34. [Google Scholar] [CrossRef]
- Tougeron-Brousseau, B.; Delcampe, A.; Gueudry, J.; Vera, L.; Doan, S.; Hoang-Xuan, T.; Muraine, M. Vision-related function after scleral lens fitting in ocular complications of stevens-johnson syndrome and toxic epidermal necrolysis. Am. J. Ophthalmol. 2009, 148, 852–859.e2. [Google Scholar] [CrossRef] [PubMed]
- Laballe, R.; Vigne, J.; Denion, E.; Lemaitre, F.; Goux, D.; Pisella, P.-J. Preclinical assessment of scleral lens as a reservoir-based ocular therapeutic system. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2016, 39, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tan, H.; Hao, L. Functional hydrogel contact lens for drug delivery in the application of oculopathy therapy. J. Mech. Behav. Biomed. Mater. 2016, 64, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Iwatsu, M.; Sado, K.; Kanai, A. Studies on the uptake and release of fluoroquinolones by disposable contact lenses. CLAO J. Off. Publ. Contact Lens Assoc. Ophthalmol. Inc. 2001, 27, 216–220. [Google Scholar]
- Hehl, E.M.; Beck, R.; Luthard, K.; Guthoff, R.; Drewelow, B. Improved penetration of aminoglycosides and fluorozuinolones into the aqueous humour of patients by means of Acuvue contact lenses. Eur. J. Clin. Pharmacol. 1999, 55, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Yokozaki, Y.; Sakabe, J.; Shimoyama, Y. Enhanced impregnation of hydrogel contact lenses with salicylic acid by addition of water in supercritical carbon dioxide. Chem. Eng. Res. Des. 2015, 104, 203–207. [Google Scholar] [CrossRef]
- Costa, V.P.; Braga, M.E.M.; Guerra, J.P.; Duarte, A.R.C.; Duarte, C.M.M.; Leite, E.O.B.; Gil, M.H.; de Sousa, H.C. Development of therapeutic contact lenses using a supercritical solvent impregnation method. J. Supercrit. Fluids 2010, 52, 306–316. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Yañez, F.; Concheiro, A. Ocular drug delivery from molecularly-imprinted contact lenses. J. Drug Deliv. Sci. Technol. 2010, 20, 237–248. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Yañez, F.; Barreiro-Iglesias, R.; Concheiro, A. Imprinted soft contact lenses as norfloxacin delivery systems. J. Controlled Release 2006, 113, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Malakooti, N.; Alexander, C.; Alvarez-Lorenzo, C. Imprinted contact lenses for sustained release of polymyxin B and related antimicrobial peptides. J. Pharm. Sci. 2015, 104, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, H.; Hosaka, S.; Kunitomo, T.; Tanzawa, H. Ocular inserts for controlled release of antibiotics. Biomaterials 1983, 4, 170–174. [Google Scholar] [CrossRef]
- Hosaka, S.; Ozawa, H.; Tanzawa, H.; Kinitomo, T.; Nichols, R.L. In vivo evaluation of ocular inserts of hydrogel impregnated with antibiotics for trachoma therapy. Biomaterials 1983, 4, 243–248. [Google Scholar] [CrossRef]
- Punch, P.I.; Costa, N.D.; Edwards, M.E.; Wilcox, G.E. The release of insoluble antibiotics from collagen ocular inserts in vitro and their insertion into the conjunctival sac of cattle. J. Vet. Pharmacol. Ther. 1987, 10, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, V.; Kaltsatos, V.; Boisramé, B.; Varesio, E.; Veuthey, J.-L.; Fathi, M.; Balant, L.P.; Gex-Fabry, M.; Gurny, R. Optimized release of dexamethasone and gentamicin from a soluble ocular insert for the treatment of external ophthalmic infections. J. Controlled Release 1998, 52, 215–220. [Google Scholar] [CrossRef]
- Di Colo, G.; Burgalassi, S.; Chetoni, P.; Fiaschi, M.P.; Zambito, Y.; Saettone, M.F. Gel-forming erodible inserts for ocular controlled delivery of ofloxacin. Int. J. Pharm. 2001, 215, 101–111. [Google Scholar] [CrossRef]
