Abstract
A renewed interest in mammalian orthoreoviruses (MRVs) has emerged since new viruses related to bat MRV type 3, detected in Europe, were identified in humans and pigs with gastroenteritis. This study reports the isolation and characterization of a novel reassortant MRV from the lesser horseshoe bat (Rhinolophus hipposideros). The isolate, here designated BatMRV1-IT2011, was first identified by electron microscopy and confirmed using PCR and virus-neutralization tests. The full genome sequence was obtained by next-generation sequencing. Molecular and antigenic characterizations revealed that BatMRV1-IT2011 belonged to serotype 1, which had not previously been identified in bats. Phylogenetic and recombination detection program analyses suggested that BatMRV1-IT2011 was a reassortant strain containing an S1 genome segment similar to those of MRV T1/bovine/Maryland/Clone23/59 and C/bovine/Indiana/MRV00304/2014, while other segments were more similar to MRVs of different hosts, origins and serotypes. The presence of neutralizing antibodies against MRVs has also been investigated in animals (dogs, pigs, bovines and horses). Preliminary results suggested that MRVs are widespread in animals and that infections containing multiple serotypes, including MRVs of serotype 1 with an S1 gene similar to BatMRV1-IT2011, are common. This paper extends the current knowledge of MRVs and stresses the importance to continue and improve MRV surveillance in bats and other mammals through the development and standardization of specific diagnostic tools.
1. Introduction
Orthoreoviruses, with mammalian orthoreovirus (MRV) as the type species, are non-enveloped viruses having a segmented dsRNA genome. MRVs have a wide geographic distribution, can infect virtually all mammals, including humans, and are responsible for symptomatic or asymptomatic infections [1]. Three MRV serotypes have been recognized based on the capacity of anti-MRV sera to neutralize viral infectivity and inhibit hemagglutination [2].
Members of the genus Orthoreovirus contain 10 genome segments, which are designed as large (L, three segments), medium (M, three segments) or small (S, four segments) based on their electrophoretic mobility [3].
Neutralization and hemagglutinin activities are restricted to a single reovirus gene segment, S1 [4]. This encodes the σ1 protein, located on the outer capsid of the virion, that is responsible for viral attachment to cellular receptors and determines the reovirus serotype [5]. An analysis of the S1 genes of MRVs has shown a strict correlation between sequence similarities and viral serotypes [3,6,7]. Conversely, the other genome segments show no correlation to viral serotype, suggesting that MRV reoviruses have evolved independently of serotypes [8].
A renewed interest in Orthoreovirus has occurred for several reasons, mainly, but not only, related to public health concerns. In the last few years, MRVs have often been determined to be responsible for severe human illnesses, including hemorrhagic enteritis, acute respiratory infections and encephalitis [9,10,11,12,13,14,15]. The zoonotic potential of reoviruses has already been described and discussed elsewhere [10,11]. Novel MRVs have recently been identified in several hosts, including bats in Italy and Germany [16,17], and a novel Orthoreovirus, with a high similarity to the MRVs found in bats in Europe, was detected in Slovenia from a child requiring hospitalization due to acute gastroenteritis [15]. Recently, a virulent MRV containing an S1 segment that was strongly homologous to bat MRV3 was detected from diarrheic pigs in the United States [18]. In addition, a bat-borne fusogenic Orthoreovirus with zoonotic potential was detected from healthy flying foxes (Pteropus vampyrus) legally imported from Indonesia to Europe [19]. Moreover, the segmented nature of Orthoreovirus genomes poses risks for the potential formation of novel reassortant viruses with unpredictable biological properties. Finally, the scientific community’s awareness of the importance of reovirological studies has increased because MRVs are being evaluated as oncolytic agents in experimental cancer therapies [20].
Bats, as the most abundant, assorted and geographically-disperse vertebrates, are increasingly known as reservoirs of viruses that can cross species barriers to infect humans and other animals [21]. In a previous study, we showed that MRVs are quite frequently detected in bats and appear genetically more differentiated in comparison with the orthoreoviruses found in other animal species [22]. However, to date, the unique MRVs identified in bats belong to serotypes 2 (Asia) and 3 (Europe) [16,17,23].
This study reports the first isolation and characterization of a new reassortant MRV strain, BatMRV1-IT2011, belonging to serotype 1 from the lesser horseshoe bat (Rhinolophus hipposideros) in Italy.
2. Results
2.1. Sampling
In total, 15 fecal samples were collected from 2009–2015 from the reproductive colony of R. hipposideros. During the sampling activities, no relevant mortality or clinical signs indicative of infectious diseases of bats were recorded, and a normal reproductive success rate (number and mortality rate of juveniles until fledging) was observed.
2.2. Virological Tests
BatMRV1-IT2011 was isolated by cell culture (Fetal monkey kidney MARC-145) from Sample 191,797 collected in August 2011. The isolate caused a clear cytopathic effects (CPE) at 4–5 days post-inoculation with granulating inclusions, shrinking and falling off. nsEM performed on the supernatant of the infected cell culture revealed a non-enveloped icosahedral virus ~75 nm in diameter, which was morphologically related to the reoviruses (Figure 1). Virus identification was first confirmed by RT-PCR specific for the MRV L1 gene.
