Functional Characterization of a Bidirectional Plant Promoter from Cotton Leaf Curl Burewala Virus Using an Agrobacterium-Mediated Transient Assay
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material and Bacterial Strain
2.2. Isolation of CLCuBuV Bidirectional Promoter
- (i)
- 5'- CCATGGTGACTTTGGTTTAGAGACAACAAC-3' and 5'- CTGCAGTAATTCCTAGCCCTTATTACCAG-3'
- (ii)
- 5'- CTGCAGTGACTTTGGTCAATTAGAGACAAC-3' and 5'- CCATGGTAATTCCTAGCCCTTATTACCAG-3'
2.3. Plasmid Construction
2.4. Sequence Analysis
2.5. Preparation of the Agrobacterium Suspension
2.6. Agrobacterium-Mediated Infiltration
2.7. Histochemical Detection of GUS Activity
2.8. Fluorometric Determination of GUS Activity
2.9. Statistical Analysis
3. Results
3.1. Structure and Sequence Analysis
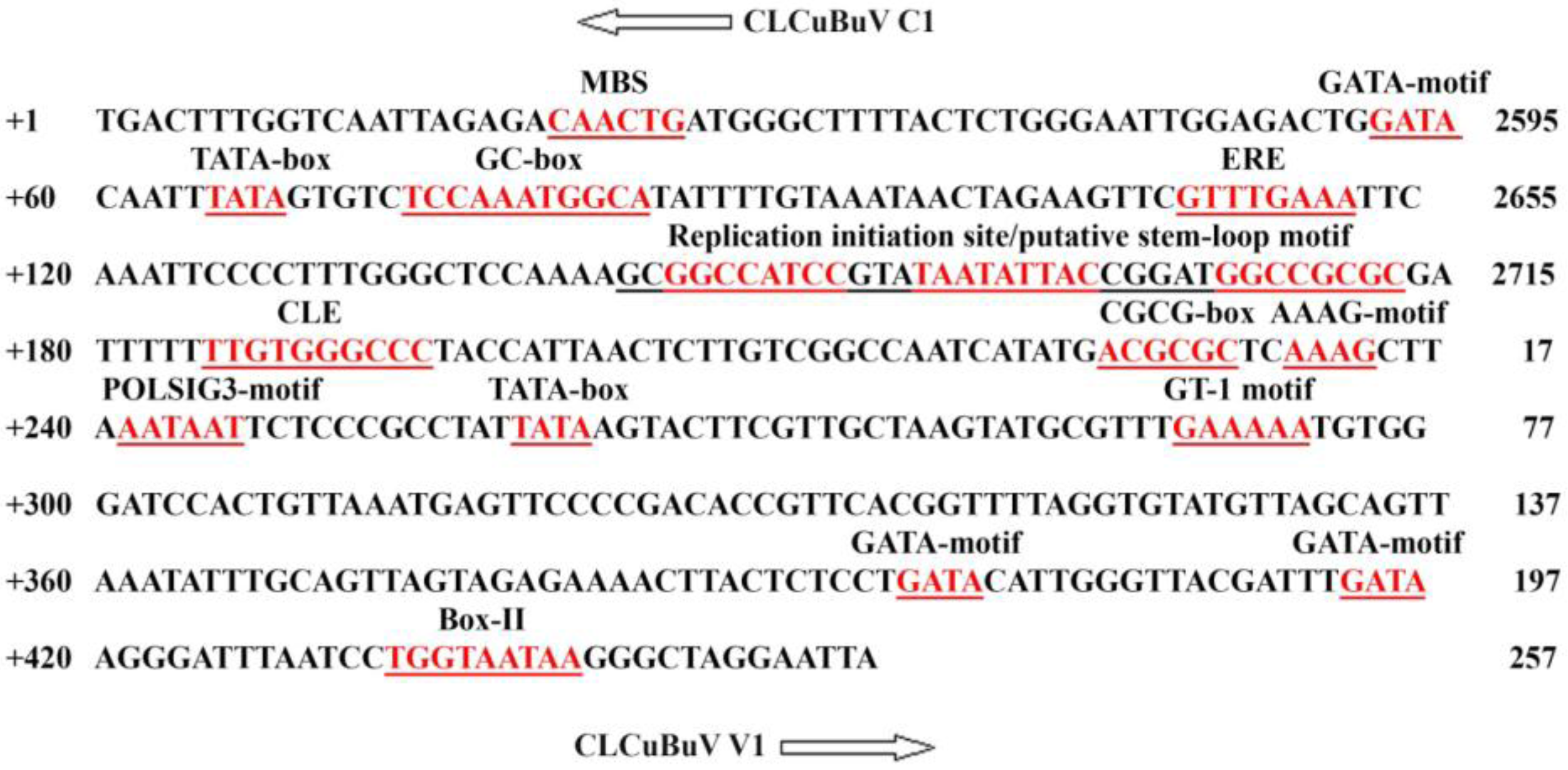
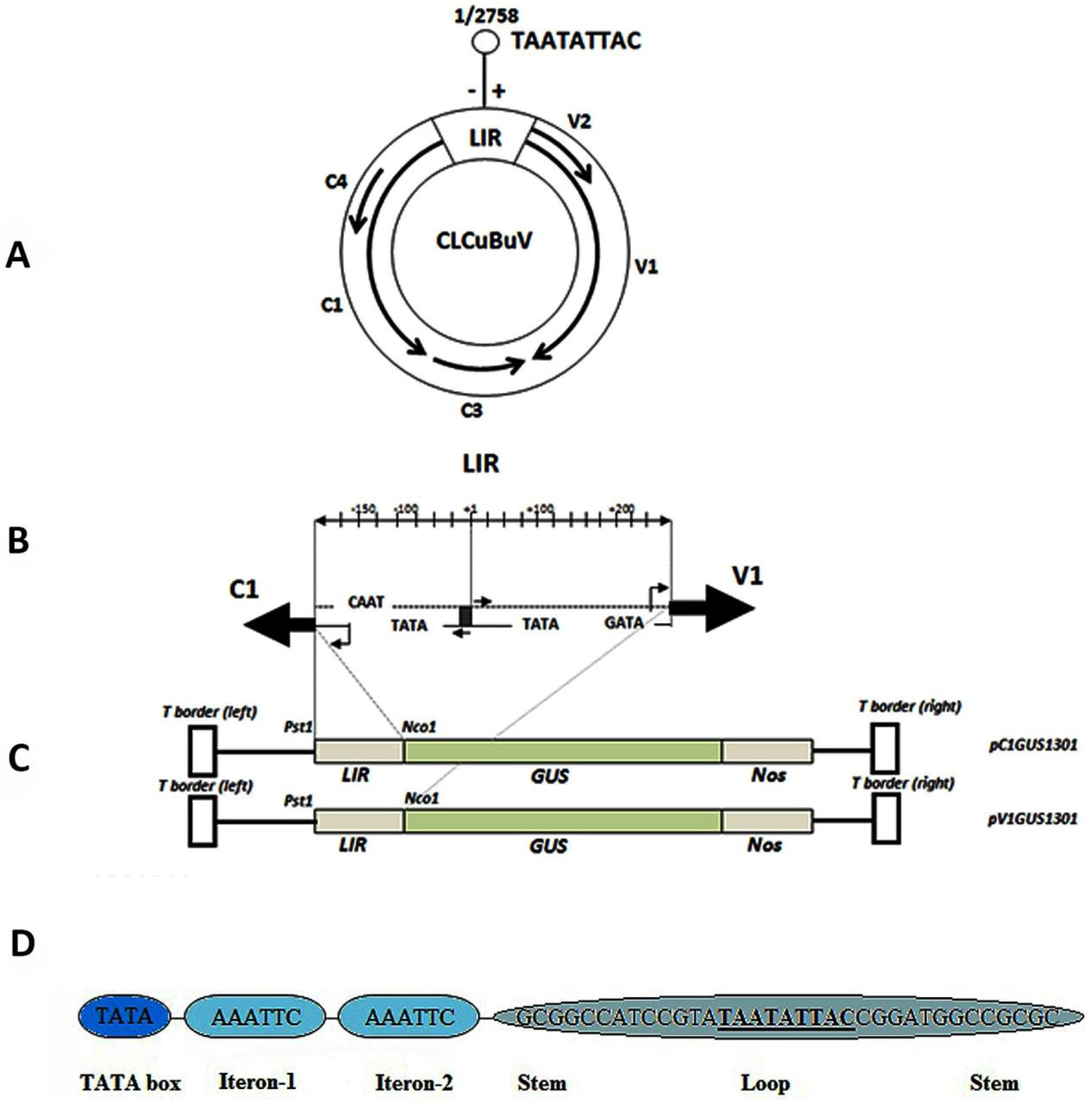
| No. | TFBs a | Function of the motif | organism | Sequence | Strand | Position |
|---|---|---|---|---|---|---|
| 1 | Box-I | LRE b | Pisum sativum | TTTCAAA | − | 110 |
| TTTCAAA | − | 286 | ||||
| 2 | Box-II | LRE | Solanum tubersum | TGGTAATAA | + | 433 |
| 3 | Box-W1 | Fungal elicitor RE | Petroselinum crispum | TTGACC | − | 7 |
| 4 | C-Repeat/DRE | Cold and dehydration RE | Arabidopsis thaliana | TGGCCCGAC | − | 209 |
| 5 | CAAT-box | Core promoter elements | many | many | +,− | many |
| 6 | CGCG-box | Signal RE | Arabidopsis thaliana | ACGCGC | + | 226 |
| GCGCGG | − | 173 | ||||
| 7 | CGTCA-motif | MeJA RE | Hordeum vulgare | CGTCA | − | 223 |
| 8 | Circadian | Circadian control | Lycopersicon esculentum | CAANNNNATC | − | 178 |
| 9 | ERE | Ethylene RE | Dianthus caryophyllus | ATTTCAAA | + | 110 |
| 10 | GATA-motif | LRE | Arabidopsis thaliana | GATA | + | many |
| 11 | G-box | LRE | Triticum aestivum | TCCACATGGCA | + | 74 |
| 12 | GC-motif | unknown | Oryza sativa | GCCGCGCCG | + | 171 |
| 13 | GT-1motif | LRE | Avena sativa | GGTAAT | − | 161 |
| Oryza sativa | GAAAAA | + | 290 | |||
| Pisum sativum | GGTAAT | + | 435 | |||
| 14 | HSE | HSE c | Brassica oleracea | AGAAAACTT | + | 380 |
| 15 | I-box | LRE | many | many | +,− | many |
| 16 | MBS | MYB drought RE | Arabidopsis thaliana | CAACTG | + | 20 |
| TAACTG | − | 355 | ||||
| TAACTG | − | 366 | ||||
| 17 | Skn-1_motif | Endosperm expression RE | Oryza sativa | GTCAT | − | 222 |
| 18 | TATA-box | Core elements located at −30 of TSS | many | many | +,− | many |
| 19 | TGACG-motif | MeJA RE | Horedeum volgare | TGACG | + | 223 |
| 20 | W-box | LRE | Arabidopsis thaliana | TTGACC | − | 7 |
| TFBs | PLACE ID | PLACE accession ID | Sequence | Copy number | Description |
|---|---|---|---|---|---|
| Cytokinin related | ARFAT(Aux RE) | S000270 | NGATT | 2 | “ARR1-binding element” found in rice andArabidopsis;ARR1 is response regulator;N = G/A/C/T [40] |
| ARR1AT | S000454 | TGTCTC | 5 | “ARF biding site” found in the promoters of primary/early response gene [41] | |
