Abstract
Prion diseases or Transmissible Spongiform Encephalopathies (TSEs) are lethal neurodegenerative disorders involving the misfolding of the host encoded cellular prion protein, PrPC. This physiological form of the protein is expressed throughout the body, and it reaches the highest levels in the central nervous system where the pathology occurs. The conversion into the pathogenic isoform denoted as prion or PrPSc is the key event in prion disorders. Prominent candidates for the treatment of prion diseases are antibodies and their derivatives. Anti-PrPC antibodies are able to clear PrPSc from cell culture of infected cells. Furthermore, application of anti-PrPC antibodies suppresses prion replication in experimental animal models. Major drawbacks of immunotherapy are immune tolerance, the risks of neurotoxic side effects, limited ability of compounds to cross the blood-brain barrier and their unfavorable pharmacokinetic. The focus of this review is to recapitulate the current understanding of the molecular mechanisms for antibody mediated anti-prion activity. Although relevant for designing immunotherapeutic tools, the characterization of key antibody parameters shaping the molecular mechanism of the PrPC to PrPSc conversion remains elusive. Moreover, this review illustrates the various attempts towards the development of anti-PrP antibody compounds and discusses therapeutic candidates that modulate PrP expression.
1. Introduction
Prion diseases, or Transmissible Spongiform Encephalopathies (TSEs), represent a group of lethal neurodegenerative diseases. In addition to humans, several mammalian species may develop TSE, including Bovine Spongioform Encelopathy (BSE) in cattle, scrapie in sheep and goat or Chronic Wasting Diseases (CWD) in deer, moose and elk [1]. Certain types of the disease can be transmitted from human to human, such as Kuru or iatrogenic CJD (iCJD); but also from animals to humans, where the most prominent example is BSE in variant Cruetzfeldt-Jacob Disease (vCJD), mostly acquired through the consumption of BSE-infected food. However, less than 5% of prion-caused diseases are acquired, 10% to 15% are defined as genetic, while the remaining majority are considered sporadic [2]. Genetic types of the disease in humans are familial CJD (fCJD), fatal familial insomnia (FFI), prion protein cerebral angiopathy (PrP CAA) and Gerstmann–Sträussler–Scheinker syndrome (GSS), while sporadic types include sporadic CJD (sCJD), sporadic fatal insomnia and variably protease-sensitive prionopathy (VPSPr) [3], the most recently identified prionopathy [4].
These diseases have a long asymptomatic incubation period and largely differ in their clinical course, which typically ranges from a few months to several years. What is common is that all are triggered by misfolding of a host encoded cellular prion protein (PrPC) [5]. All TSEs share common neurodegenerative features: aggregation of the misfolded PrPC, early synaptic dysfunction and irreversible death of neurons [6]. PrPC is physiologically expressed throughout the body and is highly expressed in the central (CNS) and peripheral (PNS) nervous system, as a normal part of the neuronal membrane. It has a complex intracellular trafficking that seems to depend on the cell type [7]. The development of TSE includes the pathological conversion of the PrPC into the toxic and infectious isoform denoted as prion or PrPSc. PrPSc faithfully replicates, aggregates and deposits in brain parenchyma and is not prone to degradation via cellular proteases [1]. From the infected cell, horizontal and vertical transmission can occur, since misfolded proteins are efficiently transmitted to the daughter cells and by the intercellular spread [8].
The transgenic mice lacking Prnp gene are resistant to prion diseases [9] suggesting that the disease progression is dependent on a pool of PrPC within the cell that can be replicated. The PrP knockout mice show no significant phenotype. Likewise, the conditional Prnp knockout showed no signs of neurodegeneration [10]. This focused the design of therapeutic approaches towards the attenuation of PrPC [11]. However, a growing body of data reveals potential physiological PrPC functions, including its neuroprotective role in the CNS, while the loss of PrPC function renders the cells more susceptible to different types of stress [12]. In spite of this, the lack of deleterious effects upon the absence or silencing of PrP, observed in relevant animal models, infers a window of opportunity that can be used for the treatment aimed at the neutralization or depletion of the PrPC. This review will focus on the role of prion-specific antibodies in the modulation of PrP biology and the development of related therapeutic applications.
2. Therapeutic Candidates that Modulate PrPC Expression or Accessibility to Conversion
A number of drugs have been tested for therapeutic intervention in patients affected by TSEs, but none significantly increase the survival of patients [13]. The hypothesis that PrPC is essential for prion replication, but dispensable for the host, resulted in two types of anti-prion compounds that target PrPC expression.
First, some drugs have been tested that are considered safe for human health, and possess the desired ability to modulate PrPC expression, either by reducing or rearranging its cellular pool. A prominent example is suramin [14] and its derivatives which modulate biochemical properties of PrPC including solubility, its half-life [15] and, according to other studies, internalization rate [16]. Another example of a PrPC modulator that inhibits formation of the scrapie isoform is the drug mevinolin [17], which has multiple generic names and is used to lower cholesterol [18]. Mevinolin reduces the surface expression of PrPC leading to its intracellular accumulation [19]. Tamoxifen, another pharmaceutical [20], and its derivative 4-hydroxytamoxifen were recently shown to redirect cholesterol to lysosomes and consequently induce PrPC as well as PrPSc degradation through enhanced lysosomal trafficking and degradation [21]. However, a list of chemotherapeutics targeting PrPC expression, PrPSc expression or the conversion, including pentosan polysulfate, quinacrine, amphotericine B and flupirtine, have already been tried in clinical trials showing no or modest treatment efficacies [22]. Recently, a comprehensive drug screening was undertaken to identify new anti-PrP agents among drugs already approved for human use [23]. Screening targeted compounds that decrease PrPC expression. The most promising candidate, astemizole, prolonged the survival of prion-infected mice via stimulated autophagy [23].
The second line of compounds specifically target PrPC and as such their mode of action in principle should not affect other aspects of cellular biology, including the cell viability. One straightforward approach to specifically decrease PrPC levels is to target the expression of the gene responsible, in humans PRNP, either with interfering RNA molecules or by introducing a dominant negative mutant [24]. Molecules that bind specifically to PrPC include nucleic acid aptamers and peptide aptamers [25], which show inhibitory effect on prion conversion. In addition, a broad range of evidence shows that antibodies targeting PrPC, as a template for the scrapie prion propagation, are effective in curing infected cells [26,27,28,29]. Anti-PrPC antibodies and their derivatives represent a range of compounds able to reduce availability of the PrPC substrate for conversion; either by minimizing PrPC expression and inducing its redistribution from the sites critical for prion conversion or by preventing the formation of the molecular complexes between PrPC and PrPSc and other potential cofactors (Figure 1 and discussed below). In addition, antibodies may act as other potential drugs that bind PrPC and tend to stabilize the PrPC molecule in order to prevent conversion [11].
Beyond PrPC many drugs target PrPSc template. The awareness of the need for combination therapy is evolving after the anti-prion drug resistance was confirmed [30] for most promising candidates obtained within a comprehensive study of more than 10,000 small molecules able to reduce PrPSc content [31]. Recently, a new battery of promising small molecules with the ability to decrease the accumulation of PrPSc was obtained in another comprehensive screening that evaluated their drug ability and pharmacokinetic parameters [32]. When targeting PrPSc template, the aim may be to promote degradation, as observed for some branched polyamines [33] or for tyrosine kinase inhibitor STI571, which promotes lysosomal degradation [34]. Alternatively, the aim may be to stabilize fibrils as was proposed for congo red [35] or luminescent conjugated polymers [36] in order to reduce the spread of low molecular weight oligomers that seem to be particularly infectious and toxic [37,38].
Antibodies so far have not been implied in the stabilization of PrPSc, but in principle they could be able to do so if they recognized epitopes of the PrPSc amyloid fibrils (Figure 1). Alternatively, antibodies can selectively target misfolded proteins while sparing native, properly folded protein [39,40,41,42,43,44,45]. An antibody that would bind specifically to PrPSc could modulate PrPSc trafficking or inhibit PrPSc interaction with other molecules analogously as described for PrPc (Figure 1). Some studies found that PrPSc recognition is a beneficial feature of a curing antibody [46]. However, a significant number of curing antibodies do not recognize PrPSc [28]. In addition, some antibodies that recognize PrPSc have weak curing properties when administrated into in vivo or in vitro settings [27,45,47,48].
