Viral Oncolysis — Can Insights from Measles Be Transferred to Canine Distemper Virus?
Abstract
:1. Introduction
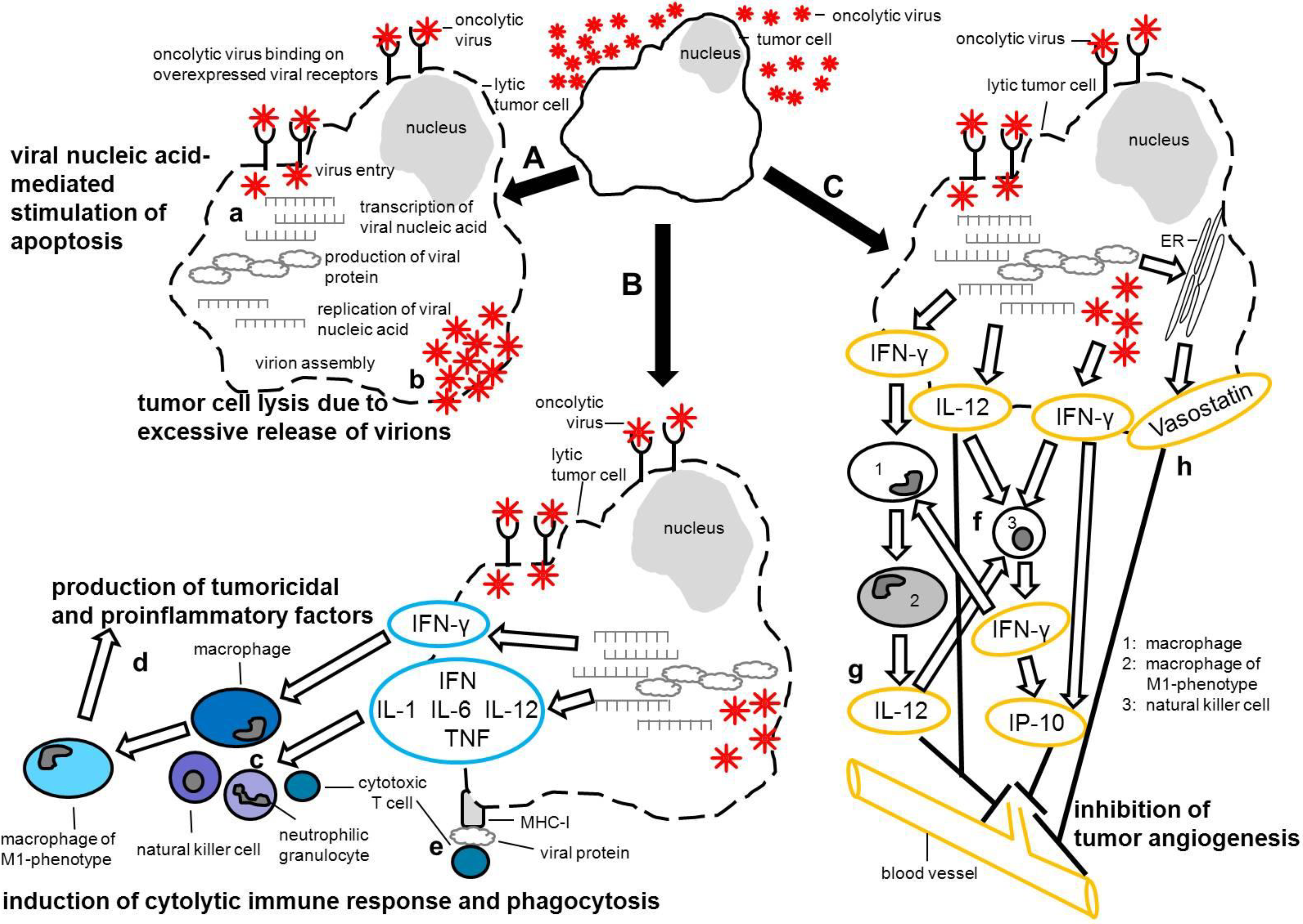
- live-attenuated naturally tumor-selective vaccine strains,
2. Viral Properties of Measles Virus and Canine Distemper Virus
2.1. Measles Virus
2.2. Canine Distemper Virus
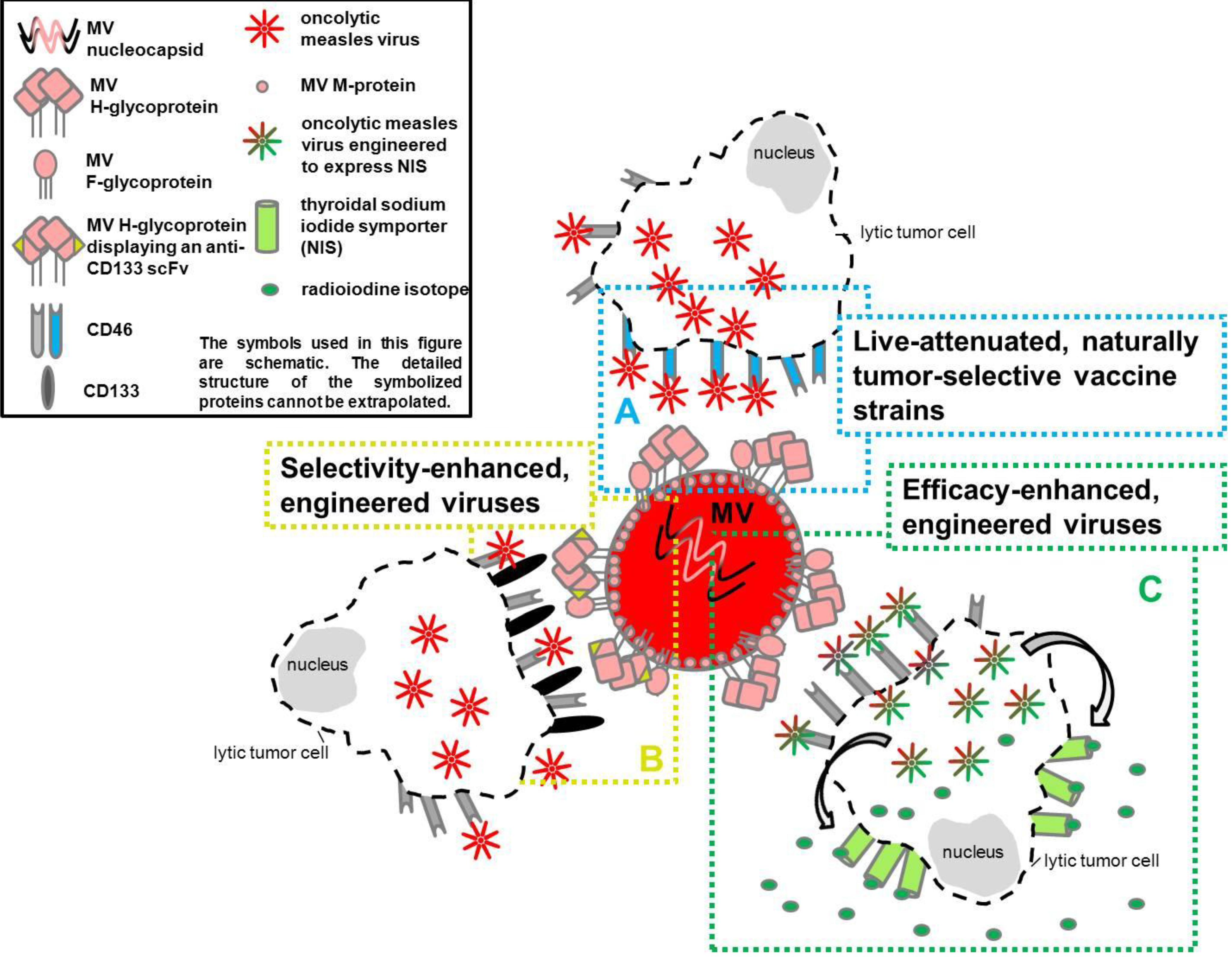
3. Measles Virus as an Oncolytic Virus
3.1. Live-Attenuated Naturally Tumor Selective Measles Vaccine Strains
3.2. Selectivity- and Efficacy-Enhanced, Engineered Measles Virus Strains
3.2.1. Oncolytic MV-Targeting
3.2.1.1. Receptor-Targeted MV
3.2.1.2. Protease-Targeted MV
3.2.1.3. miRNA-Targeted MV
3.2.2. Oncolytic MV-Monitoring
3.2.3. Efficacy-Enhanced/‘Armed’ MV (Oncolytic MV-‘Arming’)
| Virus modification | Virus strain | Tumor/tumor cell line | in vitro | in vivo | References | |
|---|---|---|---|---|---|---|
| Measles virus without modification | Edmonston | human myeloma: ARH-77 cells, RPMI 8226 cells, JJN-3 cells, MM1 cells, KAS-6/1 cells, KMS-11 cells; primary myeloma cells; | x | x | [105] | |
| Edmonston | human ovarian carcinoma: SKOV3ip.1 cells; human fibrosarcoma: HT1080 cells; human epithelial lung carcinoma: A549 cells; | x | n.d. | [49] | ||
| Edmonston Moraten | human ovarian carcinoma: OV202 cells, OV207 cells, SKOV3ip.1 cells; | x | x | [148] | ||
| Edmonston-Zagreb | human T-cell lymphoma: SeAx cells, HUT 78 cells, MyLa cells; cutaneous T-cell lymphomas (CTCL); | x | x | [149] | ||
| Schwarz | human mesothelioma: M11 cells, M13 cells, M31 cells, M47 cells, M56 cells, M61 cells; | x | n.d. | [110] | ||
| Not detailed | human B-precursor acute lymphoblastic leukemia (ALL): 697 cells, Nalm-6 cells, SEM cells, REH cells; human Burkitt's lymphoma: Raji cells, Daudi cells; human T cell leukemia: Jurkat cells; primary chronic lymphocytic leukemia (CLL) and ALL cells; | x | x | [150] | ||
| CAM-70; Schwarz MV wild-type: MV190112 | human B cell lymphoma: BJAB cells; BJAB cells; marmoset B-lymphoblastoid: B95-8 cells; human Burkitt’s lymphoma: Akata cells, BL-41 cells, BL-41/cells, Daudi cells, Mutu cells, Jijoye cells, Namalwa cells, P3HR-1 cells, Raji cells, BLCL cells, LMP1-transduced: BJAB LMP1 cells; | x | n.d. | [151] | ||
| ß-galactosidase reporter gene (MVIacZ) | Edmonston | human lymphoma: DoHH2 cells, Raji cells; | x | x | [106] | |
| CEA | Edmonston | human myeloma: RPMI 8226 cells; human fibrosarcoma: HT1080 cells; | n.d. | x | [135] | |
| Not detailed | human ovarian carcinoma: OV202 cells, OV207 cells, SKOV3ip.1 cells; | x | x | [148] | ||
| Edmonston | human breast cancer: MDA-MB-231 cells, SkBr3 cells, MCF7 cells; | x | x | [152] | ||
| Edmonston | human hepatocellular carcinoma: Hep-3B cells, HUH-7 cells; | x | x | [136] | ||
| Not detailed | human histiocytic lymphoma: U-937 cells; human Burkitt's lymphoma: Raji cells; human myeloma: KAS-6/1 cells; human ovarian carcinoma: SKOV3ip.1 cells; human hepatocellular carcinoma: HUH-7 cells; | x | x | [153] | ||
| Not detailed | human malignant glioma: U87 cells, U251 cells; primary glioblastoma multiforme: GBM12 cells; | x | x | [114] | ||
| Edmonston-NSe | human breast cancer: MDA-MB-231 cells; human ovarian carcinoma: SKOV3ip.1 cells; human cerebellar medulloblastoma: TE671 cells; | x | n.d. | [154] | ||
| Edmonston | human prostate cancer: PC-3 cells, DU-145 cells, LNCaP cells; | x | x | [155] | ||
| Edmonston | human ovarian carcinoma: SKOV3ip.1 cells; human ovarian carcinoma; | x | Phase I clinical trial | [156] | ||
| Edmonston | human hepatoblastoma (HB): Hep2G cells, HUH6 cells; | x | x | [24] | ||
| Not detailed | human glioblastoma: GBM6 cells; primary human glioblastoma cells; | x | x | [157] | ||
| Not detailed | human ovarian carcinoma: SKOV3ip.1 cells; | x | x | [158] | ||
| Edmonston | human ovarian carcinoma: SKOV3ip.1 cells, IGROV1 cells; OV202 cells; | x | x | [159] | ||
| Single chain anti-body | CD38 | Edmonston | human fibrosarcoma: HT1080 cells; | x | x | [121] |
| Edmonston | human glioblastoma: U118 MGcells; human erythroleukemia: K562 cells; human Burkitt’s lymphoma: Raji cells, Ramos cells; human ovarian carcinoma: SKOV3ip.1 cells; | x | x | [115] | ||
| CD20 | Replicating MV | human fibrosarcoma: HT1080 cells; | x | x | [122] | |
| HER2/neu | Not detailed | human ovarian carcinoma: SKOV3ip.1 cells; human medulloblastoma: TE671 cells; | x | n.d. | [160] | |
| EGFRvIII | Edmonston | human glioblastoma: U118 MG cells; human erythroleukemia: K562 cells; human Burkitt’s lymphoma: Raji cells, Ramos cells; human ovarian carcinoma: SKOV3ip.1 cells; | x | x | [115] | |
| Edmonston-NSe | human glioblastoma: U118 cells; primary glioblastoma: GBM6 cells, GBM14 cells, GBM39 cells; | x | x | [161] | ||
| PSMA | Edmonston | human prostate cancer: LNCaP cells, PC3 cells; human T cell leukemia: Jurkat cells; human Burkitt’s lymphoma: Raji cells; human multiple myeloma: KAS 6/1 cells | x | x | [162] | |
| CD133 | Not detailed | Human fibrosarcoma: HT1080 cells; Human hepatocellular: HuH7 cells; Primary glioblastoma: NCH644 cells; | x | x | [51] | |
| GM-CSF | Not detailed | human Burkitt’s lymphoma: Raji cells; | x | x | [144] | |
| NIS | Edmonston | human ovarian carcinoma: SKOV3ip.1 cells, IGROV1 cells; OV202 cells; | x | x | [159] | |
| Edmonston | Human multiple myeloma: ARH 77 cells, KAS 6/1 cells, MM1 cells; primary myeloma cells; | x | x | [53] | ||
| Not detailed | human glioblastoma: GBM6 cells; primary human glioblastoma cells; | x | x | [157] | ||
| Edmonston | human multiple myeloma: KAS-6/1 cells; | n.d. | x | [163] | ||
| Edmonston | human hepatocellular carcinoma: Hep-3B cells, HUH-7 cells; | x | x | [136] | ||
| Edmonston | human pancreatic cancer: BxPC-3 cells, MiaPaCa-2 cells, Panc-1 cells; | x | x | [164] | ||
| Edmonston | human prostate cancer: PC-3 cells, DU-145 cells, LNCaP cells; | x | x | [165] | ||
| Not detailed | human multiple myeloma: MM1 cells, KAS-6/1 cells; | x | x | [166] | ||
| Not detailed | human pancreatic cancer: BxPC-3 cells; | x | x | [167] | ||
| Edmonston | human ovarian carcinoma: SKOV3ip.1 cells; human multiple myeloma: KAS6/1 cells; | x | x | [168] | ||
| Edmonston | human malignant glioma: U87 cells, U251 cells; human primary glioblastoma: GBM6 cells, GBM10 cells, GBM12 cells, GBM39 cells, GBM43 cells, GBM44 cells; | x | x | [169] | ||
| Edmonston | human head oral squamous cell carcinoma: SCC-25 cells, SCC-15 cells; anaplastic human thyroid carcinoma: SW579 cells; human hypopharyngeal carcinoma: FaDu cells; | x | x | [170] | ||
| Edmonston | human medulloblastoma: D283med cells, UW426 cells; | x | x | [54] | ||
