Structural and Functional Insight into Canarypox Virus CNP058 Mediated Regulation of Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. CNP058 Expression and Purification
2.2. Measurement of Interactions with BH3 Peptides
2.3. CNP058 Complex Crystallization and Data Collection
2.4. Cell Culture
2.5. Transfection of HeLa Cells with GFP Constructs and Induction of Apoptosis
2.6. Flow Cytometry
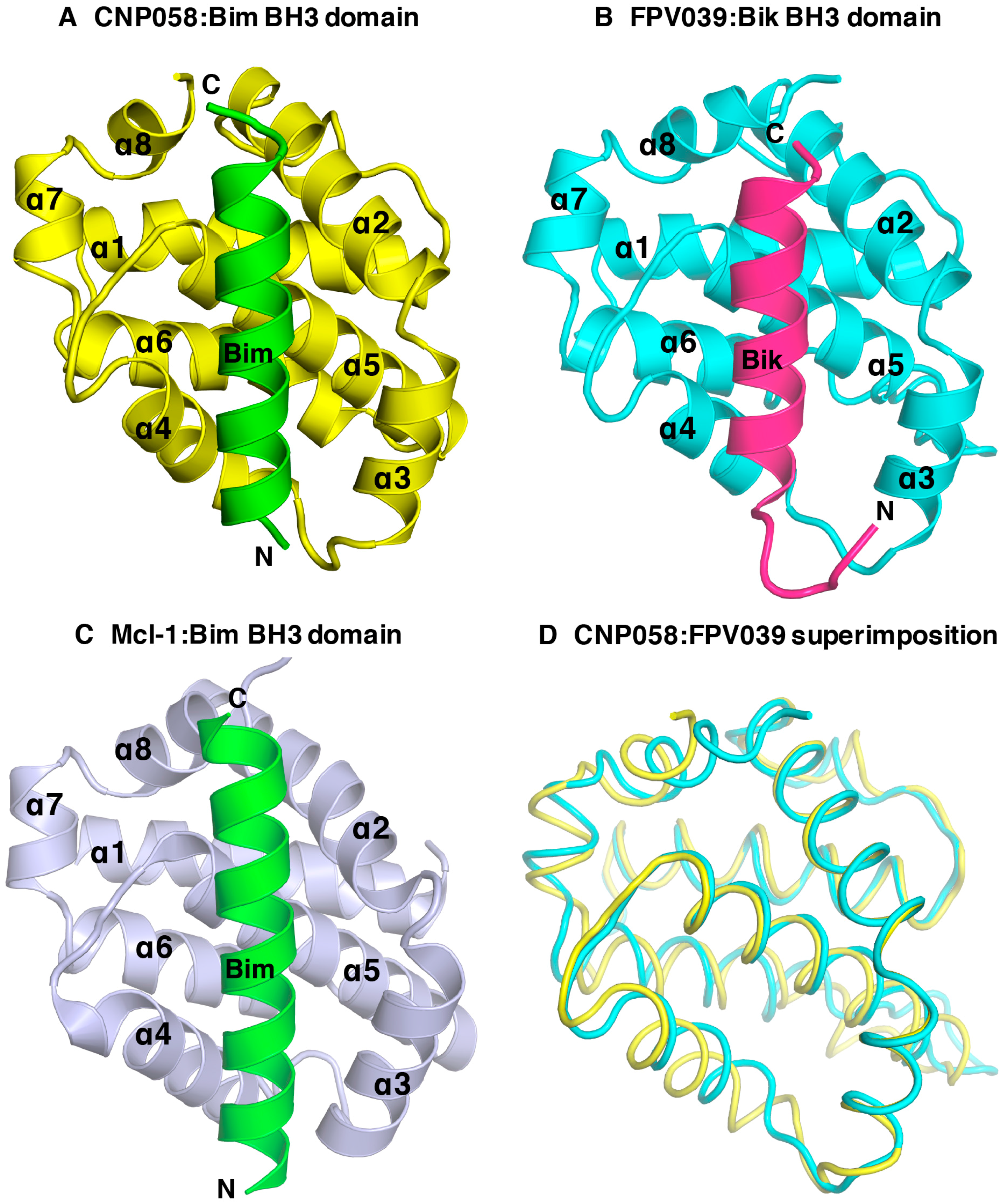
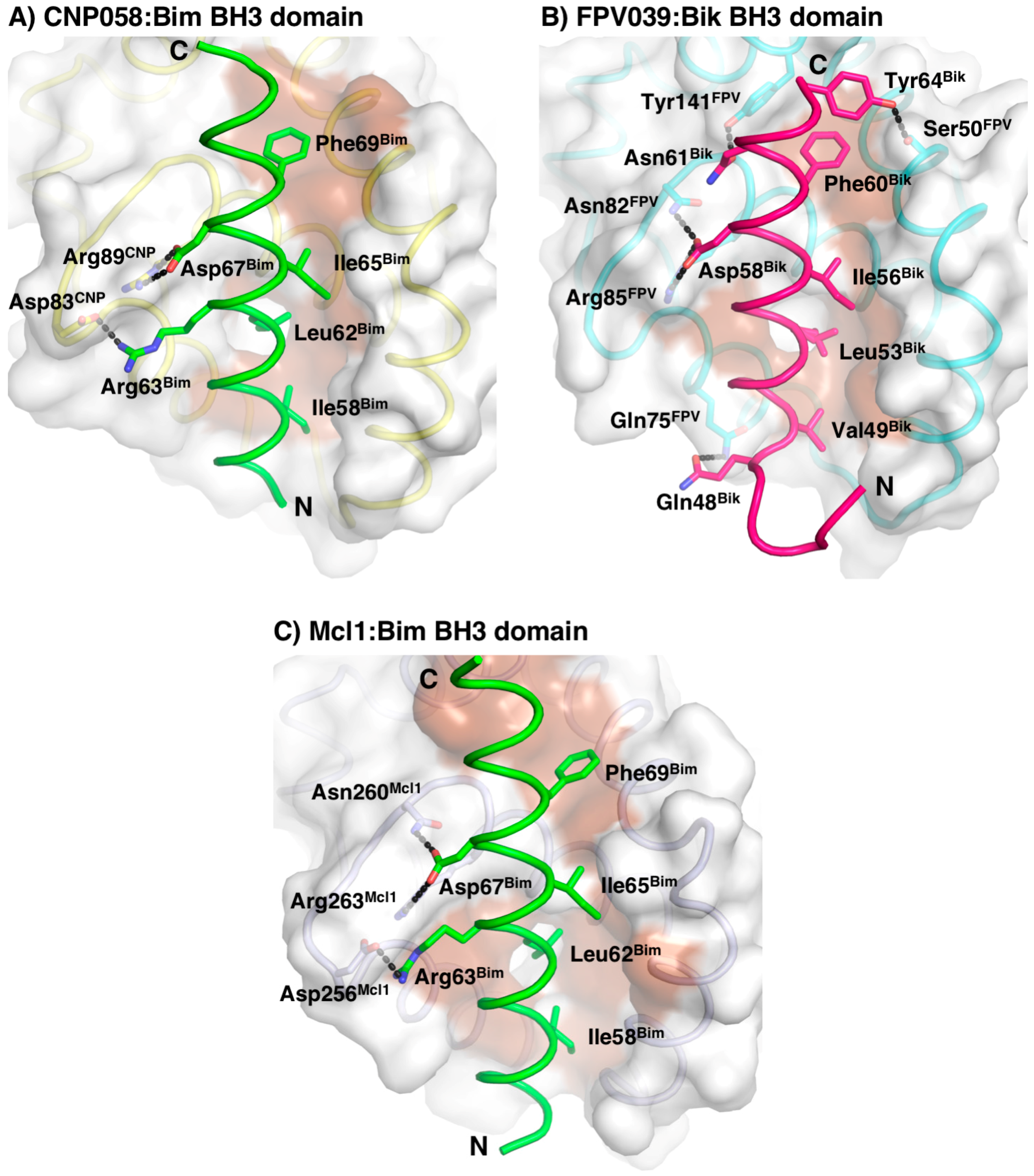
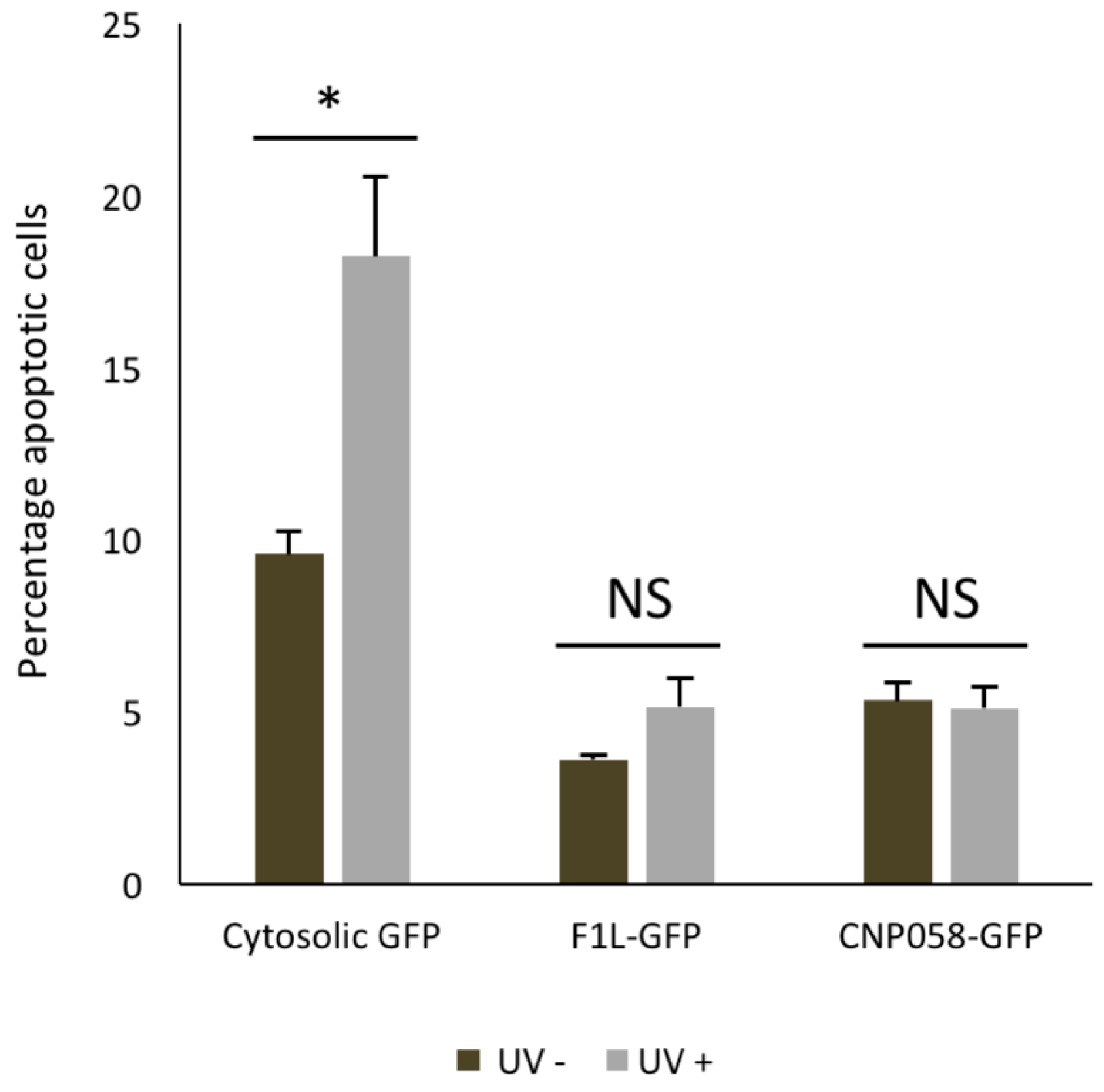
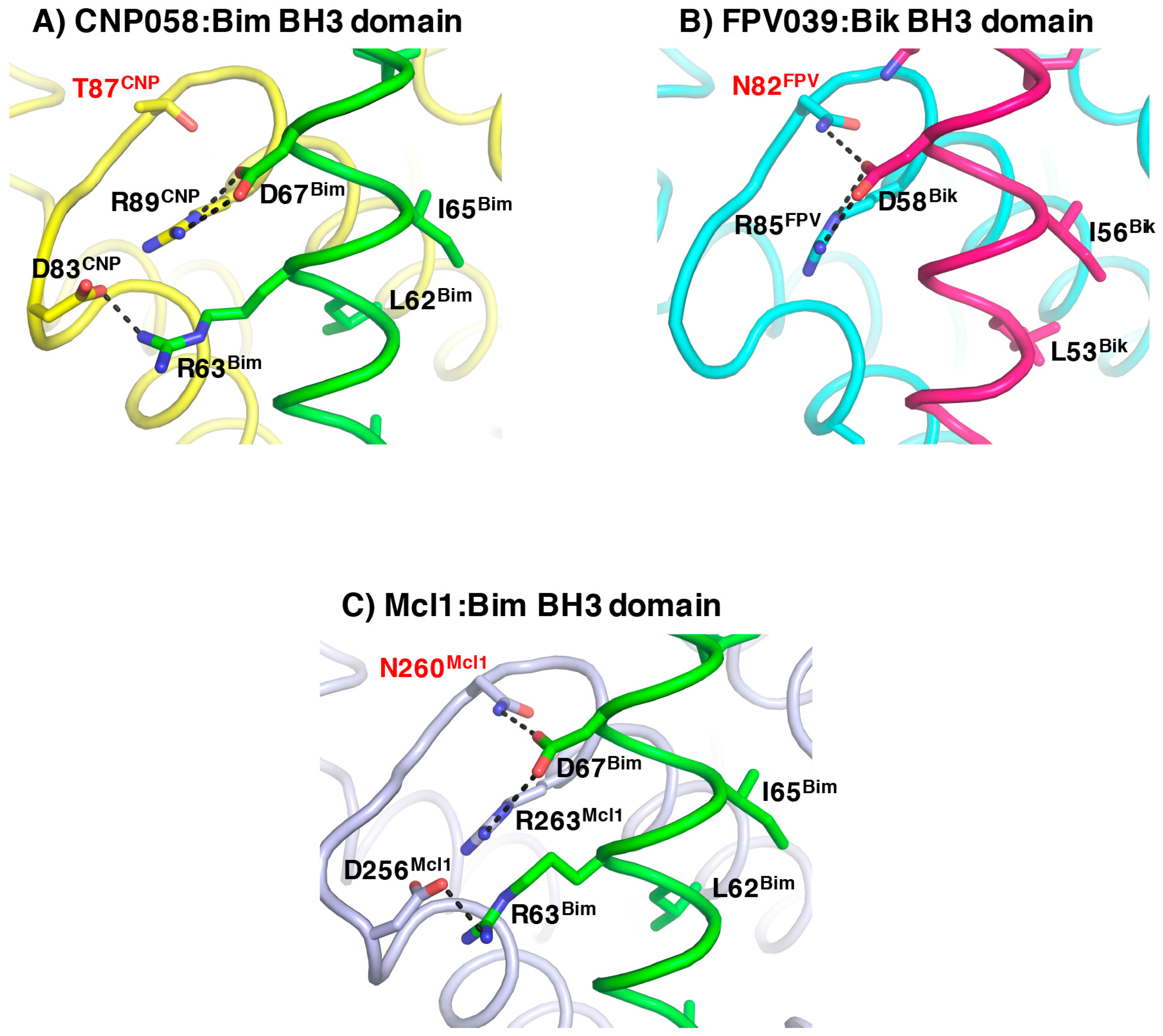
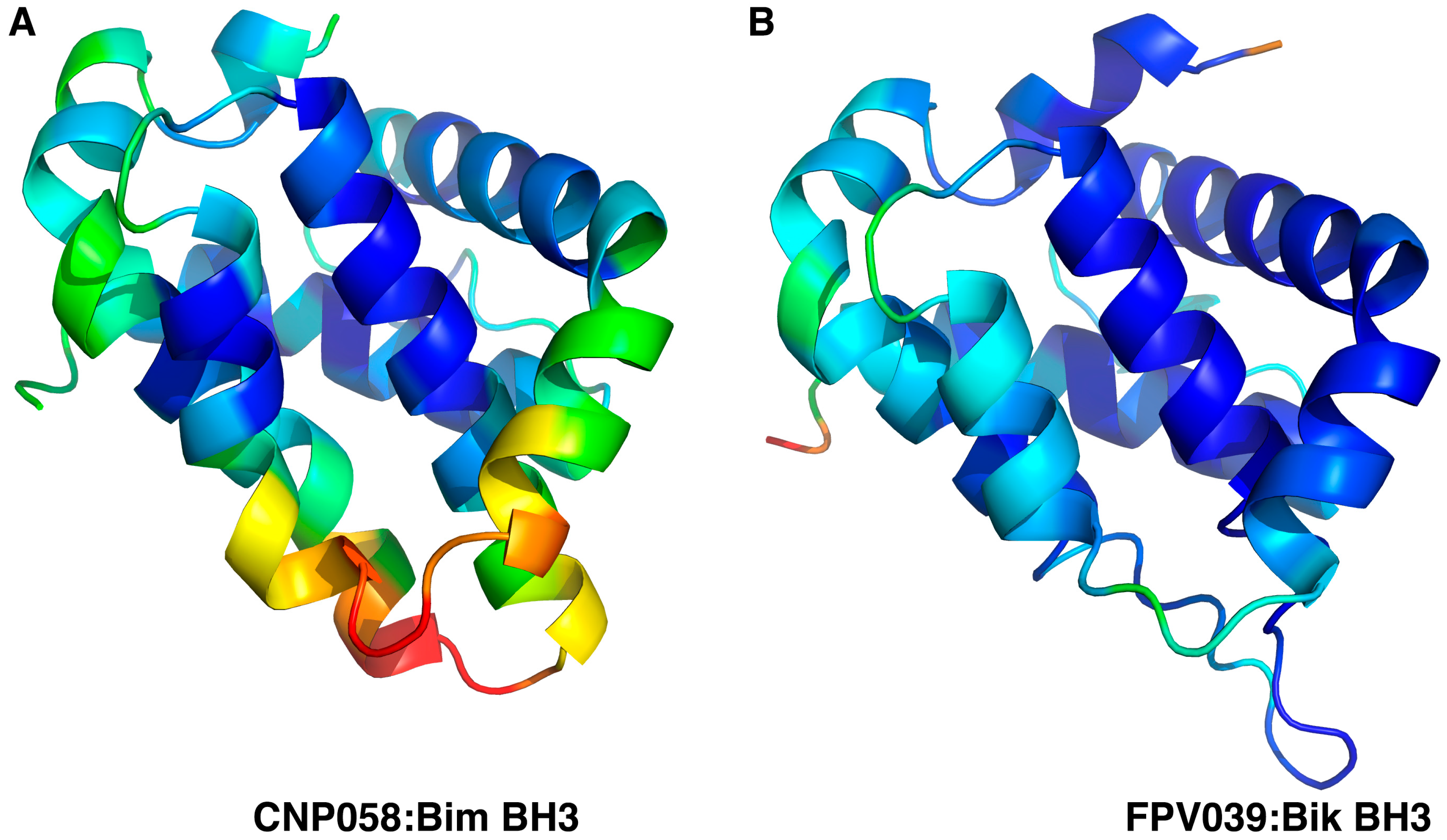
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benedict, C.A.; Norris, P.S.; Ware, C.F. To kill or be killed: Viral evasion of apoptosis. Nat. Immunol. 2002, 3, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Cuconati, A.; White, E. Viral homologs of bcl-2: Role of apoptosis in the regulation of virus infection. Genes Dev. 2002, 16, 2465–2478. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M.; Caria, S.; Hinds, M.G. The Bcl-2 family in host-virus interactions. Viruses 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. TheBcl-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M.; Hinds, M.G. The structural biology of BH3-only proteins. Methods Enzymol. 2014, 544, 49–74. [Google Scholar] [PubMed]
- Eitz Ferrer, P.; Potthoff, S.; Kirschnek, S.; Gasteiger, G.; Kastenmuller, W.; Ludwig, H.; Paschen, S.A.; Villunger, A.; Sutter, G.; Drexler, I.; et al. Induction of Noxa-mediated apoptosis by modified vaccinia virus Ankara depends on viral recognition by cytosolic helicases, leading to IRF-3/IFN-β-dependent induction of pro-apoptotic Noxa. PLoS Pathog. 2011, 7, e1002083. [Google Scholar] [CrossRef] [PubMed]
- Lomonosova, E.; Chinnadurai, G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene 2008, 27 (Suppl. 1), S2–S19. [Google Scholar] [CrossRef] [PubMed]