| Auxin related | SURECOREATSULTR11 | S000499 | GAGAC | 8 | Core of sulfur-responsive element SURE); containing ARF binding sequence GAGACA(complementary AuxRE TGTCTC)[42] |
| CATATGGMSAUR | S000370 | CATATG | 2 | Multiple auxin response modules in the soybean SAUR15A promoter [43] | |
| Mesophyll-specific | CACTFTPPCA1 | S000449 | YACT | 8 | Mesophyll-specific gene expression in C4 plant Flaveria trinervia [44]. |
| Pollen-specific | POLLEN1LELAT52 | S000245 | AGAAA | 1 | Pollen-specific expression of tomato Late52 gene [45] |
| Root-specific | ROOTMOTIFTAPOX1 | S000098 | ATATT | 5 | Root-specific motifs found in the rolD promoter [46]. |
| OSE2ROOTNODULE | S000468 | CTCTT | 1 | Nodule specificity of soybean lbc3 and N23 gene promoters [47]. |
3.2. Transient Expression of the Bidirectional Promoter
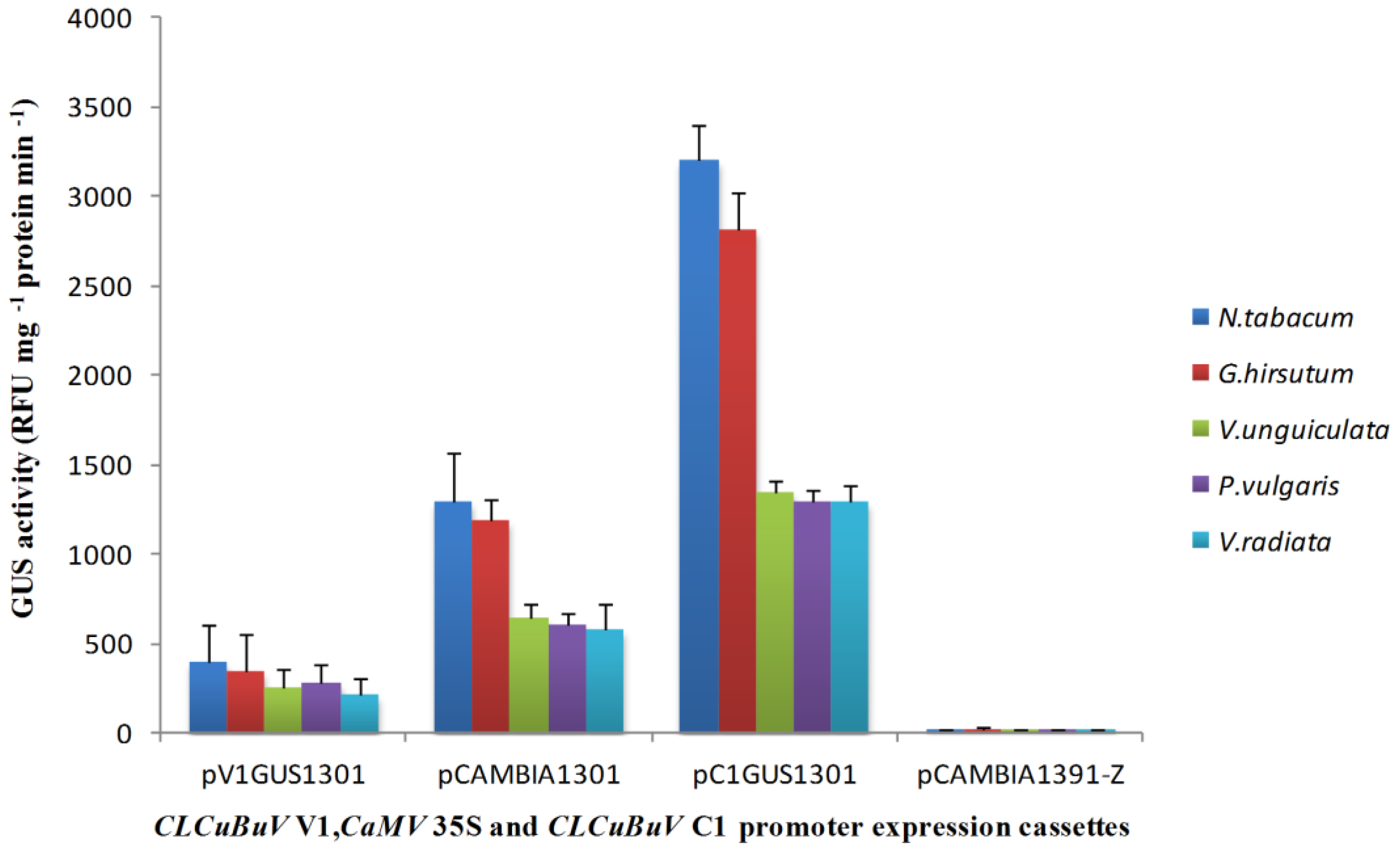
3.3. Nucleotide Sequence Comparison of the CLCuBuV LIR
| No | Virus Acronym | % identity a | LIR size (bp) | Accession IDs |
|---|---|---|---|---|
| 1 | CLCuBuV | 100 | 455 | FR837932 |
| 2 | CLCuMuV | 81.8 | 440 | AJ496287 |
| 3 | CLCuKoV | 81.6 | 447 | AJ496286 |
| 4 | CLCuShV | 81.9 | 447 | FN552004 |
| 5 | HYVMV | 77.4 | 437 | FR772082 |
| 6 | CLCuBaV | 76.4 | 444 | NC_007290 |
| 7 | MaYVCMV | 73.6 | 440 | FR715681 |
| 8 | PaLCuV | 70.1 | 478 | FM955602 |
| 9 | AEV | 69.9 | 445 | AM698011 |
| 10 | CYVMV | 69.8 | 451 | FN645926 |
| 11 | ToLCPKV | 68.1 | 456 | AM948961 |
| 12 | ChiLCMuV | 68.7 | 450 | FM149613 |
| 13 | SiLCV | 66.9 | 447 | DQ641706 |
| 14 | CLCuAaV | 65.2 | 434 | AJ002452 |
| 15 | CLCuRaV | 64.6 | 444 | JF502364 |
| 16 | CLCuGeV-PK | 32.7 | 453 | FR751142 |
| 17 | CLCuGeV-SD | 32.6 | 451 | AY036007 |
3.4. Enriched Regulatory Elements in the Geminivirus Bidirectional Promoters
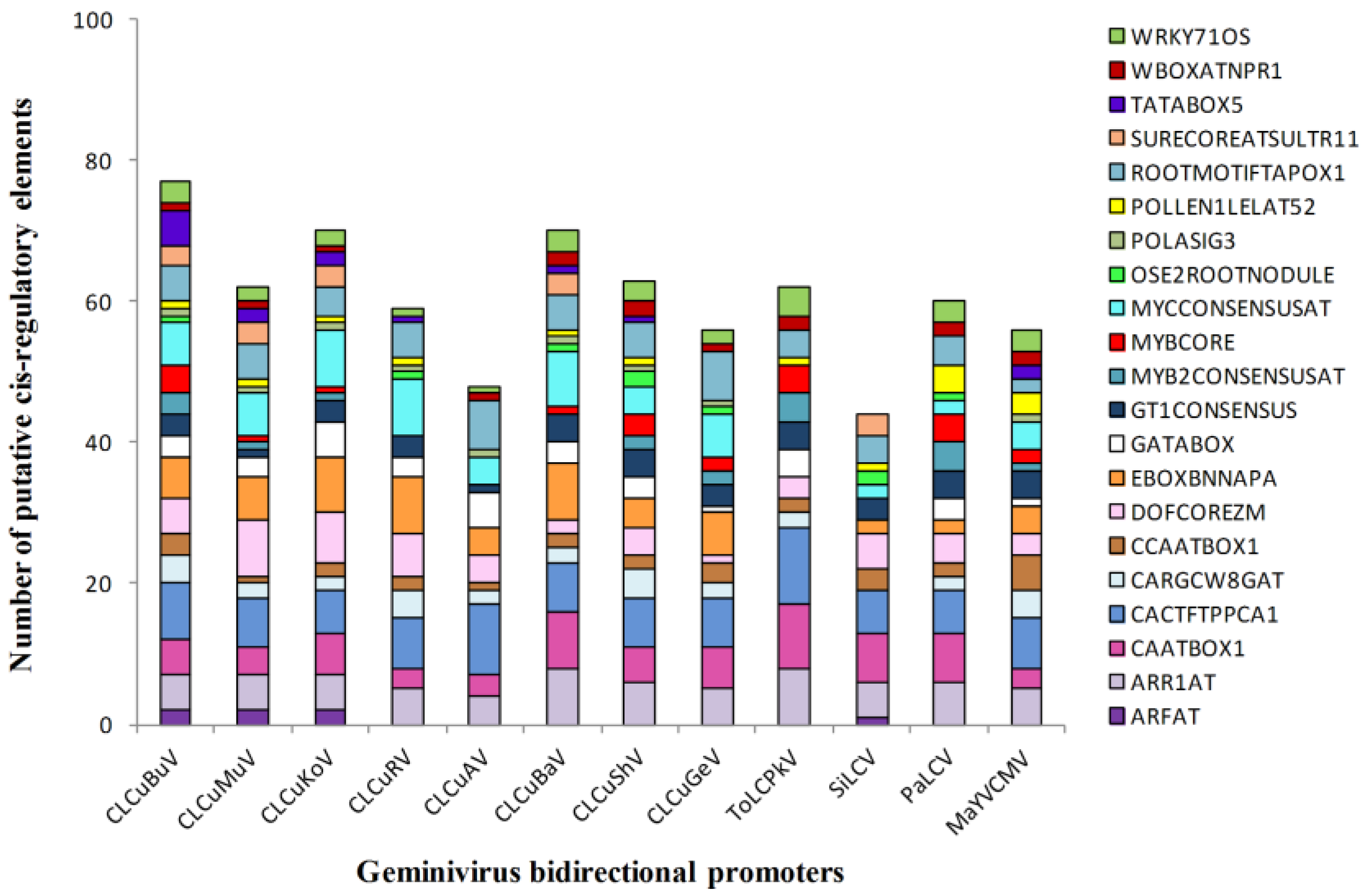
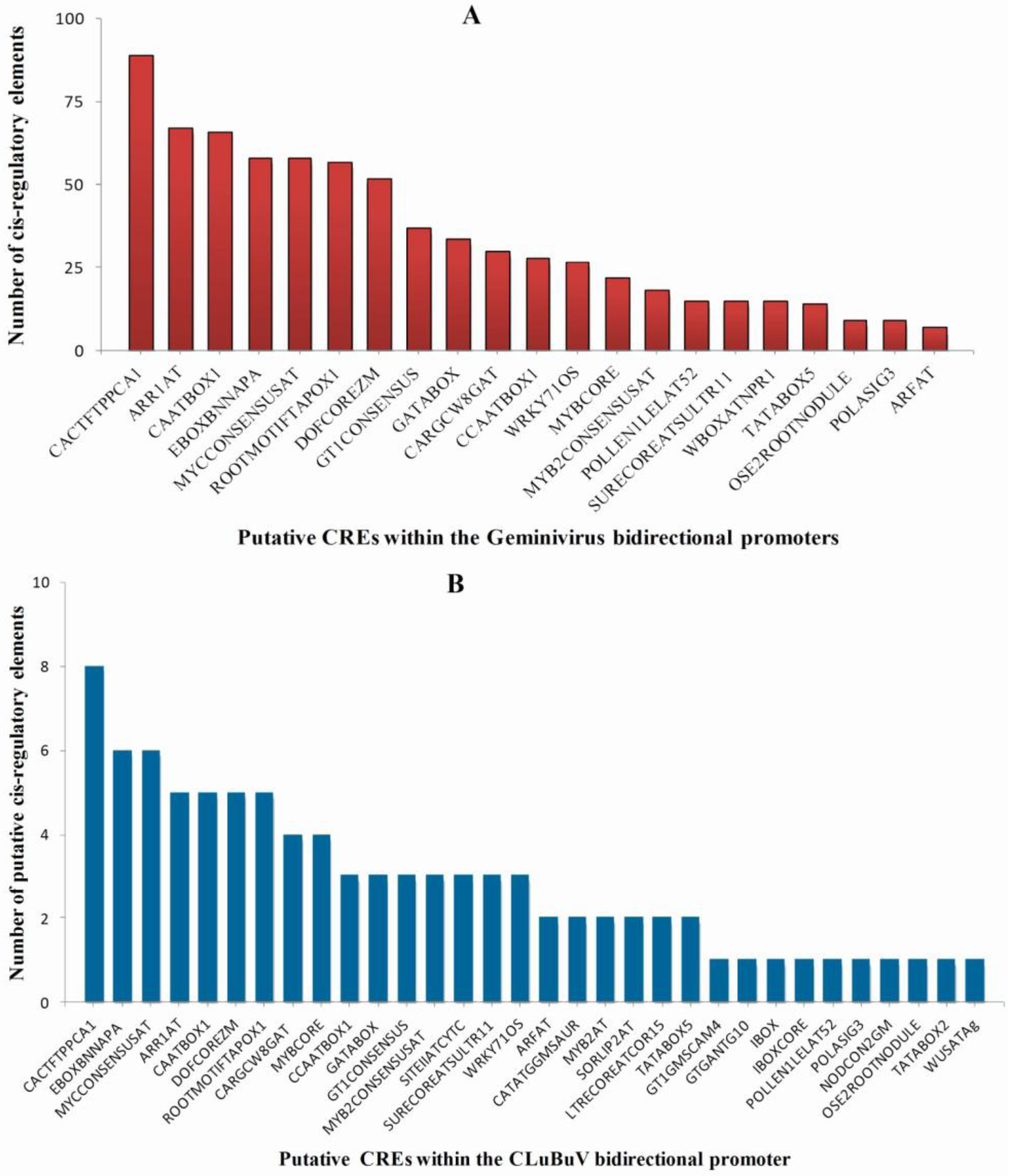
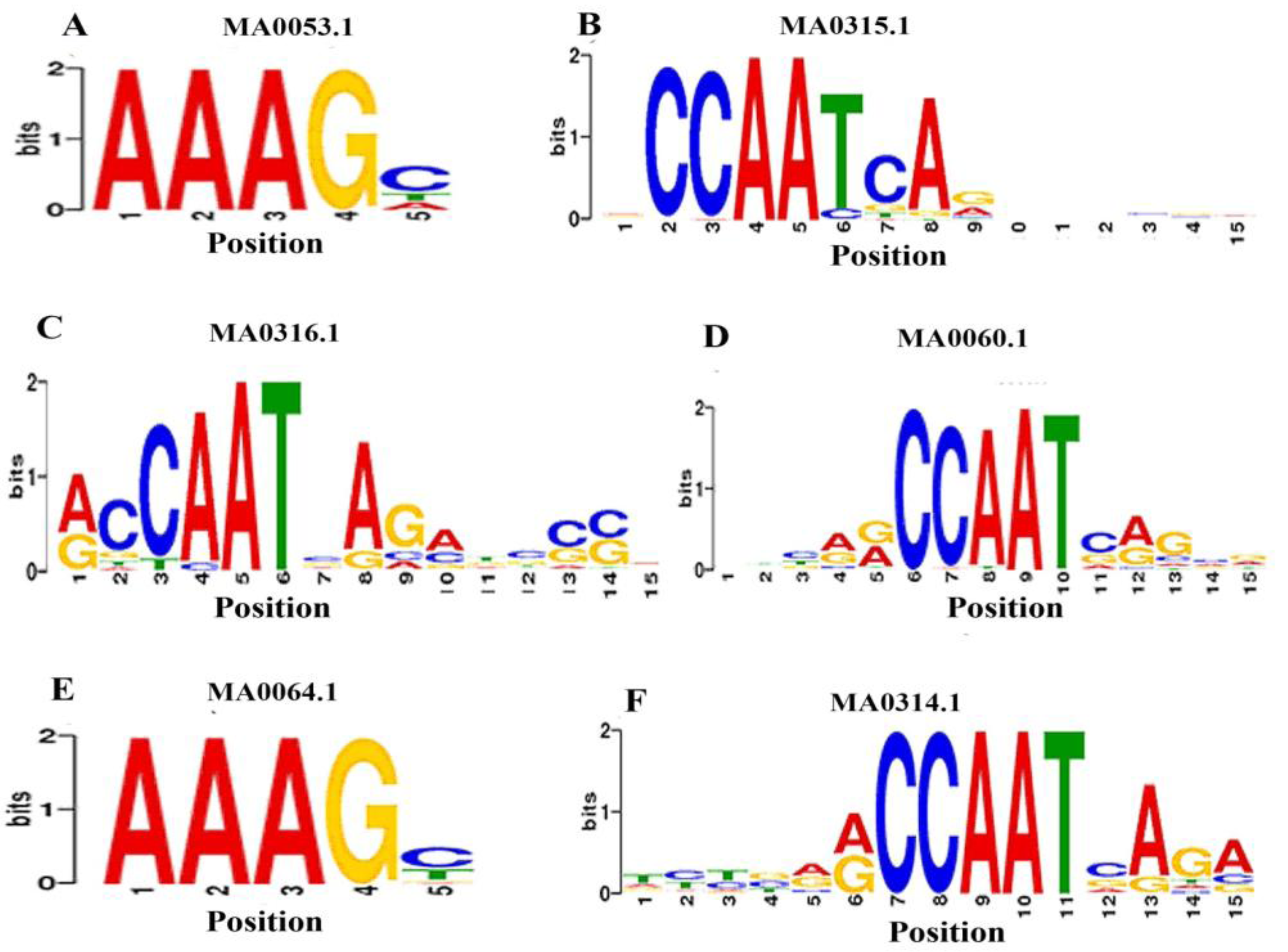
3.5. Identification of TFBs that Bind to the Promoters of ds-DNA Animal Viruses
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Fauquet, C.; Briddon, R.; Brown, J.; Moriones, E.; Stanley, J.; Zerbini, M.; Zhou, X. Geminivirus strain demarcation and nomenclature. Arch. Virol. 2008, 153, 783–821. [Google Scholar] [CrossRef]
- Stanley, J.B.D.M.; Briddon, R.W.; Brown, J.K.; Fauquet, C.M. Geminiviridae. In Virus Taxonomy, Viith Report of the Ictv; Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Elsevier/Academic Press: London, UK, 2005; pp. 301–326. [Google Scholar]
- Odell, J.T.; Nagy, F.; Chua, N.-H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35s promoter. Nature 1985, 313, 810–812. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Y.; Meng, M.; Chen, L.; Zhu, Z. Isolation and identification of a super strong plant promoter from cotton leaf curl multan virus. Plant Mol. Biol. 2003, 53, 1–14. [Google Scholar] [CrossRef]