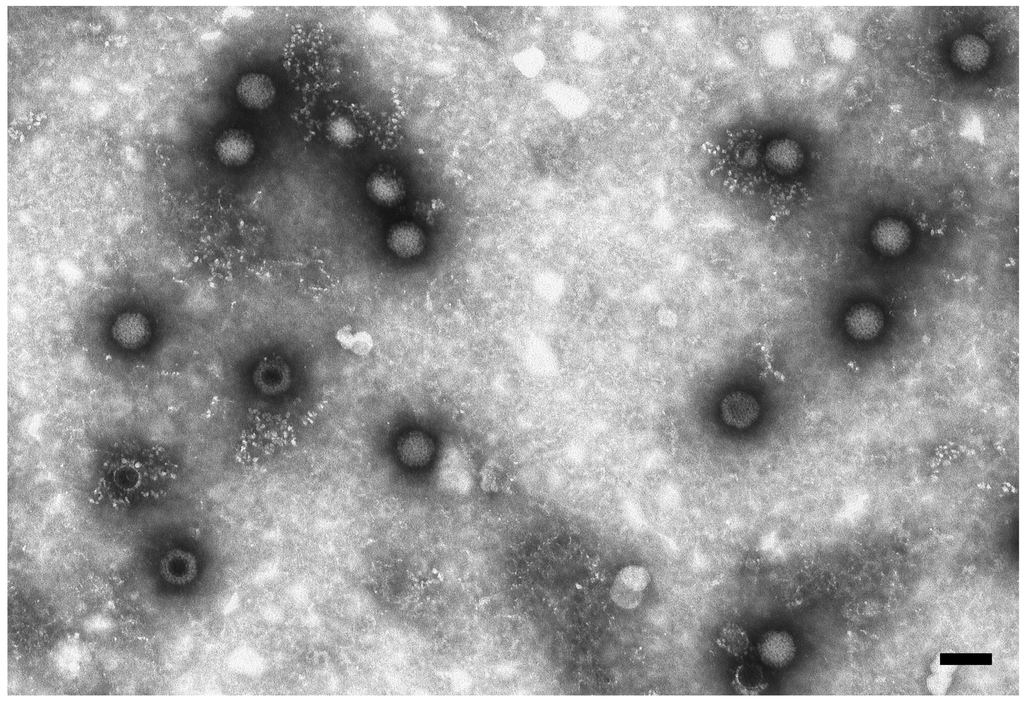
Figure 1.
Electron micrograph of reovirus particles in the supernatant of VERO cells. Negative staining (2% sodium phosphotungstate). TEM FEI Tecnai G2 Spirit, 85 kV. Bar = 100 nm.
2.3. Genome Characterization of BatMRV1-IT2011
The full genome sequence of BatMRV1-IT2011 was determined starting from the cell culture supernatants with CPEs, as previously described [15]. The nucleotide sequences of all 10 genome segments L1-L3, M1-M3 and S1-S4 were deposited in GenBank under Accession Nos. KT900695–KT900704.
A molecular analysis revealed that BatMRV1-IT2011 was a novel serotype 1 MRV, with an S1 segment similar to the bovine MRV T1/bovine/Maryland/Clone23/59 and C/bovine/Indiana/MRV00304/2014, but the other segments were more similar to MRVs of different hosts, origins and serotypes. The results of an analysis using the BLAST algorithm showing the highest similarities for each genome segment are reported in Table 1.
The results showed that BatMRV1-IT2011 contains MRV genes that are highly similar to those in viruses detected in bats, humans, cows, civets and pigs, with no date or geographical correlation. Most of the MRVs related to BaMRV1-IT2011 are associated with enteric/respiratory diseases and encephalitis in animals and humans.
The phylogenetic trees, generated by the neighbor-joining method, for each genome segment are reported in Figure 2.
A phylogenetic analysis revealed discrepancies in the clustering of the 10 genes between BatMRV1-IT2011 and the other MRV strains included in the analysis (Figure 2). In particular, BatMRV1-IT2011 clustered with MRVTou05 based on the M3 and S2 segments, but not for the S1 genomic region, where it was more similar to T1/bovine/Maryland/Clone23/59 and C/bovine/Indiana/MRV00304/2014. This reflects a pattern of topological incongruence caused by reassortment. Unfortunately, the whole genome of T1/bovine/Maryland/Clone23/59 was not available, and for this reason, the C/bovine/Indiana/MRV00304/2014 strain was included in the Recombination Detection Program RDP analysis, even though it was isolated after the putative reassortant strain described here.
