Intranasal Immunization with Influenza Virus-Like Particles Containing Membrane-Anchored Cholera Toxin B or Ricin Toxin B Enhances Adaptive Immune Responses and Protection against an Antigenically Distinct Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. Construction of Membrane-Anchored RTB or CTB Coding Sequences
2.3. Generation of Recombinant Baculoviruses and Production of cVLPs
2.4. Immunofluorescence Assay (IFA)
2.5. Western Blot Analysis
2.6. Electron Microscopy
2.7. Immunization and Challenge
2.8. Antibody Responses and Hemagglutination-Inhibition (HAI) Titers
2.9. Lung Viral Titers and Microneutralization Assay
2.10. Cytokine Assays Using ELISpot
2.11. Statistical Analysis
3. Results
3.1. Construction and Characterization of rBVs Expressing CTB or RTB
3.2. Production and Characterization of CTB-VLP or RTB-VLP
3.3. cVLPs Containing CTB or RTB Enhance Systemic Antibody Responses
3.4. cVLPs Containing CTB or RTB Enhance Mucosal Immune Responses
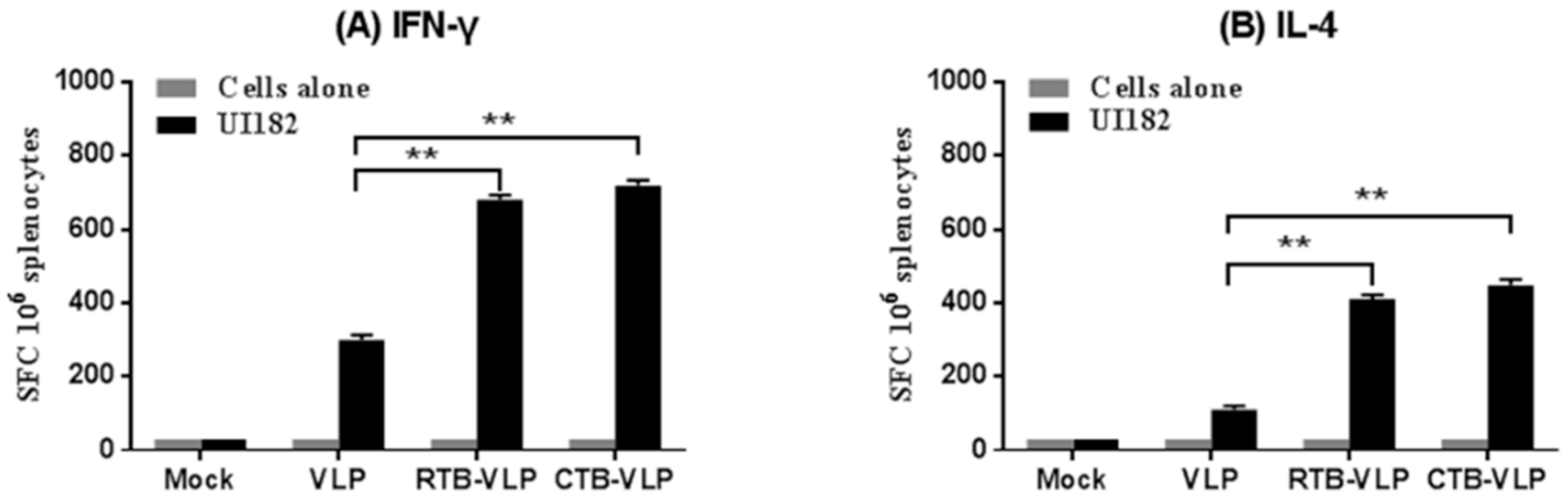
3.5. cVLPs Containing CTB or RTB Enhance Cell-Mediated Immune Responses
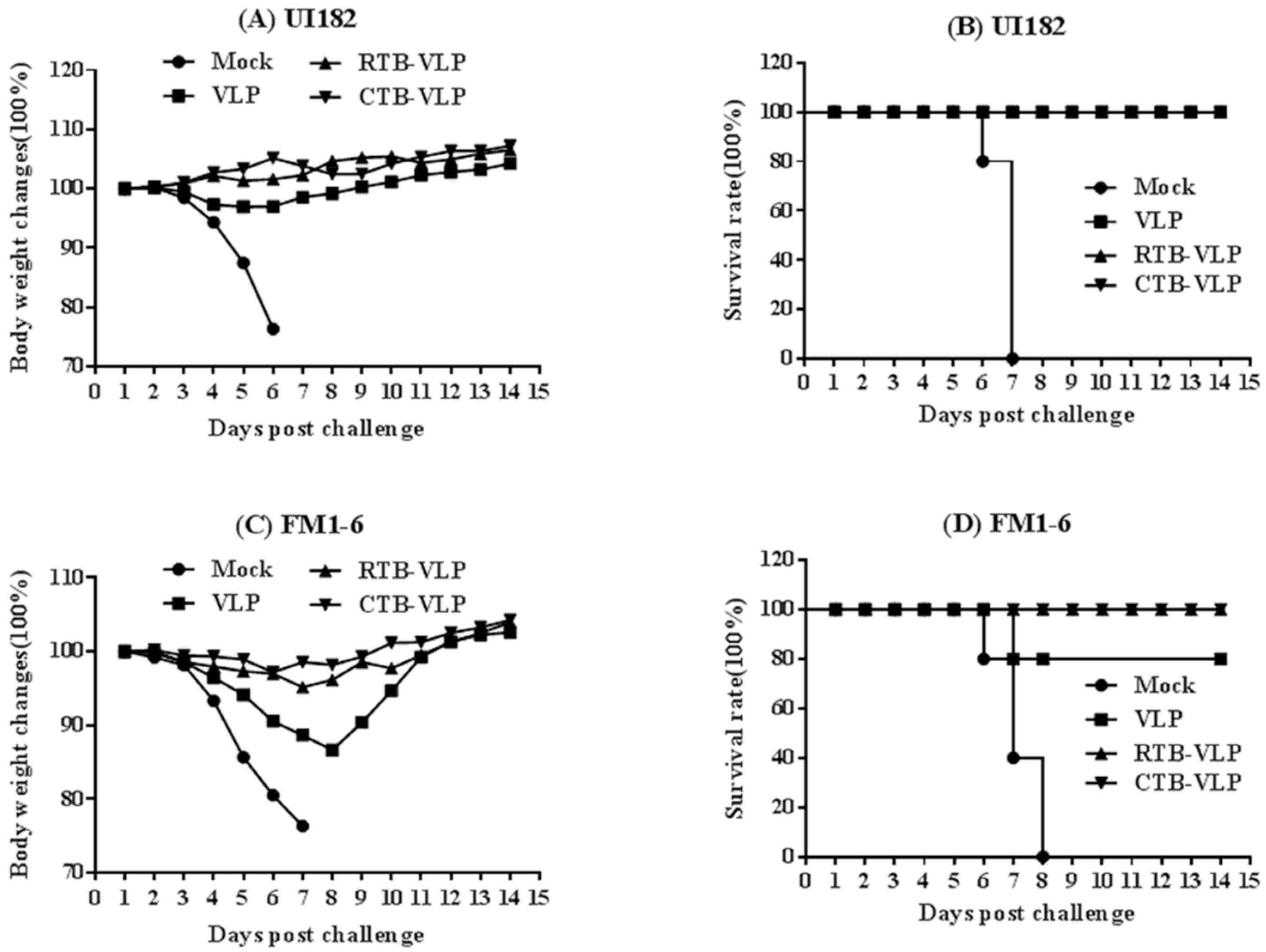
3.6. cVLPs Containing CTB or RTB Enhance Heterosubtypic Protection against Lethal Virus Challenge
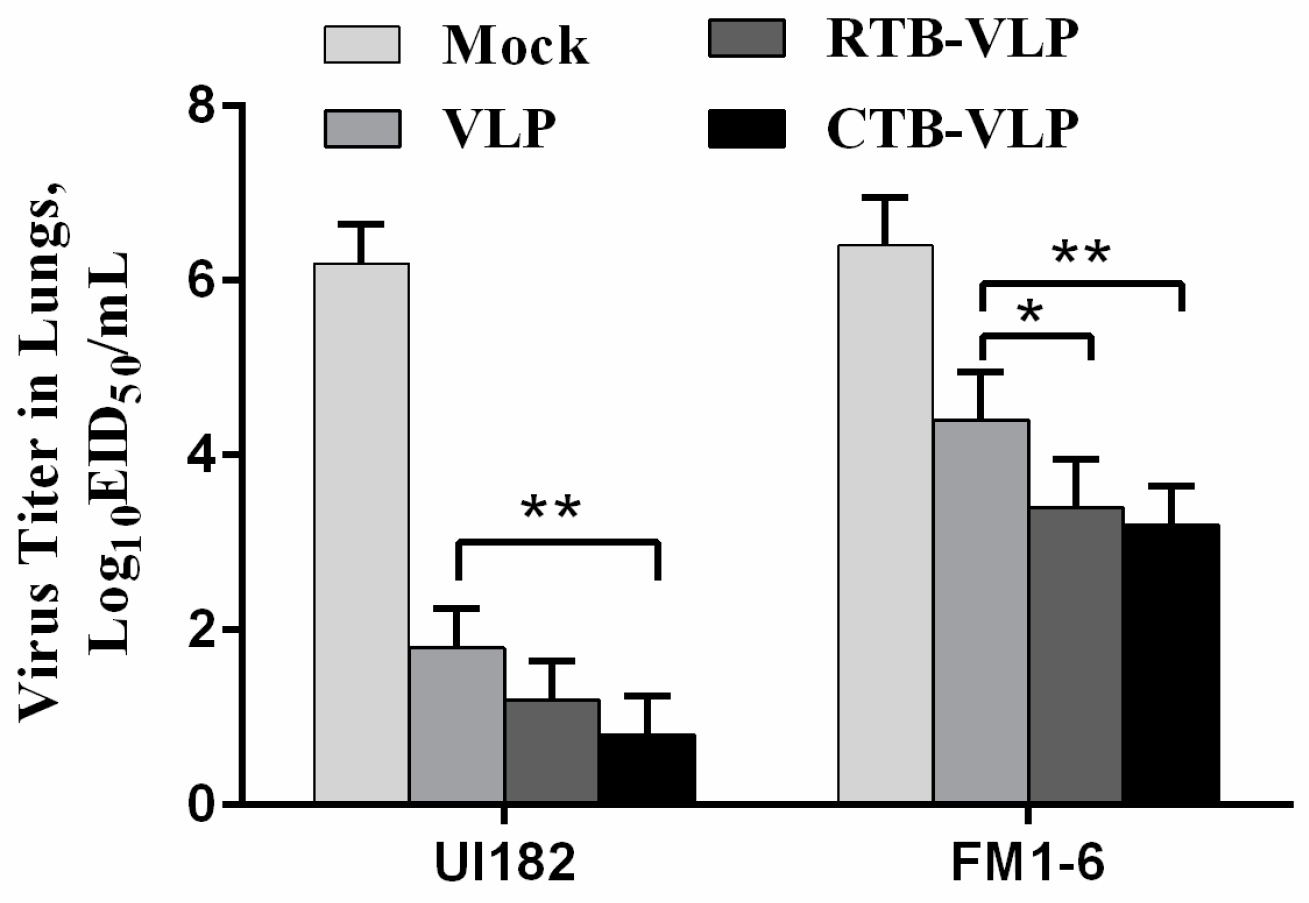
3.7. Immunization with cVLPs Containing CTB or RTB Reduces Viral Loads Following Challenge
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pence, B.D.; Hester, S.N.; Donovan, S.M.; Woods, J.A. Dietary whole glucan particles do not affect antibody or cell-mediated immune responses to influenza virus vaccination in mice. Immunol. Investig. 2012, 41, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, X.; Liu, J.; Dong, L.; Chen, Q.; Liu, J.; Kong, H.; Zhang, Q.; Qi, X.; Hou, D.; et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015, 25, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Clegg, C.H.; Roque, R.; Perrone, L.A.; Rininger, J.A.; Bowen, R.; Reed, S.G. GLA-AF, an emulsion-free vaccine adjuvant for pandemic influenza. PLoS ONE 2014, 9, e88979. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.R. Influenza virus-like particle vaccines. Expe. Rev. Vaccines 2009, 8, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Bourgeois, M.; Quan, F.S.; Lipatov, A.S.; Song, J.M.; Chen, L.M.; Compans, R.W.; York, I.; Kang, S.M.; Donis, R.O. Virus-like particle vaccine containing hemagglutinin confers protection against 2009 H1N1 pandemic influenza. Clin. Vaccine Immunol. 