The Tumor-Associated Marker, PVRL4 (Nectin-4), Is the Epithelial Receptor for Morbilliviruses
Abstract
:1. Introduction
2. Routes of Morbillivirus Entry
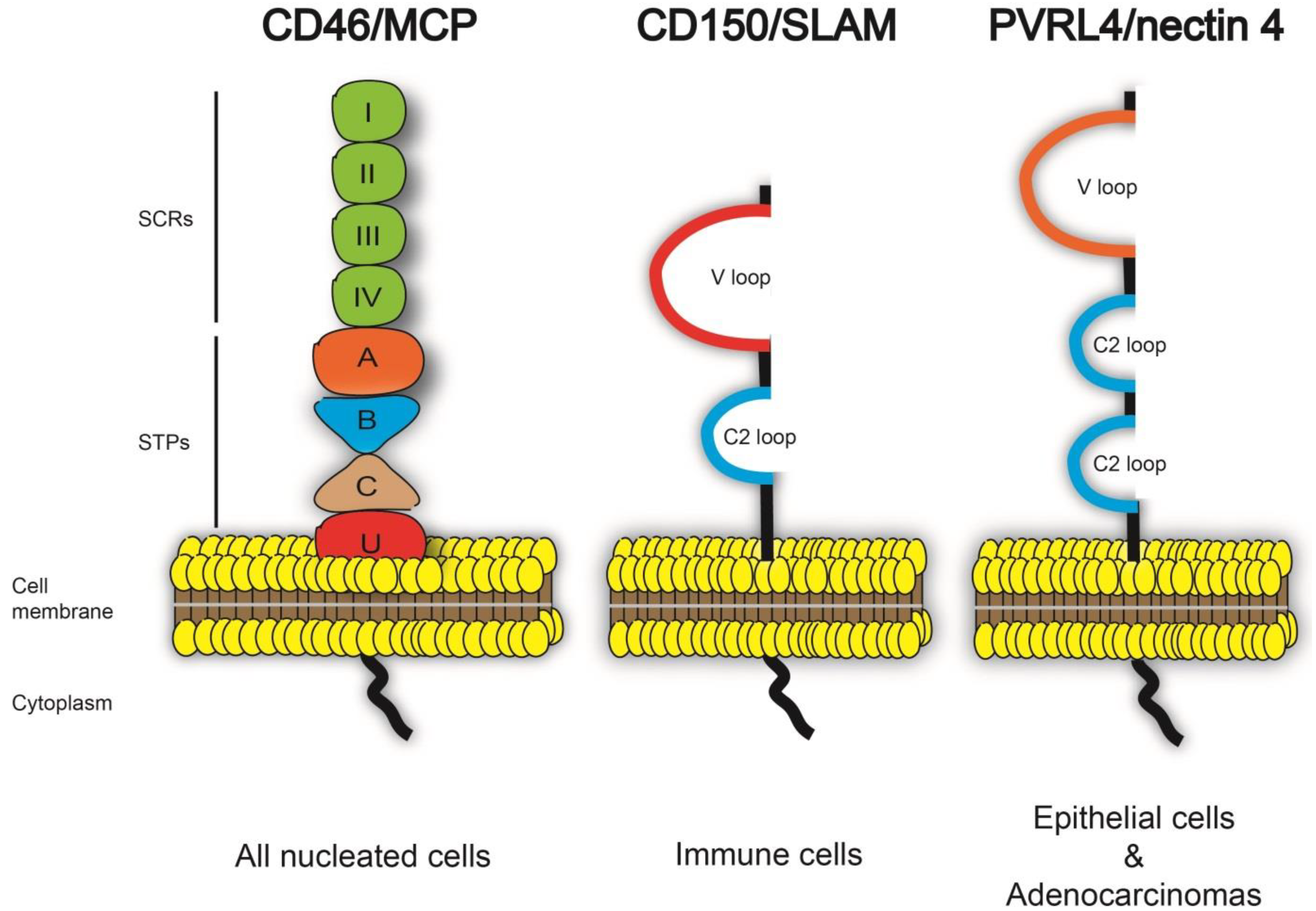

2.1. PVRL4 Expression and Distribution
3. Structural Insights into the Interaction of Morbilliviruses with PVRL4
3.1. The V Domain of PVRL4 is Essential for Morbillivirus Entry
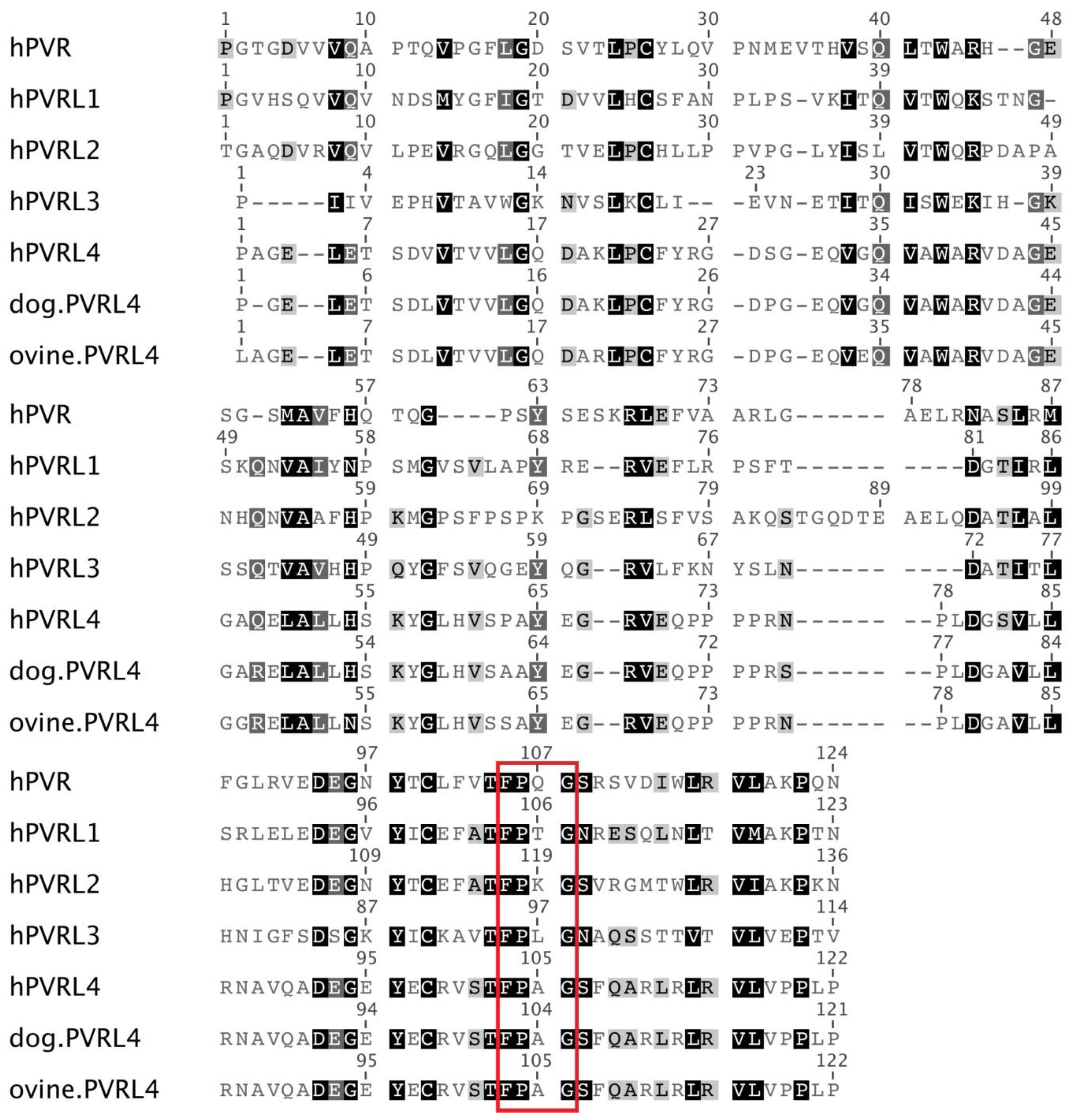
3.2. High Similarity between the PVRL4 Receptors from Different Origins Might Weaken the Species Barrier for Morbillivirus
4. The Oncolytic Potential of Measles Virus
4.1. MeV Receptor Expression in Cancer Cells

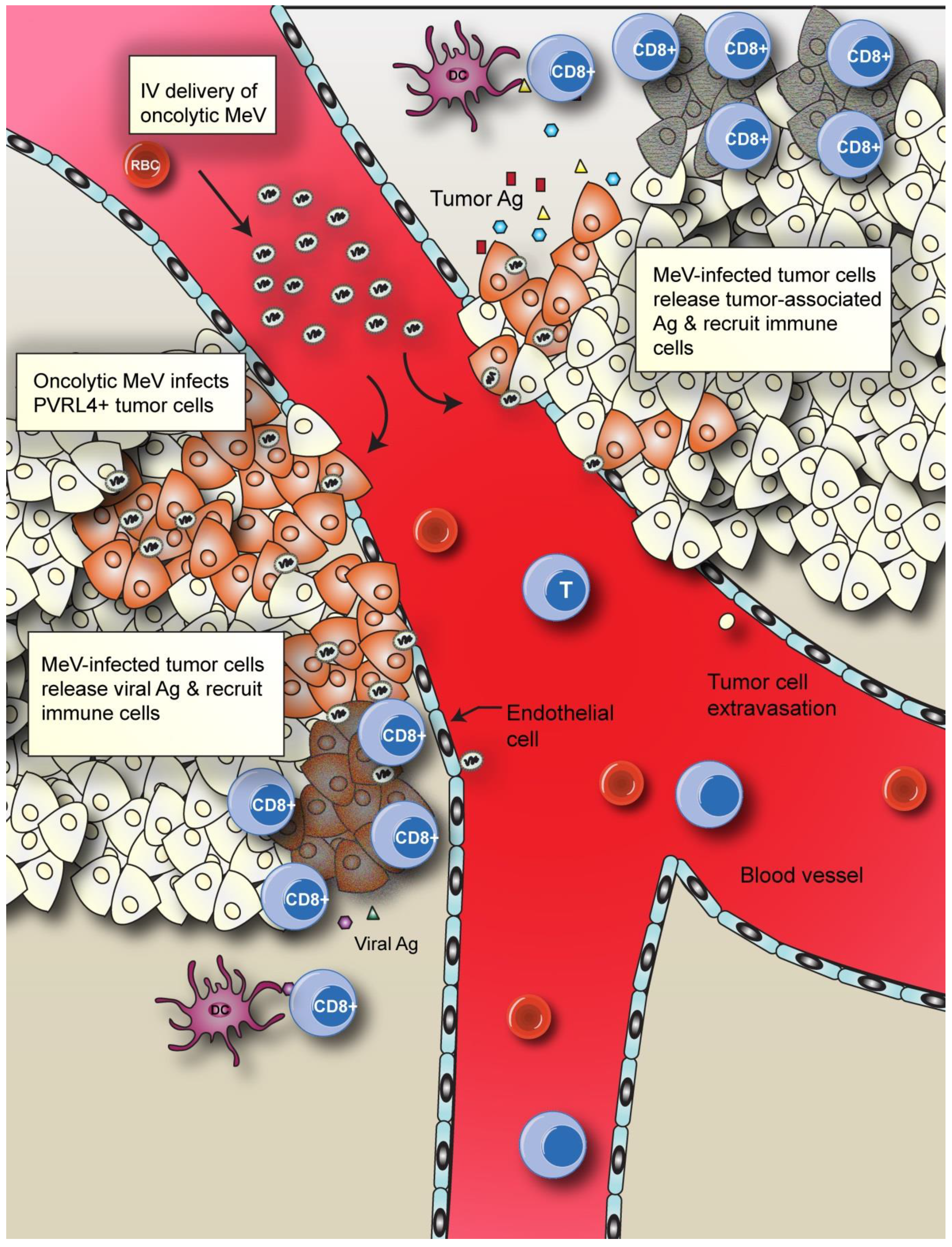
4.2. Enhancing MeV Oncolytic Activity and Anti-Tumor Immunity
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- McChesney, M.B.; Miller, C.J.; Rota, P.A.; Zhu, Y.D.; Antipa, L.; Lerche, N.W.; Ahmed, R.; Bellini, W.J. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology 1997, 233, 74–84. [Google Scholar] [CrossRef]
- Appel, M.J. Distemper pathogenesis in dogs. J. Am. Vet. Med. Assoc. 1970, 156, 1681–1684. [Google Scholar]
- Barrett, T. Morbillivirus infections, with special emphasis on morbilliviruses of carnivores. Vet. Microbiol. 1999, 69, 3–13. [Google Scholar] [CrossRef]
- Deem, S.L.; Spelman, L.H.; Yates, R.A.; Montali, R.J. Canine distemper in terrestrial carnivores: A review. J. Zoo Wildl. Med. 2000, 31, 441–451. [Google Scholar]
- Banyard, A.C.; Parida, S.; Batten, C.; Oura, C.; Kwiatek, O.; Libeau, G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J. Gen. Virol. 2010, 91, 2885–2897. [Google Scholar] [CrossRef] [Green Version]
- Griffin, D.E.; Knipe, D.M.; Lamb, R.A.; Martin, M.A.; Roizman, B.; Straus, S.E. Measles Virus. In fields Virology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1551–1586. [Google Scholar]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgartner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef]
- De Vries, R.D.; Mesman, A.W.; Geijtenbeek, T.B.; Duprex, W.P.; de Swart, R.L. The pathogenesis of measles. Curr. Opin. Virol. 2012, 2, 248–255. [Google Scholar] [CrossRef]
- Avota, E.; Gassert, E.; Schneider-Schaulies, S. Measles virus-induced immunosuppression: From effectors to mechanisms. Med. Microbiol. Immunol. 2010, 199, 227–237. [Google Scholar] [CrossRef]
- Beckford, A.P.; Kaschula, R.O.; Stephen, C. Factors associated with fatal cases of measles. A retrospective autopsy study. S Afr. Med. J. 1985, 68, 858–863. [Google Scholar]
- Cosby, S.L.; Duprex, W.P.; Hamill, L.A.; Ludlow, M.; McQuaid, S. Approaches in the understanding of morbillivirus neurovirulence. J. Neurovirol. 2002, 8 (Suppl. 2), 85–90. [Google Scholar] [CrossRef]