- Westphal, D.; Kluck, R.M.; Dewson, G. Building blocks of the apoptotic pore: How Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 2014, 21, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.N.; Strasser, A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011, 18, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M.; Hinds, M.G. Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis. 2013, 4, e909. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Cipriani, R. Specific disruption of intermediate filaments and the nuclear lamina by the 19-kDa product of the adenovirus e1b oncogene. Proc. Natl. Acad. Sci. USA 1989, 86, 9886–9890. [Google Scholar] [CrossRef] [PubMed]
- Brun, A.; Rivas, C.; Esteban, M.; Escribano, J.M.; Alonso, C. African swine fever virus gene a179l, a viral homologue of Bcl-2, protects cells from programmed cell death. Virology 1996, 225, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Oudejans, J.J.; van den Brule, A.J.C.; Jiwa, N.M.; De Bruin, P.C.; Ossenkoppele, G.J.; van der Valk, P.; Walboomers, J.M.M.; Meijer, C.J.L.M. BHRF1, the Rpstein-Barr virus (EBV) homologue of the Bcl-2 protooncogene, is transcribed in EBV-associated B-cell lymphomas and in reactive lymphocytes. Blood 1995, 86, 1893–1902. [Google Scholar] [PubMed]
- Nava, V.E.; Cheng, E.H.; Veliuona, M.; Zou, S.; Clem, R.J.; Mayer, M.L.; Hardwick, J.M. Herpesvirus saimiri encodes a functional homolog of the human Bcl-2 oncogene. J. Virol. 1997, 71, 4118–4122. [Google Scholar] [PubMed]
- Taylor, J.M.; Barry, M. Near death experiences: Poxvirus regulation of apoptotic death. Virology 2006, 344, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M.; van Delft, M.F.; Lee, E.F.; Gulbis, J.M.; Fairlie, W.D.; Huang, D.C.; Colman, P.M. A structural viral mimic of prosurvival Bcl-2: A pivotal role for sequestering proapoptotic Bax and Bak. Mol. Cell 2007, 25, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Thibault, J.; Mehta, N.; Colman, P.M.; Barry, M.; Kvansakul, M. Structural insight into BH3 domain binding of vaccinia virus antiapoptotic F1L. J. Virol. 2014, 88, 8667–8677. [Google Scholar] [CrossRef] [PubMed]
- Anasir, M.I.; Caria, S.; Skinner, M.A.; Kvansakul, M. Structural basis of apoptosis inhibition by the fowlpox virus protein FPV039. J. Biol. Chem. 2017, 292, 9010–9021. [Google Scholar] [CrossRef] [PubMed]