2011, 18, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Moron, G.; Rueda, P.; Casal, I.; Leclerc, C. CD8α-CD11b+ dendritic cells present exogenous virus-like particles to CD8+ T cells and subsequently express CD8α and CD205 molecules. J. Exp. Med. 2002, 195, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Antonis, A.F.; Bruschke, C.J.; Rueda, P.; Maranga, L.; Casal, J.I.; Vela, C.; Hilgers, L.A.; Belt, P.B.; Weerdmeester, K.; Carrondo, M.J.; et al. A novel recombinant virus-like particle vaccine for prevention of porcine parvovirus-induced reproductive failure. Vaccine 2006, 24, 5481–5490. [Google Scholar] [CrossRef] [PubMed]
- Harro, C.D.; Pang, Y.Y.; Roden, R.B.; Hildesheim, A.; Wang, Z.; Reynolds, M.J.; Mast, T.C.; Robinson, R.; Murphy, B.R.; Karron, R.A.; et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 2001, 93, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Qiao, M.; Nascimbeni, M.; Hu, Z.; Rehermann, B.; Murthy, K.; Liang, T.J. Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates. J. Virol. 2004, 78, 6995–7003. [Google Scholar] [CrossRef] [PubMed]
- LoBue, A.D.; Lindesmith, L.; Yount, B.; Harrington, P.R.; Thompson, J.M.; Johnston, R.E.; Moe, C.L.; Baric, R.S. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine 2006, 24, 5220–5234. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, C.M.; Clements, J.D.; Estes, M.K.; Conner, M.E. Rotavirus 2/6 viruslike particles administered intranasally with cholera toxin, Escherichia coli heat-labile toxin (LT), and LT-R192G induce protection from rotavirus challenge. J. Virol. 1998, 72, 3390–3393. [Google Scholar] [PubMed]
- Bright, R.A.; Carter, D.M.; Daniluk, S.; Toapanta, F.R.; Ahmad, A.; Gavrilov, V.; Massare, M.; Pushko, P.; Mytle, N.; Rowe, T.; et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 2007, 25, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Huang, C.; Compans, R.W.; Kang, S.M. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 2007, 81, 3514–3524. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Steinhauer, D.; Huang, C.; Ross, T.M.; Compans, R.W.; Kang, S.M. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine 2008, 26, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Yoo, D.G.; Song, J.M.; Clements, J.D.; Compans, R.W.; Kang, S.M. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J. Virol. 2009, 83, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Vunnava, A.; Compans, R.W.; Kang, S.M. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS ONE 2010, 5, e9161. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Hossain, J.; Yoo, D.G.; Lipatov, A.S.; Davis, C.T.; Quan, F.S.; Chen, L.M.; Hogan, R.J.; Donis, R.O.; Compans, R.W.; et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 2010, 405, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Hossain, D.M.; Mohanty, S.; Ray, P.; Das, T.; Sa, G. Tumor gangliosides and T cells: A deadly encounter. Front. Biosci. 2012, 4, 502–519. [Google Scholar] [CrossRef]
- Holmgren, J.; Svennerholm, A.M. Cholera and the immune response. Prog. Allergy 1983, 33, 106–119. [Google Scholar] [PubMed]
- Holmgren, J.; Harandi, A.M.; Czerkinsky, C. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Expert Rev. Vaccines 2003, 2, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Czerkinsky, C.; Eriksson, K.; Mharandi, A. Mucosal immunisation and adjuvants: A brief overview of recent advances and challenges. Vaccine 2003, 21, S89–S95. [Google Scholar] [CrossRef]
- Cong, Y.; Weaver, C.T.; Elson, C.O. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J. Immunol. 1997, 159, 5301–5308. [Google Scholar] [PubMed]
- Chen, K.S.; Strober, W. Cholera holotoxin and its B subunit enhance Peyer’s patch B cell responses induced by orally administered influenza virus: Disproportionate cholera toxin enhancement of the IgA B cell response. Eur. J. Immunol. 1990, 20, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, N.; Yuan, H.; Li, S.; Liu, L.; Pu, Z.; Wan, J.; Wang, H.; Chang, Y.; Li, R. Immunomodulatory activity of recombinant Ricin toxin binding Subunit B (RTB). Int. J. Mol. Sci. 2013, 14, 12401–12410. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Chen, M. Successful immunization against gastric infection with Helicobacter species: Use of a cholera toxin B-subunit-whole-cell vaccine. Infect. Immun. 1994, 62, 3594–3597. [Google Scholar] [PubMed]
- Tamura, S.; Samegai, Y.; Kurata, H.; Nagamine, T.; Aizawa, C.; Kurata, T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine 1988, 6, 409–413. [Google Scholar] [CrossRef]