| Edmonston | human head and neck cancer: HN3 cells, HN5 cells, PJ41 cells; human colorectal cancer: HCT116 cells; | x | x | [171] | ||
| NIS | Edmonston | human endometrial cancer: HEC-1-A cells, Ishikawa cells, KLE cells, RL95-2 cells, AN3CA cells; ARK-1 cells, ARK-2 cells, SPEC-2 cells; | x | x | [172] | |
| Edmonston B | human T-cell lymphoma: SeAx cells, MyLa2059 cells, HUT78 cells; | x | x | [173] | ||
| Human IL-13 at the C-terminus of the H-protein | Not detailed | human malignant glioma: U87, U118, U251 cells; | x | x | [174] | |
| MMP | Edmonston B–based parental MV strain (NSe) | human fibrosarcoma: HT1080 cells; human glioblastoma: U87mg cells; human liver resection material with primary and secondary tumors; | x | n.d. | [128] | |
| Not detailed | human fibrosarcoma: HT1080 cells; | x | x | [125] | ||
| αVβ3-integrin targeted (RGD or echistatin domains) | Edmonston | multiple myeloma xenografts; | x | x | [175] | |
| Not detailed | human multiple myeloma: KAS6/1 cells; | x | x | [176] | ||
| Human light immunoglobulin chain reporter gene | Edmonston | Human multiple myeloma: ARH-77 cells, KAS 6/1 cells; human T cell leukemia: Jurkat cells; | x | x | [177] | |
| NAP | Edmonston | human breast cancer: MCF-7 cells, MDA-MB-231 cells; | x | x | [178] | |
| Suicide gene SCD/FCU-1 | Not detailed | human ovarian carcinoma: OAW42 cells, SKOV3 cells; primary human ovarian carcinoma cells; | x | n.d. | [179] | |
| Edmonston B | human melanoma: A375M, Mel888, pMelL, and SK-MEL-28 cells; | x | x | [140] | ||
| Schwarz | human cholangiocarcinoma: RBE, HuCCT1, TFK-1 cells; | x | x | [141] | ||
| Not detailed | Primary murine and rhesus macaque hepatocytes; | x | x | [143] | ||
| Mérieux | Human cholangiocarcinoma: HuCCT-1 cells; Human hepatocellular carcinoma: Hep3B cells; Human colorectal adenocarcinoma: HCT116 and HCT15 cells; | x | n.d. | [180] | ||
| DARPins | EGFR | Edmonston | human adenocarcinoma: AU565 cells, SK-Br-3; human breast ductal carcinoma: BT-474 cells, MCF-7 cells; human colon adenocarcinoma: Caco-2 cells, HT-29 cells, SW-620; human fibrosarcoma: HT1080 cells; human ovarian carcinoma: SK-OV-3 cells; human malignant glioma: U87mg cells; | x | x | [181] |
| Her2/neu | ||||||
| EpCAM | ||||||
| MicroRNA-sensitive (containing target sites for microRNA-7 in the 3' untranslated region of the viral fusion gene) | Edmonston-B vaccine lineage | human malignant glioma: U87 cells; primary human brain tissue from the peripheral invasion front of a glioma resection; | x | x | [134] | |
3.3. Clinical Trials with Oncolytic Measles Virus
4. Canine Distemper Virus as an Oncolytic Virus
5. Summary and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Adams, V.J.; Evans, K.M.; Sampson, J.; Wood, J.L. Methods and mortality results of a health survey of purebred dogs in the UK. J. Small Anim. Pract. 2010, 51, 512–524. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Proschowsky, H.F.; Rugbjerg, H.; Ersboll, A.K. Mortality of purebred and mixed-breed dogs in denmark. Prev. Vet. Med. 2003, 58, 63–74. [Google Scholar] [CrossRef]
- Fidel, J.; Schiller, I.; Hauser, B.; Jausi, Y.; Rohrer-Bley, C.; Roos, M.; Kaser-Hotz, B. Histiocytic sarcomas in flat-coated retrievers: A summary of 37 cases (November 1998-March 2005). Vet. Comp. Oncol. 2006, 4, 63–74. [Google Scholar] [CrossRef]
- Hedan, B.; Thomas, R.; Motsinger-Reif, A.; Abadie, J.; Andre, C.; Cullen, J.; Breen, M. Molecular cytogenetic characterization of canine histiocytic sarcoma: A spontaneous model for human histiocytic cancer identifies deletion of tumor suppressor genes and highlights influence of genetic background on tumor behavior. BMC Cancer 2011, 11, 201. [Google Scholar] [CrossRef] [Green Version]
- Schlick, K.; Aigelsreiter, A.; Pichler, M.; Reitter, S.; Neumeister, P.; Hoefler, G.; Beham-Schmid, C.; Linkesch, W. Histiocytic sarcoma - targeted therapy: Novel therapeutic options? A series of 4 cases. Onkologie 2012, 35, 447–450. [Google Scholar] [CrossRef]
- Hornick, J.L.; Jaffe, E.S.; Fletcher, C.D. Extranodal histiocytic sarcoma: Clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am. J. Surg. Pathol. 2004, 28, 1133–1144. [Google Scholar] [CrossRef]
- Saboo, S.S.; Krajewski, K.M.; Shinagare, A.B.; Jagannathan, J.P.; Hornick, J.L.; Ramaiya, N. Imaging features of primary extranodal histiocytic sarcoma: Report of two cases and a review of the literature. Cancer Imaging 2012, 12, 253–258. [Google Scholar] [CrossRef]
- Coppedè, F.; Lopomo, A.; Spisni, R.; Migliore, L. Genetic and epigenetic biomarkers for diagnosis, prognosis and treatment of colorectal cancer. World J. Gastroenterol. 2014, 20, 943–956. [Google Scholar]
- Ortiz Comino, R.M.; Burgos Guadix, N.; De Los Santos de Lopez, R.L.; Romero Ortiz, A.D.; De Vega, M. Five year lung cancer survival (2004–2007) from hospital virgen de las nieves in granada. Chest 2014, 145, 327A. [Google Scholar] [CrossRef]
- Sinn, M.; Striefler, J.K.; Sinn, B.V.; Sallmon, D.; Bischoff, S.; Stieler, J.M.; Pelzer, U.; Bahra, M.; Neuhaus, P.; Dorken, B.; et al. Does long-term survival in patients with pancreatic cancer really exist? Results from the conko-001 study. J. Surg. Oncol. 2013, 108, 398–402. [Google Scholar] [CrossRef]
- Goldsmith, H.S. Clinical advances in the treatment of cancer. Am. J. Surg. 1969, 118, 368–376. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Jagsi, R. Progress and controversies: Radiation therapy for invasive breast cancer. CA Cancer J. Clin. 2013, 64, 135–152. [Google Scholar] [CrossRef]
- Lana, S.E.; Kogan, L.R.; Crump, K.A.; Graham, J.T.; Robinson, N.G. The use of complementary and alternative therapies in dogs and cats with cancer. J. Am. Anim. Hosp. Assoc. 2006, 42, 361–365. [Google Scholar]
- Patil, S.S.; Gentschev, I.; Nolte, I.; Ogilvie, G.; Szalay, A.A. Oncolytic virotherapy in veterinary medicine: Current status and future prospects for canine patients. J. Transl. Med. 2012, 10, 3. [Google Scholar] [CrossRef]
- Dervisis, N.G.; Dominguez, P.A.; Newman, R.G.; Cadile, C.D.; Kitchell, B.E. Treatment with DAV for advanced-stage hemangiosarcoma in dogs. J. Am. Anim. Hosp. Assoc. 2011, 47, 170–178. [Google Scholar] [CrossRef]
- Marconato, L. The staging and treatment of multicentric high-grade lymphoma in dogs: A review of recent developments and future prospects. Vet. J. 2011, 188, 34–38. [Google Scholar] [CrossRef]
- Westberg, S.; Sadeghi, A.; Svensson, E.; Segall, T.; Dimopoulou, M.; Korsgren, O.; Hemminki, A.; Loskog, A.S.; Totterman, T.H.; von Euler, H. Treatment efficacy and immune stimulation by AdCD40L gene therapy of spontaneous canine malignant melanoma. J. Immunother. 2013, 36, 350–358. [Google Scholar] [CrossRef]
