Effect of Bacteriophage Infection in Combination with Tobramycin on the Emergence of Resistance in Escherichia coli and Pseudomonas aeruginosa Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Bacteriophage
2.2. Antibiotic Preparation
2.3. Biofilm Growth
2.4. Determination of MOI and Antibiotic Concentration on Cell Survival
2.5. Antibiotic Treatment
2.6. Antibiotic and Bacteriophage Treatment
2.7. Data Analysis
3. Results and Discussion
3.1. Effect of Antibiotic and Phage Concentrations on Cell Survival
3.2. Effect of Tobramycin and T4 on E. coli Biofilm Cell Survival
| E. coli | P. aeruginosa | |||||
|---|---|---|---|---|---|---|
| Time (h) | Log CFU.mm–2 ± SE | |||||
| 6 (Control) | 4.39 ± 0.16 | 2.96 ± 0.15 | ||||
| 24 (Control) | 4.48 ± 0.15 | 3.63 ± 0.15 | ||||
| Treatment | Tobramycin a | T4 a | Tob + T4 a | Tobramycin b | PB-1 b | Tob + PB-1 b |
| 6 | −0.27 ± 0.55 | −0.10 ± 0.21 | −1.6 ± 0.32 | 2.7 ± 0.30 | 2.4 ± 0.21 | 2.0 ± 0.35 |
| 24 | 2.1 ± 0.66 | −0.79 ± 0.20 | −1.8 ± 0.43 | 1.8 ± 0.36 | 3.2 ± 0.17 | 1.6 ± 0.33 |

3.3. Determination of E. coli Biofilm Cell Resistance
| E. coli | P. aeruginosa | |||
|---|---|---|---|---|
| % decrease in resistance | Tobramycin a | T4 b | Tobramycin c | PB-1 d |
| >99.99% | 39% | 60% | 99% | |
3.4. Effect of Tobramycin and PB-1 on P. aeruginosa Biofilm Cell Survival
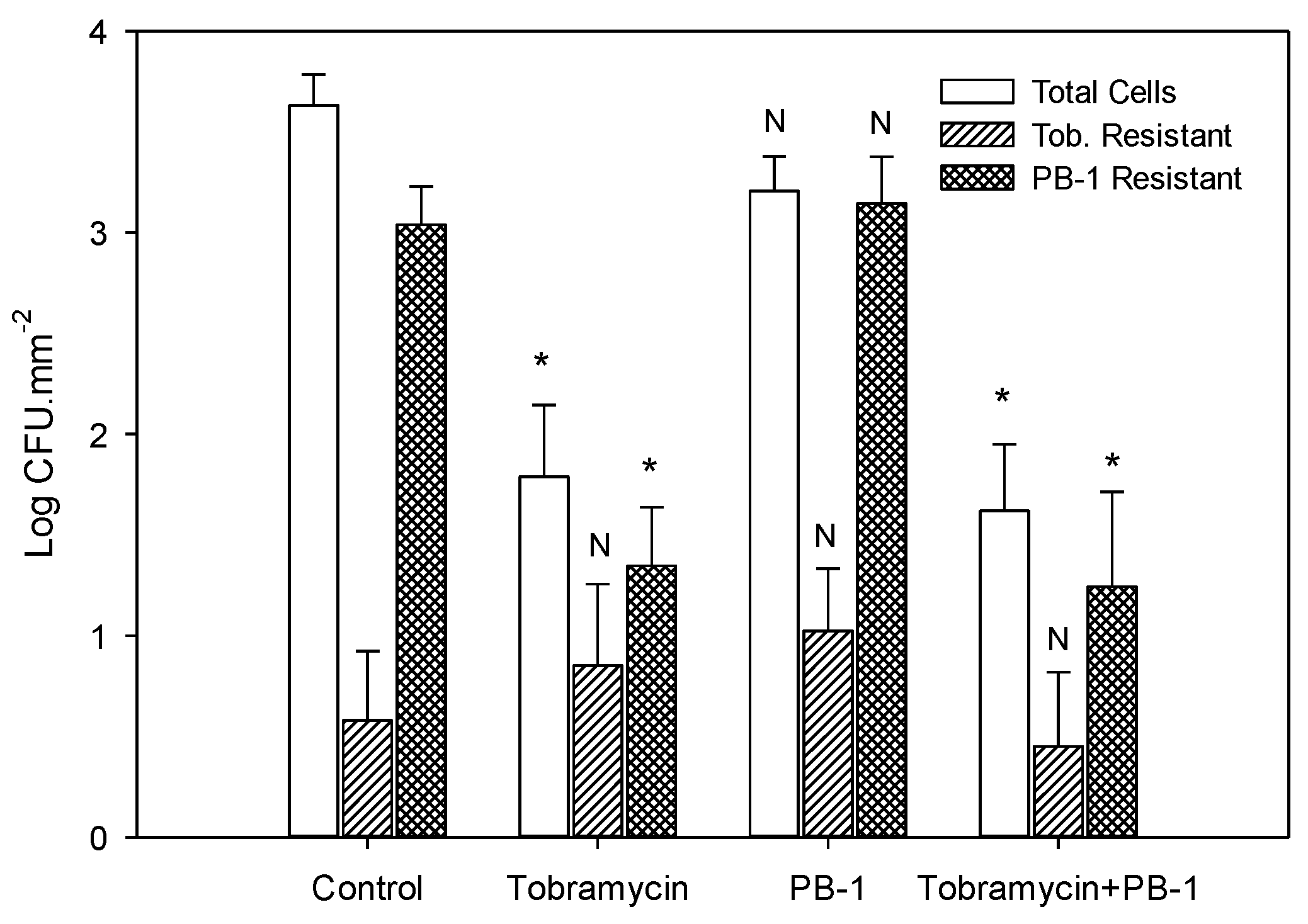
3.5. Determination of P. aeruginosa Biofilm Cell Resistance
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernandez, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- Singh, P.K.; Schaefer, A.L.; Parsek, M.R.; Moninger, T.O.; Welsh, M.J.; Greenberg, E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000, 407, 762–764. [Google Scholar] [CrossRef]
- Steinberger, R.E.; Holden, P.A. Extracellular DNA in single- and multiple-species unsaturated biofilms. Appl. Environ. Microbiol. 2005, 71, 5404–5410. [Google Scholar] [CrossRef]
- Absalon, C.; Ymele-Leki, P.; Watnick, P.I. The bacterial biofilm matrix as a platform for protein delivery. MBio 2012, 3, e00127–12. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Nickel, J.C.; Ruseska, I.; Wright, J.B.; Costerton, J.W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 1985, 27, 619–624. [Google Scholar] [CrossRef]
- Hoyle, B.D.; Alcantara, J.; Costerton, J.W. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrob. Agents Chemother. 1992, 36, 2054–2056. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Gupta, K.; Marques, C.N.H.; Petrova, O.E.; Sauer, K. Antimicrobial tolerance of Pseudomonas aeruginosa biofilms is activated during an early developmental stage and requires the two-component hybrid SagS. J. Bacteriol. 2013, 195, 4975–4981. [Google Scholar] [CrossRef]