- Wichmann, O.; Siedler, A.; Sagebiel, D.; Hellenbrand, W.; Santibanez, S.; Mankertz, A.; Vogt, G.; Treeck, U.; Krause, G. Further efforts needed to achieve measles elimination in Germany: results of an outbreak investigation. Bull. World Health Organ. 2009, 87, 108–115. [Google Scholar] [CrossRef]
- Carabin, H.; Edmunds, W.J.; Kou, U.; van den Hof, S.; Nguyen, V.H. The average cost of measles cases and adverse events following vaccination in industrialised countries. BMC Public Health 2002, 2. [Google Scholar] [CrossRef] [Green Version]
- Filia, A.; Tavilla, A.; Bella, A.; Magurano, F.; Ansaldi, F.; Chironna, M.; Nicoletti, L.; Palu, G.; Iannazzo, S.; Declich, S.; et al. Measles in Italy, July 2009 to September 2010. Euro. Surveill. 2011, 16, pii: 19925. [Google Scholar]
- Abubakar, M.; Munir, M. Peste des Petits Ruminants Virus: An Emerging Threat to Goat Farming in Pakistan. Transbound. Emerg. Dis. 2014. [Google Scholar] [CrossRef]
- Libeau, G.; Diallo, A.; Parida, S. Evolutionary genetics underlying the spread of peste des petits ruminants virus. Animal Frontiers 2014, 4, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Sanchez, I.R.; Vijayaraghavan, M.; Barskey, A.E.; Wallace, G.S. The economic burden of sixteen measles outbreaks on United States public health departments in 2011. Vaccine 2014, 32, 1311–1317. [Google Scholar] [CrossRef]
- Noyce, R.S.; Delpeut, S.; Richardson, C.D. Dog nectin-4 is an epithelial cell receptor for canine distemper virus that facilitates virus entry and syncytia formation. Virology 2013, 436, 210–220. [Google Scholar]
- Birch, J.; Juleff, N.; Heaton, M.P.; Kalbfleisch, T.; Kijas, J.; Bailey, D. Characterization of ovine Nectin-4, a novel peste des petits ruminants virus receptor. J. Virol. 2013, 87, 4756–4761. [Google Scholar]
- Pratakpiriya, W.; Seki, F.; Otsuki, N.; Sakai, K.; Fukuhara, H.; Katamoto, H.; Hirai, T.; Maenaka, K.; Techangamsuwan, S.; Lan, N.T.; et al. Nectin4 is an epithelial cell receptor for canine distemper virus and involved in neurovirulence. J. Virol. 2012, 86, 10207–10210. [Google Scholar] [CrossRef]
- Noyce, R.S.; Bondre, D.G.; Ha, M.N.; Lin, L.T.; Sisson, G.; Tsao, M.S.; Richardson, C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011, 7, e1002240. [Google Scholar] [CrossRef]
- Muhlebach, M.D.; Mateo, M.; Sinn, P.L.; Prufer, S.; Uhlig, K.M.; Leonard, V.H.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 530–533. [Google Scholar]
- Tatsuo, H.; Ono, N.; Yanagi, Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 2001, 75, 5842–5850. [Google Scholar] [CrossRef]
- Hsu, E.C.; Iorio, C.; Sarangi, F.; Khine, A.A.; Richardson, C.D. CDw150(SLAM) is a receptor for a lymphotropic strain of measles virus and may account for the immunosuppressive properties of this virus. Virology 2001, 279, 9–21. [Google Scholar] [CrossRef]