In conclusion, antibodies and their derivatives are on the list of most prominent candidates for the treatment of prion diseases [49,50] due to their effectiveness at targeting the PrPC as a reservoir for the prion conversion but also because of their potential to act on multiple and diverse levels in the prion pathogenesis.
3. The Role of Antibodies in the Molecular Mechanism of the PrPC to PrPSc Conversion
The key process behind prion diseases is the conversion of PrPC into the PrPSc isoform. In this process anti-PrP antibodies represent one of the most promising strategies for the treatment of prion diseases ever since they not only reduced, but completely cleared the pre-existing PrPSc from a culture of infected neuroblastoma cells [26,29,51]. However, the molecular mechanisms behind the conversion of PrPC into PrPSc and the role anti-PrP antibodies play remains elusive.
Regarding some aspects of the antibody-mediated process of clearing PrPSc a general consensus has been reached. There is a direct correlation between the affinity of anti-PrP antibody for the PrPC isoform and its potency to cure prion infected cells [27,28,52]. Furthermore, there is no unique epitope in the PrPC molecule that clears the disease, although not all epitopes are suitable or equally effective [28,47,52,53,54,55]. Finally, the compartment(s) of prion conversion are still the matter of debate, but the lines of evidence [56,57,58,59,60,61] including the most recent studies [62,63,64,65] largely agree that the plasma membrane and the membrane trafficking along the endocytic-recycling pathway are prominent sites where PrPC and PrPSc reside. Such localization favors the accessibility of both targets to the antibodies and warrants the maintenance of the stable antibody-target complexes. The ability of an antibody to recognize native PrPC molecules expressed on the plasma membrane may discriminate protective vs. non-protective immune responses [66,67,68]. In many cases, antibodies that have shown significant clearing capacities were internalized into the cells [28,52,69,70] suggesting their potential to exhibit additional positive effects also along the endocytic pathway.
Despite the convergence of several important issues of the antibody clearing capacity, there are a high number of mechanisms proposed for the function of anti-PrP antibodies. It is evident that these molecular mechanisms depend on the antibody epitope and in addition, different mechanisms do not necessarily exclude one another. Among them are: steric blocking or modifying the interaction of PrPC with PrPSc [26,52,71]; perturbation of PrPC trafficking, including internalization and degradation [28,52,70,72,73]; PrP sequestration on the cell surface [28,29,52,54]; increase of PrPC levels in the medium [28,52]; and neutralization of the infectious PrPSc template [52,72].
Although relevant for designing immunotherapeutic tools, the characterization of the antibody role in PrPC conversion to PrPSc is still not fully clarified. Thorough understanding of this molecular mechanism will contribute to the design of anti-prion therapeutics and to general principles of immunotherapy.
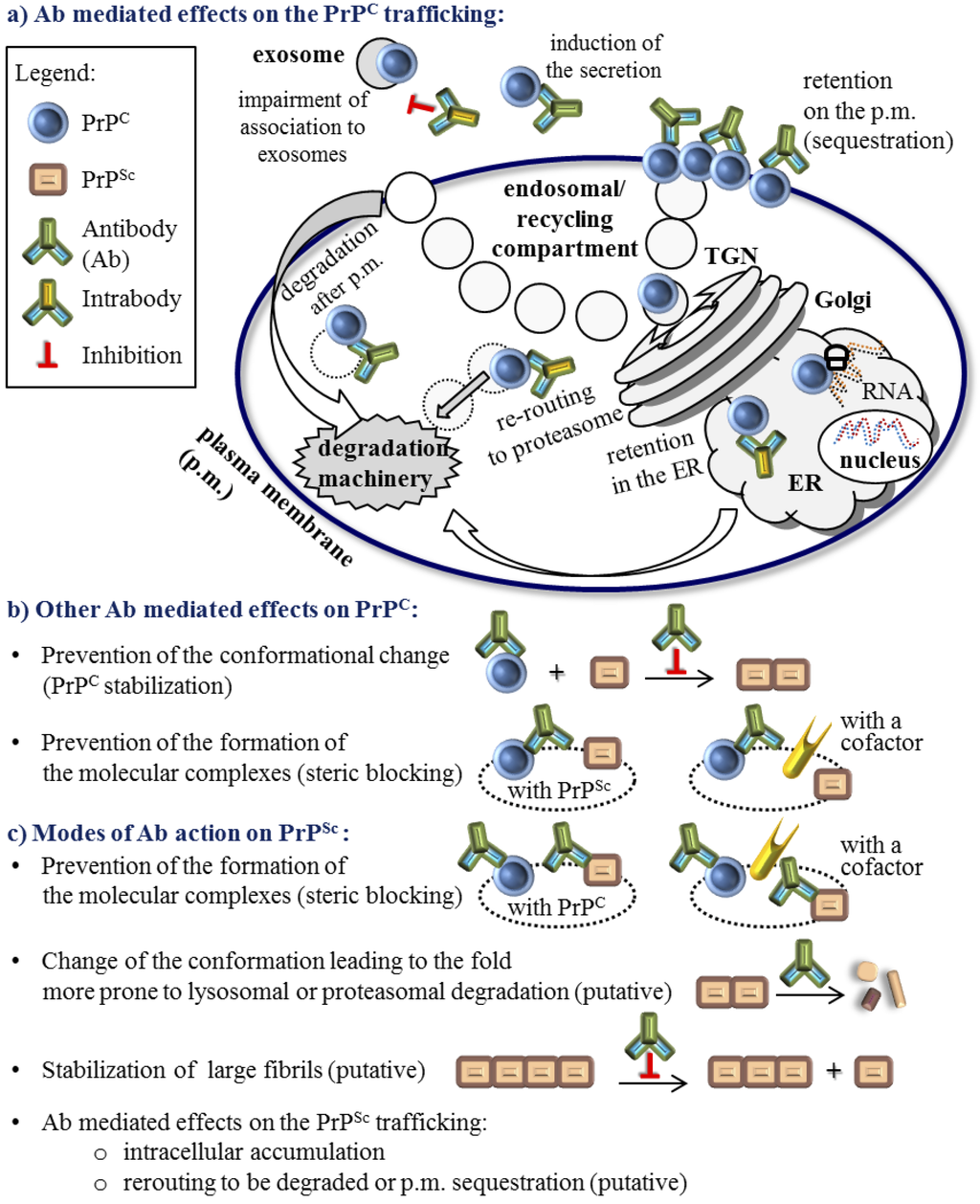
Figure 1.
Anti-prion protein antibody (Ab) modulation of the PrPC and PrPSc biology. There are no documented Ab effects on the PrPC transcription or translation. Ab effect on the immature PrPC is restricted to some intrabodies. (a) Published Ab mediated effects on the PrPC trafficking; (b) Other modes of Ab impact on mature PrPC that do not include the PrPC trafficking modulation and are not restricted to the specific compartment; (c) Modes of Ab action on the PrPSc cannot be easily separated from the modulation of PrPC because the percentage of the PrPSc in the cell is much lower and most of the Abs that recognize PrPSc do not discriminate between forms. Not fully confirmed modes of action are noted as ‘putative’. Undefined cellular compartments are depicted with a dotted line. Full IgGs and other recombinant Abs (Figure 2), except for intrabodies, are schematically represented by the same Ab symbol.
