Glucose-6-Phosphate Dehydrogenase Enhances Antiviral Response through Downregulation of NADPH Sensor HSCARG and Upregulation of NF-κB Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. G6PD Activity
2.4. Virus Preparation and Plaque Assay
2.5. Quantitative-PCR
2.6. Preparation of Cell Extracts and Western Blot Analysis
2.7. Plasmid Construction
2.8. Transfection of Plasmids or siRNAs
2.9. Electrophoretic Mobility Shift Assay
2.10. Luciferase Assay
2.11. Determination of the NADPH/NADP+ Ratio
2.12. Statistical Analysis
3. Results
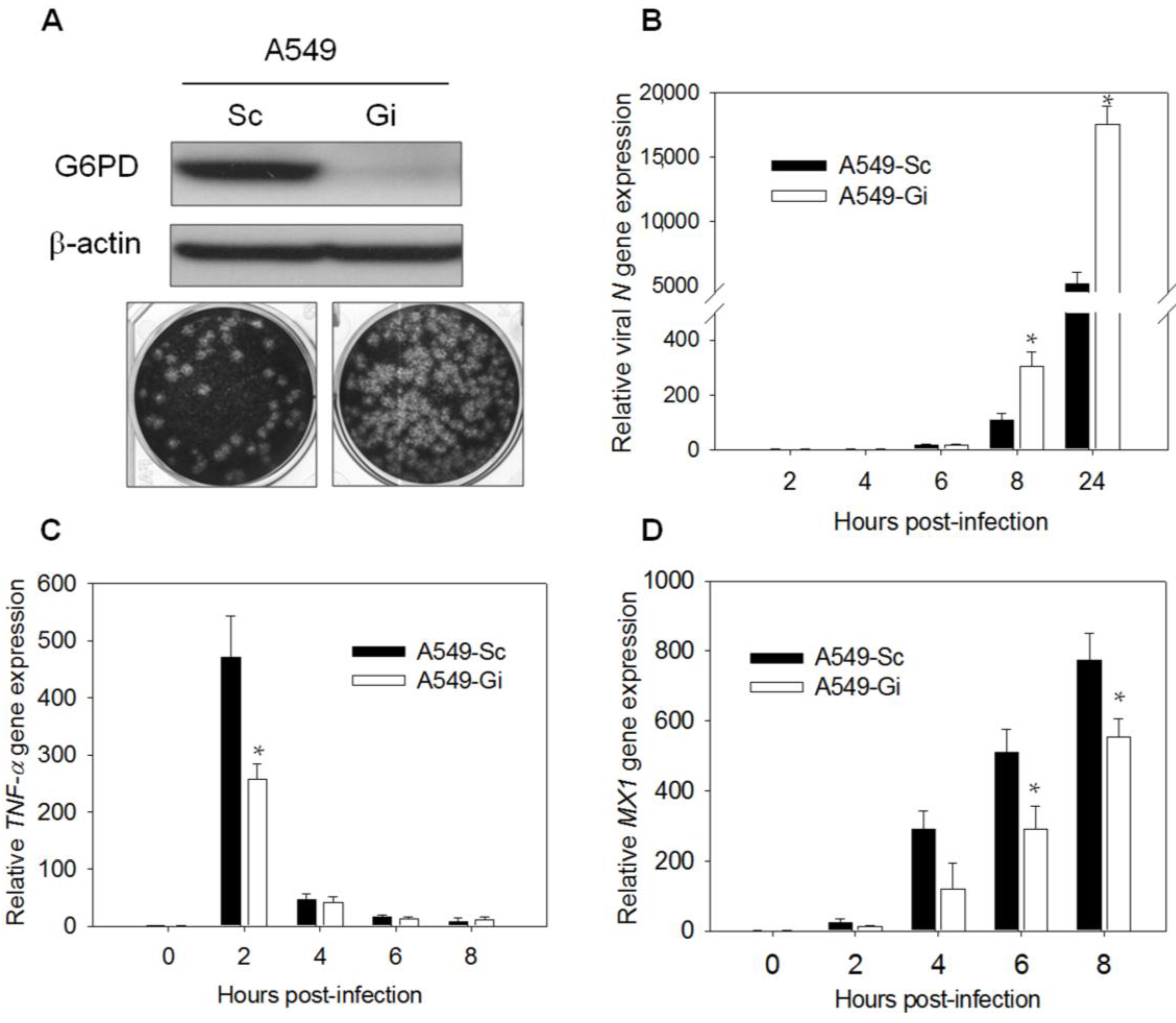
3.1. G6PD Deficiency Impairs the Expression of the Antiviral Genes, TNF-α and MX1, upon HCoV-229E or EV71 Infection
3.2. TNF-α Knockdown Enhances Viral Replication in A549 Cells

| Cell Type | Gene | Fold Increase | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | 8 h | 10 h | 24 h | ||
| A549 | HCoV 229E N gene | N.D. | 1 | 5.08 ± 1.30 | 20.92 ± 6.45 | 102.73 ± 15.24 | 946.55 ± 83.16 | 5566.24 ± 312.68 |
| TNF-α | 1 | 432.58 ± 23.35 | 71.32 ± 0.41 | 27.05 ± 0.89 | 8.39 ± 1.29 | 7.71 ± 0.75 | 236.52 ± 33.27 | |
| IFN-α | 1 | 0.89 ± 0.11 | 1.76 ± 0.83 | 1.29 ± 0.43 | 2.30 ± 1.14 | 1.90 ± 0.87 | 5.27 ± 1.73 | |
| IFN-β | 1 | 1.31 ± 0.09 | 1.17 ± 0.15 | 1.43 ± 0.27 | 0.86 ± 0.29 | 1.81 ±0.35 | 504.07 ± 42.68 | |
| OAS | 1 | 1.77 ± 0.32 | 5.49 ± 0.22 | 9.73 ± 0.42 | 11.64 ± 2.53 | 15.16 ± 2.15 | 27.1 ± 1.25 | |
| PKR | 1 | 8.99 ± 2.94 | 7.45 ± 1.48 | 7.96 ± 1.51 | 8.08 ± 1.46 | 7.47 ± 1.54 | 8.72 ± 1.97 | |
| MX1 | 1 | 123.69 ± 1.09 | 522.69 ± 50.95 | 736.82 ± 194.12 | 1390.95 ± 65.63 | 2381.91 ± 205.05 | 3709.11 ± 172.71 | |
| MRC-5 | HCoV 229E N gene | N.D. | 1 | 1.90 ± 0.12 | 6.96 ± 1.41 | 77.96 ± 14.32 | 311.13 ± 30.21 | 1145.40 ± 104.50 |
| TNF-α | 1 | 1441.36 ± 343.85 | 975.38 ± 63.78 | 303.71 ± 60.77 | 137.94 ± 24.09 | 35.49 ± 2.29 | 7955.68 ± 664.50 | |
| IFN-α | 1 | 0.78 ± 0.20 | 0.93 ± 0.32 | 1.39 ± 0.54 | 0.79 ± 0.52 | 1.63 ± 0.24 | 0.88 ± 0.43 | |
| IFN-β | 1 | 0.92 ± 0.02 | 0.89 ± 0.12 | 0.98 ± 0.29 | 0.84 ± 0.05 | 1.23 ± 0.92 | 1194.91 ± 36.47 | |
| OAS | 1 | 11.27 ± 1.33 | 93.33 ± 0.41 | 289.97 ± 27.38 | 521.57 ± 170.79 | 997.31 ± 182.12 | 4775.85 ± 620.45 | |
| PKR | 1 | 4.10 ± 1.84 | 4.18 ± 1.69 | 5.16 ± 2.13 | 4.33 ± 0.24 | 5.00 ± 1.45 | 5.07 ± 0.51 | |
| MX1 | 1 | 121.73 ± 44.20 | 512.70 ± 4.42 | 1345.64 ±105.59 | 1774.51 ± 185.55 | 1966.26 ± 333.56 | 6117.88 ± 674.95 | |
3.3. Transcription of the TNF-α and MX1 Genes Is Reduced in G6PD-Knockdown Cells Upon Virus Infection