- Bluming, A.Z.; Ziegler, J.L. Regression of Burkitt's lymphoma in association with measles infection. Lancet 1971, 2, 105–106. [Google Scholar] [CrossRef]
- Taqi, A.M.; Abdurrahman, M.B.; Yakubu, A.M.; Fleming, A.F. Regression of Hodgkin's disease after measles. Lancet 1981, 1, 1112. [Google Scholar]
- Zygiert, Z. Hodgkin's disease: Remissions after measles. Lancet 1971, 1, 593. [Google Scholar] [CrossRef]
- Zhang, S.C.; Cai, W.S.; Zhang, Y.; Jiang, K.L.; Zhang, K.R.; Wang, W.L. Engineered measles virus Edmonston strain used as a novel oncolytic viral system against human neuroblastoma through a CD46 and nectin 4-independent pathway. Cancer Lett. 2012, 325, 227–237. [Google Scholar] [CrossRef]
- Zhang, S.C.; Wang, W.L.; Cai, W.S.; Jiang, K.L.; Yuan, Z.W. Engineered measles virus Edmonston strain used as a novel oncolytic viral system against human hepatoblastoma. BMC Cancer 2012, 12, 427. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, P.; Yang, H.; Wu, Y.; Zeng, X.; Zhao, Y.; Wen, Y.; Zhao, X.; Liu, X.; Wei, Y.; et al. Live attenuated measles virus vaccine induces apoptosis and promotes tumor regression in lung cancer. Oncol. Rep. 2013, 29, 199–204. [Google Scholar]
- Parato, K.A.; Senger, D.; Forsyth, P.A.; Bell, J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer 2005, 5, 965–976. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. The yin-yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol. Rev. 2008, 222, 155–161. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: Role in tumour progression. Eur. J. Cancer 2004, 40, 1660–1667. [Google Scholar] [CrossRef]
- Schmieder, A.; Michel, J.; Schönhaar, K.; Goerdt, S.; Schledzewski, K. Differentiation and gene expression profile of tumor-associated macrophages. Semin. Cancer Biol. 2012, 22, 289–297. [Google Scholar] [CrossRef]
- Guo, C.; Buranych, A.; Sarkar, D.; Fisher, P.B.; Wang, X.Y. The role of tumor-associated macrophages in tumor vascularization. Vasc. Cell 2013, 5, 20. [Google Scholar] [CrossRef]
- Hagemann, T.; Lawrence, T.; McNeish, I.; Charles, K.A.; Kulbe, H.; Thompson, R.G.; Robinson, S.C.; Balkwill, F.R. "Re-educating" tumor-associated macrophages by targeting NF-kappaB. J. Exp. Med. 2008, 205, 1261–1268. [Google Scholar] [CrossRef]
- Dalton, D.K.; Pitts-Meek, S.; Keshav, S.; Figari, I.S.; Bradley, A.; Stewart, T.A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 1993, 259, 1739–1742. [Google Scholar]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Yao, L.; Pike, S.E.; Pittaluga, S.; Cherney, B.; Gupta, G.; Jaffe, E.S.; Tosato, G. Anti-tumor activities of the angiogenesis inhibitors interferon-inducible protein-10 and the calreticulin fragment vasostatin. Cancer Immunol. Immunother. 2002, 51, 358–366. [Google Scholar] [CrossRef]
- Yao, L.; Pike, S.E.; Setsuda, J.; Parekh, J.; Gupta, G.; Raffeld, M.; Jaffe, E.S.; Tosato, G. Effective targeting of tumor vasculature by the angiogenesis inhibitors vasostatin and interleukin-12. Blood 2000, 96, 1900–1905. [Google Scholar]
- Yao, L.; Sgadari, C.; Furuke, K.; Bloom, E.T.; Teruya-Feldstein, J.; Tosato, G. Contribution of natural killer cells to inhibition of angiogenesis by interleukin-12. Blood 1999, 93, 1612–1621. [Google Scholar]
- Angiolillo, A.L.; Sgadari, C.; Taub, D.D.; Liao, F.; Farber, J.M.; Maheshwari, S.; Kleinman, H.K.; Reaman, G.H.; Tosato, G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 1995, 182, 155–162. [Google Scholar] [CrossRef]
- Sgadari, C.; Angiolillo, A.L.; Tosato, G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood 1996, 87, 3877–3882. [Google Scholar]
- Brunner, J.M.; Plattet, P.; Doucey, M.A.; Rosso, L.; Curie, T.; Montagner, A.; Wittek, R.; Vandelvelde, M.; Zurbriggen, A.; Hirling, H.; et al. Morbillivirus glycoprotein expression induces ER stress, alters Ca2+ homeostasis and results in the release of vasostatin. PLoS One 2012, 7, e32803. [Google Scholar] [CrossRef]
- Pike, S.E.; Yao, L.; Jones, K.D.; Cherney, B.; Appella, E.; Sakaguchi, K.; Nakhasi, H.; Teruya-Feldstein, J.; Wirth, P.; Gupta, G.; et al. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J. Exp. Med. 1998, 188, 2349–2356. [Google Scholar] [CrossRef]
- Pike, S.E.; Yao, L.; Setsuda, J.; Jones, K.D.; Cherney, B.; Appella, E.; Sakaguchi, K.; Nakhasi, H.; Atreya, C.D.; Teruya-Feldstein, J.; et al. Calreticulin and calreticulin fragments are endothelial cell inhibitors that suppress tumor growth. Blood 1999, 94, 2461–2468. [Google Scholar]
- Bourke, M.G.; Salwa, S.; Harrington, K.J.; Kucharczyk, M.J.; Forde, P.F.; de Kruijf, M.; Soden, D.; Tangney, M.; Collins, J.K.; O'Sullivan, G.C. The emerging role of viruses in the treatment of solid tumours. Cancer Treat. Rev. 2011, 37, 618–632. [Google Scholar] [CrossRef]
- Chiocca, E.A. Oncolytic viruses. Nat. Rev. Cancer 2002, 2, 938–950. [Google Scholar] [CrossRef]
- Liu, T.C.; Galanis, E.; Kirn, D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007, 4, 101–117. [Google Scholar] [CrossRef]
- Msaouel, P.; Dispenzieri, A.; Galanis, E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: An overview. Curr. Opin. Mol. Ther. 2009, 11, 43–53. [Google Scholar]
- Msaouel, P.; Iankov, I.D.; Dispenzieri, A.; Galanis, E. Attenuated oncolytic measles virus strains as cancer therapeutics. Curr. Pharm. Biotechnol. 2012, 13, 1732–1741. [Google Scholar] [CrossRef]
- Msaouel, P.; Opyrchal, M.; Domingo Musibay, E.; Galanis, E. Oncolytic measles virus strains as novel anticancer agents. Expert Opin. Biol. Ther. 2013, 13, 483–502. [Google Scholar] [CrossRef]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Anderson, B.D.; Nakamura, T.; Russell, S.J.; Peng, K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004, 64, 4919–4926. [Google Scholar] [CrossRef]
- Russell, S.J.; Peng, K.W. Measles virus for cancer therapy. Curr. Top. Microbiol. Immunol. 2009, 330, 213–241. [Google Scholar]
- Bach, P.; Abel, T.; Hoffmann, C.; Gal, Z.; Braun, G.; Voelker, I.; Ball, C.R.; Johnston, I.C.; Lauer, U.M.; Herold-Mende, C.; et al. Specific elimination of CD133+ tumor cells with targeted oncolytic measles virus. Cancer Res. 2013, 73, 865–874. [Google Scholar] [CrossRef]
- Klonisch, T.; Wiechec, E.; Hombach-Klonisch, S.; Ande, S.R.; Wesselborg, S.; Schulze-Osthoff, K.; Los, M. Cancer stem cell markers in common cancers - therapeutic implications. Trends Mol. Med. 2008, 14, 450–460. [Google Scholar] [CrossRef]
- Dingli, D.; Peng, K.W.; Harvey, M.E.; Greipp, P.R.; O'Connor, M.K.; Cattaneo, R.; Morris, J.C.; Russell, S.J. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 2004, 103, 1641–1646. [Google Scholar] [CrossRef]