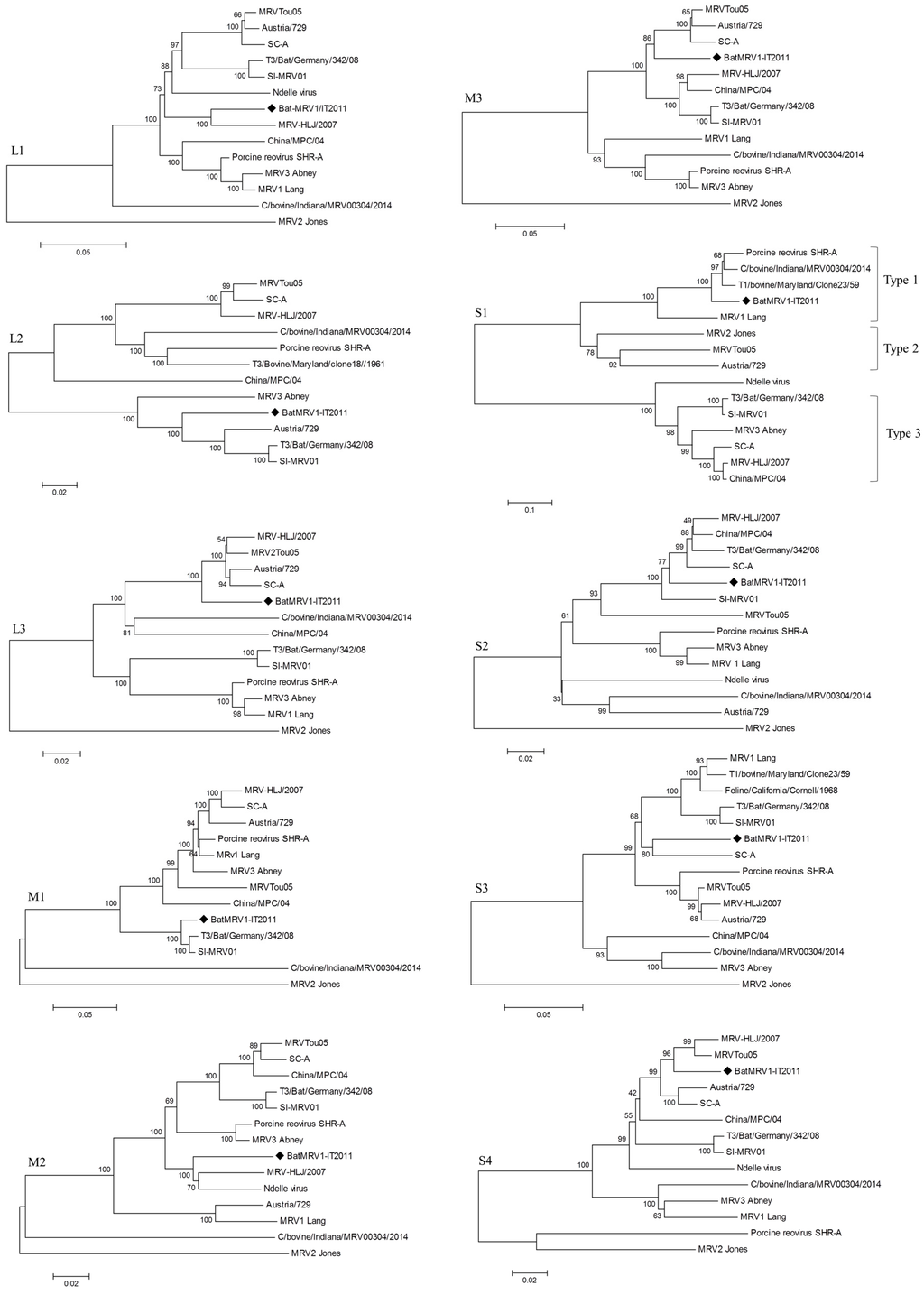
Figure 2.
Phylogenetic trees of the BatMRV1-IT2011 strain’s genome segments (♦) and the closest related whole-genome MRV strains from GenBank.

Table 1.
Highest nucleotide identities for each gene segment of the novel BatMRV1-IT2011. MRV, mammalian orthoreovirus.