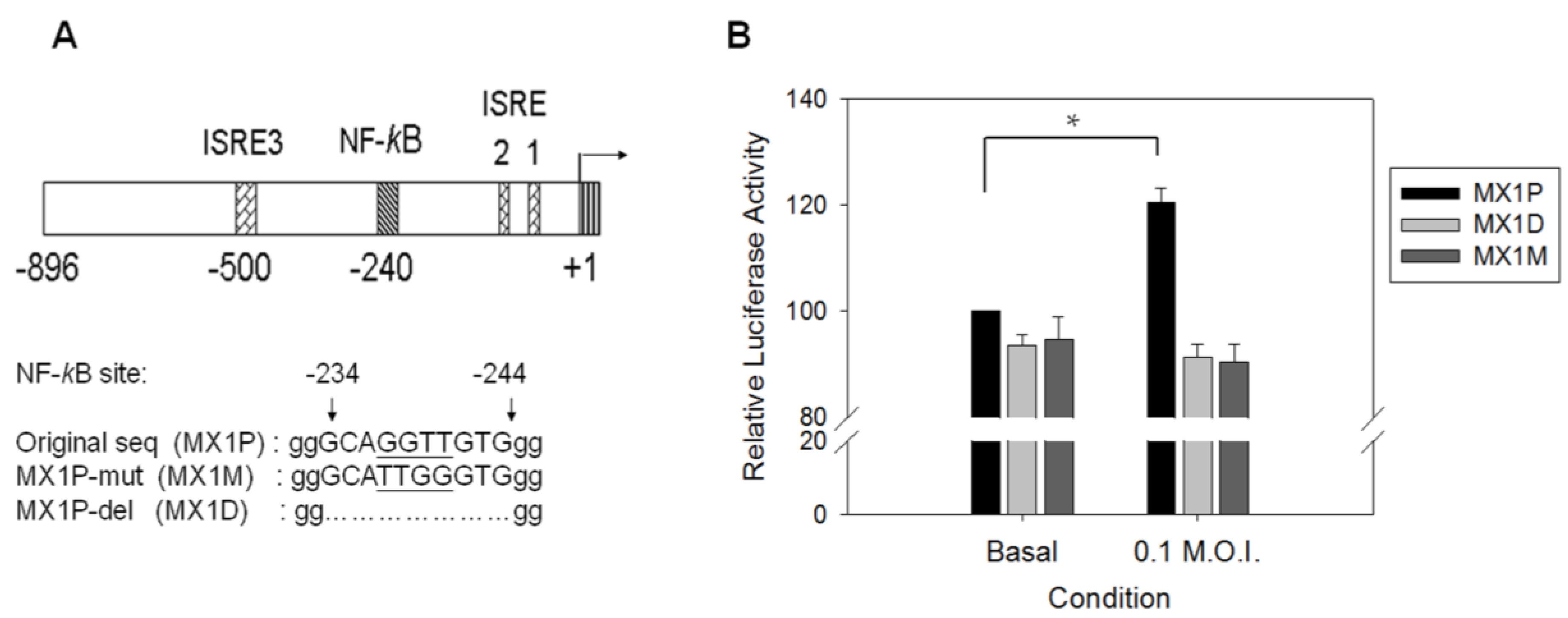
3.4. Binding Activity of NF-κB Is Diminished in Virus-Infected G6PD-Knockdown Cells
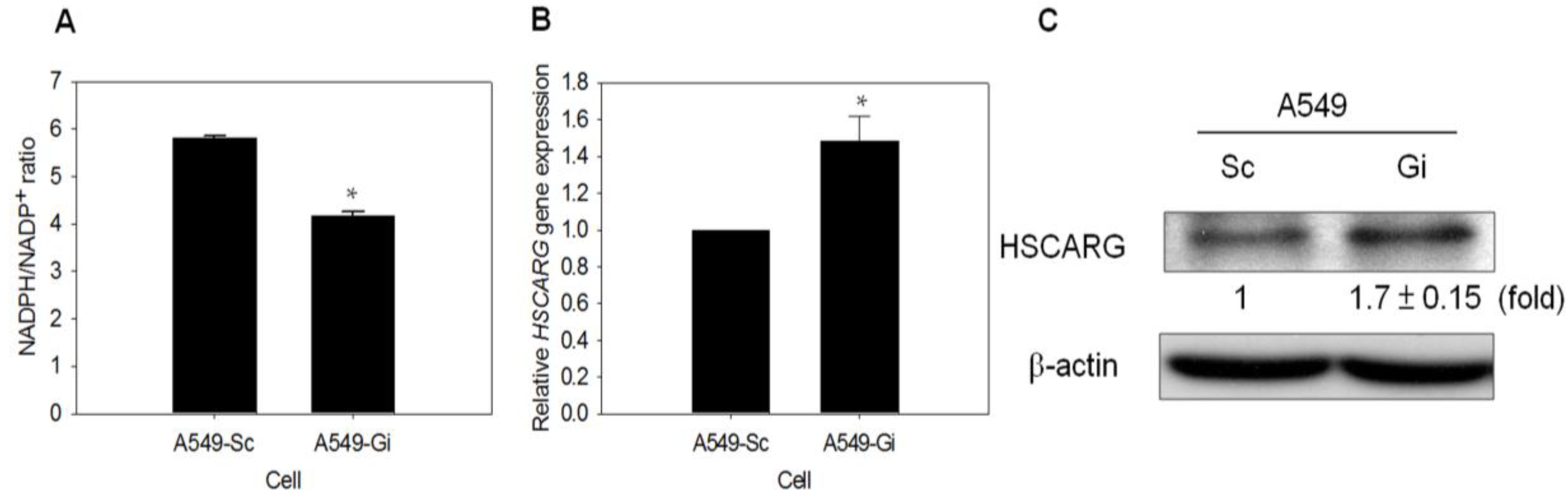
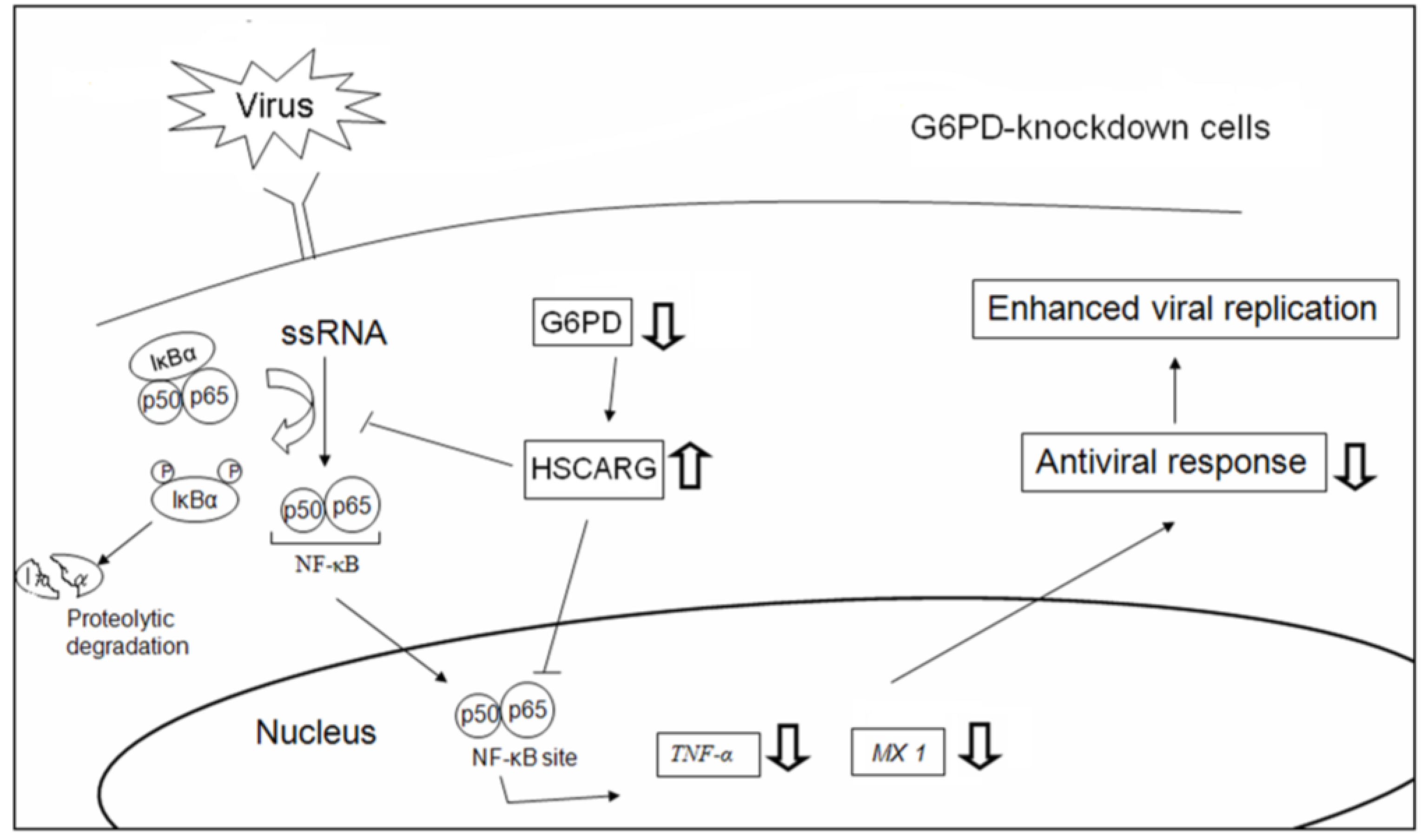
3.5. HSCARG Expression Is Enhanced in G6PD-Knockdown Cells
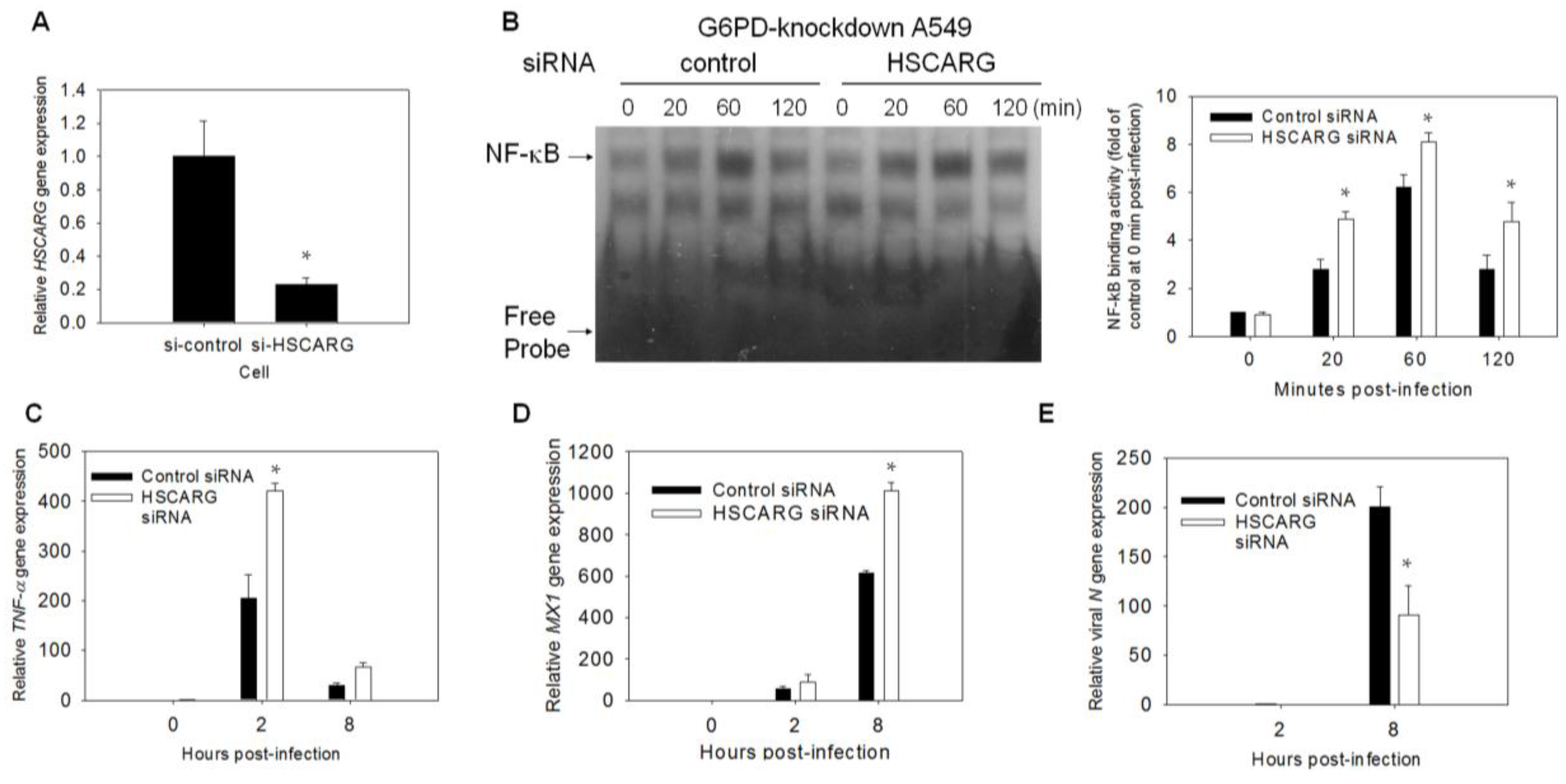
3.6. Enhancement of the Antiviral Response and Inhibition of Viral Gene Expression Are Observed in HSCARG-Knockdown Cells
3.7. Exogenous G6PD or IDH1 Expression Restores Antiviral Gene Expression and Inhibits Viral Replication

4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ho, H.Y.; Cheng, M.L.; Chiu, D.T. Glucose-6-phosphate dehydrogenase—Beyond the realm of red cell biology. Free Radic. Res. 2014, 48, 1028–1048. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Cheng, M.L.; Chiu, D.T. G6PD—An old bottle with new wine. Chang Gung Med. J. 2005, 28, 606–612. [Google Scholar] [PubMed]
- Cheng, M.L.; Ho, H.Y.; Liang, C.M.; Chou, Y.H.; Stern, A.; Lu, F.J.; Chiu, D.T. Cellular glucose-6-phosphate dehydrogenase (G6PD) status modulates the effects of nitric oxide (NO) on human foreskin fibroblasts. FEBS Lett. 2000, 475, 257–262. [Google Scholar] [CrossRef]
- Gao, L.P.; Cheng, M.L.; Chou, H.J.; Yang, Y.H.; Ho, H.Y.; Chiu, D.T. Ineffective GSH regeneration enhances G6PD-knockdown Hep G2 cell sensitivity to diamide-induced oxidative damage. Free Radic. Biol. Med. 2009, 47, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Cheng, M.L.; Lu, F.J.; Chou, Y.H.; Stern, A.; Liang, C.M.; Chiu, D.T. Enhanced oxidative stress and accelerated cellular senescence in glucose-6-phosphate dehydrogenase (G6PD)-deficient human fibroblasts. Free Radic. Biol. Med. 2000, 29, 156–169. [Google Scholar] [CrossRef]
- Lin, C.J.; Ho, H.Y.; Cheng, M.L.; You, T.H.; Yu, J.S.; Chiu, D.T. Impaired dephosphorylation renders G6PD-knockdown HepG2 cells more susceptible to H2O2-induced apoptosis. Free Radic. Biol. Med. 2010, 49, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Arese, P.; de Flora, A. Pathophysiology of hemolysis in glucose-6-phosphate dehydrogenase deficiency. Semin. Hematol. 1990, 27, 1–40. [Google Scholar] [PubMed]
- Sirdah, M.; Reading, N.S.; Perkins, S.L.; Shubair, M.; Aboud, L.; Prchal, J.T. Hemolysis and Mediterranean G6PD mutation (c.563 C>T) and c.1311 C>T polymorphism among Palestinians at Gaza Strip. Blood Cells Mol. Dis. 2012, 48, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, R.S.; Estwick, D.; Peddi, R. G6PD deficiency: Its role in the high prevalence of hypertension and diabetes mellitus. Ethn. Dis. 2001, 11, 749–754. [Google Scholar] [PubMed]