- Pattanaik, S.; Dey, N.; Bhattacharyya, S.; Maiti, I.B. Isolation of full-length transcript promoter from the strawberry vein banding virus (SVBV) and expression analysis by protoplasts transient assays and in transgenic plants. Plant Sci. 2004, 167, 427–438. [Google Scholar] [CrossRef]
- Hanley-Bowdoin, L.; Settlage, S.B.; Orozco, B.M.; Nagar, S.; Robertson, D. Geminiviruses: Models for plant DNA replication, transcription, and cell cycle regulation. Crit. Rev. Plant Sci. 1999, 18, 71–106. [Google Scholar] [CrossRef]
- Accotto, G.P.; Donson, J.; Mullineaux, P. Mapping of digitaria streak virus transcripts reveals different RNA species from the same transcription unit. EMBO J. 1989, 8, 1033–1039. [Google Scholar]
- Petty, I.; Coutts, R.; Buck, K. Transcriptional mapping of the coat protein gene of tomato golden mosaic virus. J. Gen. Virol. 1988, 69, 1359–1365. [Google Scholar] [CrossRef]
- Sunter, G.; Bisaro, D.M. Transcription map of the B genome component of tomato golden mosaic virus and comparison with a component transcripts. Virology 1989, 173, 647–655. [Google Scholar] [CrossRef]
- Sunter, G.; Hartitz, M.D.; Bisaro, D.M. Tomato golden mosaic virus leftward gene expression: Autoregulation of geminivirus replication protein. Virology 1993, 195, 275–280. [Google Scholar] [CrossRef]
- Sunter, G.; Bisaro, D.M. Identification of a minimal sequence required for activation of the tomato golden mosaic virus coat protein promoter in protoplasts. Virology 2003, 305, 452–462. [Google Scholar] [CrossRef]
- Zhan, X.; Haley, A.; Richardson, K.; Morris, B. Analysis of the potential promoter sequences of african cassava mosaic virus by transient expression of the beta-glucuronidase gene. J. Gen. Virol. 1991, 72, 2849–2852. [Google Scholar] [CrossRef]
- Haley, A.; Zhan, X.; Richardson, K.; Head, K.; Morris, B. Regulation of the activities of African cassava mosaic virus promoters by the AC1, AC2, and AC3 gene products. Virology 1992, 188, 905–909. [Google Scholar] [CrossRef]
- Hong, Y.; Stanley, J. Regulation of African cassava mosaic virus complementary-sense gene expression by N-terminal sequences of the replication-associated protein AC1. J. Gen. Virol. 1995, 76, 2415–2422. [Google Scholar] [CrossRef]
- Frey, P.M.; Schärer-Hernández, N.G.; Fütterer, J.; Potrykus, I.; Puonti-Kaerlas, J. Simultaneous analysis of the bidirectional African cassava mosaic virus promoter activity using two different luciferase genes. Virus Genes 2001, 22, 231–242. [Google Scholar] [CrossRef]
- Fenoll, C.; Black, D.M.; Howell, S.H. The intergenic region of maize streak virus contains promoter elements involved in rightward transcription of the viral genome. EMBO J. 1988, 7, 1589–1596. [Google Scholar]
- Dinant, S.; Ripoll, C.; Pieper, M.; David, C. Phloem specific expression driven by wheat dwarf geminivirus V-sense promoter in transgenic dicotyledonous species. Physiol. Plant. 2004, 121, 108–116. [Google Scholar] [CrossRef]