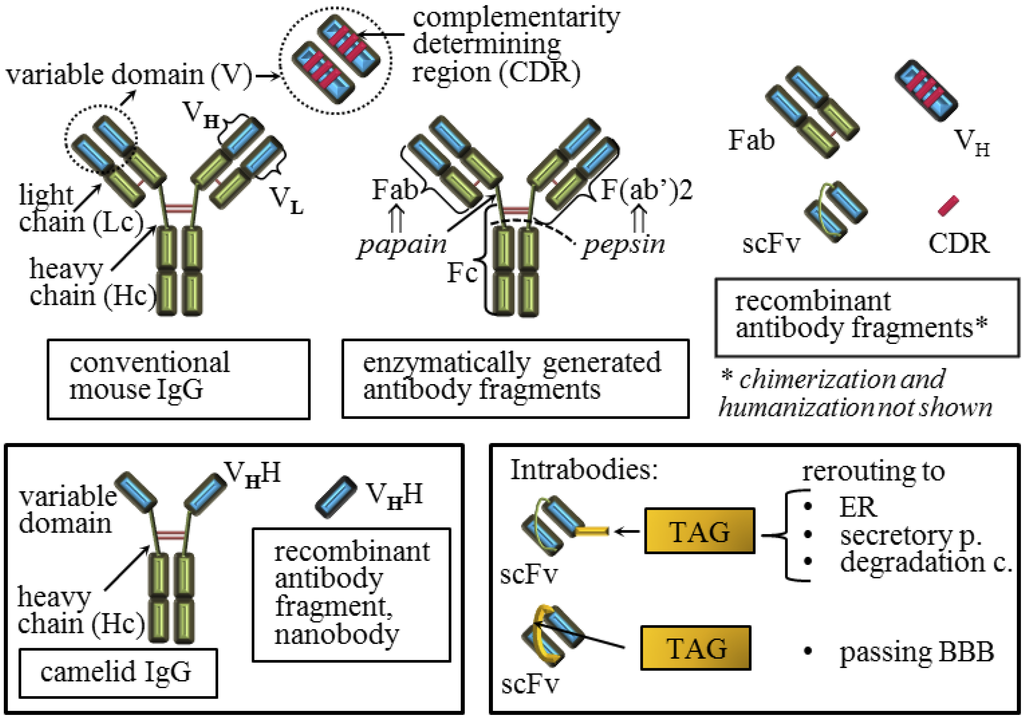
Figure 2.
Conventional and recombinant antibody compounds that have been developed against the prion protein. Schematic representation of mouse natural IgG (conventional IgG) that is composed of two light chains and two heavy chains linked together with disulfide bridges is shown. A variable domain of the conventional antibody that binds to the specific antigen is composed of the sequence on the heavy and on the light chain while each of these sequences is composed of three complementarity determining regions (CDRs). Common IgG fragments generated by enzymatic digestions or by the recombinant DNA technique are shown. The natural camelid IgG possess only two chains linked by disulfide bridges and each variable domain is composed of a single chain. Intrabodies are recombinant intracellular antibodies that are usually engineered to localize to a specific cellular compartment.
4. Active and Passive Immunotherapy Approaches
Active immunotherapy implies the production of anti-PrP antibodies by the host, mostly following vaccination, while in the passive immunotherapy a pre-made therapeutic, an antibody-based compound, is delivered directly or by the gene therapy.
Active immunotherapy suffers from the tolerance of the immune system to develop antibodies to the host protein. The prion protein is a native cellular protein and an organism is unlikely to recognize the subtle changes attributed to PrPSc as a threat especially as it is imprinted in the immune system to avoid the self-recognition. A number of approaches were undertaken in active immunization studies, including various antigens and adjuvants in order to break the tolerance against PrP [49,50]. The evaluation of essential protective immune response properties in different mouse models revealed the importance of an antigen to provoke antibodies recognizing cell-surface PrPC [66]. This finding was confirmed in the recent study identifying compounds with the best immunogenic potential, in which the protection model was further associated with depletion of mature follicular dendritic cells, which spread peripheral prion infection [67]. Encouraging results in the generation of host antibodies towards PrPC were obtained using PrP monomer peptides, multiple antigenic peptides, full PrPs, proteins resembling PrP epitopes or PrP dimers as the antigen of choice, administrated through various vector-, protein-, virus- or cell-carriers [49,50,74]. In addition, naturally occurring PrP autoantibodies were recently confirmed in cerebrospinal fluid and serum samples of healthy individuals [75]. The approach to target the PrPSc template and at the same time avoid the recognition of abundantly expressed PrPc, guided the development of the vaccine based on the PrPSc dominant epitope YYR [40]. The follow up studies were focused to (i) exclude the concern that such anti-PrPSc antibodies might by themselves induce the formation of the PrPSc template and to (ii) drive the anti-PrP antibody response towards conformations of PrP mutants associated with genetic types of the disease [76]. The animal studies show that still mucosal vaccination seems to be the most effective, although this approach would be restricted to preventing oral transmission among animals and human populations at risk [77,78].
Ubiquitous PrPC expression not only aggravates the natural expansion of anti-PrP antibodies, but also the introduction of premade antibodies recognizing PrPC via passive immunotherapeutic approaches might lead to severe immune reactions. This concern was alleviated after the groundbreaking study showing that co-expression of PrP-specific antibodies with PrPC expressed at physiological levels does not induce autoimmune responses or significant changes in various immune cell populations, which were still able to respond to other stimuli [71]. At the same time, the substantial anti-PrP antibody levels in the serum prevented scrapie pathogenesis after prion inoculation. Another study, based on subsequent and continuous passive immunizations initiated immediately following the scrapie inoculation, resulted in the prolongation of the incubation period or even prevention of disease, depending on the antibody used and the inoculum quantity [79]. The passive immunization attracted further interest after it was shown that the application of anti-PrP antibodies suppressed, in some cases even permanently, a peripheral prion replication in vivo and that such treatment was successful even after the onset of peripheral prion replication, although not after the clinical signs of illness were present [47]. In addition, administrated anti-PrP antibodies did not deplete PrP expressing immune cell populations nor was evidence for autoimmune reactions found. Recent data showed that in addition to intraventricular administration [80], a peripheral administration of antibodies could alleviate the disease progression also at the time of clinical onset [81]. The efficacy of the antibody distribution in the cerebella and thalami was in the correlation with the prolongation of survival times. Indeed, the delivery of the therapeutic to the infected brain is essential and as such new vectors that allow for the better delivery of anti-prion protein antibodies into the brain are being envisaged (discussed below, [82,83,84]). Likewise, recombinant antibody-derivatives with improved drug characteristics are being designed (discussed below). However, advancements of the numerous passive immunizations in the last decade [49] that resulted in more or less significant increase in the resistance to PrPSc, including the prolongation of the incubation period and the lifespan of treated animals, have been compromised with their possible neurotoxic effects [55,85]. These effects are still a matter of debate [86], but certainly pose additional requests to the design of the antibody-based therapeutics.
A line of evidence shows how PrPC plays an important role in the pathogenesis of other neurodegenerative diseases, such as Alzheimer’s disease (AD) [87]. Among neurodegenerative disorders, clinical development of immunotherapeutic strategies to cure AD patients is by far most advanced [88]. Unfortunately, active anti-AD immunization trials were stopped due to the severe side effects [88] while two phase III trials of anti-β-amyloid monoclonal antibody recently showed that passive immunotherapy provokes less alarming side effects, but does not improve clinical outcomes in patients with AD [89]. Regardless, new links between distinct neurodegenerative diseases will undoubtedly boost the anti-prion immunotherapeutic approaches including the especially relevant topic of better understanding of toxic side effects. A prominent example is the recent study on fully humanized anti-PrP antibody that was shown to prevent Aβ synaptotoxicity in rats without inducing obvious neurotoxicity [90]. As already mentioned, our understanding of the molecular mechanisms behind the mode of action of anti-PrP antibodies is insufficient and, correspondingly, multiple mechanisms are still proposed for the antibody-mediated plaque removal in AD [88]. The findings from the research on fundamental principles driving the immunotherapy of prion illnesses may thus provide a breakthrough in our knowledge of the more common neurodegenerative diseases.
5. Molecular Parameters that Influence the Quality of the Anti-Prion Protein Antibody Effect
In spite of the continuous progress, the major drawbacks of the passive immunization approach are still the (i) unfavorable pharmacokinetic of drugs; (ii) high amount of the drugs needed; (iii) and the inability of drugs to cross the blood-brain barrier (BBB) if they are not focused on the inhibition of peripheral prion replication and must access the central nervous system (CNS); all the above probably lead to limited success as therapeutics in vivo. Last but not the least; (iv) there is a concern that anti-PrPC antibodies might be neurotoxic. To that aim different recombinant antibody derivatives with different properties have been designed. Still, clinical immunotherapy trials in the neurodegenerative diseases used conventional full length IgG, although in the recombinant, humanized form [89]. In spite of the general complaint of IgG low potential to reach the CNS, in vivo studies showed that autoantibodies against Aβ can in fact cross the BBB [49] and peripheral administration of humanized form of IgG reached therapeutically active concentrations to prevent Aβ synaptotoxicity [90].