- Di Colo, G.; Burgalassi, S.; Chetoni, P.; Fiaschi, M.P.; Zambito, Y.; Saettone, M.F. Relevance of polymer molecular weight to the in vitro/in vivo performances of ocular inserts based on poly(ethylene oxide). Int. J. Pharm. 2001, 220, 169–177. [Google Scholar] [CrossRef]
- Di Colo, G.; Zambito, Y.; Burgalassi, S.; Serafini, A.; Saettone, M.F. Effect of chitosan on in vitro release and ocular delivery of ofloxacin from erodible inserts based on poly(ethylene oxide). Int. J. Pharm. 2002, 248, 115–122. [Google Scholar] [CrossRef]
- Üstündağ-Okur, N.; Gökçe, E.H.; Bozbıyık, D.İ.; Eğrilmez, S.; Ertan, G.; Özer, Ö. Novel nanostructured lipid carrier-based inserts for controlled ocular drug delivery: Evaluation of corneal bioavailability and treatment efficacy in bacterial keratitis. Expert Opin. Drug Deliv. 2015, 12, 1791–1807. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Y.; Aqil, M.; Ali, A. Ocular inserts for controlled delivery of pefloxacin mesylate: Preparation and evaluation. Acta Pharm. Zagreb Croat. 2005, 55, 305–314. [Google Scholar]
- Mundada, A.S.; Shrikhande, B.K. Design and evaluation of soluble ocular drug insert for controlled release of ciprofloxacin hydrochloride. Drug Dev. Ind. Pharm. 2006, 32, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Mundada, A.S.; Shrikhande, B.K. Formulation and evaluation of ciprofloxacin hydrochloride soluble ocular drug insert. Curr. Eye Res. 2008, 33, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Pawar, P.K.; Katara, R.; Majumdar, D.K. Design and evaluation of moxifloxacin hydrochloride ocular inserts. Acta Pharm. Zagreb Croat. 2012, 62, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Swami, G.; Rahman, M. Development and optimization of controlled release bioerodable anti infective ophthalmic insert. Curr. Drug Deliv. 2014, 11, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Xiong, L.; Sun, N. Different concentrations of clarithromycin ophthalmic gel for rabbits corneal ulcers induced by Staphylococcus aureus. Yan Ke Xue Bao 2008, 24, 18–22. [Google Scholar] [PubMed]
- Liu, Z.; Li, J.; Nie, S.; Liu, H.; Ding, P.; Pan, W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int. J. Pharm. 2006, 315, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassas, R.S.; El-Khatib, M.M. Ophthalmic controlled release in situ gelling systems for ciprofloxacin based on polymeric carriers. Drug Deliv. 2009, 16, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Mansour, S.; Mortada, N.D.; Abd Elhady, S.S. Ocular poloxamer-based ciprofloxacin hydrochloride in situ forming gels. Drug Dev. Ind. Pharm. 2008, 34, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Nanjwade, B.K.; Deshmukh, R.V.; Gaikwad, K.R.; Parikh, K.A.; Manvi, F.V. Formulation and evaluation of micro hydrogel of Moxifloxacin hydrochloride. Eur. J. Drug Metab. Pharmacokinet. 2012, 37, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Aqil, M.; Imam, S.S.; Ali, A. Development and evaluation of a novel in situ gel of sparfloxacin for sustained ocular drug delivery: In vitro and ex vivo characterization. Pharm. Dev. Technol. 2015, 20, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Y.; Aqil, M.; Ali, A.; Zafar, S. Evaluation of carbopol-methyl cellulose-based sustained-release ocular delivery system for pefloxacin mesylate using rabbit eye model. Pharm. Dev. Technol. 2006, 11, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, X.-G.; Li, X.; Pan, W.; Li, J. Study on the ocular pharmacokinetics of ion-activated in situ gelling ophthalmic delivery system for gatifloxacin by microdialysis. Drug Dev. Ind. Pharm. 2007, 33, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- El-Laithy, H.M.; Nesseem, D.I.; El-Adly, A.A.; Shoukry, M. Moxifloxacin-Gelrite in situ ophthalmic gelling system against photodynamic therapy for treatment of bacterial corneal inflammation. Arch. Pharm. Res. 2011, 34, 1663–1678. [Google Scholar] [CrossRef] [PubMed]
