Stem-Cell-Based Gene Therapy for HIV Infection
Abstract
:1. Introduction
2. Engineering HIV Resistant Cells
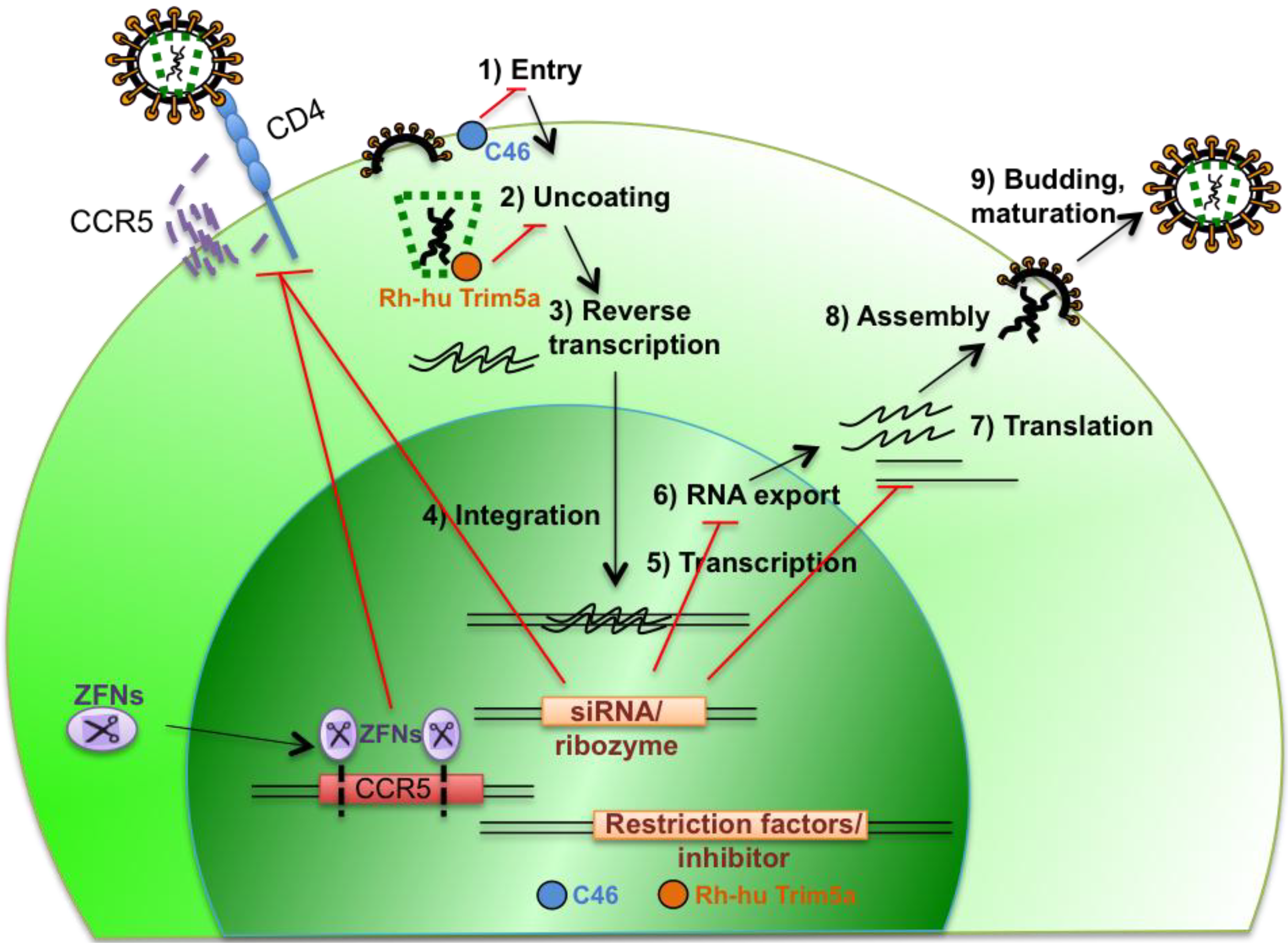
2.1. Targeting Expression of Cellular Genes that Are Essential for Viral Replication
2.2. Targeting HIV Gene Expression
2.3. Introduction of Genes that Interfere with HIV Replication
2.4. Combination Therapy to Prevent Viral Escape
3. Engineering anti-HIV Immunity
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Yukl, S.A.; Shergill, A.; McQuaid, K.; Gianella, S.; Lampiris, H.; Hare, C.B.; Pandori, M.; Sinclair, E.; Günthard, H.F.; Fischer, M.; et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS 2010, 24, 2451–2460. [Google Scholar] [CrossRef]
- Deeks, S.G. HIV infection, inflammation, immunosenescence, and aging. Medicine 2011, 62, 141–155. [Google Scholar] [CrossRef]
- Volberding, P.A.; Deeks, S.G. Antiretroviral therapy and management of HIV infection. Lancet 2010, 376, 49–62. [Google Scholar] [CrossRef]
- Yu, M.; Poeschla, E.; Wong-Staal, F. Progress towards gene therapy for HIV infection. Gene Ther. 1994, 1, 13–26. [Google Scholar]
- Piché, A. Gene therapy for HIV infections: Intracellular immunization. Can. J. Infect. Dis. 1999, 10, 307–312. [Google Scholar]
- Weiss, R.A. Thirty years on: HIV receptor gymnastics and the prevention of infection. BMC Biol. 2013, 11, e57. [Google Scholar] [CrossRef]
- Liu, R.; Paxton, W.A.; Choe, S.; Ceradini, D.; Martin, S.R.; Horuk, R.; MacDonald, M.E.; Stuhlmann, H.; Koup, R.A.; Landau, N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 1996, 86, 367–377. [Google Scholar] [CrossRef]
- De Roda Husman, A.M.; Koot, M.; Cornelissen, M.; Keet, I.P.; Brouwer, M.; Broersen, S.M.; Bakker, M.; Roos, M.T.; Prins, M.; de Wolf, F.; et al. Association between CCR5 genotype and the clinical course of HIV-1 infection. Ann. Intern. Med. 1997, 127, 882–890. [Google Scholar] [CrossRef]
- Gilliam, B.L.; Riedel, D.J.; Redfield, R.R. Clinical use of CCR5 inhibitors in HIV and beyond. J. Transl. Med. 2011, 9, S9. [Google Scholar] [CrossRef]
- Hütter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Müßig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kücherer, C.; Blau, O.; et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef]
- Allers, K.; Hütter, G.; Hofmann, J.; Loddenkemper, C.; Rieger, K.; Thiel, E.; Schneider, T. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 2011, 117, 2791–2799. [Google Scholar] [CrossRef]
- Qin, X.-F.; An, D.S.; Chen, I.S.Y.; Baltimore, D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl. Acad. Sci. USA 2003, 100, 183–188. [Google Scholar] [CrossRef]