- Hoiby, N. A personal history of research on microbial biofilms and biofilm infections. Pathog. Dis. 2014, 70, 205–211. [Google Scholar] [CrossRef]
- Krylov, V.N. Bacteriophages of Pseudomonas aeruginosa: Long-term prospects for use in phage therapy. Adv. Virus Res. 2014, 88, 227–278. [Google Scholar]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Tré-Hardy, M.; Nagant, C.; El Manssouri, N.; Vanderbist, F.; Traore, H.; Vaneechoutte, M.; Dehaye, J.P. Efficacy of the combination of tobramycin and a macrolide in an in vitro Pseudomonas aeruginosa mature biofilm model. Antimicrob. Agents Chemother. 2010, 54, 4409–4415. [Google Scholar] [CrossRef]
- Bonhoeffer, S.; Lipsitch, M.; Levin, B.R. Evaluating treatment protocols to prevent antibiotic resistance. Proc. Natl. Acad. Sci. USA 1997, 94, 12106–12111. [Google Scholar] [CrossRef]
- Parra-Ruiz, J.; Vidaillac, C.; Rose, W.E.; Rybak, M.J. Activities of high-dose daptomycin, vancomycin, and moxifloxacin alone or in combination with clarithromycin or rifampin in a novel in vitro model of Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 2010, 54, 4329–4334. [Google Scholar] [CrossRef]
- Fu, W.; Forster, T.; Mayer, O.; Curtin, J.J.; Lehman, S.M.; Donlan, R.M. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob. Agents Chemother. 2010, 54, 397–404. [Google Scholar] [CrossRef]
- Bedi, M.S.; Verma, V.; Chhibber, S. Amoxicillin and specific bacteriophage can be used together for eradication of biofilm of Klebsiella pneumoniae B5055. World J. Microbiol. Biotechnol. 2009, 25, 1145–1151. [Google Scholar] [CrossRef]
- Verma, V.; Harjai, K.; Chhibber, S. Structural changes induced by a lytic bacteriophage make ciprofloxacin effective against older biofilm of Klebsiella pneumoniae. Biofouling 2010, 26, 729–737. [Google Scholar] [CrossRef]
- Rahman, M.; Kim, S.; Kim, S.M.; Seol, S.Y.; Kim, J. Characterization of induced Staphylococcus aureus bacteriophage SAP-26 and its anti-biofilm activity with rifampicin. Biofouling 2011, 27, 1087–1093. [Google Scholar] [CrossRef]
- Bradley, D.E.; Robertson, D. The structure and infective process of a contractile Pseudomonas aeruginosa bacteriophage. J. Gen Virol 1968, 3, 247–254. [Google Scholar] [CrossRef]
- Adams, M.H. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- Corbin, B.D.; McLean, R.J.C.; Aron, G.M. Bacteriophage T4 multiplication in a glucose-limited Escherichia coli biofilm. Can J. Microbiol. 2001, 47, 680–684. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.W.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar]