- Usharani, K.; Periasamy, M.; Malathi, V. Studies on the activity of a bidirectional promoter of Mungbean yellow mosaic India virus by agroinfiltration. Virus Res. 2006, 119, 154–162. [Google Scholar] [CrossRef]
- Sunitha, S.; Mahajan, N.; Veluthambi, K. The TrAP/REn monodirectional promoter of Mungbean yellow mosaic geminivirus (MYMV) displays root-specific expression in transgenic tobacco. Plant Cell 2012, 109, 535–545. [Google Scholar]
- Eagle, P.A.; Hanley-Bowdoin, L. cis Elements that contribute to geminivirus transcriptional regulation and the efficiency of DNA replication. J. Virol. 1997, 71, 6947–6955. [Google Scholar]
- Argüello-Astorga, G.; Guevara-Gonzalez, R.; Herrera-Estrella, L.; Rivera-Bustamante, R. Geminivirus replication origins have a group-specific organization of iterative elements: A model for replication. Virology 1994, 203, 90–100. [Google Scholar] [CrossRef]
- Eagle, P.A.; Orozco, B.M.; Hanley-Bowdoin, L. A DNA sequence required for geminivirus replication also mediates transcriptional regulation. Plant Cell 1994, 6, 1157–1170. [Google Scholar]
- Amrao, L.; Amin, I.; Shahid, M.S.; Briddon, R.W.; Mansoor, S. Cotton leaf curl disease in resistant cotton is associated with a single begomovirus that lacks an intact transcriptional activator protein. Virus Res. 2010, 152, 153–163. [Google Scholar] [CrossRef]
- Nawaz-ul-Rehman, M.S.; Briddon, R.W.; Fauquet, C.M. A melting pot of old world begomoviruses and their satellites infecting a collection of Gossypium species in Pakistan. PLoS One 2012, 7, e40050. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Shahid, A.A.; Mohamed, B.B.; Dahab, A.A.; Bajwa, K.S.; Rao, A.Q.; Khan, M.A.U.; Ilyas, M.; Haider, M.S.; Husnain, T. Molecular characterization and phylogenetic analysis of a variant of highly infectious cotton leaf curl Burewala virus associated with CLCuD from Pakistan. Aust. J. Crop Sci. 2013, 7, 1113–1122. [Google Scholar]
- Mullineaux, P.M.; Rigden, J.E.; Dry, I.B.; Krake, L.R.; Rezaian, M.A. Mapping of the polycistronic RNAs of tomato leaf curl geminivirus. Virology 1993, 193, 414–423. [Google Scholar] [CrossRef]
- Dry, I.; Krake, L.; Mullineaux, P.; Rezaian, A. Regulation of tomato leaf curl viral gene expression in host tissues. Mol. Plant Microbe Interact. 2000, 13, 529–537. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; van de Peer, Y.; Rouzé, P.; Rombauts, S. Plantcare, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (place) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Wingender, E.; Dietze, P.; Karas, H.; Knüppel, R. Transfac: A database on transcription factors and their DNA binding sites. Nucleic Acids Res. 1996, 24, 238–241. [Google Scholar] [CrossRef]
