Natural History of HPV Infection across the Lifespan: Role of Viral Latency
Abstract
:1. Introduction
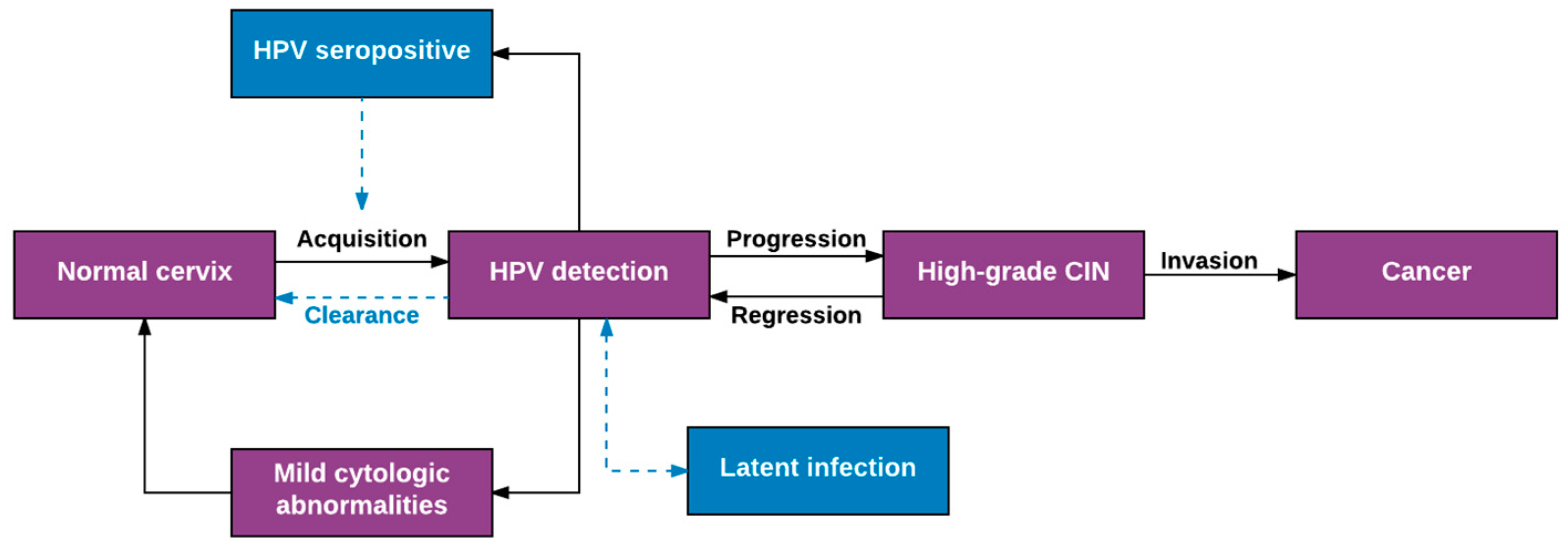
2. Overview of Well-Established Aspects of HPV Natural History
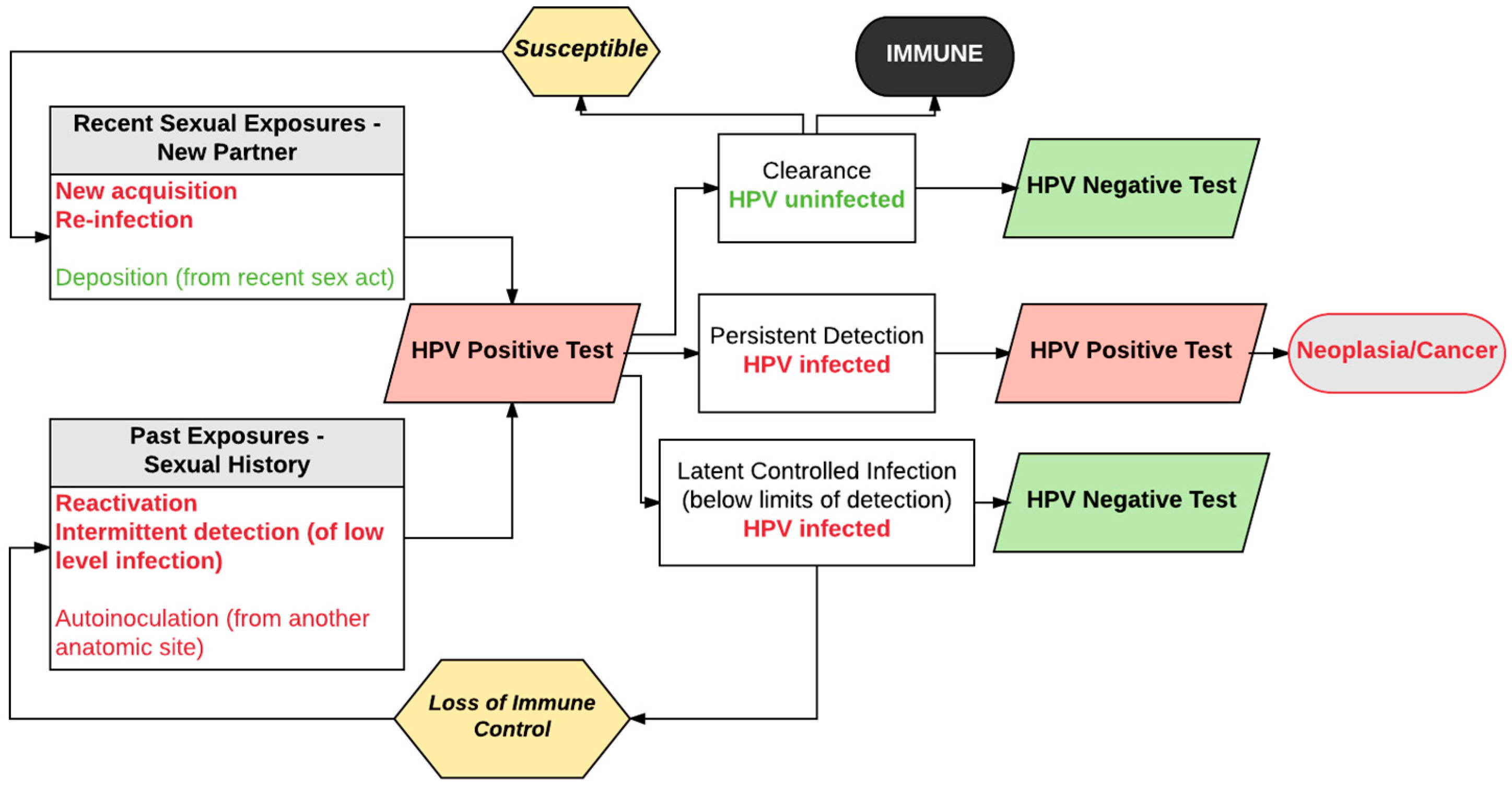
3. Expanded View of HPV Natural History
4. Evidence Supporting Re-Detection as an Important Transition from Negative to Positive HPV Tests
5. New Acquisition versus Re-Detection of Prior Infection: Evidence from Studies of Mid-Adult Women
6. Protection against Re-Infection: Role of Naturally Acquired Antibodies and Vaccine
7. Rationale for Resolving Remaining Uncertainties
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [PubMed]
- Burchell, A.N.; Winer, R.L.; de Sanjose, S.; Franco, E.L. Chapter 6: Epidemiology and Transmission Dynamics of Genital HPV Infection. Vaccine 2006, 24 (Suppl. 3), S52–S61. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Fu, T.C.; Carter, J.J.; Hughes, J.P.; Feng, Q.; Hawes, S.E.; Schwartz, S.M.; Xi, L.F.; Lasof, T.; Stern, J.E.; Galloway, D.A.; et al. Re-detection vs. new acquisition of high-risk human papillomavirus in mid-adult women. Int. J. Cancer 2016, 139, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Shew, M.L.; Ermel, A.C.; Tong, Y.; Tu, W.; Qadadri, B.; Brown, D.R. Episodic detection of human papillomavirus within a longitudinal cohort of young women. J. Med. Virol. 2015, 87, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Cummings, D.A.; Zenilman, J.M.; Gravitt, P.E.; Brotman, R.M. Characterizing the temporal dynamics of human papillomavirus DNA detectability using short-interval sampling. Cancer Epidemiol. Biomark. Prev. 2014, 23, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.J.; Koutsky, L.A.; Hughes, J.P.; Lee, S.K.; Kuypers, J.; Kiviat, N.; Galloway, D.A. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 2000, 181, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Gravitt, P.E. Evidence and impact of human papillomavirus latency. Open Virol. J. 2012, 6, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Strickler, H.D.; Burk, R.D.; Fazzari, M.; Anastos, K.; Minkoff, H.; Massad, L.S.; Hall, C.; Bacon, M.; Levine, A.M.; Watts, D.H.; et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J. Natl. Cancer Inst. 2005, 97, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.C.; Hughes, J.P.; Feng, Q.; Hulbert, A.; Hawes, S.E.; Xi, L.F.; Schwartz, S.M.; Stern, J.E.; Koutsky, L.A.; Winer, R.L. Epidemiology of Human