- Ameeduzzafar; Ali, J.; Fazil, M.; Qumbar, M.; Khan, N.; Ali, A. Colloidal drug delivery system: Amplify the ocular delivery. Drug Deliv. 2014, 23, 700–716. [Google Scholar] [CrossRef]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Nanoemulsion as a Potential Ophthalmic Delivery System for Dorzolamide Hydrochloride. AAPS PharmSciTech 2009, 10, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.-F.; Zheng, L.-Q.; Tung, C.-H. Phase behavior of the microemulsions and the stability of the chloramphenicol in the microemulsion-based ocular drug delivery system. Int. J. Pharm. 2005, 301, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Kesavan, K. Phase-transition W/O Microemulsions for ocular delivery: Evaluation of antibacterial activity in the treatment of bacterial keratitis. Ocul. Immunol. Inflamm. 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Üstündag-Okur, N.; Gökçe, E.H.; Eğrilmez, S.; Özer, Ö.; Ertan, G. Novel ofloxacin-loaded microemulsion formulations for ocular delivery. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2014, 30, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Alshamsan, A.; Aljuffali, I.A.; Mishra, A.K.; Sultana, Y. Delivery of gatifloxacin using microemulsion as vehicle: Formulation, evaluation, transcorneal permeation and aqueous humor drug determination. Drug Deliv. 2016, 23, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Müller, R.H.; Keck, C.M.; Bou-Chacra, N.A. Mucoadhesive dexamethasone acetate-polymyxin B sulfate cationic ocular nanoemulsion--novel combinatorial formulation concept. Int. J. Pharm. Sci. 2016, 71, 327–333. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobao, P.; Frigerio, C.; Sousa Lobo, J.M. Nanoparticles in Ocular Drug Delivery Systems for Topical Administration: Promises and Challenges. Curr. Pharm. Des. 2015, 21, 5212–5224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Hao, J.-L.; Wang, S.; Zheng, Y.; Zhang, W.-S. Nanoparticles in the ocular drug delivery. Int. J. Ophthalmol. 2013, 6, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Suresh, P.K.; Desmukh, R. Design of Eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomedicine Nanotechnol. Biol. Med. 2010, 6, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Alexander, K.S.; Riga, A.T. Sulfacetamide loaded Eudragit® RL100 nanosuspension with potential for ocular delivery. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2010, 13, 510–523. [Google Scholar]
- Ibrahim, H.K.; El-Leithy, I.S.; Makky, A.A. Mucoadhesive nanoparticles as carrier systems for prolonged ocular delivery of gatifloxacin/prednisolone bitherapy. Mol. Pharm. 2010, 7, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Bourgeois, S.; Almouazen, E.; Pelletier, J.; Renaud, F.; Fessi, H.; Kodjikian, L. Microencapsulation of rifampicin for the prevention of endophthalmitis: In vitro release studies and antibacterial assessment. Int. J. Pharm. 2016, 505, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, W.; Kodjikian, L.; Fessi, H. Preparation of vancomycin microparticles: Importance of preparation parameters. Int. J. Pharm. 2006, 324, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine Nanotechnol. Biol. Med. 2010, 6, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J. Drug Target. 2011, 19, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Nokhodchi, A.; Barzegar-Jalali, M.; Lotfipour, F.; Adibkia, K.; Ehyaei, N.; Valizadeh, H. Physicochemical and anti-bacterial performance characterization of clarithromycin nanoparticles as colloidal drug delivery system. Colloids Surf. B Biointerfaces 2011, 88, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Pokharkar, V.; Patil, V.; Mandpe, L. Engineering of polymer-surfactant nanoparticles of doxycycline hydrochloride for ocular drug delivery. Drug Deliv. 