- Bai, J.; Gorantla, S.; Banda, N.; Cagnon, L.; Rossi, J.; Akkina, R. Characterization of anti-CCR5 ribozyme-transduced CD34+ hematopoietic progenitor cells in vitro and in a SCID-hu mouse model in vivo. Mol. Ther. 2000, 1, 244–254. [Google Scholar] [CrossRef]
- Swan, C.H.; Bühler, B.; Steinberger, P.; Tschan, M.P.; Barbas, C.F.; Torbett, B.E. T-cell protection and enrichment through lentiviral CCR5 intrabody gene delivery. Gene Ther. 2006, 13, 1480–1492. [Google Scholar] [CrossRef]
- Schroers, R.; Davis, C.M.; Wagner, H.-J.; Chen, S.-Y. Lentiviral transduction of human T-lymphocytes with a RANTES intrakine inhibits human immunodeficiency virus type 1 infection. Gene Ther. 2002, 9, 889–897. [Google Scholar] [CrossRef]
- Shimizu, S.; Hong, P.; Arumugam, B.; Pokomo, L.; Boyer, J.; Koizumi, N.; Kittipongdaja, P.; Chen, A.; Bristol, G.; Galic, Z.; et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood 2010, 115, 1534–1544. [Google Scholar] [CrossRef]
- Bobis-Wozowicz, S.; Osiak, A.; Rahman, S.H.; Cathomen, T. Targeted genome editing in pluripotent stem cells using zinc-finger nucleases. Methods 2011, 53, 339–346. [Google Scholar] [CrossRef]
- Perez, E.E.; Wang, J.; Miller, J.C.; Jouvenot, Y.; Kim, K.A.; Liu, O.; Wang, N.; Lee, G.; Bartsevich, V.V.; Lee, Y.-L.; et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 808–816. [Google Scholar] [CrossRef]
- Holt, N.; Wang, J.; Kim, K.; Friedman, G.; Wang, X.; Taupin, V.; Crooks, G.M.; Kohn, D.B.; Gregory, P.D.; Holmes, M.C.; et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol. 2010, 28, 839–847. [Google Scholar] [CrossRef]
- Li, L.; Krymskaya, L.; Wang, J.; Henley, J.; Rao, A.; Cao, L.-F.; Tran, C.-A.; Torres-Coronado, M.; Gardner, A.; Gonzalez, N.; et al. Genomic editing of the HIV-1 coreceptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases. Mol. Ther. 2013, 21, 1259–1269. [Google Scholar] [CrossRef]
- Wilen, C.B.; Wang, J.; Tilton, J.C.; Miller, J.C.; Kim, K.A.; Rebar, E.J.; Sherrill-Mix, S.A.; Patro, S.C.; Secreto, A.J.; Jordan, A.P.O.; et al. Engineering HIV-resistant human CD4+ T cells with CXCR4-specific zinc-finger nucleases. PLoS Pathog. 2011, 7, e1002020. [Google Scholar] [CrossRef]
- Kiem, H.-P.; Jerome, K.R.; Deeks, S.G.; McCune, J.M. Hematopoietic-stem-cell-based gene therapy for HIV disease. Stem Cell 2012, 10, 137–147. [Google Scholar]
- Mitsuyasu, R.T.; Merigan, T.C.; Carr, A.; Zack, J.A.; Winters, M.A.; Workman, C.; Bloch, M.; Lalezari, J.; Becker, S.; Thornton, L.; et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat. Med. 2009, 15, 285–292. [Google Scholar] [CrossRef]