- Reese, M.G. Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput. Chem. 2001, 26, 51–56. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. Meme suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Portales-Casamar, E.; Thongjuea, S.; Kwon, A.T.; Arenillas, D.; Zhao, X.; Valen, E.; Yusuf, D.; Lenhard, B.; Wasserman, W.W.; Sandelin, A. Jaspar 2010: The greatly expanded open-access database of transcription factor binding profiles. Nucleic Acids Res. 2010, 38, D105–D110. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fenoll, C.; Schwarz, J.J.; Black, D.M.; Schneider, M.; Howell, S.H. The intergenic region of maize streak virus contains a GC-rich element that activates rightward transcription and binds maize nuclear factors. Plant Mol. Biol. 1990, 15, 865–877. [Google Scholar] [CrossRef]
- Ruiz-Medrano, R.; Guevara-Gonzalez, R.; Argüello-Astorga, G.; Monsalve-Fonnegra, Z.; Herrera-Estrella, L.; Rivera-Bustamante, R. Identification of a sequence element involved in AC2-mediated transactivation of the pepper huasteco virus coat protein gene. Virology 1999, 253, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, J.J.; Chakraborty, T.; Martin, J.; Zhou, J.; Olson, E.N. The basic region of myogenin cooperates with two transcription activation domains to induce muscle-specific transcription. Mol. Cell. Biol. 1992, 12, 266–275. [Google Scholar]
- Yanagisawa, S.; Schmidt, R.J. Diversity and similarity among recognition sequences of Dof transcription factors. Plant J. 1999, 17, 209–214. [Google Scholar] [CrossRef]
- Ross, E.J.; Stone, J.M.; Elowsky, C.G.; Arredondo-Peter, R.; Klucas, R.V.; Sarath, G. Activation of the Oryza sativa non-symbiotic haemoglobin-2 promoter by the cytokinin-regulated transcription factor, arr1. J. Exp. Botany 2004, 55, 1721–1731. [Google Scholar] [CrossRef]
- Goda, H.; Sawa, S.; Asami, T.; Fujioka, S.; Shimada, Y.; Yoshida, S. Comprehensive comparison of auxin-regulated and brassinosteroid-regulated genes in Arabidopsis. Plant Physiol. 2004, 134, 1555–1573. [Google Scholar] [CrossRef]
- Maruyama‐Nakashita, A.; Nakamura, Y.; Watanabe‐Takahashi, A.; Inoue, E.; Yamaya, T.; Takahashi, H. Identification of a novel cis-acting element conferring sulfur deficiency response in Arabidopsis roots. Plant. J. 2005, 42, 305–314. [Google Scholar] [CrossRef]
- Xu, N.; Hagen, G.; Guilfoyle, T. Multiple auxin response modules in the soybean SAUR 15A promoter. Plant Sci. 1997, 126, 193–201. [Google Scholar] [CrossRef]
- Gowik, U.; Burscheidt, J.; Akyildiz, M.; Schlue, U.; Koczor, M.; Streubel, M.; Westhoff, P. cis-Regulatory elements for mesophyll-specific gene expression in the C4 plant Flaveria trinervia, the promoter of the C4 phosphoenolpyruvate carboxylase gene. Plant Cell 2004, 16, 1077–1090. [Google Scholar] [CrossRef]
- Filichkin, S.A.; Leonard, J.M.; Monteros, A.; Liu, P.-P.; Nonogaki, H. A novel endo-β-mannanase gene in tomato LeMAN5 is associated with anther and pollen development. Plant Physiol. 2004, 134, 1080–1087. [Google Scholar] [CrossRef]
- Elmayan, T.; Tepfer, M. Evaluation in tobacco of the organ specificity and strength of therold promoter, domain a of the 35S promoter and the 35S2 promoter. Transgenic Res. 1995, 4, 388–396. [Google Scholar] [CrossRef]