- Meloni, T.; Pacifico, A.; Forteleoni, G.; Meloni, G.F. G6PD deficiency and diabetes mellitus in northern Sardinian subjects. Haematologica 1992, 77, 94–95. [Google Scholar] [PubMed]
- Hecker, P.A.; Leopold, J.A.; Gupte, S.A.; Recchia, F.A.; Stanley, W.C. Impact of glucose-6-phosphate dehydrogenase deficiency on the pathophysiology of cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H491–H500. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.H.; Tsai, S.C.; Chiu, D.T. Decreased blood activity of glucose-6-phosphate dehydrogenase associates with increased risk for diabetes mellitus. Endocrine 2002, 19, 191–195. [Google Scholar] [CrossRef]
- Ho, H.Y.; Cheng, M.L.; Weng, S.F.; Chang, L.; Yeh, T.T.; Shih, S.R.; Chiu, D.T. Glucose-6-phosphate dehydrogenase deficiency enhances Enterovirus 71 infection. J. Gen. Virol. 2008, 89, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Tseng, C.P.; Cheng, M.L.; Ho, H.Y.; Shih, S.R.; Chiu, D.T. Glucose-6-phosphate dehydrogenase deficiency enhances human Coronavirus 229E infection. J. Infect. Dis. 2008, 197, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.C.; Huang, C.S.; Lee, C.N.; Chang, S.Y.; King, C.C.; Kao, C.L. Higher infection of Dengue virus serotype 2 in human monocytes of patients with G6PD deficiency. PLoS ONE 2008, 3, e1557. [Google Scholar] [CrossRef] [PubMed]
- Goodbourn, S.; Didcock, L.; Randall, R.E. Interferons: Cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000, 81, 2341–2364. [Google Scholar] [CrossRef] [PubMed]
- McFadden, G.; Mohamed, M.R.; Rahman, M.M.; Bartee, E. Cytokine determinants of viral tropism. Nat. Rev. Immunol. 2009, 9, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, S.; Siren, J.; Tissari, J.; Veckman, V.; Pirhonen, J.; Severa, M.; Sun, Q.; Lin, R.; Meri, S.; Uze, G.; et al. Tumor necrosis factor α enhances influenza A virus-induced expression of antiviral cytokines by activating RIG-I gene expression. J. Virol. 2006, 80, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Bartee, E.; Mohamed, M.R.; McFadden, G. Tumor necrosis factor and interferon: Cytokines in harmony. Curr. Opin. Microbiol. 2008, 11, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Sadler, A.J.; Williams, B.R. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Justesen, J.; Hartmann, R.; Kjeldgaard, N.O. Gene structure and function of the 2′-5′-oligoadenylate synthetase family. Cell Mol. Life Sci. 2000, 57, 1593–1612. [Google Scholar] [CrossRef] [PubMed]
