Prevalence of Antibodies against Hantaviruses in Serum and Saliva of Adults Living or Working on Farms in Yorkshire, United Kingdom
Abstract
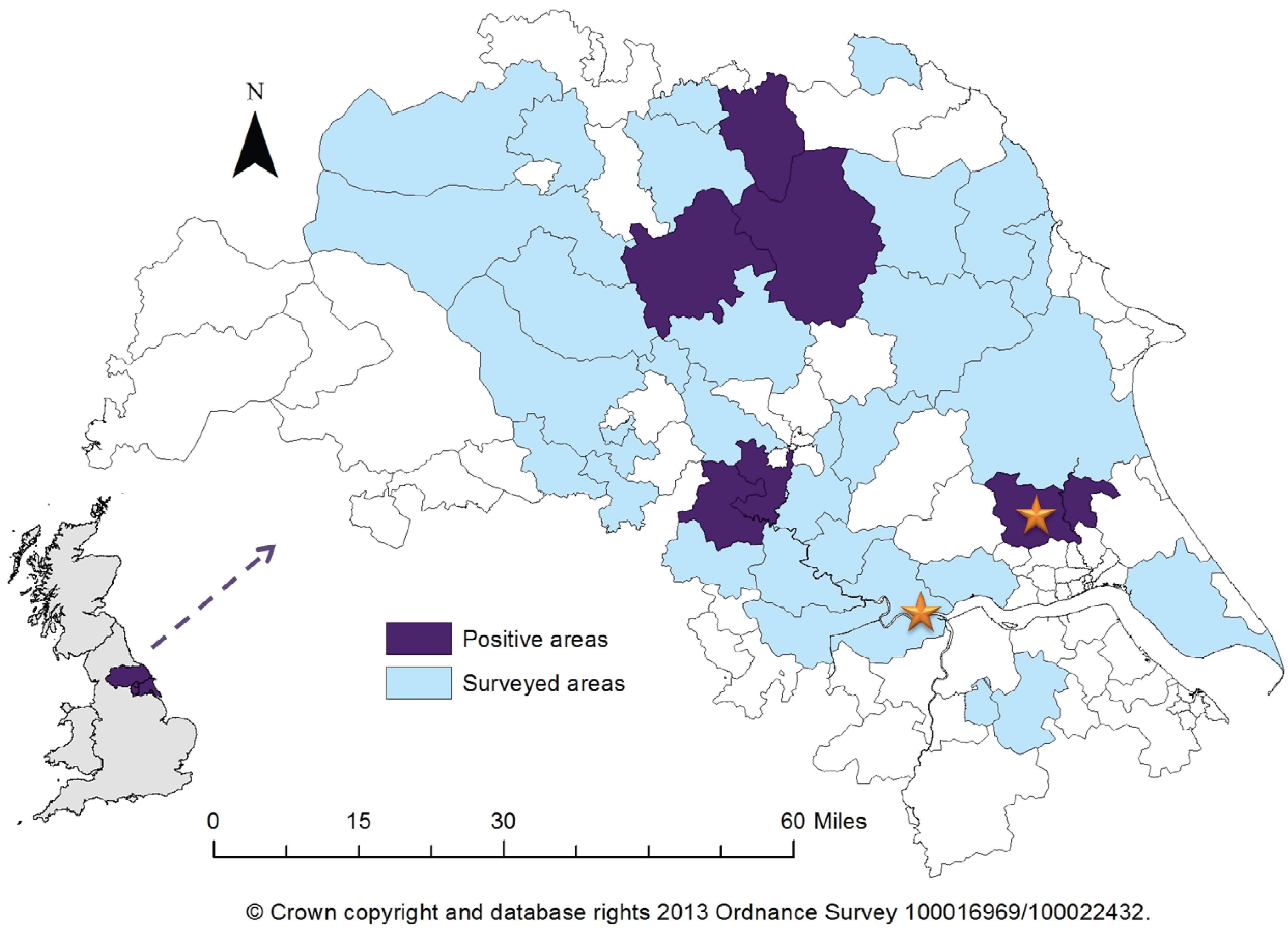
:1. Introduction
2. Results
| Sample | DOBV | HTNV | PUUV | SAAV | SNV | SEOV | Result |
|---|---|---|---|---|---|---|---|
| 1 | − (−) | − (+) | ++ (+) | − (−) | ++ (+) | + (++) | PUUV/SNV |
| 2 | − (−) | ++ (+) | − (+) | + (+) | − (+) | ++ (+) | HTNV/SEOV |
| 3 | − (−) | + (+) | + (+) | − (−) | + (+) | ++ (+) | SEOV |
| 4 | − (−) | − (+) | +++ (−) | − (−) | − (+) | + (+) | PUUV |
| 5 | − (+) | +++ (+) | − (−) | − (−) | − (+) | ++ (+) | HTNV/SEOV |
| 6 | − (+) | ++ (+) | − (−) | − (−) | − (+) | + (+) | HTNV/SEOV |
| 7 | − (−) | +++ (++) | − (+) | − (−) | − (+) | ++ (++) | HTNV/SEOV |
| 8 | − | ++ | − | − | − | + | HTNV/SEOV |
| 9 | − (+) | ++ (++) | − (+) | − (+) | − (+) | + (++) | HTNV/SEOV |
| Category | No. positive (%) | No. Negative | No. tested | Odds ratio | CI | p-value* |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| <30 | 3 (14.3) | 18 | 21 | 2.5 | 0.4 to 13.2 | 0.2 |
| ≥30 | 6 (6.1) | 92 | 98 | |||
| Median | 45 | 51 | ||||
| Gender | ||||||
| Male | 7 (7.0) | 93 | 100 | |||
| Female | 2 (10.5) | 17 | 19 | 1.6 | 0.1 to 9.2 | 0.6 |
| Farmer | ||||||
| Yes | 8 (8.2) | 90 | 98 | 1.8 | 0.2 to 82.7 | >0.99 |
| No | 1 (4.8) | 20 | 21 | |||
| Farm type | ||||||
| Animal | 4 (11.4) | 31 | 35 | 0.7 | ||
| Arable | 1 (7.1) | 13 | 14 | 1.7 | 0.1 to 88.8 | |
| Mixed | 4 (5.7) | 66 | 70 | 1.9 | 0.3 to 11 | |
| Farm classification | ||||||
| Cereal | 5 (6.3) | 75 | 80 | 0.7 | 0.1 to 3.9 | 0.7 |
| Cropping | 3 (7.5) | 37 | 40 | 1.1 | 0.2 to 5.5 | >0.99 |
| Horticulture | 0 | 4 | 4 | NA | ||
| Pig | 1 (5.9) | 16 | 17 | 0.8 | 0.02 to 6.7 | >0.99 |
| Poultry | 3 (15.0) | 17 | 20 | 3.0 | 0.4 to 15.6 | 0.1 |
| Dairy | 1 (5.6) | 17 | 18 | 0.8 | 0.02 to 6.2 | >0.99 |