| BatMRV1-IT2011 | Similarity (%) | MRV Strain | Serotype | Host | Disease | Country | GenBank Accession No. | Encoding Protein and Function |
|---|---|---|---|---|---|---|---|---|
| L1 | 93 | MRV-HLJ/2007 | 3 | Pig | Fever, respiratory illness | China | HQ642769.1 | λ3—RNA-dependent RNA polymerase |
| 90 | Porcine reovirus SHR-A | 1 | Pig | NA | China | JX415466.1 | ||
| L2 | 91 | Austria/729 | 2 | Pig | Encephalitis | Austria | JN799427.1 | λ2—Guanylyltransferase, methyltransferase |
| 90 | T3/bat/Germany/342/08 | 3 | Bat | Hemorrhagic enteritis | Germany | JQ412756.1 | ||
| 90 | SI-MRV01 | 3 | Human | Acute gastroenteritis | Slovenia | KF154725.1 | ||
| L3 | 95 | MRVTou05 | 2 | Human | Encephalitis | France | GU196308.1 | λ1—Helicase, binds dsRNA, NTPase |
| 94 | MRV-HLJ/2007 | 3 | Pig | Fever, respiratory illness | China | HQ642769.1 | ||
| M1 | 98 | T3/bat/Germany/342/08 | 3 | Bat | Hemorrhagic enteritis | Germany | JQ412758.1 | μ2—NTPase |
| 98 | SI-MRV01 | 3 | Human | Acute gastroenteritis | Slovenia | KF154727.1 | ||
| M2 | 92 | MRV-HLJ/2007 | 3 | Pig | Fever, respiratory illness | China | HQ642773.1 | μ1—Cell penetration, apoptosis |
| 92 | 4 Ndelle virus | Putative 4 | Mouse | NA | Cameroon | AF368034.1 | ||
| M3 | 93 | MRVTou05 | 2 | Human | Encephalitis | France | GU196314.1 | μNS—Nucleates viral inclusion bodies |
| 92 | Austria/729 | 2 | Pig | Encephalitis | Austria | JN799425.1 | ||
| S1 | 90 | T1/bovine/Maryland/Clone23/59 | 1 | Bovine | NA | USA | AY862134.1 | σ1, σ1s—Viral attachment |
| 88 | C/bovine/Indiana/MRV00304/2014 | 1 | Bovine | Diarrhea | USA | KJ676385.1 | ||
| S2 | 95 | China/MPC/04 | 3 | Civet | NA | China | GQ468273.1 | σ2—Inner capsid structural protein |
| 94 | T3/bat/Germany/342/08 | 3 | Bat | Hemorrhagic enteritis | Germany | JQ412762.1 | ||
| 94 | SI-MRV01 | 3 | Human | Acute gastroenteritis | Slovenia | KF154731.1 | ||
| 94 | MRV-HLJ/2007 | 3 | Pig | Fever, respiratory illness | China | HQ642776.1 | ||
| S3 | 91 | SC-A | 3 | Pig | Diarrhea | China | DQ411553.1 | σ2—ssRNA-binding |
| 91 | Feline/California/Cornell/1968 | 3 | Cat | NA | USA | U35362 | ||
| S4 | 95 | MRVTou05 | 2 | Human | Encephalitis | France | GU196313.1 | σ3—DS-RNA binding, modulation of cellular interferon response |
| 95 | MRV-HLJ/2007 | 2 | Pig | Fever, respiratory illness | China | HQ642778.1 |
Notes: L, large segments; M, medium segments; S, small segments; NA, not available.
An RDP analysis was performed on three genes (M3, S1, S2) to better describe the reassortant event. In Figure 3, it is shown that BatMRV1-IT2011 was derived from a cross-over event, which probably occurred between strains that had sequences that were highly related to MRVTou05 and C/bovine/Indiana/MRV00304/2014. Indeed, RDP4 detected well-supported breakpoints near the boundaries of the S1 segments in the concatenated genomes, suggesting that the phylogenetic incongruence was significant among the three regions. All of the algorithms indicated that the newly-reassortant strains had arisen by acquiring the S1 genome segment from a strain belonging to serotype 1 in which the S1 gene was closely related to C/bovine/Indiana/MRV00304/2014.
To better investigate the differences in BatMRV1-IT2011, we aligned the deduced amino acid (aa) sequence of the σ1 protein with those from the strains T1/bovine/Maryland/Clone23/59, C/bovine/Indiana/MRV00304/2014, porcine reovirus SHR-A and MRV type 1 Lang. The aa sequence comparison revealed a 90% identity between BatMRV1-IT2011 and the bovine strains with differences of 49 and 44 aa for C/bovine/Indiana/MRV00304/2014 and T1/bovine/Maryland/Clone23/59, respectively (Figure S1). The aa sequence comparison between BatMRV1-IT2011 and porcine reovirus SHR-A and MRV type 1 Lang revealed an 85.6% and 79.0% identity, respectively. Phylogenetic analysis of the deduced aa sequence from BatMRV1-IT2011 is reported (Figure S1).
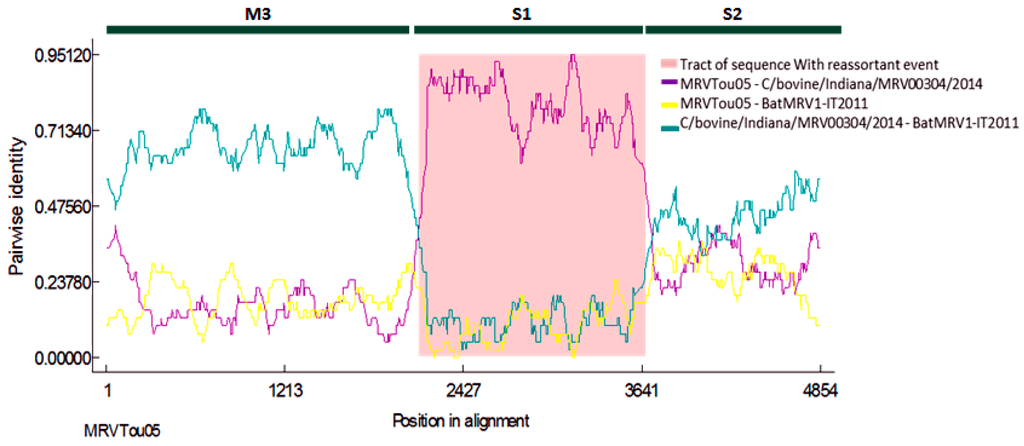
Figure 3.