Since the generation of the first immunogens, numerous anti-PrP antibodies and antibody compounds have been developed, at first polyclonal [91] and later mostly by the immunization of Prnp°/° mice [9]. Interestingly, the first antibodies obtained upon the immunization of Prnp°/° mice were generated in the active form of the recombinant antigen-binding fragment, Fab (fragment antigen-binding), by the phage display technology due to the instability of initially acquired hybridoma cell lines secreting conventional monoclonal antibodies (Figure 2) [92]. Series of conventional antibodies have been raised since then, with promising candidates able to cure PrPSc in vitro with half maximal inhibitory concentration of PrPSc (IC50) far below 1 μM [50]. The process of developing new panels of anti-PrP antibodies is still in progress [45,93,94,95,96]. A study aimed at characterizing the pharmacokinetic properties of anti-PrP antibodies with curing properties in vitro showed that their curing capacity in vivo is associated with intrinsic pharmacokinetic properties rather than their isotype, epitope or affinity [97].
Recombinant Fab fragments (Figure 2) successfully cleared prion infectivity from cell cultures of infected cells [26]. Although a difference between polyclonal IgG molecules and corresponding Fab fragments in their capacity to inhibit prion replication in infected cells was observed [51], a comparison of several full IgGs and their Fab fragments showed that they retain similar binding properties and similar curing capacity [98]. The Fab fragments should be less prone to induce neurotoxicity, but this is still disputed. Namely, two studies showing neurotoxic side effects upon the antibody injection into the brain agreed that antibody mediated neurotoxicity was not mediated by its Fc (fragment crystallizable) fragment, but by triggering PrPC and that toxicity was dependent on the dosage of the antibody treatment [55,85]. Unfortunately, these studies did not reach agreement about other antibody parameters that should be taken into consideration during drug development nor on the molecular mechanism triggering downstream neurotoxic effects. The first study proposed that the divalent antibody form is responsible for crosslinking and triggering PrPC molecules leading to cell apoptosis [85], while the other proposed that the antibody epitope within a particular PrPC domain is detrimental for calpain activation [55]. The latter study showed no significant differences between the monovalent and divalent forms of the antibodies tested. Fortunately, in both studies some antibodies escaped the neurotoxic phenotype. Taken together with studies showing no deleterious effects upon the comparable antibody administration [54,80,86], emphasizes that generalizations about the toxicity of antibodies should be avoided and strongly suggests that a therapeutic window must exist. Certainly, Fab fragments have shorter circulating half-lives, but improved production opportunities via recombinant prokaryotic expression and enhanced capacity of penetrating into the brain [98,99] when compared to the full IgGs. However, in respect to these later advantages, smaller recombinant compounds are even more promising (Figure 2, [100]) and in addition to the Fabs and IgGs, many smaller monovalent compounds have been designed.
The limitations of full IgGs: the poor influx into CNS, the putative toxicity and the complex assembly; prompted studies aimed at generation of more potent recombinant proteins on the backbone of antibodies with desirable affinity characteristics. In line with that, recombinant anti-PrP scFvs (single-chain variable fragments, Figure 2) were designed and verified in neurodegenerative disease models [101]. Here, the scFvs retained the ability to clear the PrPSc infected cell cultures [68,84,102,103,104]. Recently, a humanized anti-PrPSc scFv has been produced [105]. The acknowledged advantage of the scFvs is their expression, suitable for large scale production in the periplasmic space of E. coli. In parallel, several eukaryotic cell lines secreting scFvs have been established [68,102,103]. One of the trasnsfectants was made on the background of immortalized microglia, acknowledged brain-engraftable cells that resulted in a short prolongation of the survival times in mice [103]. Indeed, the main advantage of the scFv is its single polypeptide sequence suitable for the gene transfer-based passive immunization, the approach in which the antibody is not delivered by the direct application, but by a corresponding gene encoding the antibody later synthesized by the host. The most recognized vectors for the delivery of these antibody genes, possessing high transduction efficiency and allowing intracerebral spread, are adeno-associated virus (AAV) based vectors. Two studies on vector types AAV2 and AAV9, both carrying genes for anti-PrP scFv, have resulted in a delay in the onset of clinical signs of disease, prolonged survival time, milder neuropathological changes, reduced PrPSc burden in the brain and, importantly, no inflammatory or neurotoxic effects together with prominent neuronal transduction efficiency and spread [82,83]. However, these beneficial outcomes were not all significant and both groups in their experimental model used intracerebral injection of the vectors followed by intraperitoneal challenge at expected peak of the scFv gene expression. A study aimed to design molecules and delivery mode that might function both peripherally and within the brain, thus affecting both sites of prion replication, explored the potential of lentiviral and AAV vectors encoding anti-PrP scFv [84]. In cell culture models of PrPSc clearance the lentiviral construct represented a more efficient delivery system compared to AAV. The scFv antibody format is also prevalent for the intracellular antibody (intrabody) expression [73]. These recombinant antibodies are engineered to localize to a specific cellular compartment. The Anti-PrP scFv with ER retention signal successfully retained PrPC in the ER and prevented PrPSc formation in the corresponding transfected cell lines, while the secretory version of the same intrabody mediated re-routing of PrPC to proteasome as well as impairment of PrPC association to exosomes [73]. An interesting scFv was recently obtained by fusing anti-PrPSc antibody variable domains with an advanced linker, cell-penetrating peptide (CPP), penetratin [106]. Upon administration in the mouse tail vein, the scFv without penetratin mainly stained the endothelial cells of brain veins, while the penetratin-scFv was transported through the BBB into the brain cells. However, an unexpected localization into the nuclei was observed that might necessitate additional modifications of this promising recombinant antibody.
In addition to the scFvs, other, smaller antibody forms have been envisaged. To that aim, camelid antibodies are of great interest, since they lack light chains and consequently possess a genuine single chain variable domain. Thus, corresponding recombinant antibody fragments, called nanobodies, are significantly smaller than scFvs obtained from the conventional antibodies (Figure 2). The ability of recombinant camelid antibody fragments to abolish prion replication in infected cell lines [72,96] and to diffuse into the brain parenchyma upon peripheral administration was confirmed [69]. A variable domain of the conventional antibody is composed of the sequence on the heavy and on the light chain and each of these sequences is composed of three complementarity determining regions (CDRs, Figure 2). The heavy chain of the anti-PrP antibody, combined with unrelated light chains, retained the capacity to prevent prion pathogenesis upon peripheral scrapie challenge [71]. Furthermore, a peptide mimicking only the third CDR of the anti-PrP heavy chain domain (CDR3H) showed anti-prion capacity in vitro [104].
Many promising anti-PrP antibody compounds have been produced so far. The main concern remains that, unless an artificial amount of an antibody is supplied to the site of infection, the reduction of the PrPc content in patients might only postpone and not prevent the illness [107]. The fact that a small amount of PrPC is enough for the productive replication underscores the hypothesis [108]. Alike, the need for the improvement of diagnostic tools that could pinpoint the illness at the earliest stage goes hand in hand with the need to optimize the infection::antibody ratio. Most of the administrated antibody compounds, including the full IgGs, do not have suitable characteristics to cure the infection in brain, but at the same time the gene-based delivery routes are still providing only a short term supply [82,83,103]. Although our future might decide on the gene therapy with the smallest possible antibody based drugs, currently in the treatment of neurodegenerative diseases conventional humanized IgG approach is still mainstream.
