Abstract
Since the emergence of West Nile virus (WNV) in North America in 1999, understanding of the clinical features, spectrum of illness and eventual functional outcomes of human illness has increased tremendously. Most human infections with WNV remain clinically silent. Among those persons developing symptomatic illness, most develop a self-limited febrile illness. More severe illness with WNV (West Nile neuroinvasive disease, WNND) is manifested as meningitis, encephalitis or an acute anterior (polio) myelitis. These manifestations are generally more prevalent in older persons or those with immunosuppression. In the future, a more thorough understanding of the long-term physical, cognitive and functional outcomes of persons recovering from WNV illness will be important in understanding the overall illness burden.
1. Introduction
In the decade since the emergence of West Nile virus (WNV) in North America, our collective understanding of the clinical manifestations, prognostic factors and short- and long-term outcomes of illness has increased dramatically. Previous historic outbreaks of WNV in sub-Saharan Africa and the Middle East had typically been characterized as mild, self-limited febrile illness with few or no sequelae [1]. More recently, beginning in the 1990s, more cases of neurologic illness, particularly “neuroinvasive” disease, in which the virus directly infects the nervous system, began to be recognized [1,2]. However, the ongoing outbreak of WNV in North America has greatly expanded our understanding of the spectrum of illness associated with WNV infection in humans, and a number of previously under-recognized syndromes have been characterized [3,4,5,6].
It is generally estimated that about 80% of persons infected with WNV remain asymptomatic. Of those who develop symptoms, the vast majority develop an acute, systemic febrile illness (“West Nile fever”, WNF). Data suggest that less than 1% of infected persons develop neurologic illness, which is primarily attributed to neuroinvasive disease, in which the virus breaches the intrathecal space and produces infection of central nervous system (CNS) structures. Neuroinvasive disease includes aseptic meningitis (“West Nile meningitis”, WNM), encephalitis (“West Nile encephalitis”, WNE) or an acute poliomyelitis-like syndrome (“West Nile poliomyelitis”, WNP) [1,2]. WNM involves infection of the meninges (the outer covering of the brain and spinal cord) and makes up the largest percentage of neuroinvasive disease cases in younger age groups. WNE involves viral infection of the brain parenchyma itself and is more typically manifested in older persons or individuals with compromised immune systems. WNP results from viral infection of the anterior horn cells of the spinal cord, leading to acute flaccid limb weakness. When discussing the various manifestations of WNV infection, it is important to keep in mind that the clinical picture may not always be as clear-cut as delineated above, and sometimes, the clinical features of WNF, WNM and WNE may overlap. For example, patients may develop an altered mental status, due to severe systemic illness, without true histopathologic or radiologic evidence of cerebral inflammation or “encephalitis” in the pathophysiologic sense. Similarly, patients presenting with fever, headache and “neck stiffness” may not undergo lumbar puncture to demonstrate pleocytosis, and consequently, a diagnosis of WN “meningitis” may not be reported. Despite these limitations, the clinical syndrome in most persons with WNV illness can be diagnosed based upon clinical grounds.
2. Clinical Syndromes Associated with WNV Infection
2.1. West Nile Fever (WNF)
WNF is the predominant clinical syndrome seen in most infected persons. All ages may be affected, but data suggest that the proportion of WNF may be higher among younger individuals [5,7,8,9]. Following an incubation period of approximately 2–14 days, infected persons typically experience the abrupt onset of fever, headache, fatigue and myalgias. Gastrointestinal complaints, including nausea and vomiting, have been frequently described and may lead to dehydration.
WNF may sometimes be associated with a rash, which tends to be morbilliform, maculopapular and non-pruritic and predominates over the torso and extremities, sparing the palms and soles [10,11,12,13]. (Figure 1). The rash may be transient, lasting less than 24 h in some persons. Interestingly, this rash appears to be more frequently seen in WNF than in more severe illness manifestations (WNM or WNE) [10]. In addition, rash is more frequently observed among younger persons than among older persons [10]. These findings raise the question as to whether the presence of a rash correlates with host immune or cytokine response to infection.
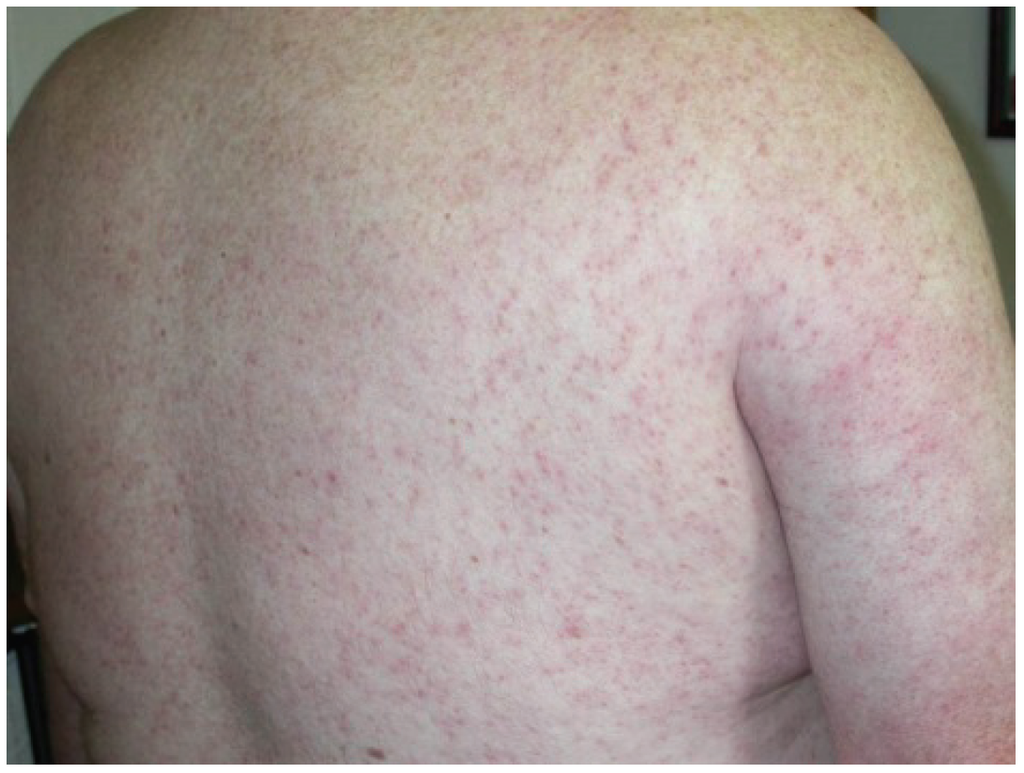
Figure 1.
Diffuse maculopapular rash associated with West Nile virus infection.
Although elderly persons with WNF may experience adverse outcomes and have a higher mortality rate than younger symptomatic persons [4,8], most patients experience complete recovery. Some otherwise healthy persons, however, may continue to experience persistent fatigue, headaches and difficulties concentrating for days or weeks following infection [14]. In particular, profound fatigue, sometimes interfering with work or school activities, may last for months among persons recovering from WNF [15]. Deaths among persons with WNF occur primarily among older persons and the immunocompromised population and are frequently attributable to cardiopulmonary complications [16].
2.2. West Nile Neuroinvasive Disease
2.2.1. West Nile Meningitis (WNM)
WNM is essentially indistinguishable from other viral meningitides (or “aseptic meningitis”). Persons developing WNM experience the abrupt onset of fever and headache and demonstrate meningeal signs, including nuchal rigidity, Kernig’s and/or Brudzinski’s signs and photophobia or phonophobia. The associated headache may be severe, requiring hospitalization for pain control; associated gastrointestinal symptoms, such as nausea, vomiting and diarrhea, may result in dehydration, exacerbating head pain and systemic symptoms [15]. WNM is generally associated with a favorable outcome, though, similar to WNF, some patients experience persistent headache, fatigue and myalgias [3,15].