| Livestock | 7 (8.1) | 79 | 86 | 1.8 | 0.3 to 18.2 | 0.7 |
| Other | 0 | 3 | 3 | NA | ||
| Farm size | ||||||
| <500 | 6 (7.2) | 77 | 83 | |||
| ≥500 | 3 (9.4) | 29 | 32 | 2.0 | 0.5 to 11.5 | 0.4 |
| Median | 330 | 296 | ||||
| Materials | ||||||
| Silage | 9 (9.8) | 83 | 92 | NA | 0.8 to Inf | 0.06 |
| Bedding | 9 (8.6) | 96 | 105 | NA | 0.4 to Inf | 0.4 |
| Feed | 9 (9.1) | 90 | 99 | NA | 0.6 to Inf | 0.2 |
| Hay | 8 (9.3) | 78 | 86 | 4.2 | 0.5 to 190.1 | 0.3 |
| Timber | 7 (6.7) | 97 | 104 | 0.8 | 0.1 to 8.4 | 0.7 |
| Coal | 6 (9.8) | 55 | 61 | 2.3 | 0.5 to 15.0 | 0.3 |
| Rodents seen | ||||||
| Mice | 6 (5.1) | 112 | 118 | 0.1 | 0.02 to 0.96 | 0.02 |
| Rats | 9 (7.6) | 109 | 118 | NA | 0.2 to Inf | >0.99 |
| During day | 2 (1.7) | 117 | 119 | 0.006 | 0.0004 to 0.05 | <0.001 |
| At night | 9 (7.6) | 109 | 118 | NA | 0.2 to Inf | >0.99 |
| Rodent control | ||||||
| Professional | 4 (14.3) | 24 | 28 | 2.8 | 0.5 to 14.2 | 0.2 |
| Self | 5 (5.6) | 85 | 90 |
3. Discussion
4. Materials and Methodology
4.1. Study Location
4.2. Study Subjects
4.3. Data Processing and Statistical Analysis
4.4. Serological Test
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Vaheri, A.; Henttonen, H.; Voutilainen, L.; Mustonen, J.; Sironen, T.; Vapalahti, O. Hantavirus infections in Europe and their impact on public health. Rev. Med. Virol. 2012. [Google Scholar] [CrossRef]
- Watson, D.C.; Sargianou, M.; Papa, A.; Chra, P.; Starakis, I.; Panos, G. Epidemiology of hantavirus infections in humans: A comprehensive, global overview. Crit. Rev. Microbiol. 2014, 40, 261–272. [Google Scholar] [CrossRef]
- Lee, H.W.; van der Groen, G. Hemorrhagic fever with renal syndrome. Prog. Med. Virol. 1989, 36, 62–102. [Google Scholar]
- Korva, M.; Duh, D.; Saksida, A.; Trilar, T.; Avšič-Županc, T. The hantaviral load in tissues of naturally infected rodents. Microb. Infect. 2009, 11, 344–351. [Google Scholar] [CrossRef]