- Banadyga, L.; Gerig, J.; Stewart, T.; Barry, M. Fowlpox virus encodes a Bcl-2 homologue that protects cells from apoptotic death through interaction with the proapoptotic protein Bak. J. Virol. 2007, 81, 11032–11045. [Google Scholar] [CrossRef] [PubMed]
- Banadyga, L.; Veugelers, K.; Campbell, S.; Barry, M. The fowlpox virus Bcl-2 homologue, FPV039, interacts with activated Bax and a discrete subset of BH3-only proteins to inhibit apoptosis. J. Virol. 2009, 83, 7085–7098. [Google Scholar] [CrossRef] [PubMed]
- Studier, F.W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M.; Czabotar, P.E. Preparing samples for crystallization of Bcl-2 family complexes. Methods Mol. Biol. 2016, 1419, 213–229. [Google Scholar] [PubMed]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 2006, 62, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with Phenix.Refine. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Morin, A.; Eisenbraun, B.; Key, J.; Sanschagrin, P.C.; Timony, M.A.; Ottaviano, M.; Sliz, P. Collaboration gets the most out of software. Elife 2013, 2, e01456. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.A.; Socias, S.; Key, J.; Ransey, E.; Tjon, E.C.; Buschiazzo, A.; Lei, M.; Botka, C.; Withrow, J.; Neau, D.; et al. Data publication with the structural biology data grid supports live analysis. Nat. Commun. 2016, 7, 10882. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Hazes, B.; Kvansakul, M.; Colman, P.; Barry, M. Vaccinia virus F1L interacts with Bak using highly divergent Bcl-2 homology domains and replaces the function of Mcl-1. J. Biol. Chem. 2010, 285, 4695–4708. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tixeira, R.; Caruso, S.; Atkin-Smith, G.K.; Baxter, A.A.; Paone, S.; Hulett, M.D.; Poon, I.K. Monitoring the progression of cell death and the disassembly of dying cells by flow cytometry. Nat. Protoc. 2016, 11, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Sabbatini, P.; Perez, D.; Rao, L.; Modha, D.; White, E. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 1995, 10, 461–477. [Google Scholar] [CrossRef]