- Tatsuo, H.; Ono, N.; Tanaka, K.; Yanagi, Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000, 406, 893–897. [Google Scholar] [CrossRef]
- Dorig, R.E.; Marcil, A.; Chopra, A.; Richardson, C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 1993, 75, 295–305. [Google Scholar] [CrossRef]
- Naniche, D.; Varior-Krishnan, G.; Cervoni, F.; Wild, T.F.; Rossi, B.; Rabourdin-Combe, C.; Gerlier, D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 1993, 67, 6025–6032. [Google Scholar]
- Baker, K.A.; Dutch, R.E.; Lamb, R.A.; Jardetzky, T.S. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell 1999, 3, 309–319. [Google Scholar] [CrossRef]
- Colman, P.M.; Lawrence, M.C. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell. Biol. 2003, 4, 309–319. [Google Scholar] [CrossRef]
- Hernandez, L.D.; Hoffman, L.R.; Wolfsberg, T.G.; White, J.M. Virus-cell and cell-cell fusion. Annu Rev. Cell Dev. Biol. 1996, 12, 627–661. [Google Scholar] [CrossRef]
- Takimoto, T.; Taylor, G.L.; Connaris, H.C.; Crennell, S.J.; Portner, A. Role of the hemagglutinin-neuraminidase protein in the mechanism of paramyxovirus-cell membrane fusion. J. Virol. 2002, 76, 13028–13033. [Google Scholar] [CrossRef]
- Cocks, B.G.; Chang, C.C.; Carballido, J.M.; Yssel, H.; de Vries, J.E.; Aversa, G. A novel receptor involved in T-cell activation. Nature 1995, 376, 260–263. [Google Scholar] [CrossRef]
- Sidorenko, S.P.; Clark, E.A. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J. Immunol 1993, 151, 4614–4624. [Google Scholar]
- Von Messling, V.; Milosevic, D.; Cattaneo, R. Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc. Natl Acad Sci USA 2004, 101, 14216–14221. [Google Scholar] [CrossRef]
- Lemon, K.; de Vries, R.D.; Mesman, A.W.; McQuaid, S.; van Amerongen, G.; Yuksel, S.; Ludlow, M.; Rennick, L.J.; Kuiken, T.; Rima, B.K.; et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 2011, 7, e1001263. [Google Scholar] [CrossRef]
- De Swart, R.L.; Ludlow, M.; de Witte, L.; Yanagi, Y.; van Amerongen, G.; McQuaid, S.; Yuksel, S.; Geijtenbeek, T.B.; Duprex, W.P.; Osterhaus, A.D. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007, 3, e178. [Google Scholar] [CrossRef]
- Sawatsky, B.; Wong, X.X.; Hinkelmann, S.; Cattaneo, R.; von Messling, V. Canine distemper virus epithelial cell infection is required for clinical disease but not for immunosuppression. J. Virol. 2012, 86, 3658–3666. [Google Scholar] [CrossRef]