Cerebrospinal fluid (CSF) examination is characterized by a modest pleocytosis (elevation of white blood cells), generally less than 500 cells/mm3. While this pleocytosis is usually lymphocytic, which is typical of viral meningitis, CSF obtained soon after the onset of symptoms may show a neutrophilic predominance [17,18]. The presence of plasma cells has been suggested to be indicative of WN virus infection [19]; however, this finding requires further substantiation.
2.2.2. West Nile Encephalitis (WNE)
WNE may range in severity from a mild, self-limited confusional state to severe encephalopathy, coma and death. This manifestation is more commonly seen in older individuals, particularly over the age of 55, as well as immunocompromised persons [20,21]. Several neurological syndromes, primarily extrapyramidal disorders, have been observed in patients with WNE [3,5,6,22] and, in the setting of known virus circulation, may be very suggestive of WNV as the etiological agent for the encephalitis [3]. Patients with WNE frequently develop a coarse bilateral tremor, particularly in the upper extremities. The tremor tends to be postural and may have a kinetic component [3,4,6,22]. Myoclonus, predominantly of the upper extremities and facial muscles, may occur and may be present during sleep. Features of parkinsonism, including hypomimia, bradykinesia and postural instability, may be seen and can be associated with falls and functional difficulties [3,23]. Cerebellar ataxia, with associated truncal instability and gait disturbance, leading to falls, has been described [6,22,24]. These abnormal movements usually follow the onset of mental status changes. Typically, these movement disorders resolve over time; however, tremor and parkinsonism may persist in patients recovering from severe encephalitis [3,5].
The development of these movement disorders in WNE is due to specific neurotropism of WNV for extrapyramidal structures; there is frequent involvement of the brainstem (particularly, the medulla and pons), the deep gray matter nuclei, particularly the substantia nigra of the basal ganglia and the thalami, and the cerebellum [25,26,27]. This clinico-pathologic correlation may be extended to the neuroimaging abnormalities seen in West Nile encephalitis.
The estimated proportion of patients with WNE who demonstrate abnormal findings in brain magnetic resonance imaging (MRI) varies considerably between studies; however, MRI findings are not ubiquitous, and even in cases of severe WNE, the MRI may be normal; or abnormal findings may not be apparent until several weeks after the onset of illness [28,29,30]. The most characteristic MRI findings in patients with WNE are bilateral signal abnormalities in the basal ganglia and thalami on T2- , fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted image sequences, indicating the viral neurotropism for these deep gray structures (Figure 2). These MRI findings, which may be seen in other flaviviral encephalitides, including encephalitis, due to Japanese encephalitis virus and St. Louis encephalitis virus, may be indicative, but not diagnostic for, WNE. Electroencephalographic (EEG) abnormalities may be present in the form of generalized slowing, frequently anteriorly or temporally predominant, and triphasic sharp waves [31,32]. These EEG abnormalities, however, are also nonspecific. Overt seizures appear to be relatively uncommon with WNE and are estimated to occur in 3%–6% of patients [33]. Similarly, increased intracranial pressure and cerebral edema appear to be uncommon in WNE. CSF abnormalities in patients with WNE are essentially the same as those seen in WNM, characterized by moderate lymphocytic pleocytosis, elevated protein and normal glucose. One large study suggested that the mean CSF white blood cell count in patients with WNE was 227 cells/mm3 (median, 90 cells/mm3) [34].

Figure 2.
Fluid-attenuated inversion recovery magnetic resonance imaging sequence of the brain in a patient with West Nile virus encephalitis with associated parkinsonism and tremor, displaying signal abnormality in the substantia nigra (short arrow), the mesial temporal lobe (long arrow) and right posterior thalamus (thick arrow).
Neuropsychiatric symptoms, including depression, anxiety and apathy, have been reported among patients recovering from WNE [35,36]. Fatality rates from WNE have ranged from between 10% and 30%, with mortality higher among older persons and immunocompromised individuals [1,2,37]. Of note, the severity of initial encephalitic illness does not necessarily predict ultimate outcome, as some individuals developing severe WNE have been observed to go on to have full recovery [3].
2.2.3. West Nile Poliomyelitis (WNP) and Other Forms of Acute Flaccid Paralysis (AFP)
Acute and abrupt onset of limb weakness may be seen in WNV infection; in most cases, this limb paresis (partial weakness) or paralysis (complete loss of muscle power) is due to viral involvement of the lower motor neurons of the spinal cord (anterior horn cells), resulting in anterior (polio) myelitis [18,38,39,40,41,42]. This syndrome, typically associated with poliovirus, may be caused by a number of other viruses [43,44]. The clinical features of WNP are characteristic and generally dramatic, allowing for differentiation from the characteristic diffuse “muscle weakness” described by many persons with severe fatigue associated with WNV infection (Table 1). WNP generally develops soon after illness onset, usually within the first 24 to 48 h. Limb weakness generally develops rapidly and may be abrupt, occasionally raising clinical concern about stroke [45,46]. The weakness is usually asymmetric and often results in monoplegia (weakness of one limb). Patients with severe and extensive spinal cord involvement develop a more symmetric dense quadriplegia. Central facial weakness, frequently bilateral, can also be seen [38]. Sensory loss or numbness is generally absent, though some patients experience intense pain in the affected limbs just before or during the onset of weakness, and this limb pain may be persistent [18].

Table 1.
Clinical and electrodiagnostic features of different types of weakness associated with West Nile virus infection.
| Characteristic | West Nile Poliomyelitis | Guillain–Barré Syndrome | Fatigue-Related “Muscle Weakness” |
|---|---|---|---|
| Timing of onset | Acute phase of infection | One to eight weeks following acute infection | Acute infection |
| Fever and leukocytosis | Present | Absent | Present |
| Weakness distribution | Asymmetric; occasional monoplegia | Generally symmetric; proximal and distal muscles | Generalized, subjective, but neurologic examination normal |
| Sensory symptoms | Absence of numbness, paresthesias or sensory loss; pain often present | Painful distal paresthesias and sensory loss | Generally absent |
| Bowel/bladder involvement | Often present | Rare | Not present |
| Concurrent encephalopathy | Often present | Generally absent | May be seen with fever, meningitis or encephalitis |
| CSF Profile | Pleocytosis and elevated protein | No pleocytosis; elevated protein (albuminocytologic dissociation) | Pleocytosis and elevated protein in the setting of meningitis/encephalitis |
The most severe manifestation of WNP is the involvement of respiratory muscle innervation, leading to diaphragmatic and intercostal muscle paralysis and resulting in neuromuscular respiratory failure, requiring emergency endotracheal intubation [18,47]. Involvement of the lower brainstem, including the motor nuclei of the vagus and glossopharyngeal nerves, is similar to that seen in poliovirus infection and appears to be the underlying pathophysiologic basis for this manifestation [33,48]. Respiratory involvement in WNP is associated with high morbidity and mortality, and among survivors, prolonged ventilatory support lasting months may be required [18]; unfortunately, in some cases, patients are unable to be weaned from mechanical ventilation, and the withdrawal of ventilator support leads to death. Patients who develop bulbar findings, such as dysarthria, dysphagia or loss of gag reflex, are at greater risk for respiratory failure and should be monitored closely.
Electrodiagnostic studies (electromyography/nerve conduction studies) will display findings consistent with a motor axonopathy with little or no demyelinating changes and preservation of sensory nerve potentials [49,50]. Spinal MRI may show signal abnormalities in the anterior spinal cord, consistent with anterior horn cell damage; ventral nerve root enhancement may be seen, as well (Figure 3).