- Kvansakul, M.; Yang, H.; Fairlie, W.D.; Czabotar, P.E.; Fischer, S.F.; Perugini, M.A.; Huang, D.C.; Colman, P.M. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008, 15, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R.; Caria, S.; Marshall, B.; Barry, M.; Kvansakul, M. Structural basis of deerpox virus-mediated inhibition of apoptosis. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Westphal, D.; Ledgerwood, E.C.; Tyndall, J.D.; Hibma, M.H.; Ueda, N.; Fleming, S.B.; Mercer, A.A. The orf virus inhibitor of apoptosis functions in a Bcl-2-like manner, binding and neutralizing a set of BH3-only proteins and active Bax. Apoptosis 2009, 14, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Campbell, S.; Mehta, N.; Thibault, J.; Colman, P.M.; Barry, M.; Huang, D.C.; Kvansakul, M. Sheeppox virus SPPV14 encodes a Bcl-2-like cell death inhibitor that counters a distinct set of mammalian proapoptotic proteins. J. Virol. 2012, 86, 11501–11511. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.; Puthalakath, H.; Caria, S.; Chugh, S.; Doerflinger, M.; Colman, P.M.; Kvansakul, M. Variola virus F1L is a Bcl-2-like protein that unlike its vaccinia virus counterpart inhibits apoptosis independent of bim. Cell Death Dis. 2015, 6, e1680. [Google Scholar] [CrossRef] [PubMed]
- Banjara, S.; Caria, S.; Dixon, L.K.; Hinds, M.G.; Kvansakul, M. Structural insight into african swine fever virus A179l-mediated inhibition of apoptosis. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M.; Wei, A.H.; Fletcher, J.I.; Willis, S.N.; Chen, L.; Roberts, A.W.; Huang, D.C.; Colman, P.M. Structural basis for apoptosis inhibition by Epstein-Barr virus BHRF1. PLoS Pathog. 2010, 6, e1001236. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, A.M.; Letai, A. BH3 domains define selective inhibitory interactions with BHRF-1 and KSHV Bcl-2. Cell Death Differ. 2008, 15, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.; Woo, J.S.; Liang, C.; Lee, K.H.; Jung, J.U.; Oh, B.H. An insight into the mechanistic role of beclin 1 and its inhibition by prosurvival Bcl-2 family proteins. Autophagy 2008, 4, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, M.; Zhai, D.; Jin, C.; Aleshin, A.E.; Stec, B.; Reed, J.C.; Liddington, R.C. Vaccinia virus N1L protein resembles a B cell lymphoma-2 (Bcl-2) family protein. Protein Sci. 2007, 16, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Willis, S.N.; Wei, A.; Smith, B.J.; Fletcher, J.I.; Hinds, M.G.; Colman, P.M.; Day, C.L.; Adams, J.M.; Huang, D.C. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 2005, 17, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Smits, C.; Czabotar, P.E.; Hinds, M.G.; Day, C.L. Structural plasticity underpins promiscuous binding of the prosurvival protein A1. Structure 2008, 16, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.N.; Chen, L.; Dewson, G.; Wei, A.; Naik, E.; Fletcher, J.I.; Adams, J.M.; Huang, D.C. Proapoptotic bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005, 19, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Meusburger, S.; Hawkins, C.J.; Riglar, D.T.; Lee, E.F.; Fairlie, W.D.; Huang, D.C.; Adams, J.M. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc. Natl. Acad. Sci. USA 2008, 105, 18081–18087. [Google Scholar] [CrossRef] [PubMed]
- Caria, S.; Hinds, M.G.; Kvansakul, M. Structural insight into an evolutionarily ancient programmed cell death regulator—The crystal structure of marine sponge BHP2 bound to LB-Bak-2. Cell Death Dis. 2017, 8, e2543. [Google Scholar] [CrossRef] [PubMed]
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Kutish, G.F.; Rock, D.L. The genome of canarypox virus. J. Virol. 2003, 78, 353–366. [Google Scholar] [CrossRef]
- Gyuranecz, M.; Foster, J.T.; Dan, A.; Ip, H.S.; Egstad, K.F.; Parker, P.G.; Higashiguchi, J.M.; Skinner, M.A.; Hofle, U.; Kreizinger, Z.; et al. Worldwide phylogenetic relationship of avian poxviruses. J. Virol. 2013, 87, 4938–4951. [Google Scholar] [CrossRef] [PubMed]
- Jarmin, S.; Manvell, R.; Gough, R.E.; Laidlaw, S.M.; Skinner, M.A. Avipoxvirus phylogenetics: Identification of a PCR length polymorphism that discriminates between the two major clades. J. Gen. Virol. 2006, 87, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M.; Hinds, M.G. The Bcl-2 family: Structures, interactions and targets for drug discovery. Apoptosis 2015, 20, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Leaman, D.W. Involvement of Noxa in cellular apoptotic responses to interferon, double-stranded RNA, and virus infection. J. Biol. Chem. 2005, 280, 15561–15568. [Google Scholar] [CrossRef] [PubMed]