- Hutzen, B.; Pierson, C.R.; Russell, S.J.; Galanis, E.; Raffel, C.; Studebaker, A.W. Treatment of medulloblastoma using an oncolytic measles virus encoding the thyroidal sodium iodide symporter shows enhanced efficacy with radioiodine. BMC Cancer 2012, 12, 508. [Google Scholar] [CrossRef]
- Chan, W.M.; Rahman, M.M.; McFadden, G. Oncolytic myxoma virus: The path to clinic. Vaccine 2013, 31, 4252–4258. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.S.; Kim, S.W.; Yun, C.O. Evolution of oncolytic adenovirus for cancer treatment. Adv. Drug Deliv. Rev. 2012, 64, 720–729. [Google Scholar] [CrossRef]
- Hwang, C.C.; Umeki, S.; Kubo, M.; Hayashi, T.; Shimoda, H.; Mochizuki, M.; Maeda, K.; Baba, K.; Hiraoka, H.; Coffey, M.; et al. Oncolytic reovirus in canine mast cell tumor. PLoS One 2013, 8, e73555. [Google Scholar] [CrossRef]
- Kaur, B.; Chiocca, E.A.; Cripe, T.P. Oncolytic hsv-1 virotherapy: Clinical experience and opportunities for progress. Curr. Pharm. Biotechnol. 2012, 13, 1842–1851. [Google Scholar] [CrossRef]
- Shirakawa, T. Clinical trial design for adenoviral gene therapy products. Drug News Perspect. 2009, 22, 140–145. [Google Scholar] [CrossRef]
- Sivendran, S.; Pan, M.; Kaufman, H.L.; Saenger, Y. Herpes simplex virus oncolytic vaccine therapy in melanoma. Expert Opin. Biol. Ther. 2010, 10, 1145–1153. [Google Scholar]
- Yu, W.; Fang, H. Clinical trials with oncolytic adenovirus in china. Curr. Cancer Drug Targets 2007, 7, 141–148. [Google Scholar] [CrossRef]
- Garber, K. China approves world's first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006, 98, 298–300. [Google Scholar] [CrossRef]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgärtner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef]
- Yanagi, Y.; Takeda, M.; Ohno, S. Measles virus: Cellular receptors, tropism and pathogenesis. J. Gen. Virol. 2006, 87, 2767–2779. [Google Scholar] [CrossRef]
- Lamb, R.A.; Kolakofsky, D. Paramyxoviridae: The Viruses and Their Replication, 4th ed.; Lippincott Williams&Wilkins: Philadelphia, PA, USA, 2001; Volume 1, pp. 1305–1443. [Google Scholar]
- Lamb, R.A.; Jardetzky, T.S. Structural basis of viral invasion: Lessons from paramyxovirus f. Curr. Opin. Struct. Biol. 2007, 17, 427–436. [Google Scholar] [CrossRef]
- Diallo, A. Morbillivirus group: Genome organisation and proteins. Vet. Microbiol. 1990, 23, 155–163. [Google Scholar] [CrossRef]
- Wiener, D.; Plattet, P.; Cherpillod, P.; Zipperle, L.; Doherr, M.G.; Vandevelde, M.; Zurbriggen, A. Synergistic inhibition in cell-cell fusion mediated by the matrix and nucleocapsid protein of canine distemper virus. Virus Res. 2007, 129, 145–154. [Google Scholar] [CrossRef]
- de Vries, R.D.; Mesman, A.W.; Geijtenbeek, T.B.; Duprex, W.P.; de Swart, R.L. The pathogenesis of measles. Curr. Opin. Virol. 2012, 2, 248–255. [Google Scholar] [CrossRef]
- Schneider-Schaulies, S.; Niewiesk, S.; Schneider-Schaulies, J.; ter Meulen, V. Measles virus induced immunosuppression: Targets and effector mechanisms. Curr. Mol. Med. 2001, 1, 163–181. [Google Scholar] [CrossRef]
- Ludlow, M.; Allen, I.; Schneider-Schaulies, J. Systemic spread of measles virus: Overcoming the epithelial and endothelial barriers. Thromb. Haemost. 2009, 102, 1050–1056. [Google Scholar]
- Erlenhoefer, C.; Wurzer, W.J.; Löffler, S.; Schneider-Schaulies, S.; ter Meulen, V.; Schneider-Schaulies, J. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J. Virol. 2001, 75, 4499–4505. [Google Scholar] [CrossRef]
- Schlender, J.; Schnorr, J.J.; Spielhoffer, P.; Cathomen, T.; Cattaneo, R.; Billeter, M.A.; ter Meulen, V.; Schneider-Schaulies, S. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc. Natl. Acad. Sci. USA 1996, 93, 13194–13199. [Google Scholar] [CrossRef]
- Tatsuo, H.; Ono, N.; Tanaka, K.; Yanagi, Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000, 406, 893–897. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Frenzke, M.; Leonard, V.H.; Welstead, G.G.; Richardson, C.D.; Cattaneo, R. Measles virus infection of alveolar macrophages and dendritic cells precedes spread to lymphatic organs in transgenic mice expressing human signaling lymphocytic activation molecule (SLAM, CD150). J. Virol. 2010, 84, 3033–3042. [Google Scholar] [CrossRef]
- Wild, T.F.; Malvoisin, E.; Buckland, R. Measles virus: Both the haemagglutinin and fusion glycoproteins are required for fusion. J. Gen. Virol. 1991, 72, 439–442. [Google Scholar] [CrossRef]
- Leonard, V.H.; Sinn, P.L.; Hodge, G.; Miest, T.; Devaux, P.; Oezguen, N.; Braun, W.; McCray, P.B., Jr.; McChesney, M.B.; Cattaneo, R. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Invest. 2008, 118, 2448–2458. [Google Scholar]
- Mühlebach, M.D.; Mateo, M.; Sinn, P.L.; Prüfer, S.; Uhlig, K.M.; Leonard, V.H.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 530–533. [Google Scholar]
- Noyce, R.S.; Bondre, D.G.; Ha, M.N.; Lin, L.T.; Sisson, G.; Tsao, M.S.; Richardson, C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011, 7, e1002240. [Google Scholar] [CrossRef]
- Dörig, R.E.; Marcil, A.; Chopra, A.; Richardson, C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 1993, 75, 295–305. [Google Scholar] [CrossRef]
- Baumgärtner, W. Virale Infektionskrankheiten bei Welpen und Junghunden unter besonderer Berücksichtigung der Staupevirusinfektion. Prakt. Tierarzt. 1993, 74, 26–32. [Google Scholar]
- Dubielzig, R.R.; Higgins, R.J.; Krakowka, S. Lesions of the enamel organ of developing dog teeth following experimental inoculation of gnotobiotic puppies with canine distemper virus. Vet. Pathol. 1981, 18, 684–689. [Google Scholar]
- Headley, S.A.; Amude, A.M.; Alfieri, A.F.; Bracarense, A.P.; Alfieri, A.A.; Summers, B.A. Molecular detection of canine distemper virus and the immunohistochemical characterization of the neurologic lesions in naturally occurring old dog encephalitis. J. Vet. Diagn. Invest. 2009, 21, 588–597. [Google Scholar] [CrossRef]
- Koutinas, A.F.; Baumgärtner, W.; Tontis, D.; Polizopoulou, Z.; Saridomichelakis, M.N.; Lekkas, S. Histopathology and immunohistochemistry of canine distemper virus-induced footpad hyperkeratosis (hard pad disease) in dogs with natural canine distemper. Vet. Pathol. 2004, 41, 2–9. [Google Scholar] [CrossRef]
- Summers, B.A.; Appel, M.J. Aspects of canine distemper virus and measles virus encephalomyelitis. Neuropathol. Appl. Neurobiol. 1994, 20, 525–534. [Google Scholar] [CrossRef]
- Appel, M.J. Distemper pathogenesis in dogs. J. Am. Vet. Med. Assoc. 1970, 156, 1681–1684. [Google Scholar]
- von Messling, V.; Milosevic, D.; Cattaneo, R. Tropism illuminated: Lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc. Natl. Acad. Sci. USA 2004, 101, 14216–14221. [Google Scholar] [CrossRef]
