Preventive Activity against Influenza (H1N1) Virus by Intranasally Delivered RNA-Hydrolyzing Antibody in Respiratory Epithelial Cells of Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Virus and Cell Culture
2.3. Virus Infection to MDCK Cells
2.4. HA RNA Transcript Preparation and RNA Hydrolyzing Activity Test
2.5. Purification of 3D8 scFv Protein
2.6. Intranasal Administration of 3D8 scFv Protein
2.7. Immunohistochemistry and Histopathological Examination
2.8. Determination of Virus Titer
2.9. Measurement of Cytokine and Chemokine Levels
| Forward | Reverse | Accession No | |
|---|---|---|---|
| GAPDH | TGG CAA AGT GGA GAT TGT TGC C | AAG ATG GTG ATG GGC TTC CCG | NM_002046 |
| 3D8 scFv | TAT GCA CTG GGT GAA GCA GA | TGA GCT CCA TGT AGG CTG TG | AF232220 & AF232221 |
| Hemagglutinin | CAC CCG TCT AGC AGT GAT GA | CTC AGT GCG AAA GCA TAC CA | U08903.1 |
| Neuraminidase | CAC TTG GAA TGC AGG ACC TT | ACC AAG CAA CCG ATT CAA AC | HQ008256.1 |
| TNF-alpha | CGT CAG CCG ATT TGC TAT CT | CGG ACT CCG CAA AGT CTA AG | NM_013693.2 |
| IL-6 | AGT TGC CTT CTT GGG ACT GA | TCC ACG ATT TCC CAG AGA AC | NM_031168.1 |
| IFN-gamma | ACT GGC AAA AGG ATG GTG AC | GAC CTG TGG GTT GTT GAC CT | NM_008337.3 |
2.10. Statistical Analysis
3. Results
3.1. Direct RNA Hydrolyzing Activity of 3D8 scFv against H1N1 Influenza Virus in MDCK Cells
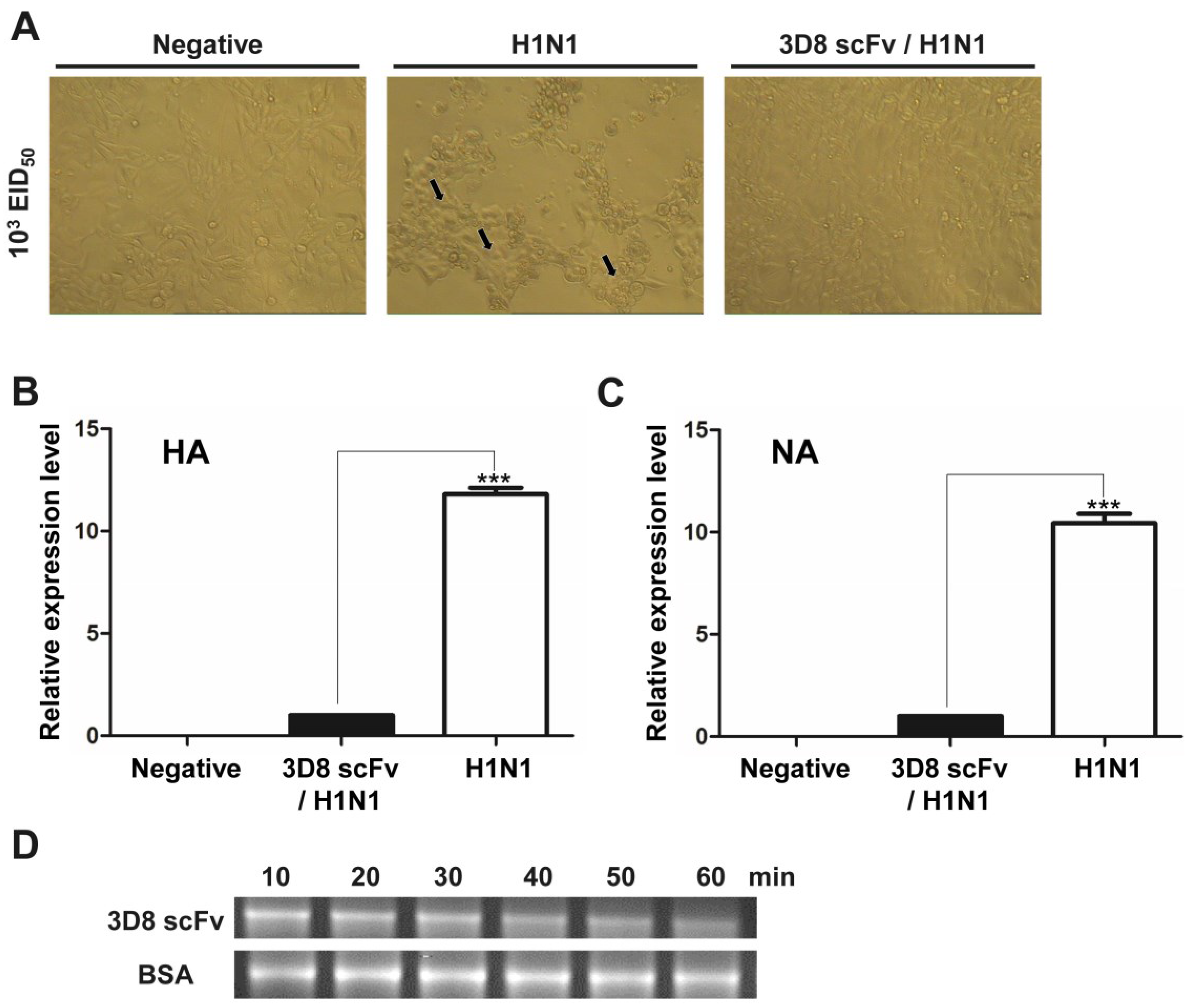
3.2. Recovery from H1N1 Infection in Mice Treated with 3D8 scFv

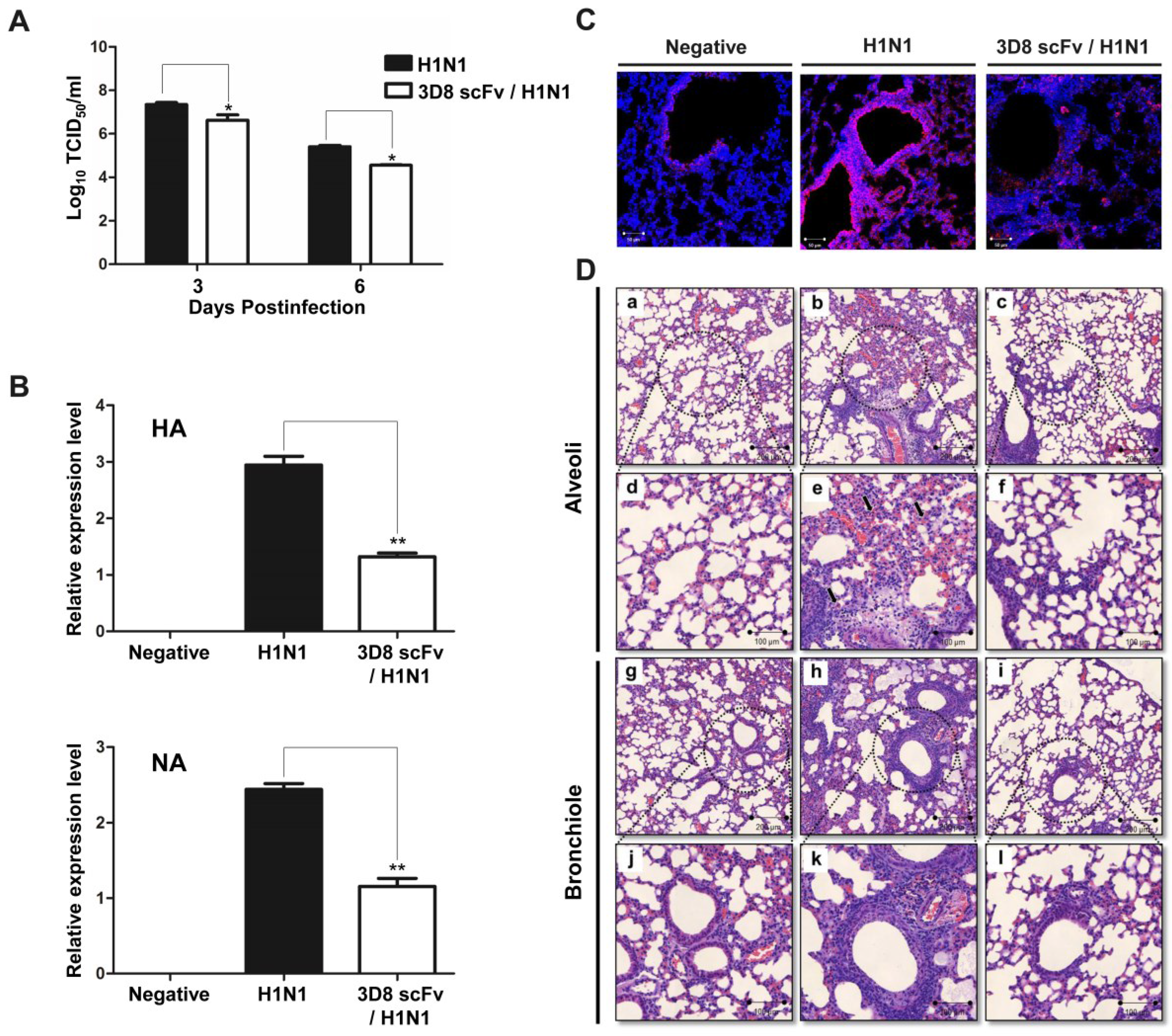
3.3. Reduced Influenza Virus Pathogenicity Due to the Preventive Effect of 3D8 scFv in the Lung
3.4. 3D8 scFv Reduced Histopathological Symptoms in Mouse Lung Tissue
3.5. 3D8 scFv Passes through the Nasal Mucosal Layer and Localizes in Epithelial Cells
3.6. The Antiviral Effect in 3D8 ScFv-treated Mice is Due to Its Catalytic Activity against Nucleic Acids