- Stougaard, J.; Jørgensen, J.-E.; Christensen, T.; Kühle, A.; Marcker, K.A. Interdependence and nodule specificity of cis-acting regulatory elements in the soybean leghemoglobin lbc 3 and N23 gene promoters. Mol. Gen. Genet. 1990, 220, 353–360. [Google Scholar] [CrossRef]
- Akbar, F.; Briddon, R.W.; Vazquez, F.; Saeed, M. Transcript mapping of cotton leaf curl burewala virus and its cognate betasatellite, cotton leaf curl multan betasatellite. Virol. J. 2012, 9. [Google Scholar] [CrossRef]
- Tjaden, G.; Edwards, J.W.; Coruzzi, G.M. cis Elements and trans-acting factors affecting regulation of a nonphotosynthetic light-regulated gene for chloroplast glutamine synthetase. Plant Physiol. 1995, 108, 1109–1117. [Google Scholar]
- Joshi, C.P. Putative polyadenylation signals in nuclear genes of higher plants: A compilation and analysis. Nucleic Acids Res. 1987, 15, 9627–9640. [Google Scholar] [CrossRef]
- O’Neill, S.D.; Kumagai, M.H.; Majumdar, A.; Huang, N.; Sutliff, T.D.; Rodriguez, R.L. The α-amylase genes in Oryza sativa: Characterization of cDNA clones and mRNA expression during seed germination. Mol. Gen. Genet. 1990, 221, 235–244. [Google Scholar]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Hartmann, U.; Sagasser, M.; Mehrtens, F.; Stracke, R.; Weisshaar, B. Differential combinatorial interactions of cis-acting elements recognized by R2R3-MYB, BZIP, and bHLH factors control light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes. Plant Mol. Biol. 2005, 57, 155–171. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Schmelzer, E.; Hahlbrock, K.; Somssich, I.E. Early nuclear events in plant defence signalling: Rapid gene activation by WRKY transcription factors. EMBO J. 1999, 18, 4689–4699. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Xie, Z.; Zou, X.; Casaretto, J.; Ho, T.-h.D.; Shen, Q.J. A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol. 2004, 134, 1500–1513. [Google Scholar] [CrossRef]
- Bovy, A.; van den Berg, C.; de Vrieze, G.; Thompson, W.F.; Weisbeek, P.; Smeekens, S. Light-regulated expression of the Arabidopsis thaliana ferredoxin gene requires sequences upstream and downstream of the transcription initiation site. Plant Mol. Biol. 1995, 27, 27–39. [Google Scholar] [CrossRef]
- Simpson, S.D.; Nakashima, K.; Narusaka, Y.; Seki, M.; Shinozaki, K.; Yamaguchi‐Shinozaki, K. Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J. 2003, 33, 259–270. [Google Scholar] [CrossRef]
- Urao, T.; Yamaguchi-Shinozaki, K.; Urao, S.; Shinozaki, K. An Arabidopsis MYB homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence. Plant Cell 1993, 5, 1529–1539. [Google Scholar]
- Park, H.C.; Kim, M.L.; Kang, Y.H.; Jeon, J.M.; Yoo, J.H.; Kim, M.C.; Park, C.Y.; Jeong, J.C.; Moon, B.C.; Lee, J.H.; et al. Pathogen- and NACL-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol. 2004, 135, 2150–2161. [Google Scholar] [CrossRef]