6. Authors’ Perspective
For more than a decade, the scientific community has been trying to envisage innovative therapeutics based on the anti-prion protein antibodies and new vaccines able to break immune tolerance against the prion protein. A remarkable pool of structural data and a considerable list of antibodies and recombinant antibody-forms generated to a single protein, the prion protein, offer a unique possibility to explore the fundamental premises of the immunotherapy. Unfortunately, few studies compared the original antibodies and their recombinant derivatives or a palette of recombinant antibodies recognizing the same epitope in thorough in vitro or in vivo studies. The influence of the size/form/valency/posttranslational modification of the antibody derivatives on the fundamental molecular mechanism triggered by their binding to the PrP molecules is still elusive. Among others, this includes the factor of antibody size on steric hindrance and blocking of PrPC conversion, the capacity of different derivatives to be internalized into the cells, the importance of their ability to crosslink the PrP molecules and induce or block endocytosis, the antibody-PrP complexes’ stability and dissociation of PrP molecules within various organelles and the molecular determinants triggering neurotoxic effects. In addition, the antibody glycosylation is very complex and its influence on the subtle differences in the antibody mode of action will be an interesting target for examination. Once rules that are more general with respect to the molecular mechanisms and the drug characteristics influencing PrPSc clearance are established, it will be easier to manipulate the functional and curative anti-PrP antibody properties. Such scientific outcomes will contribute to the understanding of general principles of recombinant antibody design and immunotherapy.
Acknowledgments
We thank Kate Pischke for proof-reading the manuscript and providing valuable comments. We would like to thank the support to T.L.R. by TALENTS FVG Fellowship Programme, FP1418521002.
Author Contributions
T.L.R and G.L. wrote this review.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar]
- Imran, M.; Mahmood, S. An overview of human prion diseases. Virol. J. 2011, 8, 559. [Google Scholar]
- Aguzzi, A.; Calella, A.M. Prions: Protein aggregation and infectious diseases. Physiol. Rev. 2009, 89, 1105–1152. [Google Scholar]
- Zou, W.Q.; Puoti, G.; Xiao, X.; Yuan, J.; Qing, L.; Cali, I.; Shimoji, M.; Langeveld, J.P.; Castellani, R.; Notari, S.; et al. Variably protease-sensitive prionopathy: A new sporadic disease of the prion protein. Ann. Neurol. 2010, 68, 162–172. [Google Scholar]
- Collinge, J.; Clarke, A.R. A general model of prion strains and their pathogenicity. Science 2007, 318, 930–936. [Google Scholar]
- Mallucci, G.R. Prion neurodegeneration: Starts and stops at the synapse. Prion 2009, 3, 195–201. [Google Scholar]
- Grassmann, A.; Wolf, H.; Hofmann, J.; Graham, J.; Vorberg, I. Cellular aspects of prion replication in vitro. Viruses 2013, 5, 374–405. [Google Scholar]
- Gousset, K.; Zurzolo, C. Tunnelling nanotubes: A highway for prion spreading? Prion 2009, 3, 94–98. [Google Scholar]
- Bueler, H.; Aguzzi, A.; Sailer, A.; Greiner, R.A.; Autenried, P.; Aguet, M.; Weissmann, C. Mice devoid of prp are resistant to scrapie. Cell 1993, 73, 1339–1347. [Google Scholar]
- Mallucci, G.R.; Ratte, S.; Asante, E.A.; Linehan, J.; Gowland, I.; Jefferys, J.G.; Collinge, J. Post-natal knockout of prion protein alters hippocampal ca1 properties, but does not result in neurodegeneration. EMBO J. 2002, 21, 202–210. [Google Scholar]
- Nicoll, A.J.; Collinge, J. Preventing prion pathogenicity by targeting the cellular prion protein. Infect. Disord. Drug Targets 2009, 9, 48–57. [Google Scholar]
- Didonna, A. Prion protein and its role in signal transduction. Cell. Mol. Biol. Lett. 2013, 18, 209–230. [Google Scholar]
- Stewart, L.A.; Rydzewska, L.H.; Keogh, G.F.; Knight, R.S. Systematic review of therapeutic interventions in human prion disease. Neurology 2008, 70, 1272–1281. [Google Scholar]
- Dressel, J.; Oesper, R. The discovery of germanin by oskar dressel and richard kothe. J. Chem. Educ. 1961, 38, 620–621. [Google Scholar]
- Nunziante, M.; Kehler, C.; Maas, E.; Kassack, M.U.; Groschup, M.; Schatzl, H.M. Charged bipolar suramin derivatives induce aggregation of the prion protein at the cell surface and inhibit prpsc replication. J. Cell Sci. 2005, 118, 4959–4973. [Google Scholar]
- Kiachopoulos, S.; Heske, J.; Tatzelt, J.; Winklhofer, K.F. Misfolding of the prion protein at the plasma membrane induces endocytosis, intracellular retention and degradation. Traffic 2004, 5, 426–436. [Google Scholar]
- Taraboulos, A.; Scott, M.; Semenov, A.; Avrahami, D.; Laszlo, L.; Prusiner, S.B. Cholesterol depletion and modification of cooh-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J. Cell Biol. 1995, 129, 121–132. [Google Scholar]
- Alberts, A.W.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E.; et al. Mevinolin: A highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme a reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 1980, 77, 3957–3961. [Google Scholar]
- Gilch, S.; Kehler, C.; Schatzl, H.M. The prion protein requires cholesterol for cell surface localization. Mol. Cell. Neurosci. 2006, 31, 346–353. [Google Scholar]
- Group, B.I.G.C.; Mouridsen, H.; Giobbie-Hurder, A.; Goldhirsch, A.; Thurlimann, B.; Paridaens, R.; Smith, I.; Mauriac, L.; Forbes, J.; Price, K.N.; et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N. Engl. J. Med. 2009, 361, 766–776. [Google Scholar]
- Marzo, L.; Marijanovic, Z.; Browman, D.; Chamoun, Z.; Caputo, A.; Zurzolo, C. 4-hydroxytamoxifen leads to prpsc clearance by conveying both prpc and prpsc to lysosomes independently of autophagy. J. Cell Sci. 2013, 126, 1345–1354. [Google Scholar]
- Sim, V.L.; Caughey, B. Recent advances in prion chemotherapeutics. Infect. Disord. Drug Targets 2009, 9, 81–91. [Google Scholar]
- Karapetyan, Y.E.; Sferrazza, G.F.; Zhou, M.; Ottenberg, G.; Spicer, T.; Chase, P.; Fallahi, M.; Hodder, P.; Weissmann, C.; Lasmezas, C.I. Unique drug screening approach for prion diseases identifies tacrolimus and astemizole as antiprion agents. Proc. Natl. Acad. Sci. USA 2013, 110, 7044–7049. [Google Scholar]
- Trevitt, C.R.; Collinge, J. A systematic review of prion therapeutics in experimental models. Brain: J. Neurol. 2006, 129, 2241–2265. [Google Scholar]
- Gilch, S.; Schatzl, H.M. Aptamers against prion proteins and prions. Cell. Mol. Life Sci.: CMLS 2009, 66, 2445–2455. [Google Scholar]