- Pratakpiriya, W.; Seki, F.; Otsuki, N.; Sakai, K.; Fukuhara, H.; Katamoto, H.; Hirai, T.; Maenaka, K.; Techangamsuwan, S.; Lan, N.T.; et al. Nectin4 is an epithelial cell receptor for canine distemper virus and involved in neurovirulence. J. Virol. 2012, 86, 10207–10210. [Google Scholar] [CrossRef]
- Suter, S.E.; Chein, M.B.; von Messling, V.; Yip, B.; Cattaneo, R.; Vernau, W.; Madewell, B.R.; London, C.A. In vitro canine distemper virus infection of canine lymphoid cells: A prelude to oncolytic therapy for lymphoma. Clin. Cancer Res. 2005, 11, 1579–1587. [Google Scholar] [CrossRef]
- Tatsuo, H.; Ono, N.; Yanagi, Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 2001, 75, 5842–5850. [Google Scholar] [CrossRef]
- Wenzlow, N.; Plattet, P.; Wittek, R.; Zurbriggen, A.; Gröne, A. Immunohistochemical demonstration of the putative canine distemper virus receptor CD150 in dogs with and without distemper. Vet. Pathol. 2007, 44, 943–948. [Google Scholar] [CrossRef]
- von Messling, V.; Oezguen, N.; Zheng, Q.; Vongpunsawad, S.; Braun, W.; Cattaneo, R. Nearby clusters of hemagglutinin residues sustain slam-dependent canine distemper virus entry in peripheral blood mononuclear cells. J. Virol. 2005, 79, 5857–5862. [Google Scholar] [CrossRef]
- Zipperle, L.; Langedijk, J.P.; Orvell, C.; Vandevelde, M.; Zurbriggen, A.; Plattet, P. Identification of key residues in virulent canine distemper virus hemagglutinin that control CD150/SLAM-binding activity. J. Virol. 2010, 84, 9618–9624. [Google Scholar] [CrossRef]
- Noyce, R.S.; Delpeut, S.; Richardson, C.D. Dog nectin-4 is an epithelial cell receptor for canine distemper virus that facilitates virus entry and syncytia formation. Virology 2013, 436, 210–220. [Google Scholar] [CrossRef]
- Watanabe, S.; Shirogane, Y.; Suzuki, S.O.; Ikegame, S.; Koga, R.; Yanagi, Y. Mutant fusion proteins with enhanced fusion activity promote measles virus spread in human neuronal cells and brains of suckling hamsters. J. Virol. 2013, 87, 2648–2659. [Google Scholar] [CrossRef]
- Baker, J.P. The first measles vaccine. Pediatrics 2011, 128, 435–437. [Google Scholar] [CrossRef]
- Naniche, D.; Varior-Krishnan, G.; Cervoni, F.; Wild, T.F.; Rossi, B.; Rabourdin-Combe, C.; Gerlier, D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 1993, 67, 6025–6032. [Google Scholar]
- Santiago, C.; Bjorling, E.; Stehle, T.; Casasnovas, J.M. Distinct kinetics for binding of the CD46 and slam receptors to overlapping sites in the measles virus hemagglutinin protein. J. Biol. Chem. 2002, 277, 32294–32301. [Google Scholar] [CrossRef]
- Schneider, U.; von Messling, V.; Devaux, P.; Cattaneo, R. Efficiency of measles virus entry and dissemination through different receptors. J. Virol. 2002, 76, 7460–7467. [Google Scholar] [CrossRef]
- Cardone, J.; Le Friec, G.; Kemper, C. CD46 in innate and adaptive immunity: An update. Clin. Exp. Immunol. 2011, 164, 301–311. [Google Scholar] [CrossRef]
- Fishelson, Z.; Donin, N.; Zell, S.; Schultz, S.; Kirschfink, M. Obstacles to cancer immunotherapy: Expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol. Immunol. 2003, 40, 109–123. [Google Scholar] [CrossRef]
- Derycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 overexpression in ovarian cancer tissues and serum: Potential role as a serum biomarker. Am. J. Clin. Pathol. 2010, 134, 835–845. [Google Scholar] [CrossRef]
- Fabre-Lafay, S.; Monville, F.; Garrido-Urbani, S.; Berruyer-Pouyet, C.; Ginestier, C.; Reymond, N.; Finetti, P.; Sauvan, R.; Adelaide, J.; Geneix, J.; et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 2007, 7, 73. [Google Scholar] [CrossRef]
- Takano, A.; Ishikawa, N.; Nishino, R.; Masuda, K.; Yasui, W.; Inai, K.; Nishimura, H.; Ito, H.; Nakayama, H.; Miyagi, Y.; et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009, 69, 6694–6703. [Google Scholar] [CrossRef]
- Peng, K.W.; Ahmann, G.J.; Pham, L.; Greipp, P.R.; Cattaneo, R.; Russell, S.J. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood 2001, 98, 2002–2007. [Google Scholar]
- Grote, D.; Russell, S.J.; Cornu, T.I.; Cattaneo, R.; Vile, R.; Poland, G.A.; Fielding, A.K. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood 2001, 97, 3746–3754. [Google Scholar] [CrossRef]
- Boisgerault, N.; Guillerme, J.B.; Pouliquen, D.; Mesel-Lemoine, M.; Achard, C.; Combredet, C.; Fonteneau, J.F.; Tangy, F.; Gregoire, M. Natural oncolytic activity of live-attenuated measles virus against human lung and colorectal adenocarcinomas. Biomed. Res. Int. 2013, 2013, 1–11. [Google Scholar]
- Melcher, A.; Parato, K.; Rooney, C.M.; Bell, J.C. Thunder and lightning: Immunotherapy and oncolytic viruses collide. Mol. Ther. 2011, 19, 1008–1016. [Google Scholar] [CrossRef]
- Donnelly, O.G.; Errington-Mais, F.; Steele, L.; Hadac, E.; Jennings, V.; Scott, K.; Peach, H.; Phillips, R.M.; Bond, J.; Pandha, H.; et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013, 20, 7–15. [Google Scholar] [CrossRef]
- Gauvrit, A.; Brandler, S.; Sapede-Peroz, C.; Boisgerault, N.; Tangy, F.; Gregoire, M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008, 68, 4882–4892. [Google Scholar] [CrossRef]
- Fonteneau, J.F.; Guillerme, J.B.; Tangy, F.; Gregoire, M. Attenuated measles virus used as an oncolytic virus activates myeloid and plasmacytoid dendritic cells. Oncoimmunology 2013, 2, e24212. [Google Scholar] [CrossRef]
- Guillerme, J.B.; Boisgerault, N.; Roulois, D.; Menager, J.; Combredet, C.; Tangy, F.; Fonteneau, J.F.; Gregoire, M. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin. Cancer Res. 2013, 19, 1147–1158. [Google Scholar] [CrossRef]
- Berchtold, S.; Lampe, J.; Weiland, T.; Smirnow, I.; Schleicher, S.; Handgretinger, R.; Kopp, H.G.; Reiser, J.; Stubenrauch, F.; Mayer, N.; et al. Innate immune defense defines susceptibility of sarcoma cells to measles vaccine virus-based oncolysis. J. Virol. 2013, 87, 3484–3501. [Google Scholar] [CrossRef]
- Liu, C.; Sarkaria, J.N.; Petell, C.A.; Paraskevakou, G.; Zollman, P.J.; Schroeder, M.; Carlson, B.; Decker, P.A.; Wu, W.; James, C.D.; et al. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin. Cancer Res. 2007, 13, 7155–7165. [Google Scholar] [CrossRef]
- Nakamura, T.; Peng, K.W.; Harvey, M.; Greiner, S.; Lorimer, I.A.; James, C.D.; Russell, S.J. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005, 23, 209–214. [Google Scholar]
- Schneider, U.; Bullough, F.; Vongpunsawad, S.; Russell, S.J.; Cattaneo, R. Recombinant measles viruses efficiently entering cells through targeted receptors. J. Virol. 2000, 74, 9928–9936. [Google Scholar] [CrossRef]