Other forms of AFP, including radiculopathy and the acute demyelinating polyradiculoneuropathy form of Guillain–Barré syndrome (GBS), have also been associated with WNV infection [51,52]. However, these syndromes appear to be far less common than poliomyelitis and may be differentiated on the basis of clinical and electrophysiologic features (Table 1). The weakness associated with GBS is usually symmetric, ascending (e.g., beginning in the legs and subsequently involving the arms and cranial nerve innervated muscles) and is associated with sensory and autonomic dysfunction. Additionally, CSF examination will generally show elevated protein in the absence of pleocytosis (‘cytoalbuminologic dissociation’), and electrodiagnostic studies may be consistent with a predominantly demyelinating polyneuropathy or prominent axonal damage (in the motor axonal variant of GBS).
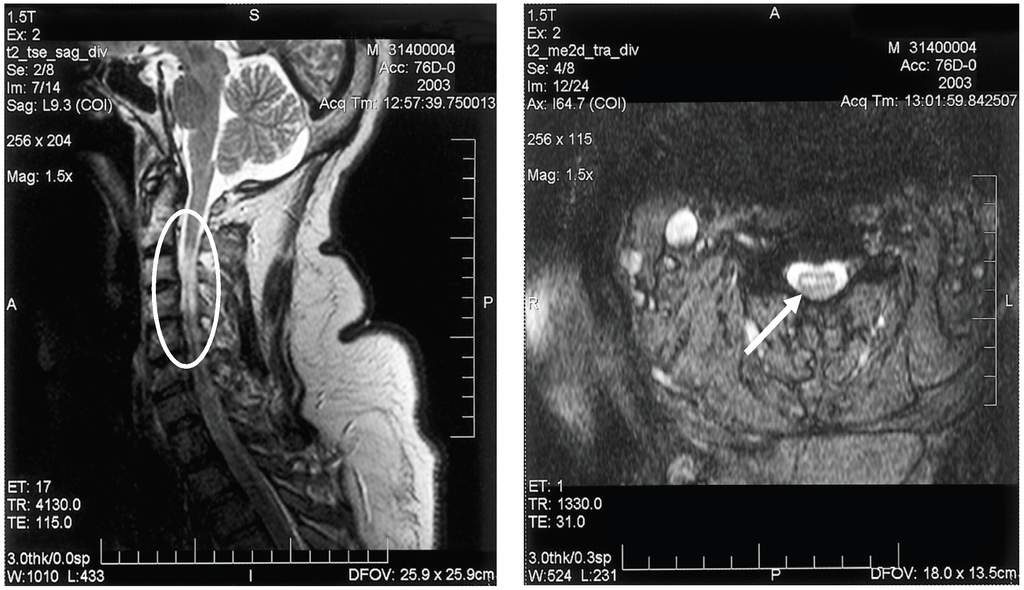
Figure 3.
Sagittal (A) and axial (B) T2-weighted magnetic resonance imaging of the cervical spinal cord of a patient with bilateral upper extremity paralysis and respiratory failure from West Nile poliomyelitis, displaying the increased signal in the anterior spinal cord (circle and arrow).
Recovery of limb strength in persons with WNP is variable [18,53]. However, persistent weakness and associated functional disability appears to be the rule, at least in the short term; prolonged physical and occupational therapy may be required. Most limb strength recovery occurs within the first 6–8 months after acute illness, following which improvement appears to plateau [53,54]. In particular, quadriplegia and respiratory failure are associated with high morbidity and mortality, and recovery is slow and incomplete [18]. More than 50% of the mortality associated with WNP occurs in patients with acute neuromuscular respiratory failure; of patients who survive respiratory failure due to WNP, a substantial number require prolonged tracheostomy or long-term supplemental oxygen [18,54]. In general, less profound initial weakness may be associated with more rapid and more complete strength recovery [18]. However, even patients with initially severe and profound paralysis may experience substantial recovery [18,53]; thus, as in encephalitis, the initial severity of paralysis is not necessarily a prognosticator of eventual outcome. This recovery phenomenon may in part be due to the involvement of a large number of motor neurons, which may initially be reversibly damaged, but are able to recover [42]. In addition, patients with WNP may be able, over time, to compensate for their motor deficits through the adaptation of motor skills, leading to improved functional recovery [55]. Electrodiagnostic studies may be useful in predicting the recovery of muscle strength, with subsequent improvement correlating with motor unit number estimate (MUNE) values [42]. Although case reports have suggested the occurrence of relapsing or delayed-onset cases of WNP [56], the long-term clinical and functional outcomes in patients with WNP is still emerging, and whether there may be the subsequent development of a delayed, “post-polio”-like syndrome years after acute illness is unknown. Subsequent prospective long-term evaluations of patients suffering from WNP will ultimately address this important question.
3. Other Clinical Manifestations of West Nile Virus Infection
3.1. Ocular Manifestations
Ocular manifestations, including chorioretinitis and vitritis, are the most commonly reported clinical manifestation of WNV infection after fever and neuroinvasive disease [57,58,59,60,61,62]. The optic nerve and retina are essentially extensions of the CNS, but will be considered ocular manifestations in this review. Chorioretinal lesions associated with WNV infection have been described as multifocal and with a “target-like” appearance [58]; retinal hemorrhages have also been noted. Lesions tend to be clustered primarily in the temporal and nasal regions of the periphery of the fundus. This distribution and appearance of the chorioretinal lesions have been suggested to be distinctive for WNV infection [57]. One study in Tunisia identified chorioretinitis in 20 (69%) of 29 patients with laboratory-confirmed, symptomatic WNV infection [63]; the authors concluded that ophthalmoscopic examination should be performed on all patients with suspected WNV disease.
An inflammatory vitritis has occurred concomitantly with the chorioretinitis and may be significant enough to obscure the optic disc. Symptomatic persons describe gradual visual blurring and loss, floaters and flashes. Although experience with management is limited, improvement both in symptoms and in underlying chorioretinal lesions has been observed following treatment with intraocular corticosteroids [58]. Occasional cases of optic neuritis (inflammation of the optic nerve) have been described in the setting of WNV infection [64]). To date, WNV has not been isolated intra-orbitally.
3.2. Other Miscellaneous Manifestations
Numerous other clinical manifestations have been described in association with WNV infection; generally, these manifestations have been described in case reports or small case series, and a definitive causal association with WNV infection is difficult to substantiate. Rhabdomyolysis has been temporally associated with WNV infection [38,45], suggesting a viral myositis, but the presence of virus in muscle tissue has not been observed. Hepatitis and pancreatitis have been reported in cases of severe WNV infection [65,66], and WNV has been identified in hepatic and pancreatic specimens at pathology, suggesting that viscerotropic WNV disease may be an infrequent manifestation of infection. Myocarditis has been seen pathologically in WNV infection, and cardiac arrhythmias have occurred in persons with WNP, suspected to be due to autonomic dysfunction [67].
A study published in 2010 identified WNV RNA in the urine of five (20%) of 25 patients at up to seven years following acute WNV illness [68]. Four of the patients reported persistent subjective symptoms, and one patient had developed renal failure after their acute WNV illness. However, one subsequent study found no evidence of WNV RNA in urine samples collected from 40 patients at 6.5 years after acute WNV illness, and another study detected WNV RNA in the urine of only one (1.6%) of 63 persons tested <5 months after initial acute WNV infection [69,70]. The frequency of the persistence of WNV RNA in urine is unknown, and the clinical implications, if any, require further substantiation.