- Fischer, C.D.; Ikuta, N.; Canal, C.W.; Makiejczuk, A.; Allgayer Mda, C.; Cardoso, C.H.; Lehmann, F.K.; Fonseca, A.S.; Lunge, V.R. Detection and differentiation of field and vaccine strains of canine distemper virus using reverse transcription followed by nested real time PCR (RT-nqPCR) and RFLP analysis. J. Virol. Methods 2013, 194, 39–45. [Google Scholar] [CrossRef]
- Kim, D.; Jeoung, S.Y.; Ahn, S.J.; Lee, J.H.; Pak, S.I.; Kwon, H.M. Comparison of tissue and fluid samples for the early detection of canine distemper virus in experimentally infected dogs. J. Vet. Med. Sci. 2006, 68, 877–879. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X.; Wang, Z.; Bao, J.; Li, L.; Zhao, Y.; Li, J. Virus excretion and antibody dynamics in goats inoculated with a field isolate of peste des petits ruminants virus. Transbound. Emerg. Dis. 2013, 60 (Suppl. 2), 63–68. [Google Scholar]
- Rota, P.A.; Khan, A.S.; Durigon, E.; Yuran, T.; Villamarzo, Y.S.; Bellini, W.J. Detection of measles virus RNA in urine specimens from vaccine recipients. J. Clin. Microbiol. 1995, 33, 2485–2488. [Google Scholar]
- Saito, T.B.; Alfieri, A.A.; Wosiacki, S.R.; Negrao, F.J.; Morais, H.S.; Alfieri, A.F. Detection of canine distemper virus by reverse transcriptase-polymerase chain reaction in the urine of dogs with clinical signs of distemper encephalitis. Res. Vet. Sci. 2006, 80, 116–119. [Google Scholar] [CrossRef]
- Scheifele, D.W.; Forbes, C.E. Prolonged giant cell excretion in severe African measles. Pediatrics 1972, 50, 867–873. [Google Scholar]
- Cleaveland, S.; Appel, M.G.; Chalmers, W.S.; Chillingworth, C.; Kaare, M.; Dye, C. Serological and demographic evidence for domestic dogs as a source of canine distemper virus infection for Serengeti wildlife. Vet. Microbiol. 2000, 72, 217–227. [Google Scholar] [CrossRef]
- Qiu, W.; Zheng, Y.; Zhang, S.; Fan, Q.; Liu, H.; Zhang, F.; Wang, W.; Liao, G.; Hu, R. Canine distemper outbreak in rhesus monkeys, China. Emerg. Infect. Dis. 2011, 17, 1541–1543. [Google Scholar]
- Sun, Z.; Li, A.; Ye, H.; Shi, Y.; Hu, Z.; Zeng, L. Natural infection with canine distemper virus in hand-feeding Rhesus monkeys in China. Vet. Microbiol. 2010, 141, 374–378. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Ochikubo, F.; Matsubara, Y.; Tsuruoka, H.; Ishii, M.; Shirota, K.; Nomura, Y.; Sugiyama, M.; Yamanouchi, K. Natural infection with canine distemper virus in a Japanese monkey (Macaca fuscata). Vet. Microbiol. 1989, 20, 193–205. [Google Scholar] [CrossRef]
- Schneider-Schaulies, J. Cellular receptors for viruses: links to tropism and pathogenesis. J. Gen. Virol. 2000, 81, 1413–1429. [Google Scholar]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; Wernerus, H.; Bjorling, L.; Ponten, F. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, pp. 1248–1250. Available online: http://www.proteinatlas.org (accessed on 12 May 2013).
