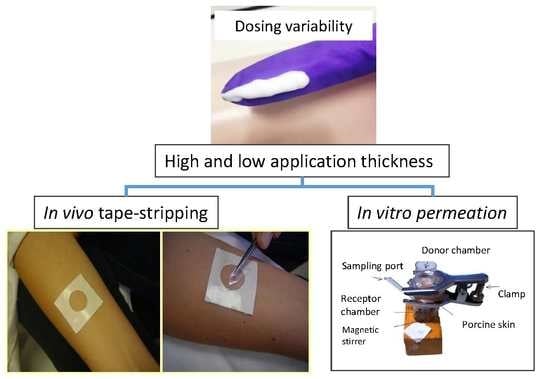
Is the Skin Absorption of Hydrocortisone Modified by the Variability in Dosing Topical Products?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
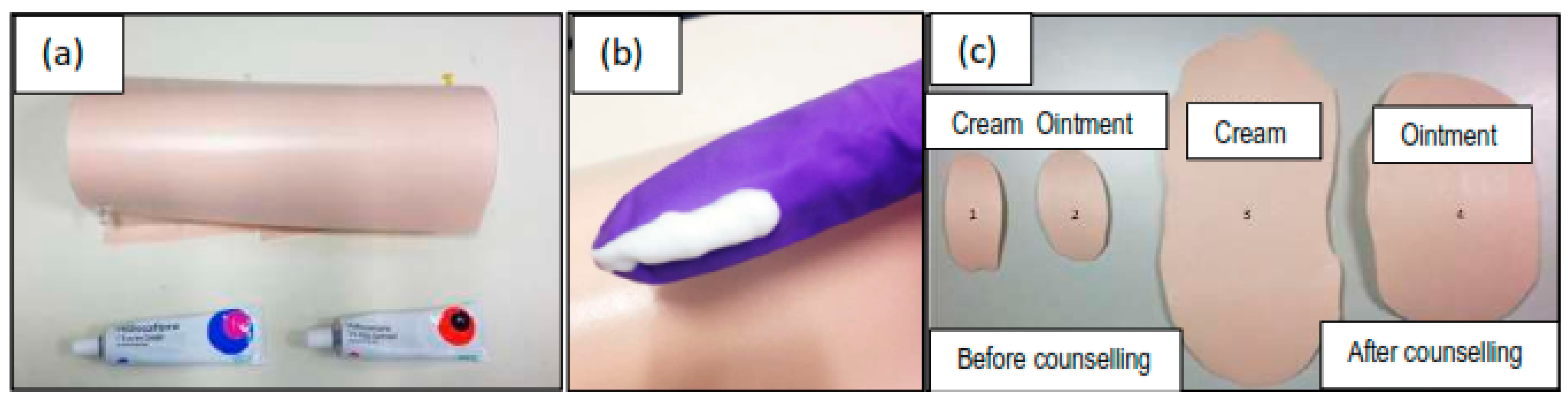
2.2. Inter-Subject Variability in Dosing Topical Products
2.3. In Vivo Tape-Stripping Experiments
2.4. In Vitro Permeation Tests (IVPT)
3. Results
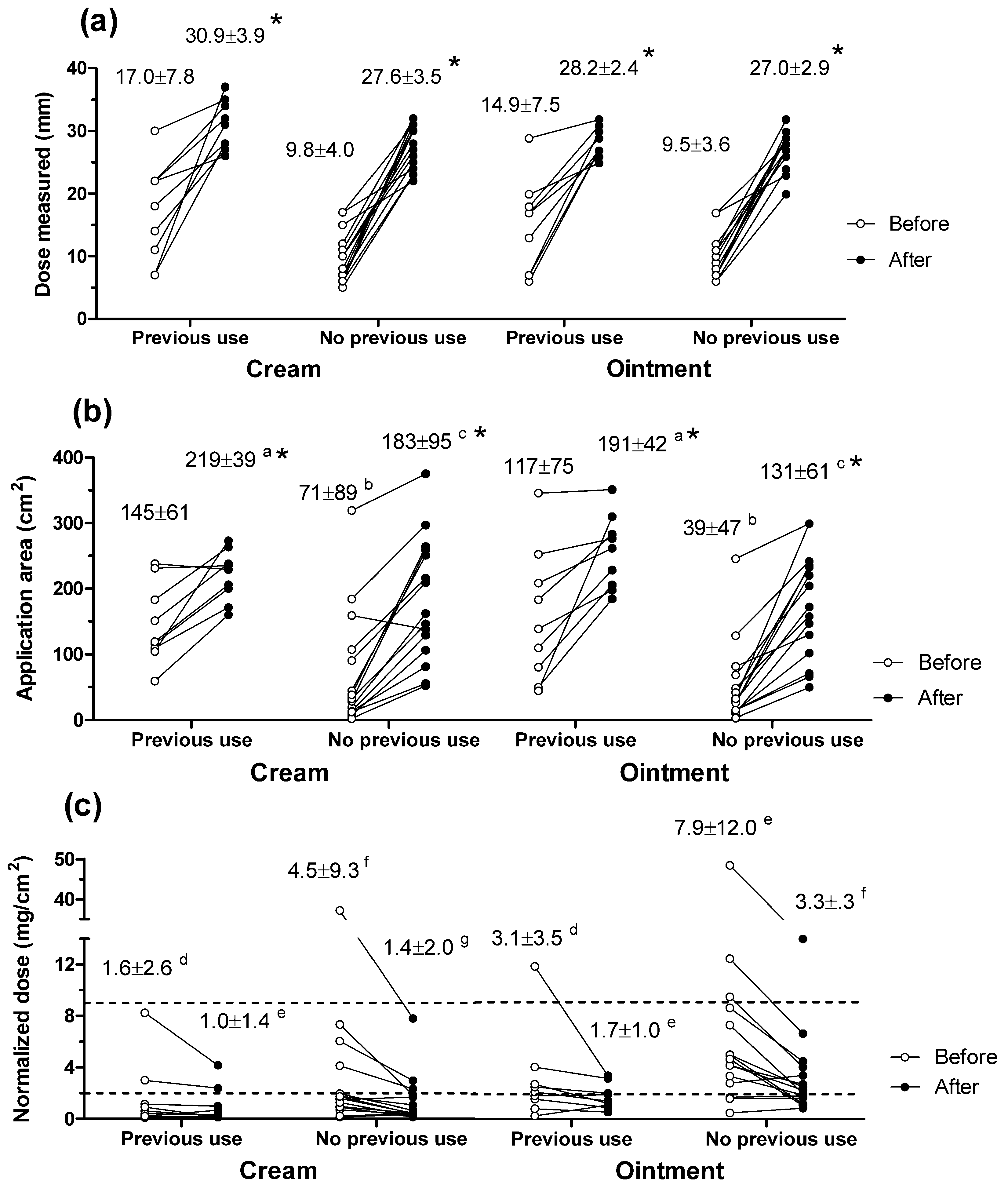
3.1. Inter-Subject Variability in Dosing Topical Products
3.2. In Vivo Tape-Stripping Experiments
3.3. In Vitro Permeation Experiments
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Savary, J.; Ortonne, J.P.; Aractingi, S. The right dose in the right place: An overview of current prescription, intruction and application modalities for topical psoriasis treatments. JEADV 2005, 19, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Bewley, A.; Dermatology Working Group. Expert consensus: Time for a change in the way we advise our patients to use topical corticosteroids. Br. J. Dermatol. 2008, 158, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Totri, C. Optimizing outcomes for paediatric atopic dermatitis. Br. J. Dermatol. 2014, 170, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Long, C.C.; Mills, C.M.; Finlay, A.Y. A practical guide to topical therapy in children. Br. J. Dermatol. 1998, 138, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Serup, J.; Lindblad, A.K.; Maroti, M.; Kjellgren, K.I.; Niklasson, E.; Ring, L.; Ahlner, J. To follow or not to follow dermatological treatment—A review of the literature. Acta Derm.-Venereol. 2008, 86, 193–197. [Google Scholar] [CrossRef] [PubMed]
- British National Formulary. Prescribing and Dispensing Information. For All Corticosteroids—(Topical). Medicines Complete. Available online: https://www.medicinescomplete.com/mc/bnf/current/DMD4928211000001101.htm#PHP56812-directionsForAdministration-topic (accessed on 13 December 2016).