4. West Nile Virus Long-Term Clinical and Functional Outcomes
As the clinical experience with WNV infection has extended over a period of years, our understanding of the short- and long-term outcomes of WNV infection has grown. However, detailed data regarding these long-term neurologic and functional outcomes of WNV is still relatively sparse. As with other viral encephalitides, initial severe neurologic illness does not necessarily correlate with eventual outcome, and some patients with initial severe encephalopathy with associated coma may experience good recovery and minimal sequelae [3]. However, others experience persistent neurologic dysfunction, including movement disorders, headaches, fatigue and cognitive complaints. Large hospital-based series suggest that patients with severe WNE frequently require assistance with daily activities following acute care discharge [4,5]. Patients frequently report functional and cognitive difficulties for over a year following acute infection, and only 37% of patients in the 1999 New York City outbreak achieved full recovery at one year [71]. Of 265 persons developing symptomatic WNV infection in Idaho between 2006 and 2008, 53% reported one or more persistent symptom six months or more following acute illness; the most frequent complaints were fatigue, muscle aches and difficulties with memory and concentration [72]. Cognitive complaints, including difficulties with attention and concentration, have been described among patients recovering from WNE and suggest a subcortical type of cognitive dysfunction based on prominent thalamic and basal ganglia involvement [3]. However, limited formal and objective neuropsychometric assessments have been performed. A few studies have shown that persons recovering from WNV illness demonstrate measurable neurocognitive deficits on standardized testing as long as one year after acute illness [73,74]. Other studies, however, have shown that persons recovering from WNV illness do not perform significantly differently on standardized neurocognitive assessments based upon the nature of clinical illness (e.g., WNF compared to WNM or WNE) or in comparison to unaffected persons [15]. However, self-reported fatigue, somatic and cognitive complaints are common among persons recovering from WNV illness, and subjective complaints and poorer performance on self-reported functionality indices have been seen in patients months or years following acute illness [71,75]. One study has suggested the normalization of self-reported symptoms within one year of acute illness [76]. As previously noted, neuropsychiatric symptoms, including depression and severe anxiety, have been reported by patients recovering from WNE [3,35,77].
5. West Nile Virus Infection in Children
Most children with symptomatic WNV infection present with WNF; of those who develop neuroinvasive disease, it most frequently manifests as meningitis [78,79,80]. However, severe and fatal encephalitis [81], poliomyelitis [82,83], rhombencephalitis [84] and hepatitis [85] have all been described in children with WNV infection. Similar to adults, immunocompromised children may be more susceptible to more severe illness [21].
6. Risk Factors for Severe West Nile Virus Illness and Death
Of all infected persons, less than 1% develop West Nile virus neuroinvasive disease (WNND). Although WNND has been reported among all ages, the proportion of persons who progress to WNND is greater among older compared to younger persons [8]. Serologic surveys in Romania and New York City indicate that WNV infection incidence is constant across all age groups during outbreaks [86,87], although serosurvey data are limited. Surveillance data from the United States indicate that age is the most important host risk factor for the development of WNND after infection. The incidence of neuroinvasive disease increases approximately 1.5-fold for each decade of life, resulting in a risk approximately 30 times greater for persons 80–90 years old compared to children younger than 10 years old [8]. During outbreaks, hospitalized persons older than 70 years of age had case fatality rates of 15% in Romania [86] and 29% in Israel [88]. Encephalitis with severe muscle weakness and change in the level of consciousness were also prominent clinical risk factors predicting fatal outcome [88,89].
Based upon a limited number of cases, patients who acquire WNV infection from infected donor organs are likely at a higher risk for severe neurologic disease and death compared with patients infected through the natural route of mosquito bite inoculation [90,91]. The risk of severe neurologic disease among other organ transplant recipients is not well-defined and may be related to the interval between infection and transplantation or the type of post-transplant immunosuppressive therapy. A seroprevalence study carried out in a Canadian outpatient transplant clinic following a WNV epidemic in 2002 indicated that the risk of neuroinvasive disease following infection was 40% (95% confidence interval 16%–80%) [92]. During that epidemic, transplant patients were approximately 40 times more likely than the general population to develop WNND [93]. However, a subsequent study assessed seropositivity and incidence of WNND among 194 solid organ transplant recipients and 195 controls and found no significant difference in seropositivity for WNV IgG between the groups and determined that the incidence of WNND among the transplant recipients was low among the seropositive transplant patients [94].
In addition to increased age and organ transplantation, hypertension, cerebrovascular disease, renal disease and diabetes have also been identified as possible risk factors for WNND, and prior immunosuppression has been associated with a fatal outcome [88,89,95,96,97,98,99]. The incidence of neuroinvasive disease and the probability of death after acquiring neuroinvasive disease are slightly higher in men than in women [8].
7. Treatment and Management
There is currently no definitive treatment for WNV infection. Prevention of infection through protection from mosquito bites is therefore critical and the single most important public health measure. In the absence of definitive antiviral treatment, management of illness due to WNV infection remains supportive. Patients with otherwise uncomplicated WNF generally do not require specific intervention, though control of headache and rehydration may sometimes be needed. However, persons with documented WN viremia and patients with WNF in which other risk factors, including older age and underlying immunosuppression, are present should be observed for progression to more severe neuroinvasive disease. Patients with severe WNM may also require pain control for severe headache, and dehydration due to associated nausea and vomiting may require hospitalization for rehydration. In patients with WNE, attention to the level of alertness and airway protection is important. While seizures and increased intracranial pressure have been infrequently reported with WNE, if present, they should be managed appropriately.
Patients with WNP may not have concurrent meningitis or encephalitis, and thus, WNV infection may not initially be suspected. This may result in the implementation of inappropriate diagnostic procedures or treatment modalities, including anticoagulation for suspected acute stroke or muscle biopsy for suspected myopathy. WNV infection should be suspected in persons developing acute asymmetric paralysis, particularly if accompanied by other signs of infection. Patients developing early dysarthria and dysphagia are at a higher risk for subsequent acute respiratory failure [18]; for this reason, hospitalization and observation of patients with poliomyelitis is prudent, and the development of dysarthria and dysphagia should be viewed with concern. Management of poliomyelitis due to poliovirus suggests that the initiation of aggressive physical activity during the acute febrile period of illness is associated with more profound and persistent weakness [100]; in the absence of additional data, the avoidance of aggressive physical activity during the acute febrile illness or during the initial 48–72 h of weakness in WNP would be a reasonable approach, with subsequent application of physical and occupational therapy.
Viremia in humans is short-lived, and WNV is usually cleared from the system by the time of clinical presentation; this fact presents a substantial theoretical obstacle for the targeting and design of specific anti-viral therapies. The recent use of several therapeutic modalities, including antiviral agents, nucleic acid analogues, missense sequences, immunomodulating agents and angiotensin-receptor blockers, has been outside the setting of carefully controlled, randomized, blinded, placebo-controlled trials; thus, anecdotal reports of the effectiveness of these agents are unsubstantiated. However, such anecdotal reports of effectiveness and the clinical desire to provide an intervention have led to empiric use.
The immunomodulating agent, interferon-α, while again showing in vitro inhibition of cytotoxicity due to WNV [101], has not been fully evaluated in animal models, and data from an open-label, non-blinded trial in the United States have not suggested a clear benefit. The use of interferon-α in the treatment of the closely related Japanese encephalitis virus suggested no benefit [102].
Animal models and anecdotal reports have suggested the efficacy of high-titer WNV-specific intravenous immune globulin (IVIG) from pooled donors (Omr-IgG-am®) [103,104,105], and humanized monoclonal WNV antibodies targeting the envelope protein of the virus (MGAWN1) [106,107,108,109]. However, animal models suggest that efficacy is greatest if these therapeutics are given prior to or very shortly after the onset of clinical illness, and attempts at human randomized clinical trials to assess the efficacy of these therapeutic agents have been unsuccessful, largely due to the challenge of enrolling a sufficient number of subjects within a likely therapeutic window. Neither of these products is licensed or available for use in the United States. As a result, the prevention of initial infection is the most critical component of any approach for the management of WNV illness.