2015, 22, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2008, 68, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.; Silva, S.; Sarmento, B.; Pintado, M. Chitosan nanoparticles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Deliv. 2015, 22, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Silva, N.C.; Sarmento, B.; Pintado, M. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2015, 34, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye–Part II—Ocular drug-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 110, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Sultana, Y.; Ali, A.; Aqil, M.; Mishra, A.K.; Chuttani, K. Preparation, characterization, and evaluation of gatifloxacin loaded solid lipid nanoparticles as colloidal ocular drug delivery system. J. Drug Target. 2010, 18, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Abul Kalam, M.; Sultana, Y.; Ali, A.; Aqil, M.; Mishra, A.K.; Aljuffali, I.A.; Alshamsan, A. Part I: Development and optimization of solid-lipid nanoparticles using Box-Behnken statistical design for ocular delivery of gatifloxacin. J. Biomed. Mater. Res. A 2013, 101, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Abul Kalam, M.; Sultana, Y.; Ali, A.; Aqil, M.; Mishra, A.K.; Chuttani, K.; Aljuffali, I.A.; Alshamsan, A. Part II: Enhancement of transcorneal delivery of gatifloxacin by solid lipid nanoparticles in comparison to commercial aqueous eye drops. J. Biomed. Mater. Res. A 2013, 101, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.S.; Ahad, A.; Aslam, M.; Imam, S.S.; Aqil, M.; Ali, A. Application of Box-Behnken design for preparation of levofloxacin-loaded stearic acid solid lipid nanoparticles for ocular delivery: Optimization, in vitro release, ocular tolerance, and antibacterial activity. Int. J. Biol. Macromol. 2016, 85, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Yousry, C.; Fahmy, R.H.; Essam, T.; El-Laithy, H.M.; Elkheshen, S.A. Nanoparticles as tool for enhanced ophthalmic delivery of vancomycin: A multidistrict-based microbiological study, solid lipid nanoparticles formulation and evaluation. Drug Dev. Ind. Pharm. 2016, 42, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Tampucci, S.; Tullio, V.; Cuffini, A.M.; Muntoni, E.; Spagnolo, R.; Zara, G.P.; Cavalli, R. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2016, 109, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Elmaradny, H.A.; Samaha, M.W. Ciprofloxacin liposomes as vesicular reservoirs for ocular delivery: Formulation, optimization, and in vitro characterization. Drug Dev. Ind. Pharm. 2009, 35, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Chetoni, P.; Monti, D.; Tampucci, S.; Matteoli, B.; Ceccherini-Nelli, L.; Subissi, A.; Burgalassi, S. Liposomes as a potential ocular delivery system of distamycin A. Int. J. Pharm. 2015, 492, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Elmaradny, H.A.; Samaha, M.W. Mucoadhesive liposomes as ocular delivery system: Physical, microbiological, and in vivo assessment. Drug Dev. Ind. Pharm. 2010, 36, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G. Ocular ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes. Pharm. Dev. Technol. 2011, 16, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Budai, L.; Hajdú, M.; Budai, M.; Gróf, P.; Béni, S.; Noszál, B.; Klebovich, I.; Antal, I. Gels and liposomes in optimized ocular drug delivery: Studies on ciprofloxacin formulations. Int. J. Pharm. 2007, 343, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M. Preparation and evaluation of thermosensitive liposomal hydrogel for enhanced transcorneal permeation of ofloxacin. AAPS PharmSciTech 2009, 10, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M. Ciprofloxacin as ocular liposomal hydrogel. AAPS Pharm. Sci. Tech. 2010, 11, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M. Optimization of gatifloxacin liposomal hydrogel for enhanced transcorneal permeation. J. Liposome Res. 2010, 20, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Hu, C.; Wei, H.; Lu, Y.; Zhang, Y.; Yang, J.; Yun, G.; Zou, W.; Song, B. Intravitreal pharmacokinetics of liposome-encapsulated amikacin in a rabbit model. Ophthalmology 1993, 100, 1640–1644. [Google Scholar] [CrossRef]