4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, J.W.; Shetty, N.; Lam, T.T.; Hon, K.L. Emerging, novel, and known influenza virus infections in humans. Infect. Dis. Clin. N. Am. 2010, 24, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Perez-Padilla, R.; de la Rosa-Zamboni, D.; Ponce de Leon, S.; Hernandez, M.; Quinones-Falconi, F.; Bautista, E.; Ramirez-Venegas, A.; Rojas-Serrano, J.; Ormsby, C.E.; Corrales, A.; et al. Pneumonia and respiratory failure from swine-origin influenza a (H1N1) in mexico. N. Engl. J. Med. 2009, 361, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Lin, Z.; Banner, D.; Leon, A.J.; Paquette, S.G.; Rubin, B.; Rubino, S.; Guan, Y.; Kelvin, D.J.; Kelvin, A.A. Immunity toward H1N1 influenza hemagglutinin of historical and contemporary strains suggests protection and vaccine failure. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boltz, D.A.; Aldridge, J.R., Jr.; Webster, R.G.; Govorkova, E.A. Drugs in development for influenza. Drugs 2010, 70, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Sheu, T.G.; Fry, A.M.; Garten, R.J.; Deyde, V.M.; Shwe, T.; Bullion, L.; Peebles, P.J.; Li, Y.; Klimov, A.I.; Gubareva, L.V. Dual resistance to adamantanes and oseltamivir among seasonal influenza A(H1N1) viruses: 2008–2010. J. Infect. Dis. 2011, 203, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.; Li, Y.; Bastien, N.; Garceau, R.; Hatchette, T.F. Oseltamivir-resistant pandemic H1N1 influenza. Can. Med. Assoc. J. 2011, 183, E420–E422. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Thaker, S.N.; Clippard, J.; Monto, A.S.; Ohmit, S.E.; Zimmerman, R.K.; Nowalk, M.P.; Gaglani, M.; Jackson, M.L.; Jackson, L.A.; et al. Interim estimates of 2013–14 seasonal influenza vaccine effectiveness—United States, February 2014. Morb. Mortal. Wkly. Rep. 2014, 63, 137–142. [Google Scholar]
- Barr, I.G.; Russell, C.; Besselaar, T.G.; Cox, N.J.; Daniels, R.S.; Donis, R.; Engelhardt, O.G.; Grohmann, G.; Itamura, S.; Kelso, A.; et al. Who recommendations for the viruses used in the 2013–2014 northern hemisphere influenza vaccine: Epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from October 2012 to January 2013. Vaccine 2014, 32, 4713–4725. [Google Scholar] [PubMed]