- Peretz, D.; Williamson, R.A.; Kaneko, K.; Vergara, J.; Leclerc, E.; Schmitt-Ulms, G.; Mehlhorn, I.R.; Legname, G.; Wormald, M.R.; Rudd, P.M.; et al. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 2001, 412, 739–743. [Google Scholar]
- Antonyuk, S.V.; Trevitt, C.R.; Strange, R.W.; Jackson, G.S.; Sangar, D.; Batchelor, M.; Cooper, S.; Fraser, C.; Jones, S.; Georgiou, T.; et al. Crystal structure of human prion protein bound to a therapeutic antibody. Proc. Natl. Acad. Sci. USA 2009, 106, 2554–2558. [Google Scholar]
- Feraudet, C.; Morel, N.; Simon, S.; Volland, H.; Frobert, Y.; Creminon, C.; Vilette, D.; Lehmann, S.; Grassi, J. Screening of 145 anti-prp monoclonal antibodies for their capacity to inhibit prpsc replication in infected cells. J. Biol. Chem. 2005, 280, 11247–11258. [Google Scholar]
- Enari, M.; Flechsig, E.; Weissmann, C. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl. Acad. Sci. USA 2001, 98, 9295–9299. [Google Scholar]
- Berry, D.B.; Lu, D.; Geva, M.; Watts, J.C.; Bhardwaj, S.; Oehler, A.; Renslo, A.R.; DeArmond, S.J.; Prusiner, S.B.; Giles, K. Drug resistance confounding prion therapeutics. Proc. Natl. Acad. Sci. USA 2013, 110, E4160–E4169. [Google Scholar]
- Ghaemmaghami, S.; May, B.C.; Renslo, A.R.; Prusiner, S.B. Discovery of 2-aminothiazoles as potent antiprion compounds. J. Virol. 2010, 84, 3408–3412. [Google Scholar]
- Ferreira, N.C.; Marques, I.A.; Conceicao, W.A.; Macedo, B.; Machado, C.S.; Mascarello, A.; Chiaradia-Delatorre, L.D.; Yunes, R.A.; Nunes, R.J.; Hughson, A.G.; et al. Anti-prion activity of a panel of aromatic chemical compounds: In vitro and in silico approaches. PLoS One 2014, 9, e84531. [Google Scholar]
- Supattapone, S.; Wille, H.; Uyechi, L.; Safar, J.; Tremblay, P.; Szoka, F.C.; Cohen, F.E.; Prusiner, S.B.; Scott, M.R. Branched polyamines cure prion-infected neuroblastoma cells. J. Virol. 2001, 75, 3453–3461. [Google Scholar]
- Ertmer, A.; Gilch, S.; Yun, S.W.; Flechsig, E.; Klebl, B.; Stein-Gerlach, M.; Klein, M.A.; Schatzl, H.M. The tyrosine kinase inhibitor sti571 induces cellular clearance of prpsc in prion-infected cells. J. Biol. Chem. 2004, 279, 41918–41927. [Google Scholar]
- Caspi, S.; Halimi, M.; Yanai, A.; Sasson, S.B.; Taraboulos, A.; Gabizon, R. The anti-prion activity of congo red. Putative mechanism. J. Biol. Chem. 1998, 273, 3484–3489. [Google Scholar]
- Margalith, I.; Suter, C.; Ballmer, B.; Schwarz, P.; Tiberi, C.; Sonati, T.; Falsig, J.; Nystrom, S.; Hammarstrom, P.; Aslund, A.; et al. Polythiophenes inhibit prion propagation by stabilizing prion protein (prp) aggregates. J. Biol. Chem. 2012, 287, 18872–18887. [Google Scholar]
- Silveira, J.R.; Raymond, G.J.; Hughson, A.G.; Race, R.E.; Sim, V.L.; Hayes, S.F.; Caughey, B. The most infectious prion protein particles. Nature 2005, 437, 257–261. [Google Scholar]
- Simoneau, S.; Rezaei, H.; Sales, N.; Kaiser-Schulz, G.; Lefebvre-Roque, M.; Vidal, C.; Fournier, J.G.; Comte, J.; Wopfner, F.; Grosclaude, J.; et al. In vitro and in vivo neurotoxicity of prion protein oligomers. PLoS Pathog. 2007, 3, e125. [Google Scholar]
- Paramithiotis, E.; Pinard, M.; Lawton, T.; LaBoissiere, S.; Leathers, V.L.; Zou, W.Q.; Estey, L.A.; Lamontagne, J.; Lehto, M.T.; Kondejewski, L.H.; et al. A prion protein epitope selective for the pathologically misfolded conformation. Nat. Med. 2003, 9, 893–899. [Google Scholar]
- Hedlin, P.D.; Cashman, N.R.; Li, L.; Gupta, J.; Babiuk, L.A.; Potter, A.A.; Griebel, P.; Napper, S. Design and delivery of a cryptic prp(c) epitope for induction of prp(sc)-specific antibody responses. Vaccine 2010, 28, 981–988. [Google Scholar]
- Jones, M.; Wight, D.; McLoughlin, V.; Norrby, K.; Ironside, J.W.; Connolly, J.G.; Farquhar, C.F.; MacGregor, I.R.; Head, M.W. An antibody to the aggregated synthetic prion protein peptide (prp106–126) selectively recognizes disease-associated prion protein (prp) from human brain specimens. Brain Pathol. 2009, 19, 293–302. [Google Scholar]
- Horiuchi, M.; Karino, A.; Furuoka, H.; Ishiguro, N.; Kimura, K.; Shinagawa, M. Generation of monoclonal antibody that distinguishes prpsc from prpc and neutralizes prion infectivity. Virology 2009, 394, 200–207. [Google Scholar]
- Curin Serbec, V.; Bresjanac, M.; Popovic, M.; Pretnar Hartman, K.; Galvani, V.; Rupreht, R.; Cernilec, M.; Vranac, T.; Hafner, I.; Jerala, R. Monoclonal antibody against a peptide of human prion protein discriminates between creutzfeldt-jacob's disease-affected and normal brain tissue. J. Biol. Chem. 2004, 279, 3694–3698. [Google Scholar]
- Korth, C.; Stierli, B.; Streit, P.; Moser, M.; Schaller, O.; Fischer, R.; Schulz-Schaeffer, W.; Kretzschmar, H.; Raeber, A.; Braun, U.; et al. Prion (prpsc)-specific epitope defined by a monoclonal antibody. Nature 1997, 390, 74–77. [Google Scholar]
- Petsch, B.; Muller-Schiffmann, A.; Lehle, A.; Zirdum, E.; Prikulis, I.; Kuhn, F.; Raeber, A.J.; Ironside, J.W.; Korth, C.; Stitz, L. Biological effects and use of prpsc- and prp-specific antibodies generated by immunization with purified full-length native mouse prions. J. Virol. 2011, 85, 4538–4546. [Google Scholar]
- Beringue, V.; Vilette, D.; Mallinson, G.; Archer, F.; Kaisar, M.; Tayebi, M.; Jackson, G.S.; Clarke, A.R.; Laude, H.; Collinge, J.; et al. Prpsc binding antibodies are potent inhibitors of prion replication in cell lines. J. Biol. Chem. 2004, 279, 39671–39676. [Google Scholar]
- White, A.R.; Enever, P.; Tayebi, M.; Mushens, R.; Linehan, J.; Brandner, S.; Anstee, D.; Collinge, J.; Hawke, S. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature 2003, 422, 80–83. [Google Scholar]
- Kubota, T.; Hamazoe, Y.; Hashiguchi, S.; Ishibashi, D.; Akasaka, K.; Nishida, N.; Katamine, S.; Sakaguchi, S.; Kuroki, R.; Nakashima, T.; et al. Direct evidence of generation and accumulation of beta-sheet-rich prion protein in scrapie-infected neuroblastoma cells with human igg1 antibody specific for beta-form prion protein. J. Biol. Chem. 2012, 287, 14023–14039. [Google Scholar]
- Roettger, Y.; Du, Y.; Bacher, M.; Zerr, I.; Dodel, R.; Bach, J.P. Immunotherapy in prion disease. Nat. Rev. Neurol. 2013, 9, 98–105. [Google Scholar]
- Bade, S.; Frey, A. Potential of active and passive immunizations for the prevention and therapy of transmissible spongiform encephalopathies. Exp. Rev. Vacc. 2007, 6, 153–168. [Google Scholar]
- Gilch, S.; Wopfner, F.; Renner-Muller, I.; Kremmer, E.; Bauer, C.; Wolf, E.; Brem, G.; Groschup, M.H.; Schatzl, H.M. Polyclonal anti-prp auto-antibodies induced with dimeric prp interfere efficiently with prpsc propagation in prion-infected cells. J. Biol. Chem. 2003, 278, 18524–18531. [Google Scholar]
- Pankiewicz, J.; Prelli, F.; Sy, M.S.; Kascsak, R.J.; Kascsak, R.B.; Spinner, D.S.; Carp, R.I.; Meeker, H.C.; Sadowski, M.; Wisniewski, T. Clearance and prevention of prion infection in cell culture by anti-prp antibodies. Eur. J. Neurosci. 2006, 23, 2635–2647. [Google Scholar]