- Kang, E.M.; de Witte, M.; Malech, H.; Morgan, R.A.; Phang, S.; Carter, C.; Leitman, S.F.; Childs, R.; Barrett, A.J.; Little, R.; et al. Nonmyeloablative conditioning followed by transplantation of genetically modified HLA-matched peripheral blood progenitor cells for hematologic malignancies in patients with acquired immunodeficiency syndrome. Blood 2002, 99, 698–701. [Google Scholar] [CrossRef]
- Bonyhadi, M.L.; Moss, K.; Voytovich, A.; Auten, J.; Kalfoglou, C.; Plavec, I.; Forestell, S.; Su, L.; Böhnlein, E.; Kaneshima, H. RevM10-expressing T cells derived in vivo from transduced human hematopoietic stem-progenitor cells inhibit human immunodeficiency virus replication. J. Virol. 1997, 71, 4707–4716. [Google Scholar]
- Podsakoff, G.M.; Engel, B.C.; Carbonaro, D.A.; Choi, C.; Smogorzewska, E.M.; Bauer, G.; Selander, D.; Csik, S.; Wilson, K.; Betts, M.R.; et al. Selective survival of peripheral blood lymphocytes in children with HIV-1 following delivery of an anti-HIV gene to bone marrow CD34+ cells. Mol. Ther. 2005, 12, 77–86. [Google Scholar] [CrossRef]
- Kohn, D.B.; Bauer, G.; Rice, C.R.; Rothschild, J.C.; Carbonaro, D.A.; Valdez, P.; Hao, Q.L.; Zhou, C.; Bahner, I.; Kearns, K.; et al. A clinical trial of retroviral-mediated transfer of a rev-responsive element decoy gene into CD34(+) cells from the bone marrow of human immunodeficiency virus-1-infected children. Blood 1999, 94, 368–371. [Google Scholar]
- Jacque, J.-M.; Triques, K.; Stevenson, M. Modulation of HIV-1 replication by RNA interference. Nature 2002, 418, 435–438. [Google Scholar] [CrossRef]
- Das, A.T.; Brummelkamp, T.R.; Westerhout, E.M.; Vink, M.; Madiredjo, M.; Bernards, R.; Berkhout, B. Human immunodeficiency virus type 1 escapes from RNA interference-mediated inhibition. J. Virol. 2004, 78, 2601–2605. [Google Scholar] [CrossRef]
- Egelhofer, M.; Brandenburg, G.; Martinius, H.; Schult-Dietrich, P.; Melikyan, G.; Kunert, R.; Baum, C.; Choi, I.; Alexandrov, A.; von Laer, D. Inhibition of human immunodeficiency virus type 1 entry in cells expressing gp41-derived peptides. J. Virol. 2004, 78, 568–575. [Google Scholar] [CrossRef]
- Van Lunzen, J.; Glaunsinger, T.; Stahmer, I.; von Baehr, V.; Baum, C.; Schilz, A.; Kuehlcke, K.; Naundorf, S.; Martinius, H.; Hermann, F.; et al. Transfer of autologous gene-modified T cells in HIV-infected patients with advanced immunodeficiency and drug-resistant virus. Mol. Ther. 2007, 15, 1024–1033. [Google Scholar]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 2004, 427, 848–853. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Stremlau, M.; Lee, M.; Sodroski, J. Removal of arginine 332 allows human TRIM5alpha to bind human immunodeficiency virus capsids and to restrict infection. J. Virol. 2006, 80, 6738–6744. [Google Scholar] [CrossRef]