8. Conclusions
Over the past decade, the understanding of the clinical spectrum of illness, as well as the immediate and longer-term outcomes associated with human WNV infection has increased substantially. However, there are remaining clinical questions that require further elucidation. Data on the long-term neurocognitive impact on patients recovering from WNE are scant, and further information is needed to ascertain long-lasting cognitive impairment following encephalitis from WNV. The parkinsonian features associated with acute WNV illness appear in most cases to be transient and resolve over time; however, recurrent- or early-onset parkinsonism in such patients due to the senescence of dopaminergic neurons remains a hypothetical possibility. Similarly, whether patients recovering from WNP will develop recurrent limb weakness in previously affected limbs years after their acute illness, akin to ‘post-polio syndrome’ seen with poliovirus, is unknown at this point, but needs assessment. In the future, additional assessment of these and other clinical manifestations of WNV infection will be critical in aiding our understanding of the pathogenesis of WNV disease and hopefully will guide management and treatment options.
Acknowledgments
The author would like to acknowledge Teresa Hammett for assistance in the development and drafting of this paper.
Conflicts of Interest
The findings and conclusions in this manuscript are those of the author and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References and Notes
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Granwehr, B.P.; Lillibridge, K.M.; Higgs, S.; Mason, P.W.; Aronson, J.F.; Campbell, G.A.; Barrett, A.D. West Nile virus: Where are we now? Lancet Infect. Dis. 2004, 4, 547–556. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Haddad, M.B.; Tierney, B.C.; Campbell, G.L.; Marfin, A.A.; van Gerpen, J.A.; Fleischauer, A.; Leis, A.A.; Stokic, D.S.; Petersen, L.R. Neurologic manifestations and outcome of West Nile virus infection. J. Am. Med. Assoc. 2003, 290, 511–515. [Google Scholar] [CrossRef]
- Emig, M.; Apple, D.J. Severe West Nile virus disease in healthy adults. Clin. Infect. Dis. 2004, 38, 289–292. [Google Scholar] [CrossRef]
- Pepperell, C.; Rau, N.; Krajden, S.; Kern, R.; Humar, A.; Mederski, B.; Simor, A.; Low, D.E.; McGeer, A.; Mazzulli, T.; et al. West Nile virus infection in 2002: Morbidity and mortality among patients admitted to hospital in southcentral Ontario. Can. Med. Assoc. J. 2003, 168, 1399–1405. [Google Scholar]
- Sayao, A.L.; Suchowersky, O.; Al-Khathaami, A.; Klassen, B.; Katz, N.R.; Sevick, R.; Tilley, P.; Fox, J.; Patry, D. Calgary experience with West Nile virus neurological syndrome during the late summer of 2003. Can. J. Neurol. Sci. 2004, 31, 194–203. [Google Scholar]
- Brown, J. West Nile virus in blood donors: Colorado cohort study, 2003. In Proceedings of the Fifth National Conference on West Nile virus in the United States, Denver, CO, USA, 3–5 February 2004.
- O’Leary, D.R.; Marfin, A.A.; Montgomery, S.P.; Kipp, A.M.; Lehman, J.A.; Biggerstaff, B.J.; Elko, V.L.; Collins, P.D.; Jones, J.E.; Campbell, G.L. The epidemic of West Nile virus in the United States, 2002. Vector Borne Zoonotic Dis. 2004, 4, 61–70. [Google Scholar] [CrossRef]
- Hayes, E.B.; Gubler, D.J. West Nile virus: Epidemiology and clinical features of an emerging epidemic in the United States. Annu. Rev. Med. 2005, 57, 181–194. [Google Scholar] [CrossRef]
- Ferguson, D.D.; Gershman, K.; LeBailly, A.; Petersen, L.R. Characteristics of the rash associated with West Nile virus fever. Clin. Infect. Dis. 2005, 41, 1204–1207. [Google Scholar] [CrossRef]
- Del Giudice, P.; Schuffenecker, I.; Zeller, H.; Grelier, M.; Vandenbos, F.; Dellamonica, P.; Counillon, E. Skin manifestations of west nile virus infection. Dermatology 2005, 211, 348–350. [Google Scholar] [CrossRef]
- Gorsche, R.; Tilley, P. The rash of West Nile virus infection. Can. Med. Assoc. J. 2005, 172. [Google Scholar] [CrossRef]
- Anderson, R.C.; Horn, K.B.; Hoang, M.P.; Gottlieb, E.; Bennin, B. Punctate exanthem of West Nile Virus infection: Report of 3 cases. J. Am. Acad. Dermatol. 2004, 51, 820–823. [Google Scholar] [CrossRef]
- Watson, J.T.; Pertel, P.E.; Jones, R.C.; Siston, A.M.; Paul, W.S.; Austin, C.C.; Gerber, S.I. Clinical characteristics and functional outcomes of West Nile Fever. Ann. Intern. Med. 2004, 141, 360–365. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Curns, A.T.; Welburg, L.; Jones, J.F.; Lundgren, L.M.; Capuron, L.; Pape, J.; Reeves, W.C.; Campbel, G.L. Neurocognitive and functional outcomes in persons recovering from West Nile virus illness. J. Neuropsychol. 2008, 2, 477–499. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Lindsey, N.P.; Campbell, G.L. Primary causes of death in reported cases of fatal West Nile Fever, United States, 2002–2006. Vector Borne Zoonotic Dis. 2011, 11, 161–164. [Google Scholar] [CrossRef]
- Crichlow, R.; Bailey, J.; Gardner, C. Cerebrospinal fluid neutrophilic pleocytosis in hospitalized West Nile virus patients. J. Am. Board Fam. Pract. 2004, 17, 470–472. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Bode, A.V.; Marfin, A.A.; Campbell, G.L.; Ewing, D.; Mazowiecki, M.; Pavot, P.V.; Schmitt, J.; Pape, J.; Biggerstaff, B.J.; et al. West Nile virus-associated flaccid paralysis. Emerg. Infect. Dis. 2005, 11, 1021–1027. [Google Scholar] [CrossRef]
- Carson, P.J.; Steidler, T.; Patron, R.; Tate, J.M.; Tight, R.; Smego, R.A., Jr. Plasma cell pleocytosis incerebrospinal fluid in patients with West Nile virus encephalitis. Clin. Infect. Dis. 2003, 37, e12–e15. [Google Scholar] [CrossRef]
- Armali, Z.; Ramadan, R.; Chlebowski, A.; Azzam, Z.S. West Nile meningo-encephalitis infection in a kidney transplant recipient. Transplant. Proc. 2003, 35, 2935–2936. [Google Scholar] [CrossRef]
- Ravindra, K.V.; Freifeld, A.G.; Kalil, A.C.; Al, E. West Nile virus-associated encephalitis in recipients of renal and pancreas transplants: Case series and literature review. Clin. Infect. Dis. 2004, 38, 1257–1260. [Google Scholar] [CrossRef]