- Tahara, M.; Takeda, M.; Shirogane, Y.; Hashiguchi, T.; Ohno, S.; Yanagi, Y. Measles virus infects both polarized epithelial and immune cells by using distinctive receptor-binding sites on its hemagglutinin. J. Virol 2008, 82, 4630–4637. [Google Scholar] [CrossRef]
- Ludlow, M.; Rennick, L.J.; Sarlang, S.; Skibinski, G.; McQuaid, S.; Moore, T.; de Swart, R.L.; Duprex, W.P. Wild-type measles virus infection of primary epithelial cells occurs via the basolateral surface without syncytium formation or release of infectious virus. J. Gen. Virol. 2010, 91, 971–979. [Google Scholar] [CrossRef]
- Leonard, V.H.; Sinn, P.L.; Hodge, G.; Miest, T.; Devaux, P.; Oezguen, N.; Braun, W.; McCray, P.B., Jr.; McChesney, M.B.; Cattaneo, R. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Invest. 2008, 118, 2448–2458. [Google Scholar]
- Sinn, P.L.; Williams, G.; Vongpunsawad, S.; Cattaneo, R.; McCray, P.B., Jr. Measles virus preferentially transduces the basolateral surface of well-differentiated human airway epithelia. J. Virol. 2002, 76, 2403–2409. [Google Scholar] [CrossRef]
- Ludlow, M.; Lemon, K.; de Vries, R.D.; McQuaid, S.; Millar, E.L.; van Amerongen, G.; Yuksel, S.; Verburgh, R.J.; Osterhaus, A.D.; de Swart, R.L.; et al. Measles virus infection of epithelial cells in the macaque upper respiratory tract is mediated by subepithelial immune cells. J. Virol. 2013, 87, 4033–4042. [Google Scholar] [CrossRef]
- Frenzke, M.; Sawatsky, B.; Wong, X.X.; Delpeut, S.; Mateo, M.; Cattaneo, R.; von Messling, V. Nectin-4-dependent measles virus spread to the cynomolgus monkey tracheal epithelium: Role of infected immune cells infiltrating the lamina propria. J. Virol. 2013, 87, 2526–2534. [Google Scholar] [CrossRef]
- Ludlow, M.; de Vries, R.D.; Lemon, K.; McQuaid, S.; Millar, E.; van Amerongen, G.; Yuksel, S.; Verburgh, R.J.; Osterhaus, A.D.; de Swart, R.L.; et al. Infection of lymphoid tissues in the macaque upper respiratory tract contributes to the emergence of transmissible measles virus. J. Gen. Virol. 2013, 94, 1933–1944. [Google Scholar] [CrossRef]
- Blau, D.M.; Compans, R.W. Entry and release of measles virus are polarized in epithelial cells. Virology 1995, 210, 91–99. [Google Scholar] [CrossRef]
- Blau, D.M.; Compans, R.W. Adaptation of measles virus to polarized epithelial cells: Alterations in virus entry and release. Virology 1997, 231, 281–289. [Google Scholar] [CrossRef]
- Maisner, A.; Klenk, H.; Herrler, G. Polarized budding of measles virus is not determined by viral surface glycoproteins. J. Virol. 1998, 72, 5276–5278. [Google Scholar]
- Naim, H.Y.; Ehler, E.; Billeter, M.A. Measles virus matrix protein specifies apical virus release and glycoprotein sorting in epithelial cells. Embo J. 2000, 19, 3576–3585. [Google Scholar] [CrossRef]
- Couacy-Hymann, E.; Bodjo, C.; Danho, T.; Libeau, G.; Diallo, A. Evaluation of the virulence of some strains of peste-des-petits-ruminants virus (PPRV) in experimentally infected West African dwarf goats. Vet. J. 2007, 173, 178–183. [Google Scholar] [CrossRef]
- Hammouchi, M.; Loutfi, C.; Sebbar, G.; Touil, N.; Chaffai, N.; Batten, C.; Harif, B.; Oura, C.; El Harrak, M. Experimental infection of alpine goats with a Moroccan strain of peste des petits ruminants virus (PPRV). Vet. Microbiol. 2012, 160, 240–244. [Google Scholar] [CrossRef]
- Ward, S.V.; George, C.X.; Welch, M.J.; Liou, L.Y.; Hahm, B.; Lewicki, H.; de la Torre, J.C.; Samuel, C.E.; Oldstone, M.B. RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 331–336. [Google Scholar] [CrossRef]
- Kurita, S.; Ogita, H.; Takai, Y. Cooperative role of nectin-nectin and nectin-afadin interactions in formation of nectin-based cell-cell adhesion. J. Biol. Chem. 2011, 286, 36297–3303. [Google Scholar] [CrossRef]
- Takai, Y.; Miyoshi, J.; Ikeda, W.; Ogita, H. Nectins and nectin-like molecules: Roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 2008, 9, 603–615. [Google Scholar] [CrossRef]
- Takai, Y.; Ikeda, W.; Ogita, H.; Rikitake, Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell Dev. Biol. 2008, 24, 309–342. [Google Scholar] [CrossRef]
- Mendelsohn, C.L.; Wimmer, E.; Racaniello, V.R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 1989, 56, 855–865. [Google Scholar] [CrossRef]
- Campadelli-Fiume, G.; Cocchi, F.; Menotti, L.; Lopez, M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 2000, 10, 305–319. [Google Scholar] [CrossRef]