- Malathi, K.; Paranjape, J.M.; Bulanova, E.; Shim, M.; Guenther-Johnson, J.M.; Faber, P.W.; Eling, T.E.; Williams, B.R.; Silverman, R.H. A transcriptional signaling pathway in the IFN system mediated by 2′-5′-oligoadenylate activation of RNAse L. Proc. Natl. Acad. Sci. USA 2005, 102, 14533–14538. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, J.; Parthoens, E.; Schepens, B.; Fiers, W.; Saelens, X. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J. Virol. 2012, 86, 13445–13455. [Google Scholar] [CrossRef] [PubMed]
- Elde, N.C.; Child, S.J.; Eickbush, M.T.; Kitzman, J.O.; Rogers, K.S.; Shendure, J.; Geballe, A.P.; Malik, H.S. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell 2012, 150, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Birdwell, L.D.; Wu, A.; Elliott, R.; Rose, K.M.; Phillips, J.M.; Li, Y.; Grinspan, J.; Silverman, R.H.; Weiss, S.R. Cell-type-specific activation of the oligoadenylate synthetase-RNAse l pathway by a murine coronavirus. J. Virol. 2013, 87, 8408–8418. [Google Scholar] [CrossRef] [PubMed]
- Zorzitto, J.; Galligan, C.L.; Ueng, J.J.; Fish, E.N. Characterization of the antiviral effects of interferon-α against a SARS-like coronoavirus infection in vitro. Cell Res. 2006, 16, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.F.; Tan, H.C.; Ooi, E.E.; Koh, D.R.; Chow, V.T. Microarray and real-time RT-PCR analyses of differential human gene expression patterns induced by severe acute respiratory syndrome (SARS) coronavirus infection of Vero cells. Microbes Infect. 2005, 7, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Ida-Hosonuma, M.; Iwasaki, T.; Yoshikawa, T.; Nagata, N.; Sato, Y.; Sata, T.; Yoneyama, M.; Fujita, T.; Taya, C.; Yonekawa, H.; et al. The α/β interferon response controls tissue tropism and pathogenicity of poliovirus. J. Virol. 2005, 79, 4460–4469. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Solomon, D.H. Tumor necrosis factor blockade and the risk of viral infection. Nat. Rev. Rheumatol. 2010, 6, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Groeger, G.; Quiney, C.; Cotter, T.G. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid. Redox Signal. 2009, 11, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Rada, B.; Leto, T.L. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol. 2008, 15, 164–187. [Google Scholar] [PubMed]