- Storm, A.; Benfeldt, E.; Andersen, S.E.; Serup, J. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J. Am. Acad. Dermatol. 2008, 59, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.R.; Horn, E.J.; Balkrishnan, R.; Basra, M.K.; Finlay, A.Y.; Mccoy, D.; Menter, A.; van de Kerkhof, P.C.M. Psoriasis: Improving adherence to topical therapy. J. Am. Acad. Dermatol. 2008, 59, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Storm, A.; Benfeldt, E.; Andersen, S.E.; Andersen, J. Basic drug information given by physicians is deficient, and patients’ knowledge low. J. Dermatol. Treat. 2009, 20, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Long, C.C.; Finlay, A.Y. The finger-tip unit—A new practical measure. Clin. Exp. Dermatol. 1991, 16, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Long, C.C.; Finlay, A.Y.; Averill, R.W. The rule of hand: 4 Hand Areas = 2 FTU = 1 g. Arch. Dermatol. 1992, 128, 1129–1130. [Google Scholar] [CrossRef] [PubMed]
- Addicks, W.J.; Flynn, G.L.; Weiner, N.; Chiang, C.M. Drug transport from thin applications of topical dosage forms: Development of methodology. Pharm. Res. 1988, 5, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Addicks, W.J.; Weiner, N.; Flynn, G.; Curl, R.; Topp, E. Topical drug delivery from thin applications: Theoretical predictions and experimental results. Pharm. Res. 1990, 7, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.H.; Hadgraft, J. A theoretical description relating skin penetration to the thickness of the applied medicament. Int. J. Pharm. 1980, 6, 321–332. [Google Scholar] [CrossRef]
- N’Dri-Stempfer, B.; Navidi, W.C.; Guy, R.H.; Bunge, A.L. Improved bioequivalence assessment of topical dermatological drug products using dermatopharmacokinetics. Pharm. Res. 2009, 26, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Wiedersberg, S.; Leopold, C.S.; Guy, R.H. Dermatopharmacokinetics of betamethasone 17-valerate: Influence of formulation viscosity and skin surface cleaning procedure. Eur. J. Pharm. Biopharm. 2009, 71, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.M.; Wiedersberg, S.; Delgado-Charro, M.B. The determination of stratum corneum thickness: An alternative approach. Eur. J. Pharm. Biopharm. 2008, 69, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Cassidy, J.M. Variations in physical dimensions and chemical composition of human stratum corneum. J. Investig. Dermatol. 1973, 61, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Gee, C.M.; Nicolazzo, J.A.; Watkinson, A.C.; Finnin, B.C. Assessment of the lateral diffusion and penetration of topically applied drugs in humans using a novel concentric tape stripping design. Pharm. Res. 2012, 29, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Caron, D.; Queille-Roussel, C.; Shah, V.P.; Schaefer, H. Correlation between the drug penetration and the blanching effect of topically applied hydrocortisone creams in human beings. J. Am. Acad. Dermatol. 1990, 23, 458–462. [Google Scholar] [CrossRef]
- Lehman, P.A.; Raney, S.G.; Franz, T.J. Topical bioequivalence: A comprehensive approach using multiple surrogate methods. In Advances in Dermatological Sciences; Chilcott, R., Brain, K.R., Eds.; RSC Publishing: Cambridge, UK, 2012; pp. 210–222. Available online: http://pubs.rsc.org/en/Content/eBook/978-1-84973-398-4 (15 November 2017).
- Raney, S.G.; Franz, T.J.; Lehman, P.A.; Lionberger, R.; Chen, M.L. Pharmacokinetics-based approaches for bioequivalence evaluation of topical dermatological drug products. Clin. Pharmacokinet. 2015, 54, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Woodford, R.; Barry, B.W. Activity and bioavailability of a new 0.1% hydrocortisone cream, egocort cream. Curr. Ther. Res. 1979, 26, 601–606. [Google Scholar]
- Woodford, R.; Barry, B.W. Alphaderm cream (1% hydrocortisone plus 10% urea)—Investigation of vasoconstrictor activity, bioavailability and application regimens in human volunteers. Curr. Ther. Res. 1984, 35, 759–767. [Google Scholar]