- Kim, Y.R.; Kim, J.S.; Lee, S.H.; Lee, W.R.; Sohn, J.N.; Chung, Y.C.; Shim, H.K.; Lee, S.C.; Kwon, M.H.; Kim, Y.S. Heavy and light chain variable single domains of an anti-DNA binding antibody hydrolyze both double- and single-stranded dnas without sequence specificity. J. Biol. Chem. 2006, 281, 15287–15295. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.H.; Lee, M.S.; Kim, K.H.; Park, S.; Shin, H.J.; Jang, Y.J.; Kim, H.I. Production and characterization of an anti-idiotypic single chain fv that recognizes an anti-DNA antibody. Immunol. Investig. 2002, 31, 205–218. [Google Scholar] [CrossRef]
- Jang, J.Y.; Jeong, J.G.; Jun, H.R.; Lee, S.C.; Kim, J.S.; Kim, Y.S.; Kwon, M.H. A nucleic acid-hydrolyzing antibody penetrates into cells via caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell. Mol. Life Sci. 2009, 66, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Yu, J.; Cho, S.; Byun, S.-J.; Kim, D.H.; Lee, T.-K.; Kwon, M.-H.; Lee, S. A nucleic-acid hydrolyzing single chain antibody confers resistance to DNA virus infection in hela cells and c57bl/6 mice. PLoS Pathog. 2014, 10, e1004208. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.R.; Pham, C.D.; Lim, S.I.; Lee, S.C.; Kim, Y.S.; Park, S.; Kwon, M.H. An rna-hydrolyzing recombinant antibody exhibits an antiviral activity against classical swine fever virus. Biochem. Biophys. Res. Commun. 2010, 395, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.M.; Cho, S.; Kim, K.E.; Byun, S.J.; Lee, T.-K.; Lee, S. Development of lactobacillus paracasei harboring nucleic acid-hydrolyzing 3d8 scfv as a preventive probiotic against murine norovirus infection. Appl. Microbiol. Biotechnol. 2015, 99, 2793–2803. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [PubMed]
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Romeo, V.D.; deMeireles, J.; Sileno, A.P.; Pimplaskar, H.K.; Behl, C.R. Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 89–116. [Google Scholar] [PubMed]
- Patton, J.S.; Platz, R.M. (D) routes of delivery: Case studies: (2) pulmonary delivery of peptides and proteins for systemic action. Adv. Drug Deliv. Rev. 1992, 8, 179–196. [Google Scholar] [CrossRef]
- Klingenberg, R.; Lebens, M.; Hermansson, A.; Fredrikson, G.N.; Strodthoff, D.; Rudling, M.; Ketelhuth, D.F.; Gerdes, N.; Holmgren, J.; Nilsson, J.; et al. Intranasal immunization with an apolipoprotein b-100 fusion protein induces antigen-specific regulatory t cells and reduces atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 946–952. [Google Scholar] [CrossRef]