- Grandvaux, N.; Soucy-Faulkner, A.; Fink, K. Innate host defense: Nox and Duox on phox’s tail. Biochimie 2007, 89, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Dolowschiak, T.; Chassin, C.; Ben Mkaddem, S.; Fuchs, T.M.; Weiss, S.; Vandewalle, A.; Hornef, M.W. Potentiation of epithelial innate host responses by intercellular communication. PLoS Pathog. 2010, 6, e1001194. [Google Scholar] [CrossRef] [PubMed]
- Soucy-Faulkner, A.; Mukawera, E.; Fink, K.; Martel, A.; Jouan, L.; Nzengue, Y.; Lamarre, D.; Vande Velde, C.; Grandvaux, N. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010, 6, e1000930. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Li, Y.; Meng, G.; Yao, S.; Zhao, Y.; Yu, Q.; Zhang, J.; Luo, M.; Zheng, X. NADPH is an allosteric regulator of HSCARG. J. Mol. Biol. 2009, 387, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Dai, X.; Zhao, Y.; Chen, Q.; Lu, F.; Yao, D.; Yu, Q.; Liu, X.; Zhang, C.; Gu, X.; et al. Restructuring of the dinucleotide-binding fold in an NADP(H) sensor protein. Proc Natl Acad Sci USA 2007, 104, 8809–8814. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Zheng, X. HSCARG regulates NF-κB activation by promoting the ubiquitination of RelA or COMMD1. J. Biol. Chem. 2009, 284, 17998–18006. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Li, T.; Hu, B.; Lian, M.; Zheng, X. HSCARG inhibits activation of NF-κB by interacting with IκB kinase-β. J. Cell Sci. 2009, 122 Pt 22, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, B.; Li, T.; Peng, Y.; Guan, J.; Lai, S.; Zheng, X. A CRM1-dependent nuclear export signal controls nucleocytoplasmic translocation of HSCARG, which regulates NF-κB activity. Traffic 2012, 13, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xu, R.; Zheng, X. HSCARG negatively regulates the cellular antiviral RIG-I like receptor signaling pathway by inhibiting TRAF3 ubiquitination via recruiting OTUB1. PLoS Pathog. 2014, 10, e1004041. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.R.; Wu, C.C.; Wu, Y.H.; Hsu, C.W.; Cheng, M.L.; Chiu, D.T. Proteome-wide dysregulation by glucose-6-phosphate dehydrogenase (G6PD) reveals a novel protective role for G6PD in aflatoxin B(1)-mediated cytotoxicity. J. Proteome Res. 2013, 12, 3434–3448. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Cheng, M.L.; Shiao, M.S.; Chiu, D.T. Characterization of global metabolic responses of glucose-6-phosphate dehydrogenase-deficient hepatoma cells to diamide-induced oxidative stress. Free Radic. Biol. Med. 2013, 54, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.K.; Jang, Y.P.; Kim, Y.C.; Kim, S.G. Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits MAP kinases and AP-1 activation via potent MKK inhibition: The role in TNF-α inhibition. Int. Immunopharmacol. 2004, 4, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Jorns, C.; Stertz, S.; Boisson-Dupuis, S.; Thimme, R.; Weidmann, M.; Casanova, J.L.; Haller, O.; Kochs, G. Induction of MxA gene expression by influenza A virus requires type I or type III interferon signaling. J. Virol. 2007, 81, 7776–7785. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Cheng, M.L.; Chiu, D.T. Glucose-6-phosphate dehydrogenase—From oxidative stress to cellular functions and degenerative diseases. Redox Rep. 2007, 12, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Tisoncik, J.R.; Billharz, R.; Burmakina, S.; Belisle, S.E.; Proll, S.C.; Korth, M.J.; Garcia-Sastre, A.; Katze, M.G. The NS1 protein of influenza A virus suppresses interferon-regulated activation of antigen-presentation and immune-proteasome pathways. J. Gen. Virol. 2011, 92, 2466. [Google Scholar] [CrossRef]
- Sharma, K.; Tripathi, S.; Ranjan, P.; Kumar, P.; Garten, R.; Deyde, V.; Katz, J.M.; Cox, N.J.; Lal, R.B.; Sambhara, S.; et al. Influenza A virus nucleoprotein exploits Hsp40 to inhibit PKR activation. PLoS ONE 2011, 6, e20215. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, G.; Zheng, D.; Cheng, G.; Tang, H. PLP2 of mouse hepatitis virus A59 (MHV-A59) targets TBK1 to negatively regulate cellular type I interferon signaling pathway. PLoS ONE 2011, 6, e17192. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Pan, J.; Tao, J.; Guo, D. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes 2011, 42, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, S.; Owens, A.P., 3rd; Baunacke, M.; Williams, J.C.; Lee, R.D.; Weithauser, A.; Sheridan, P.A.; Malz, R.; Luyendyk, J.P.; Esserman, D.A.; et al. PAR-1 contributes to the innate immune response during viral infection. J. Clin. Investig. 2013, 123, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Bendelja, K.; Vojvoda, V.; Aberle, N.; Cepin-Bogovic, J.; Gagro, A.; Mlinaric-Galinovic, G.; Rabatic, S. Decreased Toll-like receptor 8 expression and lower TNF-α synthesis in infants with acute RSV infection. Respir. Res. 2010, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, J.; Grandvaux, N.; Sharma, S.; Tenoever, B.R.; Servant, M.J.; Lin, R. Convergence of the NF-κB and interferon signaling pathways in the regulation of antiviral defense and apoptosis. Ann. N. Y. Acad. Sci. 2003, 1010, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, J. Convergence of the NF-κB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007, 18, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Akira, S. TLR signaling. Curr. Top. Microbiol. Immunol. 2006, 311, 1–16. [Google Scholar] [PubMed]