Similarity plots of BatMRV1-IT2011. M3, S1 and S2 are the three reovirus viral genes on which the RDP analysis was performed. Recombinant: IT2011; major parent: MRVTou05; minor parent: MRV00304-2014. The specific algorithms used were RDP, GENECONV, Chimaera, MaxChi, BootScan and SiScan implemented in the RDP4 software
2.4. VNTs for Virus Typing and Serology
VNTs confirmed that BatMRV1-IT2011 belongs to serotype 1, being strongly neutralized at a high titer (1/1280) from a guinea pig immune serum produced against the MRV T1L and no or low neutralization by anti-T2J and anti-T3A immune sera, respectively (Table 2).
Additionally, a high neutralizing antibody rate was found in the majority of serum samples from different mammalian species (cows, pigs, horses and dogs) against the MRVs T1L, T2J, T3A and BatMRV1-IT2011. VNTs also showed reciprocal cross-reactions between BatMRV1-IT2011 and the reference strain T1L in serum samples of all of the mammalian species (Figure S2).

Table 2.
MRV neutralizing antibody titers of rabbit and guinea pig sera produced against the MRV reference strains type 1 Lange (T1L), type 2 Jones (T2J) and type 3 Abney (T3A).
| Immune Serum | Virus (100 TCID50/25 µL) | |||
|---|---|---|---|---|
| MRV1 Lang | MRV2 Jones | MRV3 Abney | BatMRV1-IT2011 | |
| MRV negative (rabbit) | 0 | 0 | 0 | 0 |
| MRV negative (guinea pig) | 0 | 0 | 5 | 0 |
| MRV1 Lang (guinea pig) | 1280 | 80 | 40 | 1280 |
| MRV2 Jones (rabbit) | 5 | 80 | 10 | 20 |
| MRV3 Abney (guinea pig) | 0 | 0 | 320 | 20 |
Results are expressed as the reciprocal of the final serum dilution required to neutralize 100% of the inoculated cultures with a viral concentration of 100 tissue culture infectious dose 50% (TCID50)/25 µL.
3. Discussion
In our previous study, a virological survey on bat populations in Italy, we characterized 15 MRVs and determined that MRV type 3 is the most widespread among bats [16]. A similar study conducted in Germany confirmed this observation [17]. Recently, a reassortant MRV belonging to serotype 2 has been detected in bats in China [23]. Therefore, to the best of our knowledge, before this report, the only MRVs identified in bats belonged to serotypes 2 and 3. Here, we report the isolation and characterization of a new serotype 1 MRV from the lesser horseshoe bat (R. hipposideros) named BatMRV1-IT2011, with evidence of an S1 genome segment reassortment.
In addition, new original data and information on the dynamics and epidemiology of MRV infections in bats were obtained through the observation and sampling of a reproductive bat colony from May–September over a six-year (2009–2015) period. Only one sample, collected in August 2011, out of 15 total fecal samples collected during the entire study was positive for MRV. Thus, we demonstrated that the MRV infection in the lesser horseshoe bat is likely transitory.
Since the sole reassortant bat MRV was previously described in Rhinolophus pusillus and we detected a reassortant MRV in bats of the same genus, this could indicate that members of the genus Rhinolophus play a key role in the generation of new MRVs characterized by genome segment reassortments and unpredictable biological properties. The source of the MRV infection in bats has not been determined, nor has the origin of the genomically-different viruses. The transmission of reoviruses from one host to another is not limited to close contacts, but can occur due to indirect contamination. Infection through contaminated food, water or other factors in the environment is highly possible, since infective reovirus particles have been found in environmental samples [24,25,26,27]. Viral persistence outside the host is an advantageous feature that enables them to spread efficiently. As an additional possibility, MRVs could be mechanically transmitted to insectivorous bats and other mammals through insect vectors, although there is not yet evidence for this type of transmission [18].
The absence of mortality or clinical signs indicative of infectious diseases in the bat colony during the six-year observation period is not surprising, since the order Chiroptera is the most resistant of all mammals to viral infection. However, bats are known to be reservoirs and the sources of a variety of viruses with zoonotic potential.
This study also investigated the presence of neutralizing antibodies against BatMRV1-IT2011, as well as T1L, T2J and T3A in animals. The preliminary results suggested that MRVs are widespread in animals and that infections containing multiple serotypes, including MRVs of serotype 1 with a S1 gene similar to BatMRV1-IT2011, are common.
Similar trends in reovirus infections containing multiple serotypes have been previously reported in healthy dogs [28,29,30], cattle [31], swine [32] and humans [9,33,34]. Infections with multiple MRVs represent the basis for the genesis of new reassortant strains in permissive hosts, and insectivorous bats may play an important role in this genesis. For a better understanding and knowledge of the genetic diversity, origin and mechanisms involved in the genetic exchange of MRVs, researchers will need to obtain and study the whole-genome sequences of a large number of isolates collected from different hosts and countries.