- Goding, C.; Temperley, S.; Fisher, F. Multiple transcription (actors interact with the adenovirus-2 EII-late promoter: Evidence for a novel CCAAT recognition factor. Nucleic Acids Res. 1987, 15, 7761–7780. [Google Scholar] [CrossRef]
- Koikeda, S.; Ibuki, R.; Sawada, Y.; Nagata, K.; Shibata, H.; Masamune, Y.; Nakanishi, Y. Nuclear factor I stimulates transcription of the adenovirus 12 E1A gene in a cell-free system. Biochim. Biophys. Acta 1990, 1048, 85–92. [Google Scholar] [CrossRef]
- Bovolenta, C.; Tognon, M.; Liboi, E. Epidermal growth factor induces, in the EL alpha 4–2 cell line, herpes simplex virus-1 alpha 4 gene transcription in the absence of the viral trans-activator VP16. Virus Res. 1991, 19, 199–208. [Google Scholar] [CrossRef]
- Dorn, A.; Bollekens, J.; Staub, A.; Benoist, C.; Mathis, D. A multiplicity of CCAAT box-binding proteins. Cell 1987, 50, 863–872. [Google Scholar] [CrossRef]
- Cowie, A.; Kamen, R. Multiple binding sites for polyomavirus large T antigen within regulatory sequences of polyomavirus DNA. J. Virol. 1984, 52, 750–760. [Google Scholar]
- Kapila, J.; de Rycke, R.; van Montagu, M.; Angenon, G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 1997, 122, 101–108. [Google Scholar] [CrossRef]
- Leckie, B.M.; Neal Stewart, C., Jr. Agroinfiltration as a technique for rapid assays for evaluating candidate insect resistance transgenes in plants. Plant Cell Rep. 2011, 30, 325–334. [Google Scholar] [CrossRef]
- Li, J.F.; Nebenfuhr, A. FAST technique for Agrobacterium-mediated transient gene expression in seedlings of Arabidopsis and other plant species. Cold Spring Harb. Protoc. 2010, 2010. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, G.; Meng, X.; Li, Y.; Wang, Y. A versatile Agrobacterium-mediated transient gene expression system for herbaceous plants and trees. Biochem. Genet. 2012, 50, 761–769. [Google Scholar] [CrossRef]
- Baulcombe, D.C. Gene silencing: RNA makes RNA makes no protein. Curr. Biol. 1999, 9, R599–R601. [Google Scholar] [CrossRef]
- Bhaskar, P.B.; Venkateshwaran, M.; Wu, L.; Ane, J.M.; Jiang, J. Agrobacterium-mediated transient gene expression and silencing: A rapid tool for functional gene assay in potato. PLoS One 2009, 4, e5812. [Google Scholar]
- Scofield, S.R.; Tobias, C.M.; Rathjen, J.P.; Chang, J.H.; Lavelle, D.T.; Michelmore, R.W.; Staskawicz, B.J. Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 1996, 274, 2063–2065. [Google Scholar] [CrossRef]
- Frederick, R.D.; Thilmony, R.L.; Sessa, G.; Martin, G.B. Recognition specificity for the bacterial avirulence protein AvrPto is determined by Thr-204 in the activation loop of the tomato Pto kinase. Mol. Cell 1998, 2, 241–245. [Google Scholar] [CrossRef]
- Tsuda, K.; Qi, Y.; Nguyen le, V.; Bethke, G.; Tsuda, Y.; Glazebrook, J.; Katagiri, F. An efficient Agrobacterium-mediated transient transformation of Arabidopsis. Plant J. 2012, 69, 713–719. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Qi, M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 2000, 22, 543–551. [Google Scholar] [CrossRef]