- Hammond, A.L.; Plemper, R.K.; Zhang, J.; Schneider, U.; Russell, S.J.; Cattaneo, R. Single-chain antibody displayed on a recombinant measles virus confers entry through the tumor-associated carcinoembryonic antigen. J. Virol. 2001, 75, 2087–2096. [Google Scholar] [CrossRef]
- Turriziani, M.; Fantini, M.; Benvenuto, M.; Izzi, V.; Masuelli, L.; Sacchetti, P.; Modesti, A.; Bei, R. Carcinoembryonic antigen (CEA)-based cancer vaccines: Recent patents and antitumor effects from experimental models to clinical trials. Recent Pat. Anticancer Drug Discov. 2012, 7, 265–296. [Google Scholar] [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Vongpunsawad, S.; Oezgun, N.; Braun, W.; Cattaneo, R. Selectively receptor-blind measles viruses: Identification of residues necessary for SLAM- or CD46-induced fusion and their localization on a new hemagglutinin structural model. J. Virol. 2004, 78, 302–313. [Google Scholar] [CrossRef]
- Peng, K.W.; Donovan, K.A.; Schneider, U.; Cattaneo, R.; Lust, J.A.; Russell, S.J. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood 2003, 101, 2557–2562. [Google Scholar] [CrossRef]
- Bucheit, A.D.; Kumar, S.; Grote, D.M.; Lin, Y.; von Messling, V.; Cattaneo, R.B.; Fielding, A.K. An oncolytic measles virus engineered to enter cells through the CD20 antigen. Mol. Ther. 2003, 7, 62–72. [Google Scholar] [CrossRef]
- Press, O.W.; Leonard, J.P.; Coiffier, B.; Levy, R.; Timmerman, J. Immunotherapy of non-Hodgkin's lymphomas. Hematology Am. Soc. Hematol. Educ. Program. 2001, 221–240. [Google Scholar]
- Shankland, K.R.; Armitage, J.O.; Hancock, B.W. Non-Hodgkin lymphoma. Lancet 2012, 380, 848–857. [Google Scholar] [CrossRef]
- Springfeld, C.; von Messling, V.; Frenzke, M.; Ungerechts, G.; Buchholz, C.J.; Cattaneo, R. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 2006, 66, 7694–7700. [Google Scholar]
- Hadler-Olsen, E.; Winberg, J.O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumour. Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef]
- Watanabe, M.; Hirano, A.; Stenglein, S.; Nelson, J.; Thomas, G.; Wong, T.C. Engineered serine protease inhibitor prevents furin-catalyzed activation of the fusion glycoprotein and production of infectious measles virus. J. Virol. 1995, 69, 3206–3210. [Google Scholar]
- Mühlebach, M.D.; Schaser, T.; Zimmermann, M.; Armeanu, S.; Hanschmann, K.M.; Cattaneo, R.; Bitzer, M.; Lauer, U.M.; Cichutek, K.; Buchholz, C.J. Liver cancer protease activity profiles support therapeutic options with matrix metalloproteinase-activatable oncolytic measles virus. Cancer Res. 2010, 70, 7620–7629. [Google Scholar] [CrossRef]
- Edge, R.E.; Falls, T.J.; Brown, C.W.; Lichty, B.D.; Atkins, H.; Bell, J.C. A let-7 microRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol. Ther. 2008, 16, 1437–1443. [Google Scholar] [CrossRef]
- Kelly, E.J.; Hadac, E.M.; Greiner, S.; Russell, S.J. Engineering microRNA responsiveness to decrease virus pathogenicity. Nat. Med. 2008, 14, 1278–1283. [Google Scholar] [CrossRef]
- Kelly, E.J.; Nace, R.; Barber, G.N.; Russell, S.J. Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J. Virol. 2009, 84, 1550–1562. [Google Scholar]
- Lee, C.Y.; Rennie, P.S.; Jia, W.W. MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin. Cancer Res. 2009, 15, 5126–5135. [Google Scholar] [CrossRef]
- Ylösmäki, E.; Hakkarainen, T.; Hemminki, A.; Visakorpi, T.; Andino, R.; Saksela, K. Generation of a conditionally replicating adenovirus based on targeted destruction of e1a mRNA by a cell type-specific microRNA. J. Virol. 2008, 82, 11009–11015. [Google Scholar] [CrossRef]
- Leber, M.F.; Bossow, S.; Leonard, V.H.; Zaoui, K.; Grossardt, C.; Frenzke, M.; Miest, T.; Sawall, S.; Cattaneo, R.; von Kalle, C.; et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol. Ther. 2011, 19, 1097–1106. [Google Scholar] [CrossRef]
- Peng, K.W.; Facteau, S.; Wegman, T.; O'Kane, D.; Russell, S.J. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat. Med. 2002, 8, 527–531. [Google Scholar] [CrossRef]
- Blechacz, B.; Splinter, P.L.; Greiner, S.; Myers, R.; Peng, K.W.; Federspiel, M.J.; Russell, S.J.; LaRusso, N.F. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology 2006, 44, 1465–1477. [Google Scholar] [CrossRef]
- Peng, K.W.; TenEyck, C.J.; Galanis, E.; Kalli, K.R.; Hartmann, L.C.; Russell, S.J. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002, 62, 4656–4662. [Google Scholar]
- Phuong, L.K.; Allen, C.; Peng, K.W.; Giannini, C.; Greiner, S.; TenEyck, C.J.; Mishra, P.K.; Macura, S.I.; Russell, S.J.; Galanis, E.C. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003, 63, 2462–2469. [Google Scholar]
- Li, H.; Peng, K.W.; Dingli, D.; Kratzke, R.A.; Russell, S.J. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010, 17, 550–558. [Google Scholar] [CrossRef]
- Kaufmann, J.K.; Bossow, S.; Grossardt, C.; Sawall, S.; Kupsch, J.; Erbs, P.; Hassel, J.C.; von Kalle, C.; Enk, A.H.; Nettelbeck, D.M.; et al. Chemovirotherapy of malignant melanoma with a targeted and armed oncolytic measles virus. J. Invest. Dermatol. 2012, 133, 1034–1042. [Google Scholar]
- Lange, S.; Lampe, J.; Bossow, S.; Zimmermann, M.; Neubert, W.; Bitzer, M.; Lauer, U.M. A novel armed oncolytic measles vaccine virus for the treatment of cholangiocarcinoma. Hum. Gene Ther. 2013, 24, 554–564. [Google Scholar] [CrossRef]
- Lampe, J.; Bossow, S.; Weiland, T.; Smirnow, I.; Lehmann, R.; Neubert, W.; Bitzer, M.; Lauer, U.M. An armed oncolytic measles vaccine virus eliminates human hepatoma cells independently of apoptosis. Gene Ther. 2013, 20, 1033–1041. [Google Scholar] [CrossRef]
- Völker, I.; Bach, P.; Coulibaly, C.; Plesker, R.; Abel, T.; Seifried, J.; Heidmeier, S.; Mühlebach, M.D.; Lauer, U.M.; Buchholz, C.J. Intrahepatic application of suicide gene-armed measles virotherapeutics: A safety study in transgenic mice and rhesus macaques. Hum. Gene Ther. Clin. Dev. 2013, 24, 11–22. [Google Scholar] [CrossRef]
- Grote, D.; Cattaneo, R.; Fielding, A.K. Neutrophils contribute to the measles virus-induced antitumor effect: Enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003, 63, 6463–6468. [Google Scholar]
- Grossardt, C.; Engeland, C.E.; Bossow, S.; Halama, N.; Zaoui, K.; Leber, M.F.; Springfeld, C.; Jaeger, D.; von Kalle, C.; Ungerechts, G. Granulocyte-macrophage colony-stimulating factor-armed oncolytic measles virus is an effective therapeutic cancer vaccine. Hum. Gene Ther. 2013, 24, 644–654. [Google Scholar] [CrossRef]
- Garcia-Sastre, A. Mechanisms of inhibition of the host interferon alpha/beta-mediated antiviral responses by viruses. Microbes Infect. 2002, 4, 647–655. [Google Scholar] [CrossRef]
- Haralambieva, I.; Iankov, I.; Hasegawa, K.; Harvey, M.; Russell, S.J.; Peng, K.W. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol. Ther. 2007, 15, 588–597. [Google Scholar] [CrossRef]