- Santoro, M.G.; Rossi, A.; Amici, C. NF-κB and virus infection: Who controls whom. EMBO J. 2003, 22, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Siomek, A. NF-κB signaling pathway and free radical impact. Acta Biochim. Pol. 2012, 59, 323–331. [Google Scholar] [PubMed]
- Lamb, H.K.; Stammers, D.K.; Hawkins, A.R. Dinucleotide-sensing proteins: Linking signaling networks and regulating transcription. Sci. Signal. 2008, 1, pe38. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.; Biswas, B.; Mallick, D.; Ghosh, A. Acute pancreatitis—Complicating hepatitis E virus infection in a 7-year-old boy with glucose 6 phosphate dehydrogenase deficiency. Clin. Pediatr. 2009, 48, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Ngai, C.W.; Chan, W.M.; Leung, R.Y.; Chan, S.C. Hemolysis and methemoglobinemia due to hepatitis E virus infection in patient with G6PD deficiency. Ann. Hematol. 2011, 90, 1237–1238. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, A.; Jablonska-Skwiecinska, E.; Wojda, E.; Bielen, P.; Ptak, J.; Sliwinski, P.; Gorecka, D. Community-acquired pneumonia complications in a patient with hereditary glucose-6-phosphate dehydrogenase deficiency. Pneumonol. Alergol. Pol. 2007, 75, 283–288. [Google Scholar] [PubMed]
- Douzinas, E.E.; Flevari, K.; Andrianakis, I.; Betrosian, A.P. Oral atovaquone for the treatment of severe Pneumocystis jirovecii pneumonia in a patient with glucose-6-phosphate dehydrogenase deficiency. Scand. J. Infect. Dis. 2010, 42, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; Brunialti, M.K.; Rigato, O.; Machado, F.R.; Silva, E.; Salomao, R. Generation of nitric oxide and reactive oxygen species by neutrophils and monocytes from septic patients and association with outcomes. Shock 2012, 38, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Ho, H.Y.; Wu, P.R.; Chen, S.H.; Kuypers, F.A.; Cheng, M.L.; Chiu, D.T. Inability to maintain GSH pool in G6PD-deficient red cells causes futile AMPK activation and irreversible metabolic disturbance. Antioxid. Redox Signal. 2015, 22, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Cheng, M.L.; Ho, H.Y.; Chiu, D.T. The microbicidal and cytoregulatory roles of NADPH oxidases. Microbes Infect. Inst. Pasteur 2011, 13, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Serpillon, S.; Floyd, B.C.; Gupte, R.S.; George, S.; Kozicky, M.; Neito, V.; Recchia, F.; Stanley, W.; Wolin, M.S.; Gupte, S.A. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H153–H162. [Google Scholar] [CrossRef] [PubMed]