- Bi, Z.; Formenty, P.B.; Roth, C.E. Hantavirus infection: A review and global update. J. Infect. Dev. Ctries. 2008, 2, 3–23. [Google Scholar]
- Vaheri, A.; Vapalahti, O.; Plyusnin, A. How to diagnose hantavirus infections and detect them in rodents and insectivores. Rev. Med. Virol. 2008, 18, 277–288. [Google Scholar] [CrossRef]
- Stanford, C.F.; Connolly, J.H.; Ellis, W.A.; Smyth, E.T.; Coyle, P.V.; Montgomery, W.I.; Simpson, D.I. Zoonotic infections in Northern Ireland farmers. Epidemiol. Infect. 1990, 105, 565–570. [Google Scholar] [CrossRef]
- Thomas, D.R.; Salmon, R.L.; Coleman, T.J.; Morgan-Capner, P.; Sillis, M.; Caul, E.O.; Morgan, K.; Paiba, G.A.; Bennett, M.; Ribeiro, D.; et al. Occupational exposure to animals and risk of zoonotic illness in a cohort of farmers, farmworkers and their families in England. J. Agric. Saf. Health. 1999, 5, 373–382. [Google Scholar] [CrossRef]
- Jameson, L.J.; Logue, C.H.; Atkinson, B.; Baker, N.; Galbraith, S.E.; Carroll, M.W.; Brooks, T.; Hewson, R. The continued emergence of hantaviruses: Isolation of a Seoul virus implicated in human disease, United Kingdom, October 2012. Euro Surveill. 2013, 18, 4–7. [Google Scholar]
- Chiappin, S.; Antonelli, G.; Gatti, R.; De Palo, E.F. Saliva Specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta. 2007, 383, 30–40. [Google Scholar] [CrossRef]
- Mestecky, J.; Moro, I.; Kerr, M.A.; Woof, J.M. Mucosal Immunoglobulins. In Mucosal ImmunologyMestecky, J., Ogra, P.L., Bienenstock, J., Lambrecht, B.N., Lamm, M.E., Strober, W., McGhee, J.R., Mayer, L., Eds.; 3rd ed.; Elsevier Academic Press: London, UK, 2005. [Google Scholar]
- Webster, J.P. Wild brown rats (Rattus norvegicus) as a zoonotic risk on farms in England and Wales. CDR 1996, 6, R46–R49. [Google Scholar]
- Brooks, T.J.G.; Rare and Imported Pathogens Laboratory, Public Health England, Porton Down, UK. Unpublished work. 2010.