Papillomavirus Detected in the Oral Cavity and Fingernails of Mid-Adult Women. Sex. Transm. Dis. 2015, 42, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Baay, M.F.; Francois, K.; Lardon, F.; Van Royen, P.; Pauwels, P.; Vermorken, J.B.; Peeters, M.; Verhoeven, V. The presence of Y chromosomal deoxyribonucleic acid in the female vaginal swab: Possible Implications for Human Papillomavirus Testing. Cancer Epidemiol. 2011, 35, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Gravitt, P.E. The known unknowns of HPV natural history. J. Clin. Investig. 2011, 121, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Theiler, R.N.; Farr, S.L.; Karon, J.M.; Paramsothy, P.; Viscidi, R.; Duerr, A.; Cu-Uvin, S.; Sobel, J.; Shah, K.; Klein, R.S.; et al. High-risk human papillomavirus reactivation in human immunodeficiency virus-infected women: Risk Factors for Cervical Viral Shedding. Obstet. Gynecol. 2010, 115, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Rositch, A.F.; Burke, A.E.; Viscidi, R.P.; Silver, M.I.; Chang, K.; Gravitt, P.E. Contributions of recent and past sexual partnerships on incident human papillomavirus detection: Acquisition and Reactivation in Older Women. Cancer Res. 2012, 72, 6183–6190. [Google Scholar] [CrossRef] [PubMed]
- Maglennon, G.A.; McIntosh, P.; Doorbar, J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology 2011, 414, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Maglennon, G.A.; McIntosh, P.B.; Doorbar, J. Immunosuppression facilitates the reactivation of latent papillomavirus infections. J. Virol. 2014, 88, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Winer, R.L.; Hughes, J.P.; Feng, Q.; Stern, J.E.; Xi, L.F.; Koutsky, L.A. Incident Detection of High-Risk Human Papillomavirus Infections in a Cohort of High-Risk Women Aged 25–65 Years. J. Infect. Dis. 2016, 214, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Gravitt, P.E.; Rositch, A.F.; Silver, M.I.; Marks, M.A.; Chang, K.; Burke, A.E.; Viscidi, R.P. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J. Infect. Dis. 2013, 207, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Herbenick, D.; Reece, M.; Schick, V.; Sanders, S.A.; Dodge, B.; Fortenberry, J.D. Sexual behavior in the United States: Results from a National Probability Sample of Men and Women Ages 14–94. J. Sex. Med. 2010, 7 (Suppl. 5), 255–265. [Google Scholar] [CrossRef] [PubMed]