- Anderson, J.; Akkina, R. Human immunodeficiency virus type 1 restriction by human-rhesus chimeric tripartite motif 5alpha (TRIM 5alpha) in CD34(+) cell-derived macrophages in vitro and in T cells in vivo in severe combined immunodeficient (SCID-hu) mice transplanted with human fetal tissue. Human Gene Ther. 2008, 19, 217–228. [Google Scholar] [CrossRef]
- Neagu, M.R.; Ziegler, P.; Pertel, T.; Strambio-De-Castillia, C.; Grütter, C.; Martinetti, G.; Mazzucchelli, L.; Grütter, M.; Manz, M.G.; Luban, J. Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components. J. Clin. Invest. 2009, 119, 3035–3047. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Afif, H.; Cheng, S.; Martel-Pelletier, J.; Pelletier, J.-P.; Ranger, P.; Fahmi, H. Expression and regulation of microsomal prostaglandin E synthase-1 in human osteoarthritic cartilage and chondrocytes. J. Rheumatol. 2005, 32, 887–895. [Google Scholar]
- Schröfelbauer, B.; Chen, D.; Landau, N.R. A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif). Proc. Natl. Acad. Sci. USA 2004, 101, 3927–3932. [Google Scholar] [CrossRef]
- McNatt, M.W.; Zang, T.; Hatziioannou, T.; Bartlett, M.; Ben Fofana, I.; Johnson, W.E.; Neil, S.J.D.; Bieniasz, P.D.; Hope, T.J. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 2009, 5, e1000300. [Google Scholar] [CrossRef]
- DiGiusto, D.L.; Krishnan, A.; Li, L.; Li, H.; Li, S.; Rao, A.; Mi, S.; Yam, P.; Stinson, S.; Kalos, M.; et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci. Transl. Med. 2010, 2, 36ra43. [Google Scholar]
- Kiem, H.-P.; Wu, R.A.; Sun, G.; von Laer, D.; Rossi, J.J.; Trobridge, G.D. Foamy combinatorial anti-HIV vectors with MGMTP140K potently inhibit HIV-1 and SHIV replication and mediate selection in vivo. Gene Ther. 2010, 17, 37–49. [Google Scholar] [CrossRef]
- ter Brake, O.; Konstantinova, P.; Ceylan, M.; Berkhout, B. Silencing of HIV-1 with RNA interference: a multiple shRNA approach. Mol. Ther. 2006, 14, 883–892. [Google Scholar] [CrossRef]
- Chung, J.; Zhang, J.; Li, H.; Ouellet, D.L.; DiGiusto, D.L.; Rossi, J.J. Endogenous MCM7 MicroRNA cluster as a novel platform to multiplex small interfering and nucleolar RNAs for combinational HIV-1 gene therapy. Human Gene Ther. 2012, 23, 1200–1208. [Google Scholar] [CrossRef]
- Choudhary, R.; Baturin, D.; Fosmire, S.; Freed, B.; Porter, C.C. Knockdown of HPRT for selection of genetically modified human hematopoietic progenitor cells. PLoS One 2013, 8, e59594. [Google Scholar]