- Ahlm, C.; Thelin, A.; Elgh, F.; Juto, P.; Stiernstrom, E.L.; Holmberg, S.; Tarnvik, A. Prevalence of antibodies specific to Puumala virus among farmers in Sweden. Scand. J. Work Environ. Health 1998, 24, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Bennett, E.; Clement, J.; Sansom, P.; Hall, I.; Leach, S.; Medlock, J.M. Environmental and ecological potential for enzootic cycles of Puumala hantavirus in Great Britain. Epidemiol. Infect. 2010, 138, 91–98. [Google Scholar] [CrossRef]
- Pounder, K.C.; Begon, M.; Sironen, T.; Henttonen, H.; Watts, P.C.; Voutilainen, L.; Vapalahti, O.; Klempa, B.; Fooks, A.R.; McElhinney, L.M. Novel hantavirus in field vole, United Kingdom. Emerg. Infect. Dis. 2013, 19, 673–675. [Google Scholar] [CrossRef]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef]
- Harwood, R. Cell separation by gradient centrifugation. Int. Rev. Cytol. 1975, 38, 69–403. [Google Scholar]
- Lamey, P.J.; Nolan, A. The recovery of human saliva using the salivette system. Eur. J. Clin. Chem. Clin. Biochem. 1994, 32, 727–728. [Google Scholar]
- Dohoo, I.; Martin, W.; Stryhn, H. Sampling. In Methods in Epidemiologic Research; VER Inc: Charlottetown, Canada, 2012. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R foundation for statistical computing: Vienna, Austria, 2003. Available online: http://www.R-project.org/ (accessed on 13 September 2013).
- Brown, L.D.; Cai, T.T.; DasGupta, A. Interval estimation for a binomial proportion. Stat. Sci. 2001, 16, 101–123. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jameson, L.J.; Newton, A.; Coole, L.; Newman, E.N.C.; Carroll, M.W.; Beeching, N.J.; Hewson, R.; Christley, R.M. Prevalence of Antibodies against Hantaviruses in Serum and Saliva of Adults Living or Working on Farms in Yorkshire, United Kingdom. Viruses 2014, 6, 524-534. https://doi.org/10.3390/v6020524
Jameson LJ, Newton A, Coole L, Newman ENC, Carroll MW, Beeching NJ, Hewson R, Christley RM. Prevalence of Antibodies against Hantaviruses in Serum and Saliva of Adults Living or Working on Farms in Yorkshire, United Kingdom. Viruses. 2014; 6(2):524-534. https://doi.org/10.3390/v6020524
Chicago/Turabian StyleJameson, Lisa J., Autilia Newton, Louise Coole, Edmund N. C. Newman, Miles W. Carroll, Nick J. Beeching, Roger Hewson, and Robert M. Christley. 2014. "Prevalence of Antibodies against Hantaviruses in Serum and Saliva of Adults Living or Working on Farms in Yorkshire, United Kingdom" Viruses 6, no. 2: 524-534. https://doi.org/10.3390/v6020524
APA StyleJameson, L. J., Newton, A., Coole, L., Newman, E. N. C., Carroll, M. W., Beeching, N. J., Hewson, R., & Christley, R. M. (2014). Prevalence of Antibodies against Hantaviruses in Serum and Saliva of Adults Living or Working on Farms in Yorkshire, United Kingdom. Viruses, 6(2), 524-534. https://doi.org/10.3390/v6020524