- Burton, J.M.; Kern, R.Z.; Halliday, W.; Mikulis, D.; Brunton, J.; Fearon, M.; Pepperell, C.; Jaigobin, C. Neurological manifestations of West Nile virus infection. Can. J. Neurol. Sci. 2004, 31, 185–193. [Google Scholar]
- Robinson, R.L.; Shahida, S.; Madan, N.; Rao, S.; Khardori, N. Transient parkinsonism in West Nile virus encephalitis. Am. J. Med. 2003, 115, 252–253. [Google Scholar] [CrossRef]
- Kanagarajan, K.; Ganesh, S.; Alakhras, M.; Go, E.S.; Recco, R.A.; Zaman, M.M. West Nile virusinfection presenting as cerebellar ataxia and fever: Case report. South. Med. J. 2003, 96, 600–601. [Google Scholar] [CrossRef]
- Kelley, T.W.; Prayson, R.A.; Ruiz, A.I.; Isada, C.M.; Gordon, S.M. The neuropathology of West Nile virus meningoencephalitis. A report of two cases and review of the literature. Am. J. Clin. Pathol. 2003, 119, 749–753. [Google Scholar] [CrossRef]
- Guarner, J.; Shieh, W.J.; Hunter, S.; Paddock, C.D.; Morken, T.; Campbell, G.L.; Marfin, A.A.; Zaki, S.R. Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum. Pathol. 2004, 35, 983–990. [Google Scholar] [CrossRef]
- Bosanko, C.M.; Gilroy, J.; Wang, A.M.; Sanders, W.; Dulai, M.; Wilson, J.; Blum, K. West nile virus encephalitis involving the substantia nigra: Neuroimaging and pathologic findings with literature review. Arch. Neurol. 2003, 60, 1448–1452. [Google Scholar] [CrossRef]
- Brilla, R.; Block, M.; Geremia, G.; Wichter, M. Clinical and neuroradiologic features of 39 consecutive cases of West Nile Virus meningoencephalitis. J. Neurol. Sci. 2004, 220, 37–40. [Google Scholar] [CrossRef]
- Ali, M.; Safriel, Y.; Sohi, J.; Llave, A.; Weathers, S. West Nile virus infection: MR imaging findings in the nervous system. Am. J. Neuroradiol. 2005, 26, 289–297. [Google Scholar]
- Petropoulou, K.A.; Gordon, S.M.; Prayson, R.A.; Ruggierri, P.M. West Nile virus meningoencephalitis: MR imaging findings. Am. J. Neuroradiol. 2005, 26, 1986–1995. [Google Scholar]
- Rodriguez, A.J.; Westmoreland, B.F. Electroencephalographic characteristics of patients infected with West Nile virus. J. Clin. Neurophysiol. 2007, 24, 386–389. [Google Scholar] [CrossRef]
- Gandelman-Marton, R.; Kimiagar, I.; Itzhaki, A.; Klein, C.; Theitler, J.; Rabey, J.M. Electroencephalography findings in adult patients with West Nile virus-associated meningitis and meningoencephalitis. Clin. Infect. Dis. 2003, 37, 1573–1578. [Google Scholar] [CrossRef]
- Doron, S.I.; Dashe, J.F.; Adelman, L.S.; Brown, W.F.; Werner, B.G.; Hadley, S. Histopathologically proven poliomyelitis with quadriplegia and loss of brainstem function due to West Nile virus infection. Clin. Infect. Dis. 2003, 37, e74–e77. [Google Scholar] [CrossRef]
- Tyler, K.L.; Pape, J.; Goody, R.J.; Corkill, M.; Kleinschmidt-DeMasters, B.K. CSF findings in 250 patients with serologically confirmed West Nile virus meningitis and encephalitis. Neurology 2006, 66, 361–365. [Google Scholar] [CrossRef]
- Murray, K.O.; Resnick, M.; Miller, V. Depression after infection with West Nile virus. Emerg. Infect.Dis. 2007, 13, 479–481. [Google Scholar] [CrossRef]
- Nolan, M.S.; Hause, A.M.; Murray, K.O. Findings of long-term depression up to 8 years post infection from West Nile virus. J. Clin. Psychol. 2012, 68, 801–808. [Google Scholar] [CrossRef]
- Davis, L.E.; DeBiasi, R.; Goade, D.E.; Haaland, K.Y.; Harrington, J.A.; Harnar, J.B.; Pergam, S.A.; King, M.K.; DeMasters, B.K.; Tyler, K.L. West Nile virus neuroinvasive disease. Ann. Neurol. 2006, 60, 286–300. [Google Scholar] [CrossRef]
- Jeha, L.E.; Sila, C.A.; Lederman, R.J.; Prayson, R.A.; Isada, C.M.; Gordon, S.M. West Nile virus infection: A new acute paralytic illness. Neurology 2003, 61, 55–59. [Google Scholar] [CrossRef]
- Leis, A.A.; Stokic, D.S.; Polk, J.L.; Dostrow, V.; Winkelmann, M. A poliomyelitis-like syndrome from West Nile virus infection. N. Engl. J. Med. 2002, 347, 1279–1280. [Google Scholar] [CrossRef]
- Glass, J.D.; Samuels, O.; Rich, M.M. Poliomyelitis due to West Nile virus. N. Engl. J. Med. 2002, 347, 1280–1281. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Leis, A.A.; Stokic, D.S.; van Gerpen, J.A.; Marfin, A.A.; Webb, R.; Haddad, M.B.; Tierney, B.C.; Slavinski, S.A.; Polk, J.L.; et al. Acute flaccid paralysis and West Nile virus infection. Emerg. Infect. Dis. 2003, 9, 788–793. [Google Scholar] [CrossRef]
- Li, J.; Loeb, J.A.; Shy, M.E.; Shah, A.K.; Tselis, A.C.; Kupski, W.J.; Lewis, R.A. Asymmetric flaccid paralysis: A neuromuscular presentation of West Nile virus infection. Ann. Neurol. 2003, 53, 703–710. [Google Scholar] [CrossRef]
- Sejvar, J.J. West Nile virus and “poliomyelitis”. Neurology 2004, 63, 206–207. [Google Scholar] [CrossRef]
- Solomon, T.; Willison, H. Infectious causes of acute flaccid paralysis. Curr. Opin. Infect. Dis. 2003, 16, 375–381. [Google Scholar] [CrossRef]
- Kulstad, E.B.; Wichter, M.D. West Nile encephalitis presenting as a stroke. Ann. Emerg. Med. 2003, 41. [Google Scholar] [CrossRef]
- Berner, Y.N.; Lang, R.; Chowers, M.Y. Outcome of West Nile fever in older adults. J. Am. Geriatr. Soc. 2002, 50, 1844–1846. [Google Scholar] [CrossRef]
- Fan, E.; Needham, D.M.; Brunton, J.; Kern, R.Z.; Stewart, T.E. West Nile virus infection in the intensive care unit: A case series and literature review. Can. Respir. J. 2004, 11, 354–358. [Google Scholar]
- Agamanolis, D.P.; Leslie, M.J.; Caveny, E.A.; Guarner, J.; Shieh, W.J.; Zaki, S.R. Neuropathological findings in West Nile virus encephalitis: A case report. Ann. Neurol. 2003, 54, 547–551. [Google Scholar] [CrossRef]
- Leis, A.A.; Stokic, D.S.; Webb, R.M.; Slavinski, S.A.; Fratkin, J. Clinical spectrum of muscle weakness in human West Nile virus infection. Muscle Nerve 2003, 28, 302–308. [Google Scholar] [CrossRef]
- Al-Shekhlee, A.; Katirji, B. Electrodiagnostic features of acute paralytic poliomyelitis associated with West Nile virus infection. Muscle Nerve 2004, 29, 376–380. [Google Scholar] [CrossRef]
- Park, M.; Hui, J.S.; Bartt, R.E. Acute anterior radiculitis associated with West Nile virus infection. J. Neurol. Neurosurg. Psychiatry 2003, 74, 823–825. [Google Scholar] [CrossRef]
- Ahmed, S.; Libman, R.; Wesson, K.; Ahmed, F.; Einberg, K. Guillain-Barre syndrome: An unusual presentation of West Nile virus infection. Neurology 2000, 55, 144–146. [Google Scholar] [CrossRef]