- Hacke, K.; Treger, J.A.; Bogan, B.T.; Schiestl, R.H.; Kasahara, N. Genetic modification of mouse bone marrow by lentiviral vector-mediated delivery of hypoxanthine-Guanine phosphoribosyltransferase short hairpin RNA confers chemoprotection against 6-thioguanine cytotoxicity. Transplant. Proc. 2013, 45, 2040–2044. [Google Scholar] [CrossRef]
- Trono, D.; van Lint, C.; Rouzioux, C.; Verdin, E.; Barré-Sinoussi, F.; Chun, T.-W.; Chomont, N. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science 2010, 329, 174–180. [Google Scholar] [CrossRef]
- Shan, L.; Deng, K.; Shroff, N.S.; Durand, C.M.; Rabi, S.A.; Yang, H.-C.; Zhang, H.; Margolick, J.B.; Blankson, J.N.; Siliciano, R.F. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012, 36, 491–501. [Google Scholar] [CrossRef]
- Migueles, S.A.; Connors, M. Small molecules and big killers: The challenge of eliminating the latent HIV reservoir. Immunity 2012, 36, 320–321. [Google Scholar] [CrossRef]
- Garcia, F.; León, A.; Gatell, J.; Plana, M.; Gallart, T. Therapeutic vaccines against HIV infection. Hum. Vaccines 2012, 8, 569–581. [Google Scholar]
- Papagno, L.; Alter, G.; Assoumou, L.; Murphy, R.L.; Garcia, F.; Clotet, B.; Larsen, M.; Braibant, M.; Marcelin, A.-G.; Costagliola, D.; et al. Comprehensive analysis of virus-specific T-cells provides clues for the failure of therapeutic immunization with ALVAC-HIV vaccine. AIDS 2011, 25, 27–36. [Google Scholar] [CrossRef]
- Kinloch-de Loes, S.; Hoen, B.; Smith, D.E.; Autran, B.; Lampe, F.C.; Phillips, A.N.; Goh, L.-E.; Andersson, J.; Tsoukas, C.; Sonnerborg, A.; et al. Impact of therapeutic immunization on HIV-1 viremia after discontinuation of antiretroviral therapy initiated during acute infection. J. Infect. Dis. 2005, 192, 607–617. [Google Scholar] [CrossRef]
- Bozzacco, L.; Trumpfheller, C.; Siegal, F.P.; Mehandru, S.; Markowitz, M.; Carrington, M.; Nussenzweig, M.C.; Piperno, A.G.; Steinman, R.M. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc. Natl. Acad. Sci. USA 2007, 104, 1289–1294. [Google Scholar] [CrossRef]
- García, F.; Climent, N.; Guardo, A.C.; Gil, C.; León, A.; Autran, B.; Lifson, J.D.; Martínez-Picado, J.; Dalmau, J.; Clotet, B.; et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci. Transl. Med. 2013, 5, 166ra2. [Google Scholar]
- Joseph, A.; Zheng, J.H.; Chen, K.; Dutta, M.; Chen, C. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J. Virol. 2010, 84, 6645–6653. [Google Scholar] [CrossRef]