- Medina-Bolivar, F.; Wright, R.; Funk, V.; Sentz, D.; Barroso, L.; Wilkins, T.D.; Petri, W., Jr.; Cramer, C.L. A non-toxic lectin for antigen delivery of plant-based mucosal vaccines. Vaccine 2003, 21, 997–1005. [Google Scholar] [CrossRef]
- Choi, N.W.; Estes, M.K.; Langridge, W.H. Mucosal immunization with a ricin toxin B subunit-rotavirus NSP4 fusion protein stimulates a Th1 lymphocyte response. J. Biotechnol. 2006, 121, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Rutenber, E.; Ready, M.; Robertus, J.D. Structure and evolution of ricin B chain. Nature 1987, 326, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B.; Muriel, M.P.; Wantyghem, J.; Aubery, M.; Decastel, M. Ricin toxicity and intracellular routing in tumoral HT-29 cells. II. Differential ricin toxicity from the apical and basolateral surfaces of differentiated HT-29 cells. Exp. Cell Res. 1995, 221, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Ogra, P.L.; Faden, H.; Welliver, R.C. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 2001, 14, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Maeyama, J.; Isaka, M.; Yasuda, Y.; Matano, K.; Taniguchi, T.; Morokuma, K.; Ohkuma, K.; Tochikubo, K.; Goto, N. Effects of recombinant cholera toxin B subunit on IL-1beta production by macrophages in vitro. Microbiol. Immunol. 2002, 46, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Compans, R.W.; Nguyen, H.H.; Kang, S.M. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 2008, 82, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Z.; Quan, F.S.; Kang, S.M.; Bozja, J.; Skountzou, I.; Compans, R.W. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J. Virol. 2008, 82, 11813–11823. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, H.; Zheng, X.; Xue, X.; Wang, B.; Wu, H.; Zhang, K.; Fan, S.; Wang, T.; Li, N.; et al. CpG/Poly (I:C) mixed adjuvant priming enhances the immunogenicity of a DNA vaccine against eastern equine encephalitis virus in mice. Int. Immunopharmacol. 2014, 19, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Tumpey, T.M.; Bu, F.; Knell, J.; Robinson, R.; Smith, G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 2005, 23, 5751–5759. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Qi, Y.; Wang, H.; Zheng, X.; Gao, Y.; Li, N.; Yang, S.; Xia, X. Chimeric rabies virus-like particles containing membrane-anchored GM-CSF enhances the immune response against rabies virus. Viruses 2015, 7, 1134–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Z.; Xu, R.; Quan, F.S.; Kang, S.M.; Wang, L.; Compans, R.W. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS ONE 2010, 5, e13972. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ji, X.; Meng, L.; Wei, Y.; Wang, T.; Feng, N.; Zheng, X.; Wang, H.; Li, N.; Gao, X.; et al. H5N1 influenza virus-like particle vaccine protects mice from heterologous virus challenge better than whole inactivated virus. Virus Res. 2015, 200, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Tumpey, T.M.; Van Hoeven, N.; Belser, J.A.; Robinson, R.; Nathan, M.; Smith, G.; Wright, D.C.; Bright, R.A. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine 2007, 25, 4283–4290. [Google Scholar] [CrossRef] [PubMed]
- Suguitan, A.L., Jr.; McAuliffe, J.; Mills, K.L.; Jin, H.; Duke, G.; Lu, B.; Luke, C.J.; Murphy, B.; Swayne, D.E.; Kemble, G.; et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006, 3, e360. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.F.; Green, T.D.; Ross, T.M. DNA vaccines expressing soluble CD4-envelope proteins fused to C3d elicit cross-reactive neutralizing antibodies to HIV-1. Virology 2004, 328, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Kang, H.; Zheng, X.; Wang, H.; Gao, Y.; Yang, S.; Xia, X. Incorporation of membrane-anchored flagellin or Escherichia coli heat-labile enterotoxin B subunit enhances the immunogenicity of rabies virus-like particles in mice and dogs. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Inn, K.S.; Lee, G.J.; Quan, F.S. A pandemic H1N1 influenza virus-like particle vaccine induces cross-protection in mice. Immunol. Invest. 2014, 43, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Compans, R.W. Enhancement of mucosal immunization with virus-like particles of simian immunodeficiency virus. J. Virol. 2003, 77, 3615–3623. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.A.; Ahmad, A.; Veguilla, V.; Lu, X.; Smith, G.; Katz, J.M.; Pushko, P.; Tumpey, T.M. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J. Virol. 2009, 83, 5726–5734. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.C.; McKeon, R.M.; Brackin, M.N.; Miller, L.A.; Keating, R.; Brown, S.A.; Makarova, N.; Perez, D.R.; Macdonald, G.H.; McCullers, J.A. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vaccine Immunol. 2006, 13, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.C.; Lynch, J.M.; Bucher, D.J.; Le, J.; Metzger, D.W. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 2001, 166, 7381–7388. [Google Scholar] [CrossRef] [PubMed]
- Jayasekera, J.P.; Moseman, E.A.; Carroll, M.C. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 2007, 81, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Grabherr, R. Alternative influenza vaccines made by insect cells. Trends Mol. Med. 2010, 16, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Margine, I.; Martinez-Gil, L.; Chou, Y.Y.; Krammer, F. Residual baculovirus in insect cell-derived influenza virus-like particle preparations enhances immunogenicity. PLoS ONE 2012, 7, e51559. [Google Scholar] [CrossRef] [PubMed]
- Hervas-Stubbs, S.; Rueda, P.; Lopez, L.; Leclerc, C. Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J. Immunol. 2007, 178, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Macias, C.; Ferat-Osorio, E.; Tenorio-Calvo, A.; Isibasi, A.; Talavera, J.; Arteaga-Ruiz, O.; Arriaga-Pizano, L.; Hickman, S.P.; Allende, M.; Lenhard, K.; et al. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine 2011, 29, 7826–7834. [Google Scholar] [CrossRef] [PubMed]
- Ansaldi, F.; Bacilieri, S.; Durando, P.; Sticchi, L.; Valle, L.; Montomoli, E.; Icardi, G.; Gasparini, R.; Crovari, P. Cross-protection by MF59-adjuvanted influenza vaccine: Neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine 2008, 26, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Baras, B.; Stittelaar, K.J.; Simon, J.H.; Thoolen, R.J.; Mossman, S.P.; Pistoor, F.H.; van Amerongen, G.; Wettendorff, M.A.; Hanon, E.; Osterhaus, A.D. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS ONE 2008, 3, e1401. [Google Scholar] [CrossRef] [PubMed]
- Sambhara, S.; Kurichh, A.; Miranda, R.; Tumpey, T.; Rowe, T.; Renshaw, M.; Arpino, R.; Tamane, A.; Kandil, A.; James, O.; et al. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell. Immunol. 2001, 211, 143–153. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence (5′–3′) * | Restriction Enzyme Site |
|---|---|---|
| H1N1 HA F | CCGGAATTCATGAAGGCAATACTAGTAGTTCTGCTATAT | EcoR I |