- Fabre, S.; Reymond, N.; Cocchi, F.; Menotti, L.; Dubreuil, P.; Campadelli-Fiume, G.; Lopez, M. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C’-C”-D beta-strands of the nectin1 V domain. J. Biol. Chem. 2002, 277, 27006–27013. [Google Scholar]
- Satoh-Horikawa, K.; Nakanishi, H.; Takahashi, K.; Miyahara, M.; Nishimura, M.; Tachibana, K.; Mizoguchi, A.; Takai, Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 2000, 275, 10291–10299. [Google Scholar] [CrossRef]
- Reymond, N.; Fabre, S.; Lecocq, E.; Adelaide, J.; Dubreuil, P.; Lopez, M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J. Biol. Chem. 2001, 276, 43205–43215. [Google Scholar]
- Yasumi, M.; Shimizu, K.; Honda, T.; Takeuchi, M.; Takai, Y. Role of each immunoglobulin-like loop of nectin for its cell-cell adhesion activity. Biochem. Biophys. Res. Commun. 2003, 302, 61–66. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Nakanishi, H.; Kimura, K.; Matsubara, K.; Ozaki-Kuroda, K.; Katata, T.; Honda, T.; Kiyohara, Y.; Heo, K.; Higashi, M.; et al. Nectin: An adhesion molecule involved in formation of synapses. J. Cell Biol. 2002, 156, 555–565. [Google Scholar] [CrossRef]
- Ono, N.; Tatsuo, H.; Tanaka, K.; Minagawa, H.; Yanagi, Y. V domain of human SLAM (CDw150) is essential for its function as a measles virus receptor. J. Virol. 2001, 75, 1594–1600. [Google Scholar] [CrossRef]
- Delpeut, S.; Noyce, R.S.; Richardson, C.D. The V domain of dog PVRL4 (nectin-4) mediates canine distemper virus entry and virus cell-to-cell spread. Virology 2014, 454–455, 109–117. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, G.; Qi, J.; Li, Y.; He, Y.; Xu, X.; Shi, J.; Zhang, C.W.; Yan, J.; Gao, G.F. Structure of measles virus hemagglutinin bound to its epithelial receptor nectin-4. Nat. Struct. Mol. Biol. 2013, 20, 67–72. [Google Scholar]
- Harrison, O.J.; Vendome, J.; Brasch, J.; Jin, X.; Hong, S.; Katsamba, P.S.; Ahlsen, G.; Troyanovsky, R.B.; Troyanovsky, S.M.; Honig, B.; et al. Nectin ectodomain structures reveal a canonical adhesive interface. Nat. Struct. Mol. Biol. 2012, 19, 906–915. [Google Scholar]
- Drumond, A.; Ashton, B.; Buxton, S.; Cheung, M.; Heled, J.; Kearse, M.; Moir, R.; Stones-Havas, S.; Sturrock, S.; Thierer, T.; Wilson, A. Geneious v5.0. Available online: http://www.geneious.com (accessed on 1 March 2014).
- Bieringer, M.; Han, J.W.; Kendl, S.; Khosravi, M.; Plattet, P.; Schneider-Schaulies, J. Experimental adaptation of wild-type canine distemper virus (CDV) to the human entry receptor CD150. PLoS One 2013, 8, e57488. [Google Scholar]
- Sakai, K.; Yoshikawa, T.; Seki, F.; Fukushi, S.; Tahara, M.; Nagata, N.; Ami, Y.; Mizutani, T.; Kurane, I.; Yamaguchi, R.; et al. Canine distemper virus associated with a lethal outbreak in monkeys can readily adapt to use human receptors. J. Virol. 2013, 87, 7170–7175. [Google Scholar] [CrossRef]
- Otsuki, N.; Sekizuka, T.; Seki, F.; Sakai, K.; Kubota, T.; Nakatsu, Y.; Chen, S.; Fukuhara, H.; Maenaka, K.; Yamaguchi, R.; et al. Canine distemper virus with the intact C protein has the potential to replicate in human epithelial cells by using human nectin4 as a receptor. Virology 2013, 435, 485–492. [Google Scholar] [CrossRef]
- De Vries, R.D.; Ludlow, M.; Verburgh, R.J.; van Amerongen, G.; Yuksel, S.; Nguyen, D.T.; McQuaid, S.; Osterhaus, A.D.; Duprex, W.P.; de Swart, R.L. Measles Vaccination of Non-Human Primates Provides Partial Protection against Infection with Canine Distemper Virus. J. Virol. 2014, 88, 4423–4433. [Google Scholar] [CrossRef]
- Beauverger, P.; Buckland, R.; Wild, T.F. Measles virus antigens induce both type-specific and canine distemper virus cross-reactive cytotoxic T lymphocytes in mice: localization of a common Ld-restricted nucleoprotein epitope. J. Gen. Virol. 1993, 74, 2357–2363. [Google Scholar] [CrossRef]
- Beauverger, P.; Chadwick, J.; Buckland, R.; Wild, T.F. Serotype-specific and canine distemper virus cross-reactive H-2Kk-restricted cytotoxic T lymphocyte epitopes in the measles virus nucleoprotein. Virology 1994, 203, 172–177. [Google Scholar] [CrossRef]
- Stephenson, J.R.; Ter Meulen, V. Antigenic relationships between measles and canine distemper viruses: Comparison of immune response in animals and humans to individual virus-specific polypeptides. Proc. Natl. Acad. Sci. USA 1979, 76, 6601–6605. [Google Scholar] [CrossRef]
- Knuchel, M.C.; Marty, R.R.; Morin, T.N.; Ilter, O.; Zuniga, A.; Naim, H.Y. Relevance of a pre-existing measles immunity prior immunization with a recombinant measles virus vector. Hum. Vaccin. Immunother. 2013, 9, 23241. [Google Scholar]