- Cooper, L.J.; Kalos, M.; Lewinsohn, D.A.; Riddell, S.R.; Greenberg, P.D. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. J. Virol. 2000, 74, 8207–8212. [Google Scholar] [CrossRef]
- Varela-Rohena, A.; Molloy, P.E.; Dunn, S.M.; Li, Y.; Suhoski, M.M.; Carroll, R.G.; Milicic, A.; Mahon, T.; Sutton, D.H.; Laugel, B.; et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat. Med. 2008, 14, 1390–1395. [Google Scholar] [CrossRef]
- Redirected High Affinity Gag-Specific Autologous T Cells for HIV Gene Therapy. Available online: http://clinicaltrials.gov/show/NCT00991224 (accessed on 19 December 2013).
- Scholler, J.; Brady, T.L.; Binder-Scholl, G.; Hwang, W.-T.; Plesa, G.; Hege, K.M.; Vogel, A.N.; Kalos, M.; Riley, J.L.; Deeks, S.G.; et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012, 4, 132ra53. [Google Scholar]
- Mitsuyasu, R.T.; Anton, P.A.; Deeks, S.G.; Scadden, D.T.; Connick, E.; Downs, M.T.; Bakker, A.; Roberts, M.R.; June, C.H.; Jalali, S.; et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood 2000, 96, 785–793. [Google Scholar]
- Vatakis, D.N.; Arumugam, B.; Kim, S.G.; Bristol, G.; Yang, O.; Zack, J.A. Introduction of exogenous T-cell receptors into human hematopoietic progenitors results in exclusion of endogenous T-cell receptor expression. Mol. Ther. 2013, 21, 1055–1063. [Google Scholar] [CrossRef]
- Kitchen, S.G.; Levin, B.R.; Bristol, G.; Rezek, V.; Kim, S.; Aguilera-Sandoval, C.; Balamurugan, A.; Yang, O.O.; Zack, J.A. In vivo suppression of HIV by antigen specific T cells derived from engineered hematopoietic stem cells. PLoS Pathog. 2012, 8, e1002649. [Google Scholar] [CrossRef]
- Balamurugan, A.; Ng, H.L.; Yang, O.O. Rapid T cell receptor delineation reveals clonal expansion limitation of the magnitude of the HIV-1-specific CD8+ T cell response. J. Immunol. 2010, 185, 5935–5942. [Google Scholar] [CrossRef]
- Aiuti, A.; Cattaneo, F.; Galimberti, S.; Benninghoff, U.; Cassani, B.; Callegaro, L.; Scaramuzza, S.; Andolfi, G.; Mirolo, M.; Brigida, I.; et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009, 360, 447–458. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhen, A.; Kitchen, S. Stem-Cell-Based Gene Therapy for HIV Infection. Viruses 2014, 6, 1-12. https://doi.org/10.3390/v6010001
Zhen A, Kitchen S. Stem-Cell-Based Gene Therapy for HIV Infection. Viruses. 2014; 6(1):1-12. https://doi.org/10.3390/v6010001
Chicago/Turabian StyleZhen, Anjie, and Scott Kitchen. 2014. "Stem-Cell-Based Gene Therapy for HIV Infection" Viruses 6, no. 1: 1-12. https://doi.org/10.3390/v6010001
APA StyleZhen, A., & Kitchen, S. (2014). Stem-Cell-Based Gene Therapy for HIV Infection. Viruses, 6(1), 1-12. https://doi.org/10.3390/v6010001