- Chang, B.; Petersen, R.; Wisniewski, T.; Rubenstein, R. Influence of mabs on prp(sc) formation using in vitro and cell-free systems. PLoS One 2012, 7, e41626. [Google Scholar]
- Kim, C.L.; Karino, A.; Ishiguro, N.; Shinagawa, M.; Sato, M.; Horiuchi, M. Cell-surface retention of prpc by anti-prp antibody prevents protease-resistant prp formation. J. Gener. Virol. 2004, 85, 3473–3482. [Google Scholar]
- Sonati, T.; Reimann, R.R.; Falsig, J.; Baral, P.K.; O'Connor, T.; Hornemann, S.; Yaganoglu, S.; Li, B.; Herrmann, U.S.; Wieland, B.; et al. The toxicity of antiprion antibodies is mediated by the flexible tail of the prion protein. Nature 2013, 501, 102–106. [Google Scholar]
- Caughey, B.; Raymond, G.J.; Ernst, D.; Race, R.E. N-terminal truncation of the scrapie-associated form of prp by lysosomal protease(s): Implications regarding the site of conversion of prp to the protease-resistant state. J. Virol. 1991, 65, 6597–6603. [Google Scholar]
- Caughey, B.; Raymond, G.J. The scrapie-associated form of prp is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J. Biol. Chem. 1991, 266, 18217–18223. [Google Scholar]
- Taraboulos, A.; Raeber, A.J.; Borchelt, D.R.; Serban, D.; Prusiner, S.B. Synthesis and trafficking of prion proteins in cultured cells. Mol. Biol. Cell 1992, 3, 851–863. [Google Scholar]
- Borchelt, D.R.; Taraboulos, A.; Prusiner, S.B. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J. Biol. Chem. 1992, 267, 16188–16199. [Google Scholar]
- Shyng, S.L.; Huber, M.T.; Harris, D.A. A prion protein cycles between the cell surface and an endocytic compartment in cultured neuroblastoma cells. J. Biol. Chem. 1993, 268, 15922–15928. [Google Scholar]
- Marijanovic, Z.; Caputo, A.; Campana, V.; Zurzolo, C. Identification of an intracellular site of prion conversion. PLoS Pathog. 2009, 5, e1000426. [Google Scholar]
- Yamasaki, T.; Baron, G.S.; Suzuki, A.; Hasebe, R.; Horiuchi, M. Characterization of intracellular dynamics of inoculated prp-res and newly generated prp(sc) during early stage prion infection in neuro2a cells. Virology 2014, 450–451, 324–335. [Google Scholar]
- Goold, R.; McKinnon, C.; Rabbanian, S.; Collinge, J.; Schiavo, G.; Tabrizi, S.J. Alternative fates of newly formed prpsc upon prion conversion on the plasma membrane. J. Cell Sci. 2013, 126, 3552–3562. [Google Scholar]
- Rouvinski, A.; Karniely, S.; Kounin, M.; Moussa, S.; Goldberg, M.D.; Warburg, G.; Lyakhovetsky, R.; Papy-Garcia, D.; Kutzsche, J.; Korth, C.; et al. Live imaging of prions reveals nascent prpsc in cell-surface, raft-associated amyloid strings and webs. J. Cell Biol. 2014, 204, 423–441. [Google Scholar]
- Uchiyama, K.; Muramatsu, N.; Yano, M.; Usui, T.; Miyata, H.; Sakaguchi, S. Prions disturb post-golgi trafficking of membrane proteins. Nat. Commun. 2013, 4, 1846. [Google Scholar]
- Polymenidou, M.; Heppner, F.L.; Pellicioli, E.C.; Urich, E.; Miele, G.; Braun, N.; Wopfner, F.; Schatzl, H.M.; Becher, B.; Aguzzi, A. Humoral immune response to native eukaryotic prion protein correlates with anti-prion protection. Proc. Natl. Acad. Sci. USA 2004, 101, 14670–14676. [Google Scholar]
- Xanthopoulos, K.; Lagoudaki, R.; Kontana, A.; Kyratsous, C.; Panagiotidis, C.; Grigoriadis, N.; Yiangou, M.; Sklaviadis, T. Immunization with recombinant prion protein leads to partial protection in a murine model of tses through a novel mechanism. PLoS One 2013, 8, e59143. [Google Scholar]
- Shimizu, Y.; Kaku-Ushiki, Y.; Iwamaru, Y.; Muramoto, T.; Kitamoto, T.; Yokoyama, T.; Mohri, S.; Tagawa, Y. A novel anti-prion protein monoclonal antibody and its single-chain fragment variable derivative with ability to inhibit abnormal prion protein accumulation in cultured cells. Microbiol. Immunol. 2010, 54, 112–121. [Google Scholar]
- David, M.A.; Jones, D.R.; Tayebi, M. Potential candidate camelid antibodies for the treatment of protein-misfolding diseases. J. Neuroimmunol. 2014, 272, 76–85. [Google Scholar]
- Perrier, V.; Solassol, J.; Crozet, C.; Frobert, Y.; Mourton-Gilles, C.; Grassi, J.; Lehmann, S. Anti-prp antibodies block prpsc replication in prion-infected cell cultures by accelerating prpc degradation. J. Neurochem. 2004, 89, 454–463. [Google Scholar]
- Heppner, F.L.; Musahl, C.; Arrighi, I.; Klein, M.A.; Rulicke, T.; Oesch, B.; Zinkernagel, R.M.; Kalinke, U.; Aguzzi, A. Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies. Science 2001, 294, 178–182. [Google Scholar]
- Jones, D.R.; Taylor, W.A.; Bate, C.; David, M.; Tayebi, M. A camelid anti-prp antibody abrogates prp replication in prion-permissive neuroblastoma cell lines. PLoS One 2010, 5, e9804. [Google Scholar]
- Cardinale, A.; Biocca, S. Gene-based antibody strategies for prion diseases. Int. J. Cell Biol. 2013, 2013, 710406. [Google Scholar]
- Gilch, S.; Nunziante, M.; Ertmer, A.; Schatzl, H.M. Strategies for eliminating prp(c) as substrate for prion conversion and for enhancing prp(sc) degradation. Vet. Microbiol. 2007, 123, 377–386. [Google Scholar]
- Wei, X.; Roettger, Y.; Tan, B.; He, Y.; Dodel, R.; Hampel, H.; Wei, G.; Haney, J.; Gu, H.; Johnstone, B.H.; et al. Human anti-prion antibodies block prion peptide fibril formation and neurotoxicity. J. Biol. Chem. 2012, 287, 12858–12866. [Google Scholar]
- Madampage, C.A.; Maattanen, P.; Marciniuk, K.; Brownlie, R.; Andrievskaia, O.; Potter, A.; Cashman, N.R.; Lee, J.S.; Napper, S. Binding of bovine t194a prp(c) by prp(sc)-specific antibodies: Potential implications for immunotherapy of familial prion diseases. Prion 2013, 7, 301–311. [Google Scholar]
- Goni, F.; Knudsen, E.; Schreiber, F.; Scholtzova, H.; Pankiewicz, J.; Carp, R.; Meeker, H.C.; Rubenstein, R.; Brown, D.R.; Sy, M.S.; et al. Mucosal vaccination delays or prevents prion infection via an oral route. Neuroscience 2005, 133, 413–421. [Google Scholar]
- Bade, S.; Baier, M.; Boetel, T.; Frey, A. Intranasal immunization of balb/c mice against prion protein attenuates orally acquired transmissible spongiform encephalopathy. Vaccine 2006, 24, 1242–1253. [Google Scholar]
- Sigurdsson, E.M.; Sy, M.S.; Li, R.; Scholtzova, H.; Kascsak, R.J.; Kascsak, R.; Carp, R.; Meeker, H.C.; Frangione, B.; Wisniewski, T. Anti-prion antibodies for prophylaxis following prion exposure in mice. Neurosci. Lett. 2003, 336, 185–187. [Google Scholar]
- Song, C.H.; Furuoka, H.; Kim, C.L.; Ogino, M.; Suzuki, A.; Hasebe, R.; Horiuchi, M. Effect of intraventricular infusion of anti-prion protein monoclonal antibodies on disease progression in prion-infected mice. J. Gener. Virol. 2008, 89, 1533–1544. [Google Scholar]