The cooperation among veterinary virologists, bat experts and public health bodies with highly specialized diagnostic laboratories is an essential point for collaboration on topics concerning both animal and human health that are aimed to establish an innovative and suitable intervention in the case of emerging infections.
In conclusion, our results extend the current knowledge on bat MRVs and stress the importance of continued and improved Orthoreovirus surveillance in bats and other mammals, along with the development and standardization of specific diagnostic tools.
4. Materials and Methods
4.1. Sampling
Fresh fecal samples were collected for virological investigations from a known and georeferenced reproductive colony (44°17′46,36′′N, 11°7′3,79′′E) of lesser horseshoe bat (Rhinolophus hipposideros), which roosts in an old chapel located in the Vergato Province of Bologna, northern Italy (Figure 4). The bat species was identified based on morphologic characteristics according to European bat identification keys [9]. Samples were collected during the period of bat activity from 2009–2015 using a clean plastic sheet placed under the roost site for ~24 h before sampling. The survey did not encompass any direct manipulations of the bats and relied entirely on the collection of fecal samples, which were submitted to the laboratory and stored at −80 °C until processing.

Figure 4.
(A) Reproductive colony of lesser horseshoe bats (Rhinolophus hipposideros) sampled in the study and (B) its building roost.
4.2. Viral Isolation
Fecal samples were homogenized in minimal essential medium (1 g/10 mL) containing antibiotics and clarified by centrifugation at 3000× g for 15 min. Samples were inoculated in confluent monolayers of VERO (African green monkey) and MARC-145 (Fetal monkey) kidney cells, incubated at 37 °C with 5% CO2 and observed daily for 7 d to highlight the development of (CPEs). In the absence of CPEs, the cryolysates were sub-cultured twice onto fresh monolayers.
4.3. Electron Microscopy
Supernatant fluids from cell cultures showing CPEs were submitted for negative-staining electron microscopy (nsEM) using the Airfuge method [35]. Grids were stained with 2% NaPT, pH 6.8, for 1.5 min and examined at 19,000–30,000× using a Tecnai G2 Spirit transmission electron microscope (FEI, Eindhoven, NL, The Netherlands) operating at 85 kV and equipped with an Olympus Veleta digital camera. Viral particles were identified based on their morphological characteristics.
4.4. Molecular Testing and Analysis
RT-PCR with specific primers for a conserved region of the L1 viral gene, which is common to different MRV serotypes, was used for viral identification [8]. To obtain the whole genome sequence of the isolate, the Ion Torrent next-generation sequencing platform was used as previously described [15]. Searches using the BLAST algorithm were performed on the NCBI server [36] with the available database. The sequence alignments of the 10 gene segments (L1–L3, M1–M3 and S1–S4) were compared with those of reference strains and other orthoreoviruses downloaded from GenBank using the program ClustalW. Phylogenetic trees for each genome segment were generated using the neighbor-joining method with the Kimura 2-parameter model. Branch support was assessed by the bootstrap analysis of 1000 replicates. Putative reassortant viruses were preliminarily identified by their topological incongruities among all of the phylogenies. The reassortant viruses were further investigated using a small set of the full genome sequences of the closest related MRVs available from GenBank. Only strains for which the complete genome was available in GenBank were included. The 10 gene segment alignments were concatenated in the order of their length to generate a single alignment of complete genome sequences, which was further analyzed using a recombination detection program, RDP4. The specific algorithms used were RDP, GENECONV, Chimaera, MaxChi, BootScan and SiScan implemented in the RDP4 software using default settings [37]. We used more than one method to analyze the data, because evaluations of these recombination detection methods, using both simulated and empirical data, have shown that the results from only a single method are not very reliable [38]. The hypothesis of reassortment was supported when the recombinant breakpoints were detected near the junctions when the genome segments were manually concatenated.
4.5. Virus Neutralization Test
The Virus neutralization test (VNT) was carried out in 96-well microplates using BatMRV1-IT2011 grown on MARC-145 cells, and rabbit and guinea pig immune sera produced against the MRV prototype strains, type 1 Lang (T1L), type 2 Jones (T2J) and type 3 Abney (T3A).
Serial two-fold dilutions of each immune serum (2 wells/dilution) in 25 μL of serum-free culture medium were added to each well and incubated for 1 h at 37 °C with an equal volume of tissue culture fluid containing 100 tissue culture infectious dose 50% (TCID50) of BatMRV1-IT2011. Virus back titrations of the working virus dilution were included, using six wells per 10-fold dilution, to confirm the validity of the test results. A 50 μL volume of cells at log5 cells/mL in medium containing 10% fetal calf serum was added to each well. After incubation for 5–6 days at 37 °C with 5% CO2, wells were scored for CPEs, and neutralizing titers were expressed as the reciprocal of the final serum dilution required to neutralize 90% of the inoculated cultures.
For a preliminary verification of the eventual circulation and diffusion of this and other MRVs in other animals, we tested 50 serum samples collected from different species (20 cows, 10 pigs, 10 horses and 10 dogs) for the presence of neutralizing antibodies against BatMRV1-IT2011 and T1L, T2J or T3A. The serum samples were randomly collected from animals residing within 200 km of the site of the bat roost.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1999-4915/7/11/2908/s1.