- Mercer, C.H.; Tanton, C.; Prah, P.; Erens, B.; Sonnenberg, P.; Clifton, S.; Macdowall, W.; Lewis, R.; Field, N.; Datta, J.; et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: Findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013, 382, 1781–1794. [Google Scholar] [CrossRef]
- Ryser, M.D.; Rositch, A.; Gravitt, P.E. Age and sexual behavior indicates an increasing trend of HPV infection following the sexual revolution. J. Infect. Dis. 2017, 3, 46–49. [Google Scholar]
- Strickler, H.D.; Schiffman, M.H.; Shah, K.V.; Rabkin, C.S.; Schiller, J.T.; Wacholder, S.; Clayman, B.; Viscidi, R.P. A survey of human papillomavirus 16 antibodies in patients with epithelial cancers. Eur. J. Cancer Prev. 1998, 7, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.J.; Koutsky, L.A.; Wipf, G.C.; Christensen, N.D.; Lee, S.K.; Kuypers, J.; Kiviat, N.; Galloway, D.A. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J. Infect. Dis. 1996, 174, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Schiffman, M.; Herrero, R.; Carreon, J.; Hildesheim, A.; Rodriguez, A.C.; Bratti, M.C.; Sherman, M.E.; Morales, J.; Guillen, D.; et al. Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10 000 women in Costa Rica. Br. J. Cancer 2004, 91, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Studentsov, Y.Y.; Bierman, R.; Burk, R.D. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol. Biomark. Prev. 2004, 13, 110–116. [Google Scholar] [CrossRef]
- Gage, J.C.; Katki, H.A.; Schiffman, M.; Fetterman, B.; Poitras, N.E.; Lorey, T.; Cheung, L.C.; Castle, P.E.; Kinney, W.K. Age-stratified 5-year risks of cervical precancer among women with enrollment and newly detected HPV infection. Int. J. Cancer 2015, 136, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, M.; Porras, C.; Schiffman, M.; Rodriguez, A.C.; Wacholder, S.; Gonzalez, P.; Quint, W.; Van Doorn, L.J.; Sherman, M.E.; Xhenseval, V.; et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J. Natl. Cancer Inst. 2010, 102, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Studentsov, Y.; Hall, C.B.; Bierman, R.; Beardsley, L.; Lempa, M.; Burk, R.D. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J. Infect. Dis. 2002, 186, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.A.; Hailpern, S.M.; Burk, R.D. Persistent antibodies to HPV virus-like particles following natural infection are protective against subsequent cervicovaginal infection with related and unrelated HPV. Viral Immunol. 