| H1N1 HA R | AAATATGCGGCCGCTTAAATACATATTCTACACTGTAGAGACCC | Not I |
| H1N1 NA F | CCGGAATTCATGAATCCAAACCAAAAGATAATAACCATT | EcoR I |
| H1N1 NA R | AAATATGCGGCCGCTTACTTGTCAATGGTAAATGGCAACTCAGC | Not I |
| H1N1 M1 F | CCGGAATTCATGAGTCTTCTAACCGAGGTCGAAACGTAC | EcoR I |
| H1N1 M1 R | AAATATGCGGCCGCTCACTTGAATCGCTGCATCTGCACTCCCAT | Not I |
| MSP-CTB-TMCT(HA) F | CCGGAATTCATGAAGTTCCTGGTGAACGTGGCTCTGGTG | EcoR I |
| MSP-CTB-TMCT(HA) R | AAATATGCGGCCGCTTAGATGCAGATGCGGCACTGCAGGGAACC | Not I |
| MSP-RTB-TMCT(HA) F | CCGGAATTCATGAAGTTCCTGGTGAACGTCGCCTTGGTC | EcoR I |
| MSP-RTB-TMCT(HA) R | AAATATGCGGCCGCTTAGATGCAGATACGGCACTGCAAGCTACC | Not I |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Ren, Z.; Xu, N.; Meng, L.; Yu, Z.; Feng, N.; Sang, X.; Li, S.; Li, Y.; Wang, T.; et al. Intranasal Immunization with Influenza Virus-Like Particles Containing Membrane-Anchored Cholera Toxin B or Ricin Toxin B Enhances Adaptive Immune Responses and Protection against an Antigenically Distinct Virus. Viruses 2016, 8, 115. https://doi.org/10.3390/v8040115
Ji X, Ren Z, Xu N, Meng L, Yu Z, Feng N, Sang X, Li S, Li Y, Wang T, et al. Intranasal Immunization with Influenza Virus-Like Particles Containing Membrane-Anchored Cholera Toxin B or Ricin Toxin B Enhances Adaptive Immune Responses and Protection against an Antigenically Distinct Virus. Viruses. 2016; 8(4):115. https://doi.org/10.3390/v8040115
Chicago/Turabian StyleJi, Xianliang, Zhiguang Ren, Na Xu, Lingnan Meng, Zhijun Yu, Na Feng, Xiaoyu Sang, Shengnan Li, Yuanguo Li, Tiecheng Wang, and et al. 2016. "Intranasal Immunization with Influenza Virus-Like Particles Containing Membrane-Anchored Cholera Toxin B or Ricin Toxin B Enhances Adaptive Immune Responses and Protection against an Antigenically Distinct Virus" Viruses 8, no. 4: 115. https://doi.org/10.3390/v8040115
APA StyleJi, X., Ren, Z., Xu, N., Meng, L., Yu, Z., Feng, N., Sang, X., Li, S., Li, Y., Wang, T., Zhao, Y., Wang, H., Zheng, X., Jin, H., Li, N., Yang, S., Cao, J., Liu, W., Gao, Y., & Xia, X. (2016). Intranasal Immunization with Influenza Virus-Like Particles Containing Membrane-Anchored Cholera Toxin B or Ricin Toxin B Enhances Adaptive Immune Responses and Protection against an Antigenically Distinct Virus. Viruses, 8(4), 115. https://doi.org/10.3390/v8040115