- Myers, R.; Greiner, S.; Harvey, M.; Soeffker, D.; Frenzke, M.; Abraham, K.; Shaw, A.; Rozenblatt, S.; Federspiel, M.J.; Russell, S.J.; et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005, 12, 593–599. [Google Scholar] [CrossRef]
- Heinzerling, L.; Künzi, V.; Oberholzer, P.A.; Kündig, T.; Naim, H.; Dummer, R. Oncolytic measles virus in cutaneous t-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood 2005, 106, 2287–2294. [Google Scholar] [CrossRef]
- Patel, B.; Dey, A.; Ghorani, E.; Kumar, S.; Malam, Y.; Rai, L.; Steele, A.J.; Thomson, J.; Wickremasinghe, R.G.; Zhang, Y.; et al. Differential cytopathology and kinetics of measles oncolysis in two primary B-cell malignancies provides mechanistic insights. Mol. Ther. 2011, 19, 1034–1040. [Google Scholar] [CrossRef]
- Takeda, S.; Kanbayashi, D.; Kurata, T.; Yoshiyama, H.; Komano, J. Enhanced susceptibility of B lymphoma cells to measles virus by Epstein-Barr virus type III latency that upregulates CD150/signaling lymphocytic activation molecule. Cancer Sci. 2013, 105, 211–218. [Google Scholar]
- McDonald, C.J.; Erlichman, C.; Ingle, J.N.; Rosales, G.A.; Allen, C.; Greiner, S.M.; Harvey, M.E.; Zollman, P.J.; Russell, S.J.; Galanis, E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res. Treat. 2006, 99, 177–184. [Google Scholar] [CrossRef]
- Iankov, I.D.; Blechacz, B.; Liu, C.; Schmeckpeper, J.D.; Tarara, J.E.; Federspiel, M.J.; Caplice, N.; Russell, S.J. Infected cell carriers: A new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol. Ther. 2007, 15, 114–122. [Google Scholar] [CrossRef]
- Liu, C.; Erlichman, C.; McDonald, C.J.; Ingle, J.N.; Zollman, P.; Iankov, I.; Russell, S.J.; Galanis, E. Heat shock protein inhibitors increase the efficacy of measles virotherapy. Gene Ther. 2008, 15, 1024–1034. [Google Scholar] [CrossRef]
- Msaouel, P.; Iankov, I.D.; Allen, C.; Aderca, I.; Federspiel, M.J.; Tindall, D.J.; Morris, J.C.; Koutsilieris, M.; Russell, S.J.; Galanis, E. Noninvasive imaging and radiovirotherapy of prostate cancer using an oncolytic measles virus expressing the sodium iodide symporter. Mol. Ther. 2009, 17, 2041–2048. [Google Scholar] [CrossRef]
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J., Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882. [Google Scholar] [CrossRef]
- Allen, C.; Opyrchal, M.; Aderca, I.; Schroeder, M.A.; Sarkaria, J.N.; Domingo, E.; Federspiel, M.J.; Galanis, E. Oncolytic measles virus strains have significant antitumor activity against glioma stem cells. Gene Ther. 2013, 20, 444–449. [Google Scholar] [CrossRef]
- Peng, K.W.; Hadac, E.M.; Anderson, B.D.; Myers, R.; Harvey, M.; Greiner, S.M.; Soeffker, D.; Federspiel, M.J.; Russell, S.J. Pharmacokinetics of oncolytic measles virotherapy: Eventual equilibrium between virus and tumor in an ovarian cancer xenograft model. Cancer Gene Ther. 2006, 13, 732–738. [Google Scholar] [CrossRef]
- Hasegawa, K.; Pham, L.; O'Connor, M.K.; Federspiel, M.J.; Russell, S.J.; Peng, K.W. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin. Cancer Res. 2006, 12, 1868–1875. [Google Scholar] [CrossRef]
- Hasegawa, K.; Hu, C.; Nakamura, T.; Marks, J.D.; Russell, S.J.; Peng, K.W. Affinity thresholds for membrane fusion triggering by viral glycoproteins. J. Virol. 2007, 81, 13149–13157. [Google Scholar]
- Allen, C.; Vongpunsawad, S.; Nakamura, T.; James, C.D.; Schroeder, M.; Cattaneo, R.; Giannini, C.; Krempski, J.; Peng, K.W.; Goble, J.M.; et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006, 66, 11840–11850. [Google Scholar] [CrossRef]
- Liu, C.; Hasegawa, K.; Russell, S.J.; Sadelain, M.; Peng, K.W. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate 2009, 69, 1128–1141. [Google Scholar]
- Myers, R.M.; Greiner, S.M.; Harvey, M.E.; Griesmann, G.; Kuffel, M.J.; Buhrow, S.A.; Reid, J.M.; Federspiel, M.; Ames, M.M.; Dingli, D.; et al. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin. Pharmacol. Ther. 2007, 82, 700–710. [Google Scholar] [CrossRef]
- Carlson, S.K.; Classic, K.L.; Hadac, E.M.; Dingli, D.; Bender, C.E.; Kemp, B.J.; Russell, S.J. Quantitative molecular imaging of viral therapy for pancreatic cancer using an engineered measles virus expressing the sodium-iodide symporter reporter gene. Am. J. Roentgenol. 2009, 192, 279–287. [Google Scholar] [CrossRef]
- Msaouel, P.; Iankov, I.D.; Allen, C.; Morris, J.C.; von Messling, V.; Cattaneo, R.; Koutsilieris, M.; Russell, S.J.; Galanis, E. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate 2009, 69, 82–91. [Google Scholar] [CrossRef]
- Liu, C.; Russell, S.J.; Peng, K.W. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol. Ther. 2010, 18, 1155–1164. [Google Scholar] [CrossRef]
- Penheiter, A.R.; Wegman, T.R.; Classic, K.L.; Dingli, D.; Bender, C.E.; Russell, S.J.; Carlson, S.K. Sodium iodide symporter (NIS)-mediated radiovirotherapy for pancreatic cancer. Am. J. Roentgenol. 2010, 195, 341–349. [Google Scholar] [CrossRef]
- Liu, Y.P.; Tong, C.; Dispenzieri, A.; Federspiel, M.J.; Russell, S.J.; Peng, K.W. Polyinosinic acid decreases sequestration and improves systemic therapy of measles virus. Cancer Gene Ther 2012, 19, 202–211. [Google Scholar] [CrossRef]
- Opyrchal, M.; Allen, C.; Iankov, I.; Aderca, I.; Schroeder, M.; Sarkaria, J.; Galanis, E. Effective radiovirotherapy for malignant gliomas by using oncolytic measles virus strains encoding the sodium iodide symporter (MV-NIS). Hum. Gene Ther. 2012, 23, 419–427. [Google Scholar] [CrossRef]
- Li, H.; Peng, K.W.; Russell, S.J. Oncolytic measles virus encoding thyroidal sodium iodide symporter for squamous cell cancer of the head and neck radiovirotherapy. Hum. Gene Ther. 2012, 23, 295–301. [Google Scholar] [CrossRef]
- Touchefeu, Y.; Khan, A.A.; Borst, G.; Zaidi, S.H.; McLaughlin, M.; Roulstone, V.; Mansfield, D.; Kyula, J.; Pencavel, T.; Karapanagiotou, E.M.; et al. Optimising measles virus-guided radiovirotherapy with external beam radiotherapy and specific checkpoint kinase 1 inhibition. Radiother. Oncol. 2013, 108, 24–31. [Google Scholar] [CrossRef]
- Liu, Y.P.; Steele, M.B.; Suksanpaisan, L.; Federspiel, M.J.; Russell, S.J.; Peng, K.W.; Bakkum-Gamez, J.N. Oncolytic measles and vesicular stomatitis virotherapy for endometrial cancer. Gynecol. Oncol. 2014, 132, 194–202. [Google Scholar] [CrossRef]
- Künzi, V.; Oberholzer, P.A.; Heinzerling, L.; Dummer, R.; Naim, H.Y. Recombinant measles virus induces cytolysis of cutaneous T-cell lymphoma in vitro and in vivo. J. Invest. Dermatol. 2006, 126, 2525–2532. [Google Scholar] [CrossRef]