Acknowledgments
This research was supported by the Italian Ministry of Health (WFR GR-2011-023505919). Thanks also to Science Docs for the English language editing of this manuscript.
Author Contributions
D.L. designed and coordinated the study and drafted the manuscript; A.M. performed the molecular genetic studies and helped to draft the manuscript; A.S., T.N. and C.C. performed the next-generation sequencing and data analysis. Additionally, they participated in the molecular genetic studies and provided extensive technical advice; A.P., F.F. and E.S. performed the virological analyses; A.L. performed electron microscopy, participated in study coordination and helped to draft the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- References Tyler, K.L. Mammalian Reoviruses. In Fields Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 1729–1745. [Google Scholar]
- Sabin, A.B. Reoviruses: A new group of respiratory and enteric viruses formerly classified as ECHO type 10 is described. Science 1959, 130, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Nibert, M.L.; Dermody, T.S.; Fields, B.N. Structure of the reovirus cell-attachment protein; a model for the domain organization of sigma 1. J. Virol. 1990, 64, 2976–2989. [Google Scholar] [PubMed]
- Weiner, H.L.; Fields, B.N. Neutralization of reovirus: The gene responsible for the neutralization antigen. J. Exp. Med. 1977, 146, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Bassel-Duby, R.; Spriggs, D.R.; Tyler, K.L.; Fields, B.N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J. Virol. 1986, 60, 64–67. [Google Scholar] [PubMed]
- Dermody, T.S.; Nibert, M.L.; Bassel-Duby, R.; Fields, B.N. Sequence diversity in S1 genes and S1 translation products of 11 serotype 3 reovirus strains. J. Virol. 1990, 64, 4842–4850. [Google Scholar] [PubMed]
- Duncan, R. Extensive sequence divergence and phylogenetic relationships between the fusogenic and nonfusogenic orthoreovirus: A species proposal. Virology 1999, 260, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Leary, T.P.; Erker, J.C.; Chalmers, M.L.; Wetzel, J.D.; Desai, S.M.; Mushahwar, I.K.; Dermody, T.S. Detection of reovirus by reverse transcription-polymerase chain reaction using primers corresponding to conserved regions of the viral L1 genome segment. J. Clin. Microbiol. 2002, 40, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, L.A.; Barin, F.; Barthez, M.A.; Bonnaud, B.; Roingeard, P.; Goudeau, A.; Castelnau, P.; Vernet, G.; Paranhos-Baccala, G.; Komurian-Pradel, F. Novel human reovirus isolated from children with acute necrotizing encephalopathy. Emerg. Infect. Dis. 2011, 17, 1436–1444. [Google Scholar] [CrossRef]
- Chua, K.B.; Voon, K.; Crameri, G.; Tan, H.S.; Rosli, J.; McEachern, J.A.; Suluraju, S.; Yu, M.; Wang, L.F. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PLoS ONE 2008, 3, e3803. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Voon, K.; Yu, M.; Keniscope, C.; Abdul Rasid, K.; Wang, L.F. Investigation of a potential zoonotic transmission of orthoreovirus associated with acute influenza-like illness in an adult patient. PLoS ONE 2011, 6, e25434. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Lau, C.S.; Lai, A.; Ho, E.; Leung, P.; Chan, F.; Wong, A.; Lim, W. A novel reovirus isolated from a patient with acute respiratory disease. J. Clin. Virol. 2009, 45, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Hermann, L.; Embree, J.; Hazelton, P.; Wells, B.; Coombs, R.T. Reovirus type 2 isolated from cerebrospinal fluid. Pediatr. Infect. Dis. J. 2004, 373, 373–375. [Google Scholar] [CrossRef]
- Tyler, K.L.; Barton, E.S.; Ibach, M.L.; Robinson, C.; Campbell, J.A.; O’Donnell, S.M.; Valyi-Nagy, T.; Clarke, P.; Wetzel, J.D.; Dermody, T.S. Isolation and molecular characterization of novel type 3 reovirus from a child with meningitis. J. Infect. Dis. 2004, 189, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Steyer, A.; Gutiérrez-Aguire, I.; Kolenc, M.; Koren, S.; Kutnjak, D.; Pokorn, M.; Poljšak-Prijatelj, M.; Racki, N.; Ravnikar, M.; Sagadin, M.; et al. High similarity of novel orthoreovirus detected in a child hospitalized with acute gastroenteritis to mammalian orthoreoviruses found in bats in Europe. J. Clin. Microbiol. 2013, 51, 3818–3825. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Moreno, A.; Lavazza, A.; Bresaola, M.; Canelli, E.; Boniotti, M.B.; Cordioli, P. Identification of Mammalian orthoreovirus type 3 in Italian bats. Zoonoses Public Health 2013, 60, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kohl, C.; Lesnik, R.; Brinkmann, A.; Ebinger, A.; Radonic’, A.; Nitsche1, A.; Mu hldorfer, K.; Wibbelt, G.; Kurth, A. Isolation and characterization of three Mammalian orthoreoviruses from European bats. PLoS ONE 2012, 7, e43106. [Google Scholar] [CrossRef] [PubMed]