- Lorin, C.; Mollet, L.; Delebecque, F.; Combredet, C.; Hurtrel, B.; Charneau, P.; Brahic, M.; Tangy, F. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J. Virol. 2004, 78, 146–157. [Google Scholar] [CrossRef]
- Msaouel, P.; Opyrchal, M.; Domingo Musibay, E.; Galanis, E. Oncolytic measles virus strains as novel anticancer agents. Expert Opin. Biol. Ther. 2013, 13, 483–502. [Google Scholar] [CrossRef]
- Rager-Zisman, B.; Bazarsky, E.; Skibin, A.; Chamney, S.; Belmaker, I.; Shai, I.; Kordysh, E.; Griffin, D.E. The effect of measles-mumps-rubella (MMR) immunization on the immune responses of previously immunized primary school children. Vaccine 2003, 21, 2580–2588. [Google Scholar] [CrossRef]
- Singh, M.; Cattaneo, R.; Billeter, M.A. A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice. J. Virol. 1999, 73, 4823–4828. [Google Scholar]
- Wong-Chew, R.M.; Beeler, J.A.; Audet, S.; Santos, J.I. Cellular and humoral immune responses to measles in immune adults re-immunized with measles vaccine. J. Med. Virol. 2003, 70, 276–280. [Google Scholar] [CrossRef]
- Nakamura, T.; Russell, S.J. Oncolytic measles viruses for cancer therapy. Expert Opin. Biol. Ther 2004, 4, 1685–1692. [Google Scholar] [CrossRef]
- Bazan-Peregrino, M.; Sainson, R.C.; Carlisle, R.C.; Thoma, C.; Waters, R.A.; Arvanitis, C.; Harris, A.L.; Hernandez-Alcoceba, R.; Seymour, L.W. Combining virotherapy and angiotherapy for the treatment of breast cancer. Cancer Gene Ther. 2013, 20, 461–468. [Google Scholar]
- Bluming, A.Z.; Ziegler, J.L. Regression of Burkitt's lymphoma in association with measles infection. Lancet 1971, 2, 105–106. [Google Scholar] [CrossRef]
- Taqi, A.M.; Abdurrahman, M.B.; Yakubu, A.M.; Fleming, A.F. Regression of Hodgkin’s disease after measles. Lancet 1981, 1. [Google Scholar] [CrossRef]
- Nagy, N.; Cerboni, C.; Mattsson, K.; Maeda, A.; Gogolak, P.; Sumegi, J.; Lanyi, A.; Szekely, L.; Carbone, E.; Klein, G.; et al. SH2D1A and SLAM protein expression in human lymphocytes and derived cell lines. Int. J. Cancer 2000, 88, 439–447. [Google Scholar] [CrossRef]
- Parrula, C.; Fernandez, S.A.; Zimmerman, B.; Lairmore, M.; Niewiesk, S. Measles virotherapy in a mouse model of adult T-cell leukaemia/lymphoma. J. Gen. Virol. 2011, 92, 1458–1466. [Google Scholar] [CrossRef]
- Grote, D.; Russell, S.J.; Cornu, T.I.; Cattaneo, R.; Vile, R.; Poland, G.A.; Fielding, A.K. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood 2001, 97, 3746–3754. [Google Scholar] [CrossRef]
- Seya, T.; Taniguchi, M.; Matsumoto, M. [Membrane cofactor protein (MCP, CD46)]. Nihon Rinsho 1999, 57, 75–78. [Google Scholar]
- Ong, H.T.; Timm, M.M.; Greipp, P.R.; Witzig, T.E.; Dispenzieri, A.; Russell, S.J.; Peng, K.W. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp. Hematol. 2006, 34, 713–720. [Google Scholar] [CrossRef]
- Bjorge, L.; Hakulinen, J.; Wahlstrom, T.; Matre, R.; Meri, S. Complement-regulatory proteins in ovarian malignancies. Int. J. Cancer 1997, 70, 14–25. [Google Scholar] [CrossRef]
- Varsano, S.; Rashkovsky, L.; Shapiro, H.; Radnay, J. Cytokines modulate expression of cell-membrane complement inhibitory proteins in human lung cancer cell lines. Am. J. Respir. Cell Mol. Biol. 1998, 19, 522–529. [Google Scholar] [CrossRef]
- Blok, V.T.; Daha, M.R.; Tijsma, O.M.; Weissglas, M.G.; van den Broek, L.J.; Gorter, A. A possible role of CD46 for the protection in vivo of human renal tumor cells from complement-mediated damage. Lab. Invest. 2000, 80, 335–344. [Google Scholar]
- Simpson, K.L.; Jones, A.; Norman, S.; Holmes, C.H. Expression of the complement regulatory proteins decay accelerating factor (DAF, CD55), membrane cofactor protein (MCP, CD46) and CD59 in the normal human uterine cervix and in premalignant and malignant cervical disease. Am. J. Pathol. 1997, 151, 1455–1467. [Google Scholar]
- Kinugasa, N.; Higashi, T.; Nouso, K.; Nakatsukasa, H.; Kobayashi, Y.; Ishizaki, M.; Toshikuni, N.; Yoshida, K.; Uematsu, S.; Tsuji, T. Expression of membrane cofactor protein (MCP, CD46) in human liver diseases. Br. J. Cancer 1999, 80, 1820–1825. [Google Scholar] [CrossRef]
- Juhl, H.; Helmig, F.; Baltzer, K.; Kalthoff, H.; Henne-Bruns, D.; Kremer, B. Frequent expression of complement resistance factors CD46, CD55, and CD59 on gastrointestinal cancer cells limits the therapeutic potential of monoclonal antibody 17–1A. J. Surg. Oncol. 1997, 64, 222–230. [Google Scholar] [CrossRef]