- Wiedersberg, S.; Naik, A.; Leopold, C.S.; Guy, R.H. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: Assessment of topical bioavailability. Br. J. Dermatol. 2009, 160, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Cordery, S.F.; Pensado, A.; Chiu, W.S.; Shehab, M.Z.; Bunge, A.L.; Delgado-Charro, M.B.; Guy, R.H. Topical bioavailability of diclofenac from locally-acting, dermatological formulations. Int. J. Pharm. 2017, 529, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.P.; Lu, C.C.; Liao, P.C.; Chou, C.H.; Sheu, H.M.; Tsai, J.C. Detection and distribution of endogenous steroids in human stratum corneum. Dermatol. Sin. 2014, 32, 19–24. [Google Scholar] [CrossRef]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Ibuprofen transport into and through skin from topical formulations: In vitro-in vivo comparison. J. Investig. Dermatol. 2007, 127, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Marttin, E.; NeelissenSubnel, M.T.A.; DeHaan, F.H.N.; Bodde, H.E. A critical comparison of methods to quantify stratum corneum removed by tape stripping. Skin Pharmacol. 1996, 9, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Surber, C.; Schwarb, F.P.; Smith, E.W. Tape-stripping technique. J. Toxicol. Cutan. Ocul. Toxicol. 2001, 20, 461–474. [Google Scholar] [CrossRef]
- Bucks, D.A.; McMaster, J.R.; Maibach, H.I.; Guy, R.H. Bioavailability of topically administered steroids: A “mass balance” technique. J. Investig. Dermatol. 1998, 91, 29–33. [Google Scholar] [CrossRef]


| Descriptor | FTU Dosing Study | Tape-Stripping Study |
|---|---|---|
| Age (years) | 27.2 ± 10.3 | 26.0 ± 3.7 |
| Gender | 14 male; 10 female | 3 female; 2 male |
| Fingertip length (mm) | 28 ± 3 | Not applicable |
| Previous use of TCS | 9 yes; 15 no | No recent use |
| PIL offered? | 12 yes; 12 no | Not applicable |
| If yes; PIL read? | 8 yes; 4 no |
| Formulation | Uptake | Clearance | ||||
|---|---|---|---|---|---|---|
| ND (mg/cm2) | HC in SC (µg) | SC Depth (µm) | ND (mg/cm2) | HC in SC (µg) | SC Depth (µm) | |
| Cream | 2.3 ± 0.2 | 4.11 ± 3.36 | 11.1 ± 3.6 | 2.3 ± 0.2 | 3.36 ± 3.23 | 9.9 ± 4.4 |
| 9.2 ± 0.5 | 5.30 ± 5.17 | 11.4 ± 2.4 | 9.3 ± 0.7 | 4.97 ± 4.60 | 10.1 ± 2.4 | |
| Ointment | 2.1 ± 0.1 | 4.60 ± 2.45 | 10.8 ± 3.7 | 2.2 ± 0.1 | 5.27 ± 3.87 | 11.5 ± 4.0 |
| 8.9 ± 0.4 | 6.70 ± 3.53 | 8.0 ± 1.9 | 9.4 ± 0.2 | 10.6 ± 7.43 | 11.7 ± 4.5 | |
| Formulation | Normalized Dose (mg/cm2) | Cumulative HC in Receptor (µg) | |||
|---|---|---|---|---|---|
| 6 h | 22 h | 24 h | 26 h | ||
| Cream | 2.3 ± 0.2 | Not detected | 0.19 ± 045 | 0.15 ± 0.38 | 0.17 ± 0.40 γ |
| 9.8 ± 1.2 | 0.16 ± 0.19 | 1.22 ± 1.42 | 1.27 ± 1.47 | 1.31 ± 1.52 γ | |
| Ointment | 2.4 ± 0.4 | Not detected | Not detected | Not detected | Not detected |
| 9.4 ± 0.5 | ND | 2.22 ± 3.17 | 2.27 ± 3.22 | 2.41 ± 3.38 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterson, D.A.; Hallier, J.; Jenkins, E.; Cordery, S.F.; Delgado-Charro, M.B. Is the Skin Absorption of Hydrocortisone Modified by the Variability in Dosing Topical Products? Pharmaceutics 2018, 10, 9. https://doi.org/10.3390/pharmaceutics10010009
Paterson DA, Hallier J, Jenkins E, Cordery SF, Delgado-Charro MB. Is the Skin Absorption of Hydrocortisone Modified by the Variability in Dosing Topical Products? Pharmaceutics. 2018; 10(1):9. https://doi.org/10.3390/pharmaceutics10010009
Chicago/Turabian StylePaterson, Daniel A., Jacqueline Hallier, Elizabeth Jenkins, Sarah F. Cordery, and M. Begoña Delgado-Charro. 2018. "Is the Skin Absorption of Hydrocortisone Modified by the Variability in Dosing Topical Products?" Pharmaceutics 10, no. 1: 9. https://doi.org/10.3390/pharmaceutics10010009
APA StylePaterson, D. A., Hallier, J., Jenkins, E., Cordery, S. F., & Delgado-Charro, M. B. (2018). Is the Skin Absorption of Hydrocortisone Modified by the Variability in Dosing Topical Products? Pharmaceutics, 10(1), 9. https://doi.org/10.3390/pharmaceutics10010009