- Cao, N.J.; Ranganathan, C.; Kupsky, W.J.; Li, J. Recovery and prognosticators of paralysis in West Nile virus infection. J. Neurol. Sci. 2005, 236, 73–80. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Bode, A.V.; Marfin, A.A.; Campbell, G.L.; Pape, J.; Biggerstaff, B.J.; Petersen, L.R. West Nile Virus-associated flaccid paralysis outcome. Emerg. Infect. Dis. 2006, 12, 514–516. [Google Scholar] [CrossRef]
- Sejvar, J.J. The long-term outcomes of human West Nile virus infection. Clin. Infect. Dis. 2007, 44, 1617–1624. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Davis, L.E.; Szabados, E.; Jackson, A.C. Delayed-onset and recurrent limb weakness associated with West Nile virus infection. J. Neurovirol. 2010, 16, 93–100. [Google Scholar] [CrossRef]
- Hershberger, V.S.; Augsburger, J.J.; Hutchins, R.K.; Miller, S.A.; Horwitz, J.A.; Bergmann, M. Chorioretinal lesions in nonfatal cases of West Nile virus infection. Ophthalmology 2003, 110, 1732–1736. [Google Scholar] [CrossRef]
- Adelman, R.A.; Membreno, J.H.; Afshari, N.A.; Stoessel, K.M. West Nile virus chorioretinitis. Retina 2003, 23, 100–101. [Google Scholar] [CrossRef]
- Bains, H.S.; Jampol, L.M.; Caughron, M.C.; Parnell, J.R. Vitritis and chorioretinitis in a patient with West Nile virus infection. Arch. Ophthalmol. 2003, 121, 205–207. [Google Scholar] [CrossRef]
- Kuchtey, R.W.; Kosmorsky, G.S.; Martin, D.; Lee, M.S. Uveitis associated with West Nile virus infection. Arch. Ophthalmol. 2003, 121, 1648–1649. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.; Trese, M.T. West Nile virus chorioretinitis. Br. J. Ophthalmol. 2004, 88, 1599–1600. [Google Scholar] [CrossRef]
- Vandenbelt, S.; Shaikh, S.; Capone, A., Jr.; Williams, G.A. Multifocal choroiditis associated with West Nile virus encephalitis. Retina 2003, 23, 97–99. [Google Scholar] [CrossRef]
- Khairallah, M.; Ben Yahia, S.; Ladjimi, A.; Zeghidi, H.; Ben Romdhane, F.; Besbes, L.; Zaouali, S.; Messaoud, R. Chorioretinal involvement in patients with West Nile virus infection. Ophthalmology 2004, 111, 2065–2070. [Google Scholar] [CrossRef]
- Bakri, S.J.; Kaiser, P.K. Ocular manifestations of West Nile virus. Curr. Opin. Ophthalmol. 2004, 15, 537–540. [Google Scholar] [CrossRef]
- Sampson, B.A.; Ambrosi, C.; Charlot, A.; Reiber, K.; Veress, J.F.; Armbrustmacher, V. The pathology of human West Nile Virus infection. Hum. Pathol. 2000, 31, 527–531. [Google Scholar] [CrossRef]
- Perelman, A.; Stern, J. Acute pancreatitis in West Nile Fever. Am. J. Trop. Med. Hyg. 1974, 23, 1150–1152. [Google Scholar]
- Fratkin, J.D.; Leis, A.A.; Stokic, D.S.; Slavinski, S.A.; Geiss, R.W. Spinal cord neuropathology in human West Nile virus infection. Arch. Pathol. Lab. Med. 2004, 128, 533–537. [Google Scholar]
- Murray, K.; Walker, C.; Herrington, E.; Lewis, J.A.; McCormick, J.; Beasley, D.W.; Tesh, R.B.; Fisher-Hoch, S. Persistent infection with West Nile virus years after initial infection. J. Infect. Dis. 2010, 201, 2–4. [Google Scholar] [CrossRef]
- Gibney, K.B.; Lanciotti, R.S.; Sejvar, J.J.; Nugent, C.T.; Linnen, J.M.; Delorey, M.J.; Lehman, J.A.; Boswell, E.N.; Staples, J.E.; Fischer, M. West nile virus RNA not detected in urine of 40 people tested 6 years after acute West Nile virus disease. J. Infect. Dis. 2011, 203, 344–347. [Google Scholar] [CrossRef]
- Baty, S.A.; Gibney, K.B.; Staples, J.E.; Patterson, A.B.; Levy, C.; Lehman, J.; Wadleigh, T.; Feld, J.; Lanciotti, R.; Nugent, C.T.; et al. Evaluation for West Nile Virus (WNV) RNA in urine of patients within 5 months of WNV infection. J. Infect. Dis. 2012, 205, 1476–1477. [Google Scholar] [CrossRef]
- Klee, A.L.; Maidin, B.; Edwin, B.; Poshni, I.; Mostashari, F.; Fine, A.; Layton, M.; Nash, D. Long-term prognosis for clinical West Nile virus infection. Emerg. Infect. Dis. 2004, 10, 1405–1411. [Google Scholar] [CrossRef]
- Cook, R.L.; Xu, X.; Yablonsky, E.J.; Sakata, N.; Tripp, J.H.; Hess, R.; Piazza, P.; Rinaldo, C.R. Demographic and clinical factors associated with persistent symptoms after West Nile virus infection. Am. J. Trop. Med. Hyg. 2010, 83, 1133–1136. [Google Scholar] [CrossRef]
- Haaland, S.J.; Pergam, S.; Echevarria, L.A.; Davis, L.E.; Goade, D.; Harnar, J.; Nfchissey, R.A.; Sewel, C.M.; Ettestad, P. Mental status after West Nile virus infection. Emerg. Infect. Dis. 2006, 12, 1260–1262. [Google Scholar] [CrossRef]
- Sadek, J.R.; Pergam, S.A.; Harrington, J.A.; Echevarria, L.A.; Davis, L.E.; Goade, D.; Harnar, J.; Nofchissey, R.A.; Sewell, C.M.; Ettestad, P.; et al. Persistent neuropsychological impairment associated with West Nile virus infection. J. Clin. Exp. Neuropsychol. 2010, 32, 81–87. [Google Scholar] [CrossRef]
- Carson, P.J.; Konewko, P.; Wold, K.S.; Mariani, P.; Goli, S.; Bergloff, P.; Crosby, R.D. Long-term clinical and neuropsychological outcomes of West Nile virus infection. Clin. Infect. Dis. 2006, 43, 723–730. [Google Scholar] [CrossRef]
- Loeb, M.; Hanna, S.; Nicolle, L.; Eyles, J.; Elliott, S.; Rathbone, M.; Drebot, M.; Neupane, B.; Fearon, M.; Mahony, J. Prognosis after West Nile virus infection. Ann. Intern. Med. 2008, 149, 232–241. [Google Scholar] [CrossRef]
- Berg, P.J.; Smallfield, S.; Svien, L. An investigation of depression and fatigue post West Nile virus infection. S. D. Med. 2010, 63, 127–133. [Google Scholar]
- Lindsey, N.P.; Hayes, E.B.; Staples, J.E.; Fischer, M. West Nile virus disease in children, United States, 1999–2007. Pediatrics 2009, 123, e1084–e1089. [Google Scholar] [CrossRef]
- Civen, R.; Villacorte, F.; Robles, D.T.; Dassey, D.E.; Croker, C.; Borenstein, L.; Harvey, S.M.; Mascola, L. West Nile Virus Infection in the Pediatric Population. Pediatr. Infect. Dis. J. 2006, 25, 75–78. [Google Scholar] [CrossRef]
- Hayes, E.B.; O’Leary, D.R. West Nile virus infection: a pediatric perspective. Pediatrics 2004, 113, 1375–1381. [Google Scholar] [CrossRef]
- Carey, D.E.; Rodrigues, F.M.; Myers, R.M.; Webb, J.K. Arthropod-borne viral infections in children in Vellore, South India, with particular reference to dengue and West Nile viruses. Indian Pediatr. 1968, 5, 285–296. [Google Scholar]