- Anderson, B.D.; Nakamura, T.; Russell, S.J.; Peng, K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004, 64, 4919–4926. [Google Scholar] [CrossRef]
- Peng, K.W.; TenEyck, C.J.; Galanis, E.; Kalli, K.R.; Hartmann, L.C.; Russell, S.J. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002, 62, 4656–4662. [Google Scholar]
- Fabre-Lafay, S.; Garrido-Urbani, S.; Reymond, N.; Goncalves, A.; Dubreuil, P.; Lopez, M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 2005, 280, 19543–19550. [Google Scholar]
- Takano, A.; Ishikawa, N.; Nishino, R.; Masuda, K.; Yasui, W.; Inai, K.; Nishimura, H.; Ito, H.; Nakayama, H.; Miyagi, Y.; et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009, 69, 6694–6703. [Google Scholar] [CrossRef]
- Derycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 overexpression in ovarian cancer tissues and serum: Potential role as a serum biomarker. Am. J. Clin Pathol 2011, 134, 835–845. [Google Scholar]
- Pavlova, N.N.; Pallasch, C.; Elia, A.E.; Braun, C.J.; Westbrook, T.F.; Hemann, M.; Elledge, S.J. A role for PVRL4-driven cell-cell interactions in tumorigenesis. Elife 2013, 2, e00358. [Google Scholar] [CrossRef]
- Sugiyama, T.; Yoneda, M.; Kuraishi, T.; Hattori, S.; Inoue, Y.; Sato, H.; Kai, C. Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther. 2013, 20, 338–347. [Google Scholar] [CrossRef]
- Cao, L.; Si, J.; Wang, W.; Zhao, X.; Yuan, X.; Zhu, H.; Wu, X.; Zhu, J.; Shen, G. Intracellular localization and sustained prodrug cell killing activity of TAT-HSVTK fusion protein in hepatocelullar carcinoma cells. Mol. Cells 2006, 21, 104–111. [Google Scholar]
- Fillat, C.; Carrio, M.; Cascante, A.; Sangro, B. Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: fifteen years of application. Curr. Gene Ther. 2003, 3, 13–26. [Google Scholar]
- Hermiston, T.W.; Kuhn, I. Armed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Ther. 2002, 9, 1022–1035. [Google Scholar] [CrossRef]
- Hastie, E.; Grdzelishvili, V.Z. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J. Gen. Virol. 2012, 93, 2529–2545. [Google Scholar] [CrossRef]
- Liu, Y.P.; Russell, S.P.; Ayala-Breton, C.; Russell, S.J.; Peng, K.W. Ablation of Nectin4 Binding Compromises CD46 Usage by a Hybrid Vesicular Stomatitis Virus/Measles Virus. J. Virol. 2014, 88, 2195–2204. [Google Scholar] [CrossRef]
- Naik, S.; Russell, S.J. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin. Biol. Ther. 2009, 9, 1163–1176. [Google Scholar] [CrossRef]
- Stojdl, D.F.; Lichty, B.; Knowles, S.; Marius, R.; Atkins, H.; Sonenberg, N.; Bell, J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000, 6, 821–825. [Google Scholar] [CrossRef]
- Diaz, R.M.; Galivo, F.; Kottke, T.; Wongthida, P.; Qiao, J.; Thompson, J.; Valdes, M.; Barber, G.; Vile, R.G. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007, 67, 2840–2848. [Google Scholar]
- Grote, D.; Cattaneo, R.; Fielding, A.K. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003, 63, 6463–6468. [Google Scholar]
- Guillerme, J.B.; Boisgerault, N.; Roulois, D.; Menager, J.; Combredet, C.; Tangy, F.; Fonteneau, J.F.; Gregoire, M. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin. Cancer Res. 2013, 19, 1147–58. [Google Scholar] [CrossRef]
- Ong, H.T.; Hasegawa, K.; Dietz, A.B.; Russell, S.J.; Peng, K.W. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007, 14, 324–333. [Google Scholar] [CrossRef]
- Miest, T.S.; Cattaneo, R. New viruses for cancer therapy: meeting clinical needs. Nat. Rev. Microbiol. 2014, 12, 23–34. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Delpeut, S.; Noyce, R.S.; Richardson, C.D. The Tumor-Associated Marker, PVRL4 (Nectin-4), Is the Epithelial Receptor for Morbilliviruses. Viruses 2014, 6, 2268-2286. https://doi.org/10.3390/v6062268
Delpeut S, Noyce RS, Richardson CD. The Tumor-Associated Marker, PVRL4 (Nectin-4), Is the Epithelial Receptor for Morbilliviruses. Viruses. 2014; 6(6):2268-2286. https://doi.org/10.3390/v6062268
Chicago/Turabian StyleDelpeut, Sebastien, Ryan S. Noyce, and Christopher D. Richardson. 2014. "The Tumor-Associated Marker, PVRL4 (Nectin-4), Is the Epithelial Receptor for Morbilliviruses" Viruses 6, no. 6: 2268-2286. https://doi.org/10.3390/v6062268
APA StyleDelpeut, S., Noyce, R. S., & Richardson, C. D. (2014). The Tumor-Associated Marker, PVRL4 (Nectin-4), Is the Epithelial Receptor for Morbilliviruses. Viruses, 6(6), 2268-2286. https://doi.org/10.3390/v6062268