- Sloat, B.R.; Cui, Z. Evaluation of the immune response induced by a nasal anthrax vaccine based on the protective antigen protein in anaesthetized and non-anaesthetized mice. J. Pharm. Pharmacol. 2006, 58, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.N.; Lee, D.H.; Lee, Y.N.; Park, J.K.; Yuk, S.S.; Yang, S.Y.; Lee, H.J.; Woo, S.H.; Kim, H.M.; Lee, J.B.; et al. Intranasal administration of live lactobacillus species facilitates protection against influenza virus infection in mice. Antivir. Res. 2012, 93, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A. Technical aspects of immunohistochemistry. Vet. Pathol. 2005, 42, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the www for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: Berlin, Germany, 2002. [Google Scholar]
- Catania, J.; Que, L.G.; Govert, J.A.; Hollingsworth, J.W.; Wolfe, C.R. High intensive care unit admission rate for 2013–2014 influenza is associated with a low rate of vaccination. Am. J. Respir. Crit. Care Med. 2014, 189, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.S. Nasal vaccines. Adv. Drug Deliv. Rev. 2001, 51, 21–42. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery: New developments and strategies. Drug Discov. Today 2002, 7, 1184–1189. [Google Scholar] [CrossRef]
- Abraham, W.M.; Sielczak, M.W.; Ahmed, A.; Cortes, A.; Lauredo, I.T.; Kim, J.; Pepinsky, B.; Benjamin, C.D.; Leone, D.R.; Lobb, R.R.; et al. Alpha 4-integrins mediate antigen-induced late bronchial responses and prolonged airway hyperresponsiveness in sheep. J. Clin. Investig. 1994, 93, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Shardonofsky, F.R.; Venzor, J., 3rd; Barrios, R.; Leong, K.P.; Huston, D.P. Therapeutic efficacy of an anti-il-5 monoclonal antibody delivered into the respiratory tract in a murine model of asthma. J. Allergy Clin. Immunol. 1999, 104, 215–221. [Google Scholar] [CrossRef]