- Heresi, G.P.; Mancias, P.; Mazur, L.J.; Butler, I.J.; Murphy, J.R.; Cleary, T.G. Poliomyelitis-like syndrome in a child with West Nile virus infection. Pediatr. Infect. Dis. J. 2004, 23, 788–789. [Google Scholar] [CrossRef]
- Vidwan, G.; Bryant, K.K.; Puri, V.; Stover, B.H.; Rabalais, G.P. West Nile virus encephalitis in a child with left-side weakness. Clin. Infect. Dis. 2003, 37, e91–e94. [Google Scholar] [CrossRef]
- Nichter, C.A.; Pavlakis, S.G.; Shaikh, U.; Cherian, K.A.; Dobrosyzcki, J.; Porricolo, M.E.; Chatturvedi, I. Rhombencephalitis caused by West Nile fever virus. Neurology 2000, 55. [Google Scholar] [CrossRef]
- Yim, R.; Posfay-Barbe, K.M.; Nolt, D.; Fatula, G.; Wald, E.R. Spectrum of clinical manifestations of West Nile virus infection in children. Pediatrics 2004, 114, 1673–1675. [Google Scholar] [CrossRef]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Mostashari, F.; Bunning, M.L.; Kitsutani, P.T.; Singer, D.A.; Nash, D.; Cooper, M.J.; Katz, N.; Liljebjelke, K.A.; Biggerstaff, B.J.; Fine, A.D.; et al. Epidemic West Nile encephalitis, New York, 1999: Results of a household-based seroepidemiological survey. Lancet 2001, 358, 261–264. [Google Scholar] [CrossRef]
- Chowers, M.Y.; Lang, R.; Nassar, F.; Ben-David, D.; Giladi, M.; Rubinshtein, E.; Itzhaki, A.; Mishal, J.; Siegman-Igra, Y.; Kitzes, R.; et al. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg. Infect. Dis. 2001, 7, 675–678. [Google Scholar] [CrossRef]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef]
- Rhee, C.; Eaton, E.F.; Concepcion, W.; Blackburn, B.G. West Nile virus encephalitis acquired via liver transplantation and clinical response to intravenous immunoglobulin: Case report and review of the literature. Transpl. Infect. Dis. 2011, 13, 312–317. [Google Scholar] [CrossRef]
- Nett, R.J.; Kuehnert, M.J.; Ison, M.G.; Orlowski, J.P.; Fischer, M.; Staples, J.E. Current practices and evaluation of screening solid organ donors for West Nile virus. Transpl. Infect. Dis. 2012, 14, 268–277. [Google Scholar] [CrossRef]
- Kumar, D.; Drebot, M.A.; Wong, S.J.; Lim, G.; Artsob, H.; Buck, P.; Humar, A. A seroprevalence study of west nile virus infection in solid organ transplant recipients. Am. J. Transplant. 2004, 4, 1883–1888. [Google Scholar] [CrossRef]
- Kumar, D.; Prasad, G.V.; Zaltzman, J.; Levy, G.A.; Humar, A. Community-acquired West Nile virus infection in solid-organ transplant recipients. Transplantation 2004, 77, 399–402. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Meza, J.; Schweitzer, B.; Shafer, L.; Kalil, A.C.; Sambol, A.R. Seroprevalence of West Nile virus infection in solid organ transplant recipients. Transpl. Infect. Dis. 2010, 12, 120–126. [Google Scholar] [CrossRef]
- Murray, K.O.; Koers, E.; Baraniuk, S.; Herrington, E.; Carter, H.; Sierra, M.; Kilborn, C.; Arafat, R. Risk factors for encephalitis from West Nile Virus: A matched case-control study using hospitalized controls. Zoonoses Public Health 2009, 56, 370–375. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Harmon, H.; Vogt, R.L. Follow-up of 2003 human West Nile virus infections, Denver, Colorado. Emerg. Infect. Dis. 2006, 12, 1129–1131. [Google Scholar] [CrossRef]
- Jean, C.M.; Honarmand, S.; Louie, J.K.; Glaser, C.A. Risk factors for West Nile virus neuroinvasive disease, California, 2005. Emerg. Infect. Dis. 2007, 13, 1918–1920. [Google Scholar] [CrossRef]
- Murray, K.; Baraniuk, S.; Resnick, M.; Arafat, R.; Kilborn, C.; Cain, K.; Shallenberger, R.; York, T.L.; Martinez, D.; Hellums, J.S.; et al. Risk factors for encephalitis and death from West Nile virus infection. Epidemiol. Infect. 2006, 134, 1325–1332. [Google Scholar] [CrossRef]
- Bode, A.V.; Sejvar, J.J.; Pape, W.J.; Campbell, G.L.; Marfin, A.A. West Nile virus disease: A descriptive study of 228 patients hospitalized in a 4-county region of Colorado in 2003. Clin. Infect. Dis. 2006, 42, 1234–1240. [Google Scholar] [CrossRef]
- Guyton, A. Reaction of the body to poliomyelitis and the recovery process. Arch. Int. Med. 1949, 83, 27–47. [Google Scholar] [CrossRef]
- Morrey, J.D.; Day, C.W.; Julander, J.G.; Blatt, L.M.; Smee, D.F.; Sidwell, R.W. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir. Chem. Chemother. 2004, 15, 101–109. [Google Scholar]
- Solomon, T.; Dung, N.M.; Wills, B.; Kneen, R.; Gainsborough, M.; Diet, T.V.; Thuy, T.T.; Loan, H.T.; Khanh, V.C.; Vaughn, D.W.; et al. Interferon alfa-2a in Japanese encephalitis: A randomised double-blind placebo-controlled trial. Lancet 2003, 361, 821–826. [Google Scholar] [CrossRef]
- Diamond, M.S.; Shrestha, B.; Marri, A.; Mahan, D.; Engle, M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 2003, 77, 2578–2586. [Google Scholar] [CrossRef]
- Shimoni, Z.; Niven, M.J.; Pitlick, S.; Bulvik, S. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg. Infect. Dis. 2001, 7. [Google Scholar] [CrossRef]
- Agrawal, A.G.; Petersen, L.R. Human immunoglobulin as a treatment for West Nile virus infection. J. Infect. Dis. 2003, 188, 1–4. [Google Scholar] [CrossRef]
- Oliphant, T.; Engle, M.; Nybakken, G.E.; Doane, C.; Johnson, S.; Huang, L.; Gorlatov, S.; Mehlhop, E.; Marri, A.; Chung, K.M.; et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 2005, 11, 522–530. [Google Scholar] [CrossRef]
- Beigel, J.H.; Nordstrom, J.L.; Pillemer, S.R.; Roncal, C.; Goldwater, D.R.; Li, H.; Holland, P.C.; Johnson, S.; Stein, K.; Koenig, S. Safety and pharmacokinetics of single intravenous dose of MGAWN1, a novel monoclonal antibody to West Nile virus. Antimicrob. Agents Chemother. 2010, 54, 2431–2436. [Google Scholar] [CrossRef]
- Morrey, J.D.; Siddharthan, V.; Olsen, A.L.; Roper, G.Y.; Wang, H.; Baldwin, T.J.; Koenig, S.; Johnson, S.; Nordstrom, J.L.; Diamond, M.S. Humanized monoclonal antibody against West Nile virus envelope protein administered after neuronal infection protects against lethal encephalitis in hamsters. J. Infect. Dis. 2006, 194, 1300–1308. [Google Scholar] [CrossRef]
- Smeraski, C.A.; Siddharthan, V.; Morrey, J.D. Treatment of spatial memory impairment in hamsters infected with West Nile virus using a humanized monoclonal antibody MGAWN1. Antivir. Res. 2011, 91, 43–49. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).