- Narayanappa, A.T.; Sooryanarain, H.; Deventhiran, J.; Cao, D.; Venkatachalam, B.A.; Kambiranda, D.; LeRoith, T.; Heffron, C.L.; Lindstrom, N.; Hall, K.; et al. A Novel pathogenic Mammalian orthoreovirus from diarrheic pigs and swine blood meal in the United States. mBio 2015, 6, e00593–15. [Google Scholar] [PubMed]
- Lorusso, A.; Teodori, L.; Leone, A.; Marcacci, M.; Mangone, I.; Orsini, M.; Capobianco-Dondona, A.; Camma’, C.; Monaco, F.; Savini, G. A new member of the pteropine orthoreovirus species isolated from fruit bats imported to Italy. Infect. Genet. Evol. 2015, 30, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Van den Wollenberg, D.J.M.; Dautzenberg, J.C.I.; van den Hengel, S.K.; Cramer, S.J.; de Groot, R.J.; Hoeben, R.C. Isolation of reovirus T3D mutants capable of infecting human tumor cells independent of junction adhesion molecule-A. PLoS ONE 2012, 7, e48064. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging virus. Clin. Microbiol. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Moreno, A.; Prosperi, A.; Lavazza, A.; Boniotti, B.; Raffini, E.; Cordioli, P. Molecular characterization of orthoreovirus isolated from bats, dogs and cats. In Proceedings of the 8th Annual Meeting Epizone, Copenhagen, Denmark, 23–25 September 2014.
- Wang, L.; Fu, S.; Cao, L.; Lei, W.; Cao, Y.; Song, J.; Tang, Q.; Zhang, H.; Feng, Y.; Yang, W.; et al. Isolation and identification of a natural reassortant Mammalian orthoreovirus from least horseshoe bat in china. PLoS ONE 2015, 10, e0118598. [Google Scholar] [CrossRef] [PubMed]
- Lodder, W.J.; de Roda Husman, A.M. Presence of noroviruses and other enteric viruses in sewage and surface waters in The Netherlands. Appl. Environ. Microbiol. 2005, 71, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Lodder, W.J.; van den Berg, H.H.; Rutjes, S.A.; de Roda Husman, A.M. Presence of enteric viruses in source waters for drinking water production in The Netherlands. Appl. Environ. Microbiol. 2010, 76, 5965–5971. [Google Scholar] [CrossRef] [PubMed]
- Spinner, M.L.; di Giovanni, G.D. Detection and identification of mammalian reoviruses in surface water by combined cell culture and reverse transcription-PCR. Appl. Environ. Microbiol. 2001, 67, 3016–3020. [Google Scholar] [CrossRef] [PubMed]
- Irving, L.G.; Smith, F.A. One-year survey of enteroviruses, adenoviruses, and reoviruses isolated from effluent at an activated-sludge purification plant. Appl. Environ. Microbiol. 1981, 41, 51–59. [Google Scholar] [PubMed]
- Fukumi, H.; Takeuchi, Y.; Ishida, M.; Saito, H. Serological epidemiology of reovirus infection. J. Med. Sci. 1969, 22, 13–21. [Google Scholar]
- Murakami, T.; Kato, H. Reovirus antibody patterns in dogs: A trial for the application of principal component analysis to seroepidemiology. Natl. Inst. Anim. Health Q 1975, 15, 8–14. [Google Scholar]
- Osterhaus, A.; Berghuis-De Vries, J.; Steur, K. Antiviral antibodies in dogs in the Netherlands. Zentralbl. Veterinärmed. B. 1977, 24, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.; Abinanti, F.R.; Hovis, J.F. Further observations on the natural infection of cattle with reoviruses. Am. J. Hyg. 1963, 77, 38–48. [Google Scholar] [PubMed]
- Hirahara, T.; Yasuhara, H.; Matsui, O.; Kodama, K.; Nakai, M.; Sasaki, N. Characteristics of reovirus type 1 from the respiratory tract of pigs in Japan. J. Vet. Sci. 1988, 50, 353–361. [Google Scholar] [CrossRef]
- Pal, S.R.; Agarwal, S.C. Sero-epidemiological study of reovirus infection amongst the normal population of the Chandigarh area-northern India. J. Hyg. 1968, 66, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson, D. Respiratory virus antibodies in human sera from different regions of the world. Bull. World Health Organ. 1965, 32, 833–847. [Google Scholar] [PubMed]
- Lavazza, A.; Pascucci, S.; Gelmetti, D. Rod-shaped virus-like particles in intestinal contents of three avian species. Vet. Rec. 1990, 126, 581. [Google Scholar] [PubMed]
- NCBI server. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 July 2015).
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evolution. 2015, pp. 1–5. Available online: http://web.cbio.uct.ac.za/~darren/rdp.html (accessed on 20 July 2015). [CrossRef]
- Posada, D. Evaluation of methods for detecting recombination from DNA sequences: Empirical data. Mol. Biol. Evol. 2002, 19, 708–717. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).