- Wiechens, B.; Krausse, R.; Grammer, J.B.; Neumann, D.; Pleyer, U.; Duncker, G.I. Clearance of liposome-incorporated ciprofloxacin after intravitreal injection in rabbit eyes. Klin. Monatsbl. Augenheilkd. 1998, 213, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.M.; Imai, H.; Haakenson, J.K.; Brucklacher, R.M.; Fox, T.E.; Shanmugavelandy, S.S.; Unrath, K.A.; Pedersen, M.M.; Dai, P.; Freeman, W.M.; et al. Nanoliposomal minocycline for ocular drug delivery. Nanomedicine Nanotechnol. Biol. Med. 2013, 9, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Guinedi, A.S.; Mortada, N.D.; Mansour, S.; Hathout, R.M. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int. J. Pharm. 2005, 306, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Pal, D.; Mitra, A.K.; Kaur, I.P. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation, by microdialysis sampling of aqueous humor. Int. J. Pharm. 2007, 338, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.; El-Gendy, N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Akbari, V.; Abedi, D.; Pardakhty, A.; Sadeghi-Aliabadi, H. Release studies on ciprofloxacin loaded non-ionic surfactant vesicles. Avicenna J. Med. Biotechnol. 2015, 7, 69–75. [Google Scholar] [PubMed]

| Type | Polymers | References |
|---|---|---|
| Thermosensitive gels | Negative: Pluronics, poly(N-isopropyl acrylamide) Positive: poly(acrylic acid), polyacrylamide, Reversible: poloxamer, chitosan, hydroxyl propyl méthyl cellulose | [30,31,32,33] |
| pH-sensitive gels | Cellulose acetate and derivatives Carbomer Magrogol Pseudolatex Polymethacrylic acid | [29] [34] [35] |
| Ion-sensitive gels | Alginate sodium gellan gum (Gelrite®) | [3] [29] |
| Formulation | Antibiotic | Anterior (AS) or Posterior (PS) Segment | Disease Targeted | References |
|---|---|---|---|---|
| Microemulsion | Chloramphenicol | AS | Trachoma Keratitis | [138] |
| Moxifloxacin | AS | Bacterial keratitis | [139] | |
| Nanoemulsion | Polymixin B | AS | Ophthalmic infection | [142] |
| Nanoparticles | Tobramycin | AS + PS | Bacterial infection Pseudomonas aeruginosa | [163] |
| Levofloxacin | AS | Bacterial infection S. aureus and E. coli | [161] | |
| Liposomes | Ciprofloxacin | PS | Bacterial endophthalmitis | [173] |
| Distamycin A | AS | Herpes simplex virus | [165] | |
| Niosomes | Acetazolamide | AS | Glaucoma | [175] |
| Ciprofloxacin | AS | Conjunctiva + corneal ulcer | [178] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubald, M.; Bourgeois, S.; Andrieu, V.; Fessi, H. Ophthalmic Drug Delivery Systems for Antibiotherapy—A Review. Pharmaceutics 2018, 10, 10. https://doi.org/10.3390/pharmaceutics10010010
Dubald M, Bourgeois S, Andrieu V, Fessi H. Ophthalmic Drug Delivery Systems for Antibiotherapy—A Review. Pharmaceutics. 2018; 10(1):10. https://doi.org/10.3390/pharmaceutics10010010
Chicago/Turabian StyleDubald, Marion, Sandrine Bourgeois, Véronique Andrieu, and Hatem Fessi. 2018. "Ophthalmic Drug Delivery Systems for Antibiotherapy—A Review" Pharmaceutics 10, no. 1: 10. https://doi.org/10.3390/pharmaceutics10010010
APA StyleDubald, M., Bourgeois, S., Andrieu, V., & Fessi, H. (2018). Ophthalmic Drug Delivery Systems for Antibiotherapy—A Review. Pharmaceutics, 10(1), 10. https://doi.org/10.3390/pharmaceutics10010010