- Wasilenko, S.T.; Stewart, T.L.; Meyers, A.F.; Barry, M. Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 14345–14350. [Google Scholar] [CrossRef] [PubMed]
- Caria, S.; Marshall, B.; Burton, R.L.; Campbell, S.; Pantaki-Eimany, D.; Hawkins, C.J.; Barry, M.; Kvansakul, M. The N terminus of the vaccinia virus protein F1L is an intrinsically unstructured region that is not involved in apoptosis regulation. J. Biol. Chem. 2016, 291, 14600–14608. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.F.; Ludwig, H.; Holzapfel, J.; Kvansakul, M.; Chen, L.; Huang, D.C.; Sutter, G.; Knese, M.; Hacker, G. Modified Vaccinia virus ankara protein F1L is a novel BH3-domain-binding protein and acts together with the early viral protein E3l to block virus-associated apoptosis. Cell Death Differ. 2006, 13, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Rautureau, G.J.; Yabal, M.; Yang, H.; Huang, D.C.; Kvansakul, M.; Hinds, M.G. The restricted binding repertoire of Bcl-b leaves Bim as the universal BH3-only prosurvival Bcl-2 protein antagonist. Cell Death Dis. 2012, 3, e443. [Google Scholar] [CrossRef] [PubMed]
- Rautureau, G.J.; Day, C.L.; Hinds, M.G. The structure of Boo/Diva reveals a divergent Bcl-2 protein. Proteins 2010, 78, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E.; Corbett, K.D.; Berger, J.M.; McFadden, G.; Handel, T.M. Structure of M11L: A myxoma virus structural homolog of the apoptosis inhibitor, Bcl-2. Protein Sci. 2007, 16, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Neidel, S.; Maluquer de Motes, C.; Mansur, D.S.; Strnadova, P.; Smith, G.L.; Graham, S.C. Vaccinia virus protein A49 is an unexpected member of the B-cell lymphoma (Bcl)-2 protein family. J. Biol. Chem. 2015, 290, 5991–6002. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.C.; Bahar, M.W.; Cooray, S.; Chen, R.A.; Whalen, D.M.; Abrescia, N.G.; Alderton, D.; Owens, R.J.; Stuart, D.I.; Smith, G.L.; et al. Vaccinia virus proteins A52 and B14 share a Bcl-2-like fold but have evolved to inhibit NF-κB rather than apoptosis. PLoS Pathog. 