2009, 22, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Wentzensen, N.; Wacholder, S.; Kinney, W.; Gage, J.C.; Castle, P.E. Human papillomavirus testing in the prevention of cervical cancer. J. Natl. Cancer Inst. 2011, 103, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Trottier, H.; Ferreira, S.; Thomann, P.; Costa, M.C.; Sobrinho, J.S.; Prado, J.C.; Rohan, T.E.; Villa, L.L.; Franco, E.L. Human papillomavirus infection and reinfection in adult women: The Role of Sexual Activity and Natural Immunity. Cancer Res. 2010, 70, 8569–8577. [Google Scholar] [CrossRef] [PubMed]
- Viscidi, R.P.; Schiffman, M.; Hildesheim, A.; Herrero, R.; Castle, P.E.; Bratti, M.C.; Rodriguez, A.C.; Sherman, M.E.; Wang, S.; Clayman, B.; et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: Results from a Population-based Study in Costa Rica. Cancer Epidemiol. Biomark. Prev. 2004, 13, 324–327. [Google Scholar] [CrossRef]
- Beachler, D.C.; Jenkins, G.; Safaeian, M.; Kreimer, A.R.; Wentzensen, N. Natural Acquired Immunity Against Subsequent Genital Human Papillomavirus Infection: A Systematic Review and Meta-analysis. J. Infect. Dis. 2016, 213, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Velicer, C.; Zhu, X.; Vuocolo, S.; Liaw, K.L.; Saah, A. Prevalence and incidence of HPV genital infection in women. Sex. Transm. Dis. 2009, 36, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.F.; Koutsky, L.A.; Castle, P.E.; Edelstein, Z.R.; Hulbert, A.; Schiffman, M.; Kiviat, N.B. Human papillomavirus type 16 variants in paired enrollment and follow-up cervical samples: Implications for a Proper Understanding of Type-specific Persistent Infections. J. Infect. Dis. 2010, 202, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, C.M.; Skinner, S.R.; Del Rosario-Raymundo, M.R.; Garland, S.M.; Chatterjee, A.; Lazcano-Ponce, E.; Salmerón, J.; McNeil, S.; Stapleton, J.T.; Bouchard, C.; et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year Follow-up of the Phase 3, Double-blind, Randomised Controlled Viviane Study. Lancet Infect. Dis. 2016, 16, 1154–1168. [Google Scholar] [CrossRef]
- Castellsague, X.; Munoz, N.; Pitisuttithum, P.; Ferris, D.; Monsonego, J.; Ault, K.; Luna, J.; Myers, E.; Mallary, S.; Bautista, O.M.; et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br. J. Cancer 2011, 105, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hildesheim, A.; Herrero, R.; Wacholder, S.; Rodriguez, A.C.; Solomon, D.; Bratti, M.C.; Schiller, J.T.; Gonzalez, P.; Dubin, G.; Porras, C.; et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: A Randomized Trial. JAMA 2007, 298, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Scherer, E.M.; Smith, R.A.; Gallego, D.F.; Carter, J.J.; Wipf, G.C.; Hoyos, M.; Stern, M.; Thurston, T.; Trinklein, N.D.; Wald, A.; et al. A Single Human Papillomavirus Vaccine Dose Improves B Cell Memory in Previously Infected Subjects. EBioMedicine 2016, 10, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Polman, N.J.; Veldhuijzen, N.J.; Heideman, D.A.M.; Snijders, P.J.F.; Meijer, C.J.L.M.; Berkhof, J. HPV-positive women with normal cytology remain at increased risk of CIN3 after a negative repeat HPV test. Br. J. Cancer 2017. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Baussano, I. Naturally acquired immunity against human papillomavirus (HPV): Why It Matters in the HPV Vaccine Era. J. Infect. Dis. 2014, 210, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Robles, C.; Diaz, M.; Arbyn, M.; Baussano, I.; Clavel, C.; Ronco, G.; Dillner, J.; Lehtinen, M.; Petry, K.U.; et al. HPV-FASTER: Broadening the Scope for Prevention of HPV-related Cancer. Nat. Rev. Clin. Oncol. 2016, 13, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Brotman, R.M.; Zenilman, J.M.; Gravitt, P.E.; Cummings, D.A. Menstrual cycle and detectable human papillomavirus in reproductive-age women: A Time Series Study. J. Infect. Dis. 2013, 208, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Corey, L. Current Concepts for Genital Herpes Simplex Virus Infection: Diagnostics and Pathogenesis of Genital Tract Shedding. Clin. Microbiol. Rev. 2016, 29, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, J.T.; Abu-Raddad, L.; Mark, K.E.; Zhu, J.; Selke, S.; Koelle, D.M.; Wald, A.; Corey, L.; et al. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc. Natl. Acad. Sci. USA 2010, 107, 18973–18978. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gravitt, P.E.; Winer, R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses 2017, 9, 267. https://doi.org/10.3390/v9100267
Gravitt PE, Winer RL. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses. 2017; 9(10):267. https://doi.org/10.3390/v9100267
Chicago/Turabian StyleGravitt, Patti E., and Rachel L. Winer. 2017. "Natural History of HPV Infection across the Lifespan: Role of Viral Latency" Viruses 9, no. 10: 267. https://doi.org/10.3390/v9100267
APA StyleGravitt, P. E., & Winer, R. L. (2017). Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses, 9(10), 267. https://doi.org/10.3390/v9100267