- Allen, C.; Paraskevakou, G.; Iankov, I.; Giannini, C.; Schroeder, M.; Sarkaria, J.; Schroeder, M.; Puri, R.K.; Russell, S.J.; Galanis, E. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol. Ther. 2008, 16, 1556–1564. [Google Scholar] [CrossRef]
- Hallak, L.K.; Merchan, J.R.; Storgard, C.M.; Loftus, J.C.; Russell, S.J. Targeted measles virus vector displaying echistatin infects endothelial cells via alpha(v)beta3 and leads to tumor regression. Cancer Res. 2005, 65, 5292–5300. [Google Scholar] [CrossRef]
- Ong, H.T.; Trejo, T.R.; Pham, L.D.; Oberg, A.L.; Russell, S.J.; Peng, K.W. Intravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol. Ther. 2009, 17, 1012–1021. [Google Scholar]
- Iankov, I.D.; Hillestad, M.L.; Dietz, A.B.; Russell, S.J.; Galanis, E. Converting tumor-specific markers into reporters of oncolytic virus infection. Mol. Ther. 2009, 17, 1395–1403. [Google Scholar] [CrossRef]
- Iankov, I.D.; Allen, C.; Federspiel, M.J.; Myers, R.M.; Peng, K.W.; Ingle, J.N.; Russell, S.J.; Galanis, E. Expression of immunomodulatory neutrophil-activating protein of helicobacter pylori enhances the antitumor activity of oncolytic measles virus. Mol. Ther. 2012, 20, 1139–1147. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Bossow, S.; Lampe, J.; Zimmermann, M.; Taran, F.A.; Wallwiener, D.; Fehm, T.; Bitzer, M.; Lauer, U.M. Enhanced killing of ovarian carcinoma using oncolytic measles vaccine virus armed with a yeast cytosine deaminase and uracil phosphoribosyltransferase. Gynecol. Oncol. 2013, 130, 362–368. [Google Scholar] [CrossRef]
- Yurttas, C.; Berchtold, S.; Malek, N.; Bitzer, M.; Lauer, U.M. "PULSED" versus "CONTINUOUS" application of the prodrug 5-FC for enhancing oncolytic effectiveness of a measles vaccine virus armed with a suicide gene. Hum. Gene Ther. Clin. Dev. 2014. [Google Scholar] [CrossRef]
- Friedrich, K.; Hanauer, J.R.; Prufer, S.; Munch, R.C.; Volker, I.; Filippis, C.; Jost, C.; Hanschmann, K.M.; Cattaneo, R.; Peng, K.W.; et al. DARPin-targeting of measles virus: Unique bispecificity, effective oncolysis, and enhanced safety. Mol. Ther. 2013, 21, 849–859. [Google Scholar] [CrossRef]
- ClincialTrial.gov. Available online: http://www.clinicaltrials.gov/ (accessed on 13 May 2014).
- Russell, S.J.; Federspiel, M.J.; Peng, K.W.; Tong, C.; Dingli, D.; Morice, W.G.; Lowe, V.; O'Connor, M.K.; Kyle, R.A.; Leung, N.; et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin. Proc. 2014. [Google Scholar] [CrossRef]
- Schneider-Schaulies, J.; Schneider-Schaulies, S. Receptor interactions, tropism, and mechanisms involved in morbillivirus-induced immunomodulation. Adv. Virus Res. 2008, 71, 173–205. [Google Scholar] [CrossRef]
- Bankamp, B.; Takeda, M.; Zhang, Y.; Xu, W.; Rota, P.A. Genetic characterization of measles vaccine strains. J. Infect. Dis. 2011, 204, S533–S548. [Google Scholar] [CrossRef]
- Dietzel, E.; Anderson, D.E.; Castan, A.; von Messling, V.; Maisner, A. Canine distemper virus matrix protein influences particle infectivity, particle composition, and envelope distribution in polarized epithelial cells and modulates virulence. J. Virol. 2011, 85, 7162–7168. [Google Scholar] [CrossRef]
- Del Puerto, H.L.; Martins, A.S.; Milsted, A.; Souza-Fagundes, E.M.; Braz, G.F.; Hissa, B.; Andrade, L.O.; Alves, F.; Rajao, D.S.; Leite, R.C.; et al. Canine distemper virus induces apoptosis in cervical tumor derived cell lines. Virol. J. 2011, 8, 334. [Google Scholar] [CrossRef]
- Puff, C.; Krudewig, C.; Imbschweiler, I.; Baumgärtner, W.; Alldinger, S. Influence of persistent canine distemper virus infection on expression of RECK, matrix-metalloproteinases and their inhibitors in a canine macrophage/monocytic tumour cell line (DH82). Vet. J. 2009, 182, 100–107. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Kojimoto, A.; Sakai, H.; Uchida, K.; Sugano, S.; Tateyama, S. Growth characteristics of canine distemper virus in a new cell line CCT cells originated from canine malignant histiocytosis. J. Vet. Med. Sci. 2005, 67, 203–206. [Google Scholar] [CrossRef]
- Gröne, A.; Fonfara, S.; Baumgärtner, W. Cell type-dependent cytokine expression after canine distemper virus infection. Viral. Immunol. 2002, 15, 493–505. [Google Scholar] [CrossRef]
- Bourboulia, D.; Stetler-Stevenson, W.G. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin. Cancer Biol. 2010, 20, 161–168. [Google Scholar]
- Nagini, S. Recking mmp: Relevance of reversion-inducing cysteine-rich protein with Kazal motifs as a prognostic marker and therapeutic target for cancer (a review). Anticancer Agents Med. Chem. 2012, 12, 718–725. [Google Scholar] [CrossRef]
- Noda, M.; Oh, J.; Takahashi, R.; Kondo, S.; Kitayama, H.; Takahashi, C. Reck: A novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis. Rev. 2003, 22, 167–175. [Google Scholar] [CrossRef]
- Vihinen, P.; Kahari, V.M. Matrix metalloproteinases in cancer: Prognostic markers and therapeutic targets. Int. J. Cancer 2002, 99, 157–166. [Google Scholar] [CrossRef]
- Mao, X.; Liu, L.; Zhang, B.; Zhang, D. Reversion-inducing cysteine-rich protein with Kazal motifs gene expression and its clinical significance in peripheral T-cell lymphoma. Oncol. Lett. 2013, 5, 1867–1871. [Google Scholar]
- Stenzinger, A.; von Winterfeld, M.; Rabien, A.; Warth, A.; Kamphues, C.; Dietel, M.; Weichert, W.; Klauschen, F.; Wittschieber, D. Reversion-inducing cysteine-rich protein with kazal motif (RECK) expression: An independent prognostic marker of survival in colorectal cancer. Hum. Pathol. 2012, 43, 1314–1321. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, S.; Jiang, L.; Zhang, S.; Li, W.; Chen, Z.; Zhang, D. Expression of RECK and MMP-2 in salivary adenoid cystic carcinoma: Correlation with tumor progression and patient prognosis. Oncol. Lett. 2014, 7, 1549–1555. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lapp, S.; Pfankuche, V.M.; Baumgärtner, W.; Puff, C. Viral Oncolysis — Can Insights from Measles Be Transferred to Canine Distemper Virus? Viruses 2014, 6, 2340-2375. https://doi.org/10.3390/v6062340
Lapp S, Pfankuche VM, Baumgärtner W, Puff C. Viral Oncolysis — Can Insights from Measles Be Transferred to Canine Distemper Virus? Viruses. 2014; 6(6):2340-2375. https://doi.org/10.3390/v6062340
Chicago/Turabian StyleLapp, Stefanie, Vanessa M. Pfankuche, Wolfgang Baumgärtner, and Christina Puff. 2014. "Viral Oncolysis — Can Insights from Measles Be Transferred to Canine Distemper Virus?" Viruses 6, no. 6: 2340-2375. https://doi.org/10.3390/v6062340
APA StyleLapp, S., Pfankuche, V. M., Baumgärtner, W., & Puff, C. (2014). Viral Oncolysis — Can Insights from Measles Be Transferred to Canine Distemper Virus? Viruses, 6(6), 2340-2375. https://doi.org/10.3390/v6062340