- Ohsawa, N.; Song, C.H.; Suzuki, A.; Furuoka, H.; Hasebe, R.; Horiuchi, M. Therapeutic effect of peripheral administration of an anti-prion protein antibody on mice infected with prions. Microbiol. Immunol. 2013, 57, 288–297. [Google Scholar]
- Wuertzer, C.A.; Sullivan, M.A.; Qiu, X.; Federoff, H.J. Cns delivery of vectored prion-specific single-chain antibodies delays disease onset. Mol. Ther. 2008, 16, 481–486. [Google Scholar]
- Moda, F.; Vimercati, C.; Campagnani, I.; Ruggerone, M.; Giaccone, G.; Morbin, M.; Zentilin, L.; Giacca, M.; Zucca, I.; Legname, G.; et al. Brain delivery of aav9 expressing an anti-prp monovalent antibody delays prion disease in mice. Prion 2012, 6, 383–390. [Google Scholar]
- Campana, V.; Zentilin, L.; Mirabile, I.; Kranjc, A.; Casanova, P.; Giacca, M.; Prusiner, S.B.; Legname, G.; Zurzolo, C. Development of antibody fragments for immunotherapy of prion diseases. Biochem. J. 2009, 418, 507–515. [Google Scholar]
- Solforosi, L.; Criado, J.R.; McGavern, D.B.; Wirz, S.; Sanchez-Alavez, M.; Sugama, S.; DeGiorgio, L.A.; Volpe, B.T.; Wiseman, E.; Abalos, G.; et al. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 2004, 303, 1514–1516. [Google Scholar]
- Klohn, P.C.; Farmer, M.; Linehan, J.M.; O’Malley, C.; Fernandez de Marco, M.; Taylor, W.; Farrow, M.; Khalili-Shirazi, A.; Brandner, S.; Collinge, J. Prp antibodies do not trigger mouse hippocampal neuron apoptosis. Science 2012, 335, 52. [Google Scholar]
- Kellett, K.A.; Hooper, N.M. Prion protein and alzheimer disease. Prion 2009, 3, 190–194. [Google Scholar]
- Brody, D.L.; Holtzman, D.M. Active and passive immunotherapy for neurodegenerative disorders. Annu. Rev. Neurosc. 2008, 31, 175–193. [Google Scholar]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two phase 3 trials of bapineuzumab in mild-to-moderate alzheimer's disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar]
- Klyubin, I.; Nicoll, A.J.; Khalili-Shirazi, A.; Farmer, M.; Canning, S.; Mably, A.; Linehan, J.; Brown, A.; Wakeling, M.; Brandner, S.; et al. Peripheral administration of a humanized anti-prp antibody blocks alzheimer's disease abeta synaptotoxicity. J. Neurosci. 2014, 34, 6140–6145. [Google Scholar]
- Bendheim, P.E.; Barry, R.A.; DeArmond, S.J.; Stites, D.P.; Prusiner, S.B. Antibodies to a scrapie prion protein. Nature 1984, 310, 418–421. [Google Scholar]
- Williamson, R.A.; Peretz, D.; Smorodinsky, N.; Bastidas, R.; Serban, H.; Mehlhorn, I.; DeArmond, S.J.; Prusiner, S.B.; Burton, D.R. Circumventing tolerance to generate autologous monoclonal antibodies to the prion protein. Proc. Natl. Acad. Sci. USA 1996, 93, 7279–7282. [Google Scholar]
- McCutcheon, S.; Langeveld, J.P.; Tan, B.C.; Gill, A.C.; de Wolf, C.; Martin, S.; Gonzalez, L.; Alibhai, J.; Blanco, A.R.; Campbell, L.; et al. Prion protein-specific antibodies that detect multiple tse agents with high sensitivity. PLoS One 2014, 9, e91143. [Google Scholar]
- Stanker, L.H.; Serban, A.V.; Cleveland, E.; Hnasko, R.; Lemus, A.; Safar, J.; DeArmond, S.J.; Prusiner, S.B. Conformation-dependent high-affinity monoclonal antibodies to prion proteins. J. Immunol. 2010, 185, 729–737. [Google Scholar]
- Polymenidou, M.; Moos, R.; Scott, M.; Sigurdson, C.; Shi, Y.Z.; Yajima, B.; Hafner-Bratkovic, I.; Jerala, R.; Hornemann, S.; Wuthrich, K.; et al. The pom monoclonals: A comprehensive set of antibodies to non-overlapping prion protein epitopes. PLoS One 2008, 3, e3872. [Google Scholar]
- Abskharon, R.N.; Giachin, G.; Wohlkonig, A.; Soror, S.H.; Pardon, E.; Legname, G.; Steyaert, J. Probing the N-terminal beta-sheet conversion in the crystal structure of the human prion protein bound to a nanobody. J. Am. Chem. Soc. 2014, 136, 937–944. [Google Scholar]
- Feraudet-Tarisse, C.; Andreoletti, O.; Morel, N.; Simon, S.; Lacroux, C.; Mathey, J.; Lamourette, P.; Relano, A.; Torres, J.M.; Creminon, C.; et al. Immunotherapeutic effect of anti-prp monoclonal antibodies in transmissible spongiform encephalopathy mouse models: Pharmacokinetic and pharmacodynamic analysis. J. Gener. Virol. 2010, 91, 1635–1645. [Google Scholar]
- Alexandrenne, C.; Hanoux, V.; Dkhissi, F.; Boquet, D.; Couraud, J.Y.; Wijkhuisen, A. Curative properties of antibodies against prion protein: A comparative in vitro study of monovalent fragments and divalent antibodies. J. Neuroimmunol. 2009, 209, 50–56. [Google Scholar]
- Weir, A.N.; Nesbitt, A.; Chapman, A.P.; Popplewell, A.G.; Antoniw, P.; Lawson, A.D. Formatting antibody fragments to mediate specific therapeutic functions. Biochem. Soc. Trans. 2002, 30, 512–516. [Google Scholar]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar]
- Huang, L.; Su, X.; Federoff, H.J. Single-chain fragment variable passive immunotherapies for neurodegenerative diseases. Int. J. Mol. Sci. 2013, 14, 19109–19127. [Google Scholar]
- Donofrio, G.; Heppner, F.L.; Polymenidou, M.; Musahl, C.; Aguzzi, A. Paracrine inhibition of prion propagation by anti-prp single-chain fv miniantibodies. J. Virol. 2005, 79, 8330–8338. [Google Scholar]
- Fujita, K.; Yamaguchi, Y.; Mori, T.; Muramatsu, N.; Miyamoto, T.; Yano, M.; Miyata, H.; Ootsuyama, A.; Sawada, M.; Matsuda, H.; et al. Effects of a brain-engraftable microglial cell line expressing anti-prion scfv antibodies on survival times of mice infected with scrapie prions. Cell. Mol. Neurobiol. 2011, 31, 999–1008. [Google Scholar]
- Muller-Schiffmann, A.; Petsch, B.; Leliveld, S.R.; Muyrers, J.; Salwierz, A.; Mangels, C.; Schwarzinger, S.; Riesner, D.; Stitz, L.; Korth, C. Complementarity determining regions of an anti-prion protein scfv fragment orchestrate conformation specificity and antiprion activity. Mol. Immunol. 2009, 46, 532–540. [Google Scholar]
- Skrlj, N.; Vranac, T.; Popovic, M.; Curin Serbec, V.; Dolinar, M. Specific binding of the pathogenic prion isoform: Development and characterization of a humanized single-chain variable antibody fragment. PLoS One 2011, 6, e15783. [Google Scholar]
- Skrlj, N.; Drevensek, G.; Hudoklin, S.; Romih, R.; Curin Serbec, V.; Dolinar, M. Recombinant single-chain antibody with the trojan peptide penetratin positioned in the linker region enables cargo transfer across the blood-brain barrier. Appl. Biochem. Biotechnol. 2013, 169, 159–169. [Google Scholar]
- Prusiner, S.B.; Groth, D.; Serban, A.; Koehler, R.; Foster, D.; Torchia, M.; Burton, D.; Yang, S.L.; DeArmond, S.J. Ablation of the prion protein (prp) gene in mice prevents scrapie and facilitates production of anti-prp antibodies. Proc. Natl. Acad. Sci. USA 1993, 90, 10608–10612. [Google Scholar]
- Vorberg, I.; Raines, A.; Story, B.; Priola, S.A. Susceptibility of common fibroblast cell lines to transmissible spongiform encephalopathy agents. J. Infect. Dis. 2004, 189, 431–439. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).