- Zahur, M.; Maqbool, A.; Irfan, M.; Barozai, M.Y.K.; Rashid, B.; Riazuddin, S.; Husnain, T. Isolation and functional analysis of cotton universal stress protein promoter in response to phytohormones and abiotic stresses. Mol. Biol. 2009, 43, 578–585. [Google Scholar] [CrossRef]
- Zahur, M.; Maqbool, A.; Irfan, M.; Barozai, M.Y.K.; Qaiser, U.; Rashid, B.; Husnain, T.; Riazuddin, S. Functional analysis of cotton small heat shock protein promoter region in response to abiotic stresses in tobacco using Agrobacterium-mediated transient assay. Mol. Biol. Rep. 2009, 36, 1915–1921. [Google Scholar] [CrossRef]
- Sunter, G.; Hartitz, M.D.; Hormuzdi, S.G.; Brough, C.L.; Bisaro, D.M. Genetic analysis of tomato golden mosaic virus: ORF AL2 is required for coat protein accumulation while ORF AL3 is necessary for efficient DNA replication. Virology 1990, 179, 69–77. [Google Scholar] [CrossRef]
- Hong, Y.; Saunders, K.; Hartley, M.R.; Stanley, J. Resistance to geminivirus infection by virus-induced expression of dianthin in transgenic plants. Virology 1996, 220, 119–127. [Google Scholar] [CrossRef]
- Hofer, J.; Dekker, E.L.; Reynolds, H.V.; Woolston, C.J.; Cox, B.S.; Mullineaux, P.M. Coordinate regulation of replication and virion sense gene expression in wheat dwarf virus. Plant Cell 1992, 4, 213–223. [Google Scholar]
- Shivaprasad, P.; Akbergenov, R.; Trinks, D.; Rajeswaran, R.; Veluthambi, K.; Hohn, T.; Pooggin, M.M. Promoters, transcripts, and regulatory proteins of Mungbean yellow mosaic geminiviru. J. Virol. 2005, 79, 8149–8163. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ashraf, M.A.; Shahid, A.A.; Rao, A.Q.; Bajwa, K.S.; Husnain, T. Functional Characterization of a Bidirectional Plant Promoter from Cotton Leaf Curl Burewala Virus Using an Agrobacterium-Mediated Transient Assay. Viruses 2014, 6, 223-242. https://doi.org/10.3390/v6010223
Ashraf MA, Shahid AA, Rao AQ, Bajwa KS, Husnain T. Functional Characterization of a Bidirectional Plant Promoter from Cotton Leaf Curl Burewala Virus Using an Agrobacterium-Mediated Transient Assay. Viruses. 2014; 6(1):223-242. https://doi.org/10.3390/v6010223
Chicago/Turabian StyleAshraf, Muhammad Aleem, Ahmad Ali Shahid, Abdul Qayyum Rao, Kamran Shehzad Bajwa, and Tayyab Husnain. 2014. "Functional Characterization of a Bidirectional Plant Promoter from Cotton Leaf Curl Burewala Virus Using an Agrobacterium-Mediated Transient Assay" Viruses 6, no. 1: 223-242. https://doi.org/10.3390/v6010223
APA StyleAshraf, M. A., Shahid, A. A., Rao, A. Q., Bajwa, K. S., & Husnain, T. (2014). Functional Characterization of a Bidirectional Plant Promoter from Cotton Leaf Curl Burewala Virus Using an Agrobacterium-Mediated Transient Assay. Viruses, 6(1), 223-242. https://doi.org/10.3390/v6010223