2008, 4, e1000128. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Schroder, M.; Khan, A.R. Structural basis for targeting of human RNA helicase DDX3 by poxvirus protein K7. Structure 2009, 17, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
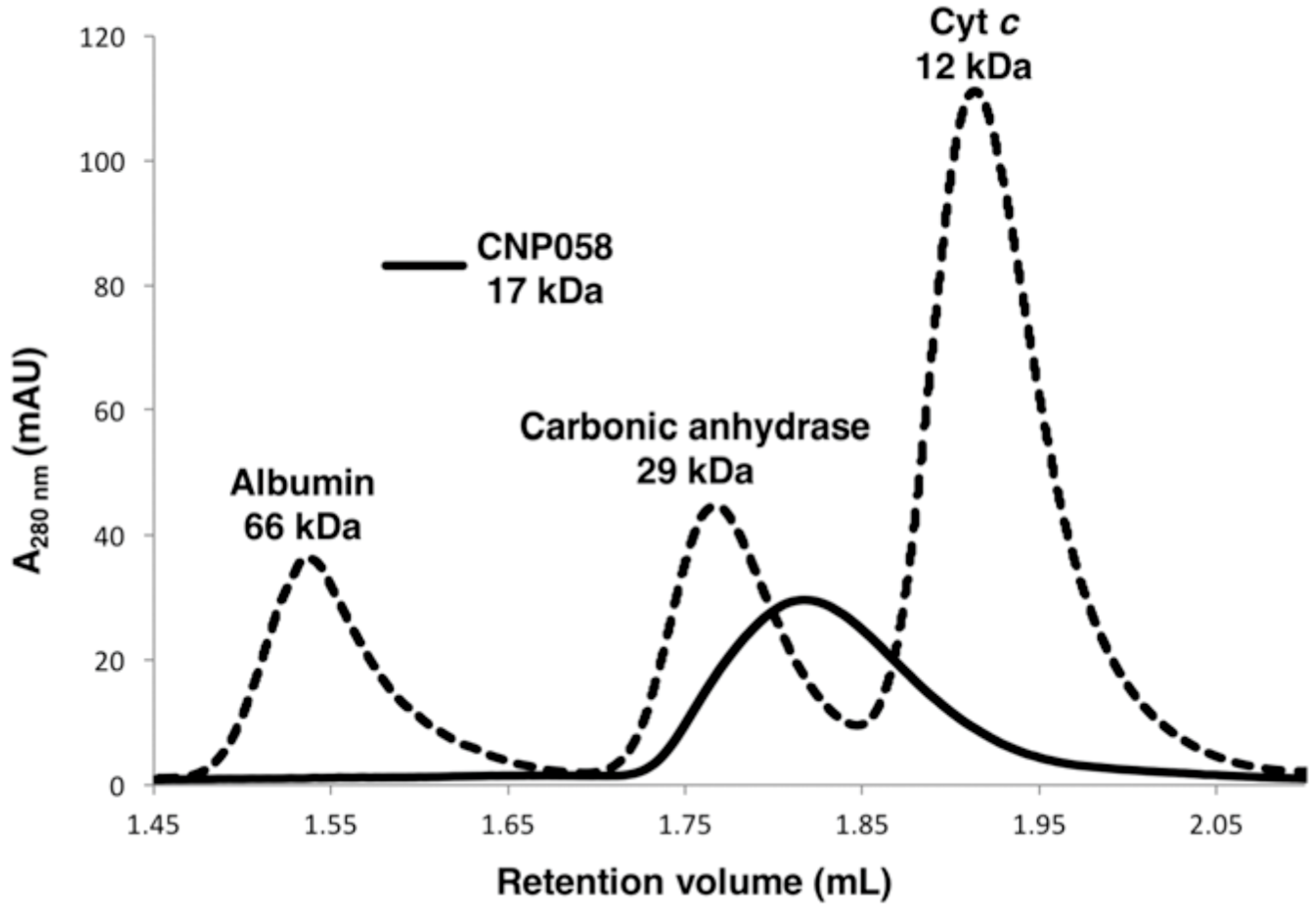
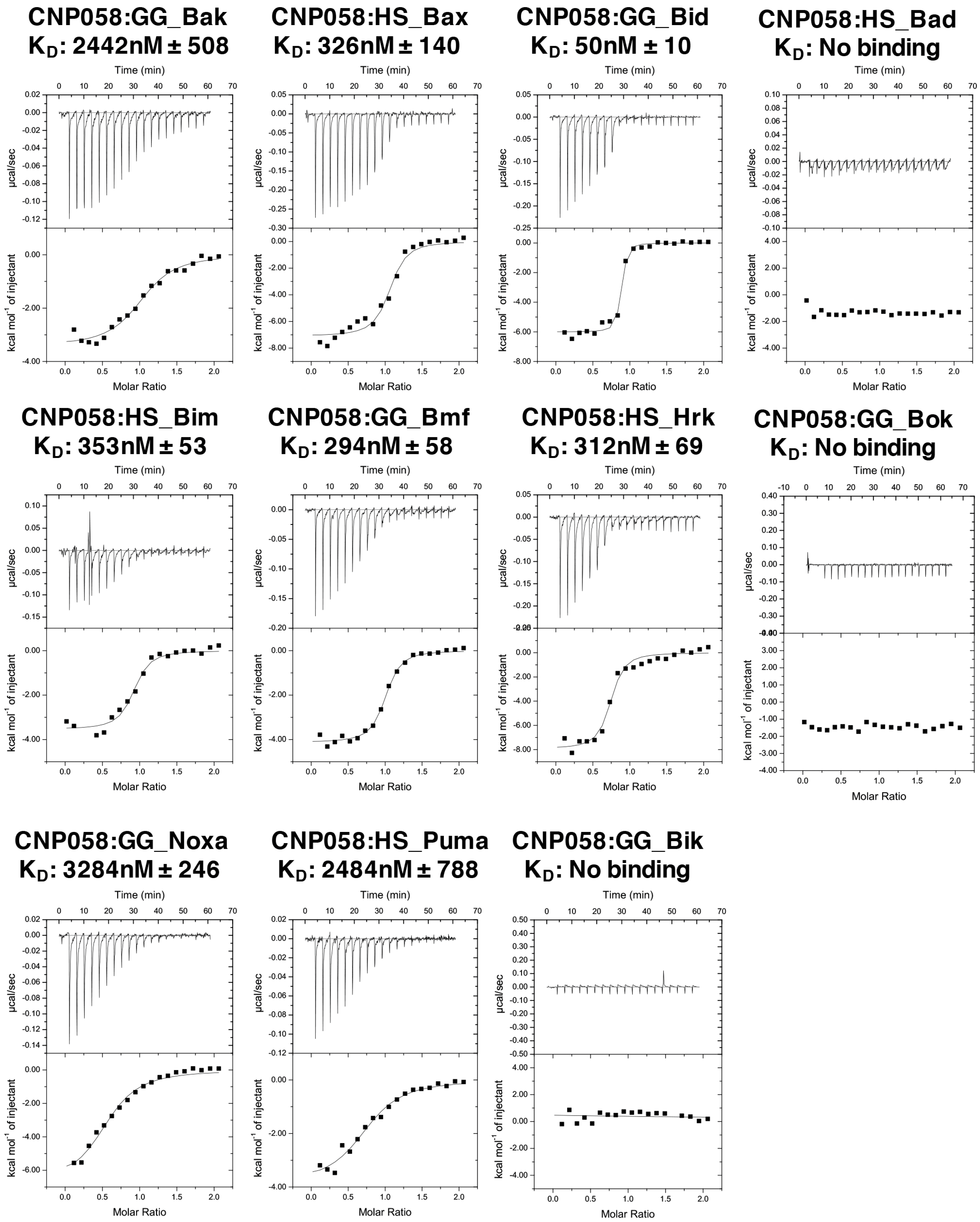
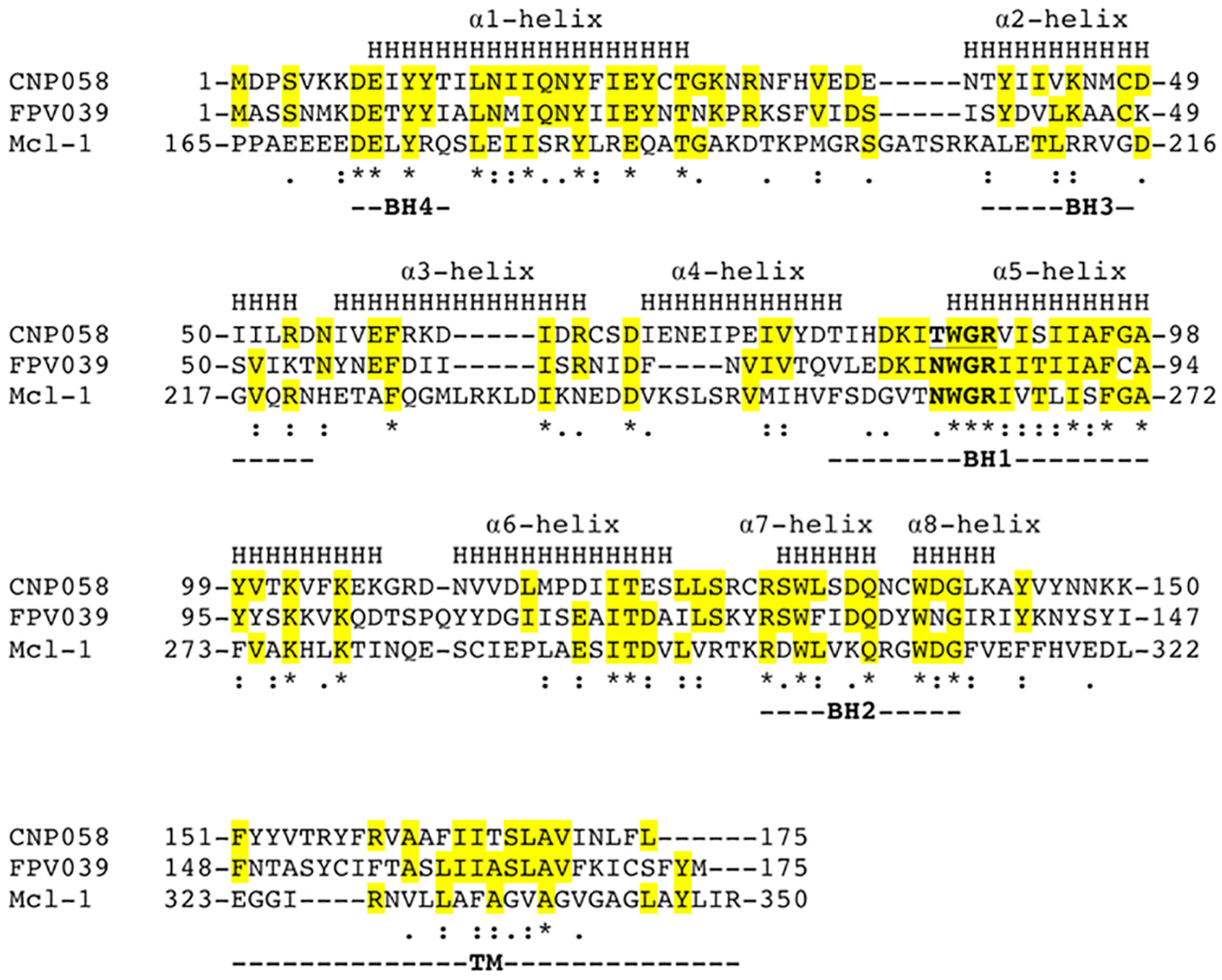
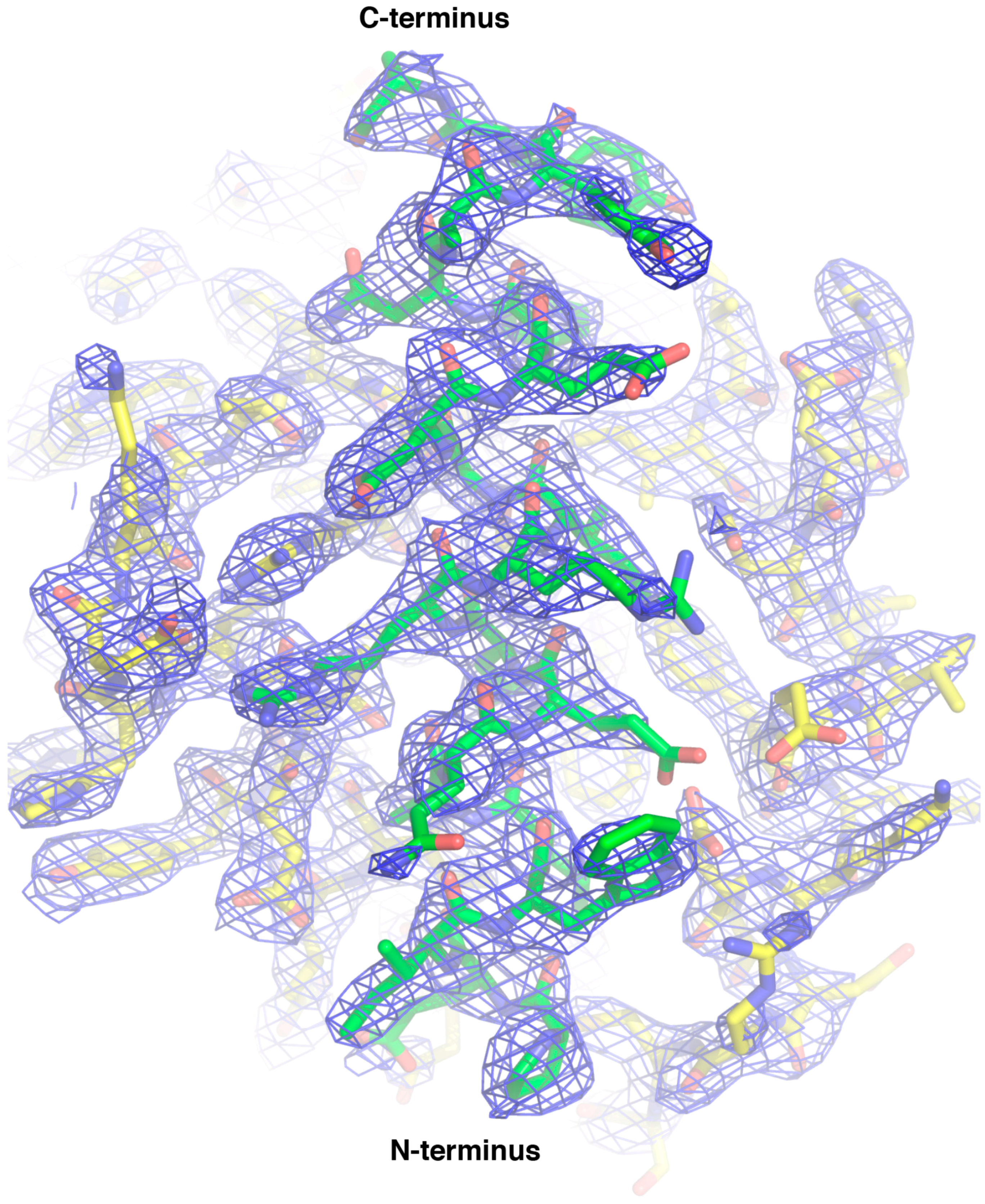
| Data Collection and Refinement Statistics (Molecular Replacement) | |
|---|---|
| CNP058-Bim BH3 domain | |
| Data collection | |
| Space group | C121 |
| No. of molecules in asymmetric unit | 1 + 1 |
| Cell dimensions | |
| a, b, c (Å) | 73.79, 34.67, 71.73 |
| α, β, γ, (°) | 90.00, 114.92, 90.00 |
| Wavelength (Å) | 0.9537 |
| Resolution (Å) | 33.46–2.45 (2.55–2.45) |
| No. unique reflections | 6021 (703) |
| Rsym or Rmerge | 0.097 (0.716) |
| I/σI | 6.1 (1.1) |
| CC1/2 | 0.99 (0.45) |
| Wilson B-factor | 40.9 |
| Completeness (%) | 97.2 (98.4) |
| Redundancy | 2.7 (2.7) |
| Refinement | |
| Resolution (Å) | 2.45 |
| No. reflections | 6017 |
| Rwork/Rfree | 0.2126/0.2453 |
| No. atoms | |
| Protein | 1340 |
| Water | 10 |
| B-factors | |
| Protein | 52.91 |
| Water | 56.11 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.003 |
| Bond angles (°) | 0.56 |
| Ramachandran statistics (%) | |
| Favored | 97.42 |
| Allowed | 2.58 |
| Disallowed | 0.00 |
| Poxviral Bcl-2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pro-Death | SPPV14 | M11L | MVA_F1 | VAR_F1 | DPV022 | FPV039 | CNP058 | N1 |
| Bad | >2000 | >1000 | NB | NB | NB | 653 | NB | >1000 |
| Bid | 341 | 100 | NB | 3200 | NB | 2 | 50 | 152 |
| Bik | >2000 | >1000 | NB | NB | NB | 30 | NB | n/a |
| Bim | 26 | 5 | 250 | NB | 340 | 10 | 353 | 72 |
| Bmf | 67 | 100 | NB | NB | NB | 16 | 294 | n/a |
| Hrk | 63 | >1000 | NB | NB | NB | 24 | 312 | n/a |
| Noxa | >2000 | >1000 | NB | NB | NB | 28 | 3284 | n/a |
| Puma | 65 | >1000 | NB | NB | NB | 24 | 2484 | n/a |
| Bak | 46 | 50 | 4300 | 2640 | 6930 | 76 | 508 | 71 |
| Bax | 32 | 75 | 1850 | 960 | 4040 | 76 | 326 | n/a |