- SigmaPlot, version 12.5; Systat Software Inc.: San José, CA, USA, 2013.
- Coulter, L.B. Synergistic effect of antibiotics and bacteriophage infection on Escherichia coli and Pseudomonas aeruginosa mixed biofilm communities. MS Thesis, Texas State University, San Marcos, TX, USA, 2012. [Google Scholar]
- Hanlon, G.W.; Denyer, S.P.; Olliff, C.J.; Ibrahim, L.J. Reduction in exopolysaccharide viscosity as an aid to bacteriophage penetration through Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 2001, 67, 2746–2753. [Google Scholar] [CrossRef]
- Sutherland, I.W.; Hughes, K.A.; Skillman, L.C.; Tait, K. The interaction of phage and biofilms. FEMS Microbiol. Lett. 2004, 232, 1–6. [Google Scholar] [CrossRef]
- Korgaonkar, A.; Trivedi, U.; Rumbaugh, K.; Whiteley, M. Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc. Natl. Acad. Sci. USA 2012, 110, 1059–1064. [Google Scholar] [CrossRef]
- Watters, C.; Everett, J.A.; Haley, C.; Clinton, A.; Rumbaugh, K. Insulin treatment modulates the host immune system to enhance Pseudomonas aeruginosa wound biofilms. Infect. Immun. 2014, 82, 92–100. [Google Scholar] [CrossRef]
- Kay, M.K.; Erwin, T.C.; McLean, R.J.C.; Aron, G.M. Bacteriophage ecology in Escherichia coli and Pseudomonas aeruginosa mixed biofilm communities. Appl. Environ. Microbiol. 2011, 77, 821–829. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coulter, L.B.; McLean, R.J.C.; Rohde, R.E.; Aron, G.M. Effect of Bacteriophage Infection in Combination with Tobramycin on the Emergence of Resistance in Escherichia coli and Pseudomonas aeruginosa Biofilms. Viruses 2014, 6, 3778-3786. https://doi.org/10.3390/v6103778
Coulter LB, McLean RJC, Rohde RE, Aron GM. Effect of Bacteriophage Infection in Combination with Tobramycin on the Emergence of Resistance in Escherichia coli and Pseudomonas aeruginosa Biofilms. Viruses. 2014; 6(10):3778-3786. https://doi.org/10.3390/v6103778
Chicago/Turabian StyleCoulter, Lindsey B., Robert J. C. McLean, Rodney E. Rohde, and Gary M. Aron. 2014. "Effect of Bacteriophage Infection in Combination with Tobramycin on the Emergence of Resistance in Escherichia coli and Pseudomonas aeruginosa Biofilms" Viruses 6, no. 10: 3778-3786. https://doi.org/10.3390/v6103778
APA StyleCoulter, L. B., McLean, R. J. C., Rohde, R. E., & Aron, G. M. (2014). Effect of Bacteriophage Infection in Combination with Tobramycin on the Emergence of Resistance in Escherichia coli and Pseudomonas aeruginosa Biofilms. Viruses, 6(10), 3778-3786. https://doi.org/10.3390/v6103778