- Gupte, R.S.; Floyd, B.C.; Kozicky, M.; George, S.; Ungvari, Z.I.; Neito, V.; Wolin, M.S.; Gupte, S.A. Synergistic activation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase by Src kinase elevates superoxide in type 2 diabetic, Zucker fa/fa, rat liver. Free Radic. Biol. Med. 2009, 47, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Gupte, S.A. Glucose-6-phosphate dehydrogenase: A novel therapeutic target in cardiovascular diseases. Curr. Opin. Investig. Drugs 2008, 9, 993–1000. [Google Scholar] [PubMed]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.J.; Hung, I.J.; Chow, C.K.; Stern, A.; Chao, S.S.; Chiu, D.T. Impaired production of nitric oxide, superoxide, and hydrogen peroxide in glucose 6-phosphate-dehydrogenase-deficient granulocytes. FEBS Lett. 1998, 436, 411–414. [Google Scholar] [CrossRef]
- Cheng, M.L.; Ho, H.Y.; Lin, H.Y.; Lai, Y.C.; Chiu, D.T. Effective NET formation in neutrophils from individuals with G6PD Taiwan-Hakka is associated with enhanced NADP+ biosynthesis. Free Radic. Res. 2013, 47, 699–709. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-H.; Chiu, D.T.-Y.; Lin, H.-R.; Tang, H.-Y.; Cheng, M.-L.; Ho, H.-Y. Glucose-6-Phosphate Dehydrogenase Enhances Antiviral Response through Downregulation of NADPH Sensor HSCARG and Upregulation of NF-κB Signaling. Viruses 2015, 7, 6689-6706. https://doi.org/10.3390/v7122966
Wu Y-H, Chiu DT-Y, Lin H-R, Tang H-Y, Cheng M-L, Ho H-Y. Glucose-6-Phosphate Dehydrogenase Enhances Antiviral Response through Downregulation of NADPH Sensor HSCARG and Upregulation of NF-κB Signaling. Viruses. 2015; 7(12):6689-6706. https://doi.org/10.3390/v7122966
Chicago/Turabian StyleWu, Yi-Hsuan, Daniel Tsun-Yee Chiu, Hsin-Ru Lin, Hsiang-Yu Tang, Mei-Ling Cheng, and Hung-Yao Ho. 2015. "Glucose-6-Phosphate Dehydrogenase Enhances Antiviral Response through Downregulation of NADPH Sensor HSCARG and Upregulation of NF-κB Signaling" Viruses 7, no. 12: 6689-6706. https://doi.org/10.3390/v7122966
APA StyleWu, Y.-H., Chiu, D. T.-Y., Lin, H.-R., Tang, H.-Y., Cheng, M.-L., & Ho, H.-Y. (2015). Glucose-6-Phosphate Dehydrogenase Enhances Antiviral Response through Downregulation of NADPH Sensor HSCARG and Upregulation of NF-κB Signaling. Viruses, 7(12), 6689-6706. https://doi.org/10.3390/v7122966