| Beclin-1 | n/a | n/a | n/a | n/a | NB | n/a | n/a | n/a |
| Asfarviral Bcl-2 | Herpesviral Bcl-2 | |||
|---|---|---|---|---|
| A179L | BHRF1 | Ks-Bcl-2 | M11 | |
| Bad | 258 | >2000 | >1000 | NB |
| Bid | 26 | 109 | 112 | 232 |
| Bik | 190 | >2000 | >1000 | NB |
| Bim | 6 | 18 | 29 | 131 |
| Bmf | 254 | >2000 | >1000 | 300 |
| Hrk | 1487 | >1000 | >1000 | 719 |
| Noxa | 1575 | >2000 | >1000 | 132 |
| Puma | 31 | 70 | 69 | 370 |
| Bak | 29 | 150 | <50 | 76.3 |
| Bax | 26 | 1400 | 980 | 690 |
| Beclin-1 | n/a | n/a | 40 | |
| Human Bcl-2 | Sponge Bcl-2 | |||||
|---|---|---|---|---|---|---|
| Bcl-2 | Bcl-w | Bcl-xL | Mcl-1 | A1 | BHP2 | |
| Bad | 16 | 30 | 5.3 | >100,000 | 15,000 | NB |
| Bid | 6800 | 40 | 82 | 2100 | 1 | NB |
| Bik | 850 | 12 | 43 | 1700 | 58 | NB |
| Bim | 2.6 | 4.3 | 4.6 | 2.4 | 1 | NB |
| Bmf | 3 | 9.8 | 9.7 | 1100 | 180 | NB |
| Hrk | 320 | 49 | 3.7 | 370 | 46 | 3760 |
| Noxa | >100,000 | >100,000 | >100,000 | 24 | 20 | NB |
| Puma | 3.3 | 5.1 | 6.3 | 5 | 1 | NB |
| Bak | >1000 | 500 | 50 | 10 | 3 | 66 |
| Bax | 100 | 58 | 130 | 12 | n/a | NB |
| Beclin-1 | n/a | n/a | 2300 | n/a | n/a | n/a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anasir, M.I.; Baxter, A.A.; Poon, I.K.H.; Hulett, M.D.; Kvansakul, M. Structural and Functional Insight into Canarypox Virus CNP058 Mediated Regulation of Apoptosis. Viruses 2017, 9, 305. https://doi.org/10.3390/v9100305
Anasir MI, Baxter AA, Poon IKH, Hulett MD, Kvansakul M. Structural and Functional Insight into Canarypox Virus CNP058 Mediated Regulation of Apoptosis. Viruses. 2017; 9(10):305. https://doi.org/10.3390/v9100305
Chicago/Turabian StyleAnasir, Mohd Ishtiaq, Amy A. Baxter, Ivan K. H. Poon, Mark D. Hulett, and Marc Kvansakul. 2017. "Structural and Functional Insight into Canarypox Virus CNP058 Mediated Regulation of Apoptosis" Viruses 9, no. 10: 305. https://doi.org/10.3390/v9100305
APA StyleAnasir, M. I., Baxter, A. A., Poon, I. K. H., Hulett, M. D., & Kvansakul, M. (2017). Structural and Functional Insight into Canarypox Virus CNP058 Mediated Regulation of Apoptosis. Viruses, 9(10), 305. https://doi.org/10.3390/v9100305