- Anik, S.T.; McRae, G.; Nerenberg, C.; Worden, A.; Foreman, J.; Hwang, J.Y.; Kushinsky, S.; Jones, R.E.; Vickery, B. Nasal absorption of nafarelin acetate, the decapeptide [d-nal(2)6)]lhrh, in rhesus monkeys. I. J. Pharm. Sci. 1984, 73, 684–685. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.; Chau, T.N.; Hoang, D.M.; Chau, N.V.; Khanh, T.H.; Dong, V.C.; et al. Fatal outcome of human influenza a (h5n1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Le, T.Q.; Kurihara, N.; Chida, J.; Cisse, Y.; Yano, M.; Kido, H. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J. Infect. Dis. 2010, 202, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Suliman, H.B.; Ryan, L.K.; Bishop, L.; Folz, R.J. Prevention of influenza-induced lung injury in mice overexpressing extracellular superoxide dismutase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L69–L78. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Youn, H.-N.; Hoang, P.M.; Cho, S.; Kim, K.-E.; Kil, E.-J.; Lee, G.; Cho, M.-J.; Hong, J.; Byun, S.-J.; et al. Preventive Activity against Influenza (H1N1) Virus by Intranasally Delivered RNA-Hydrolyzing Antibody in Respiratory Epithelial Cells of Mice. Viruses 2015, 7, 5133-5144. https://doi.org/10.3390/v7092863
Cho S, Youn H-N, Hoang PM, Cho S, Kim K-E, Kil E-J, Lee G, Cho M-J, Hong J, Byun S-J, et al. Preventive Activity against Influenza (H1N1) Virus by Intranasally Delivered RNA-Hydrolyzing Antibody in Respiratory Epithelial Cells of Mice. Viruses. 2015; 7(9):5133-5144. https://doi.org/10.3390/v7092863
Chicago/Turabian StyleCho, Seungchan, Ha-Na Youn, Phuong Mai Hoang, Sungrae Cho, Kee-Eun Kim, Eui-Joon Kil, Gunsup Lee, Mun-Ju Cho, Juhyun Hong, Sung-June Byun, and et al. 2015. "Preventive Activity against Influenza (H1N1) Virus by Intranasally Delivered RNA-Hydrolyzing Antibody in Respiratory Epithelial Cells of Mice" Viruses 7, no. 9: 5133-5144. https://doi.org/10.3390/v7092863
APA StyleCho, S., Youn, H.-N., Hoang, P. M., Cho, S., Kim, K.-E., Kil, E.-J., Lee, G., Cho, M.-J., Hong, J., Byun, S.-J., Song, C.-S., & Lee, S. (2015). Preventive Activity against Influenza (H1N1) Virus by Intranasally Delivered RNA-Hydrolyzing Antibody in Respiratory Epithelial Cells of Mice. Viruses, 7(9), 5133-5144. https://doi.org/10.3390/v7092863





