Ribavirin Protects Syrian Hamsters against Lethal Hantavirus Pulmonary Syndrome — After Intranasal Exposure to Andes Virus
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Analyses of Ribavirin Inhibition in a Novel Viral Spread Assay and Titer Reduction Assay

| Concentration | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 |
|---|---|---|---|---|---|---|---|
| ANDV alone | − | +++ | +++ | +++ | +++ | +++ | +++ |
| 10 µg/mL | − | − | − | ++ | ++ | ++ | ++ |
| 20 µg/mL | − | − | − | + | + | ++ | ++ |
| 30 µg/mL | − | − | − | − | − | + | + |
| 40 µg/mL | − | − | − | − | − | − | − |
| 50 µg/mL | − | − | − | − | − | − | − |
| 60 µg/mL | − | − | − | − | − | − | − |
| 70 µg/mL | − | − | − | − | − | − | − |
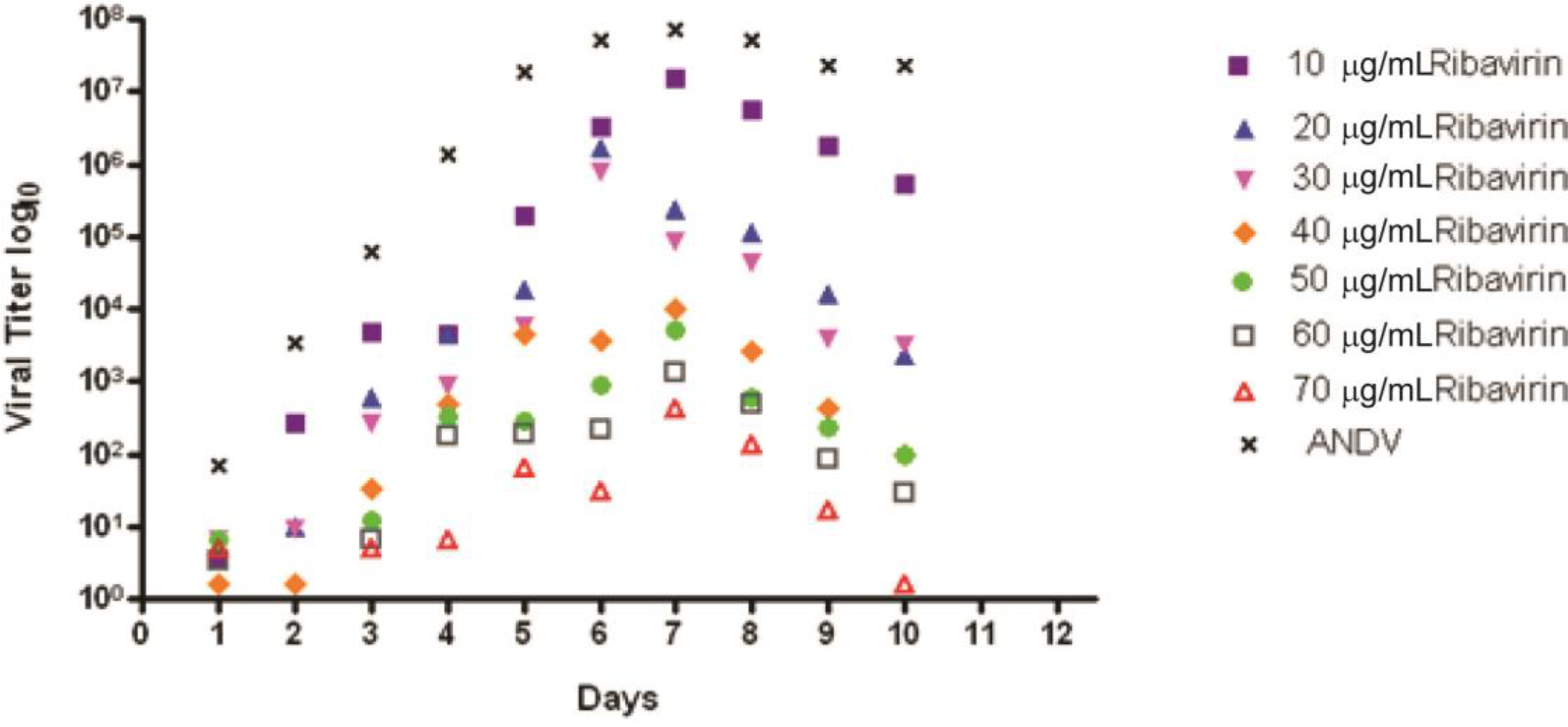
2.2. Ribavirin Efficacy in the Syrian Hamster Model of HPS Following Intranasal Challenge
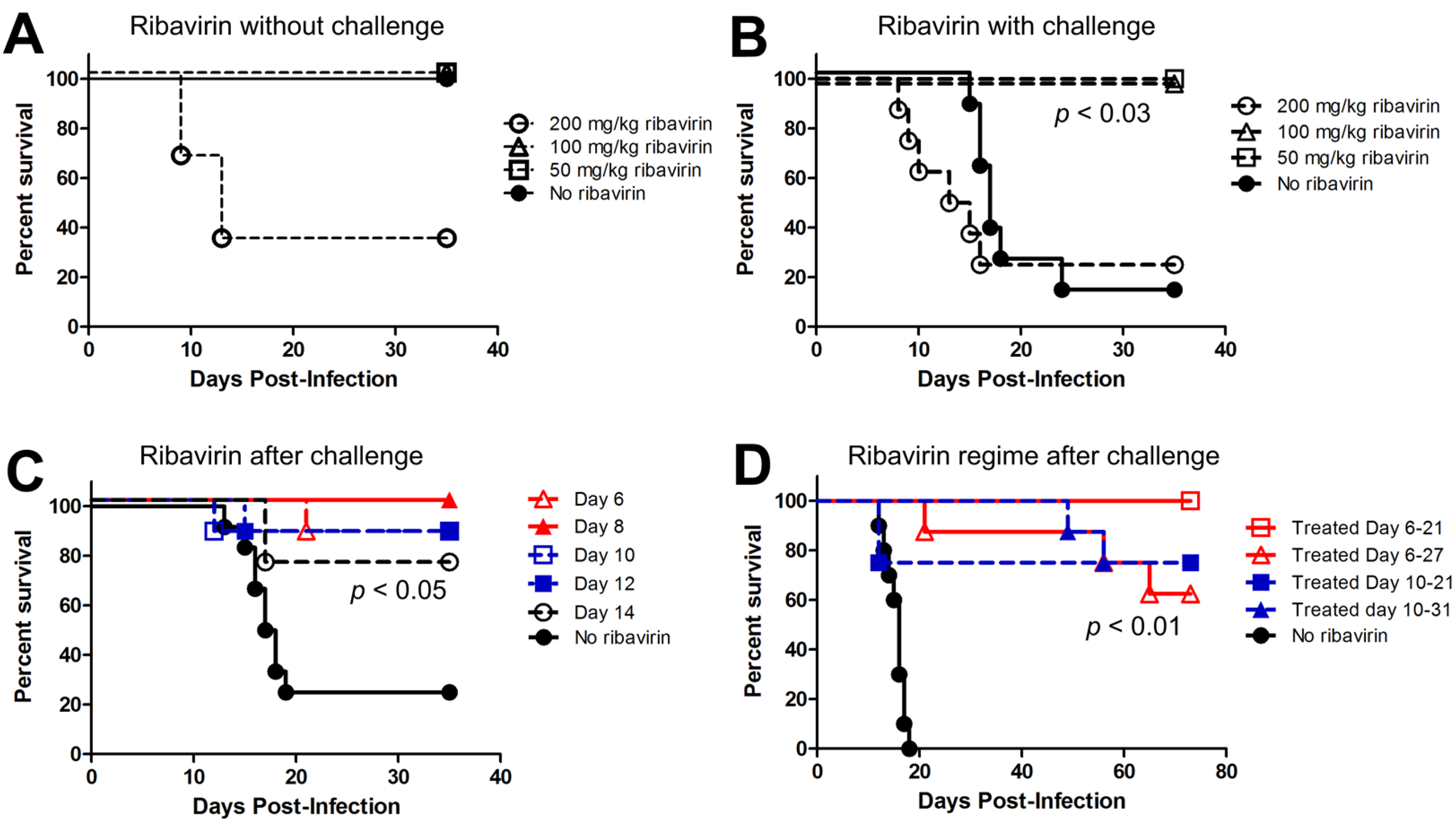
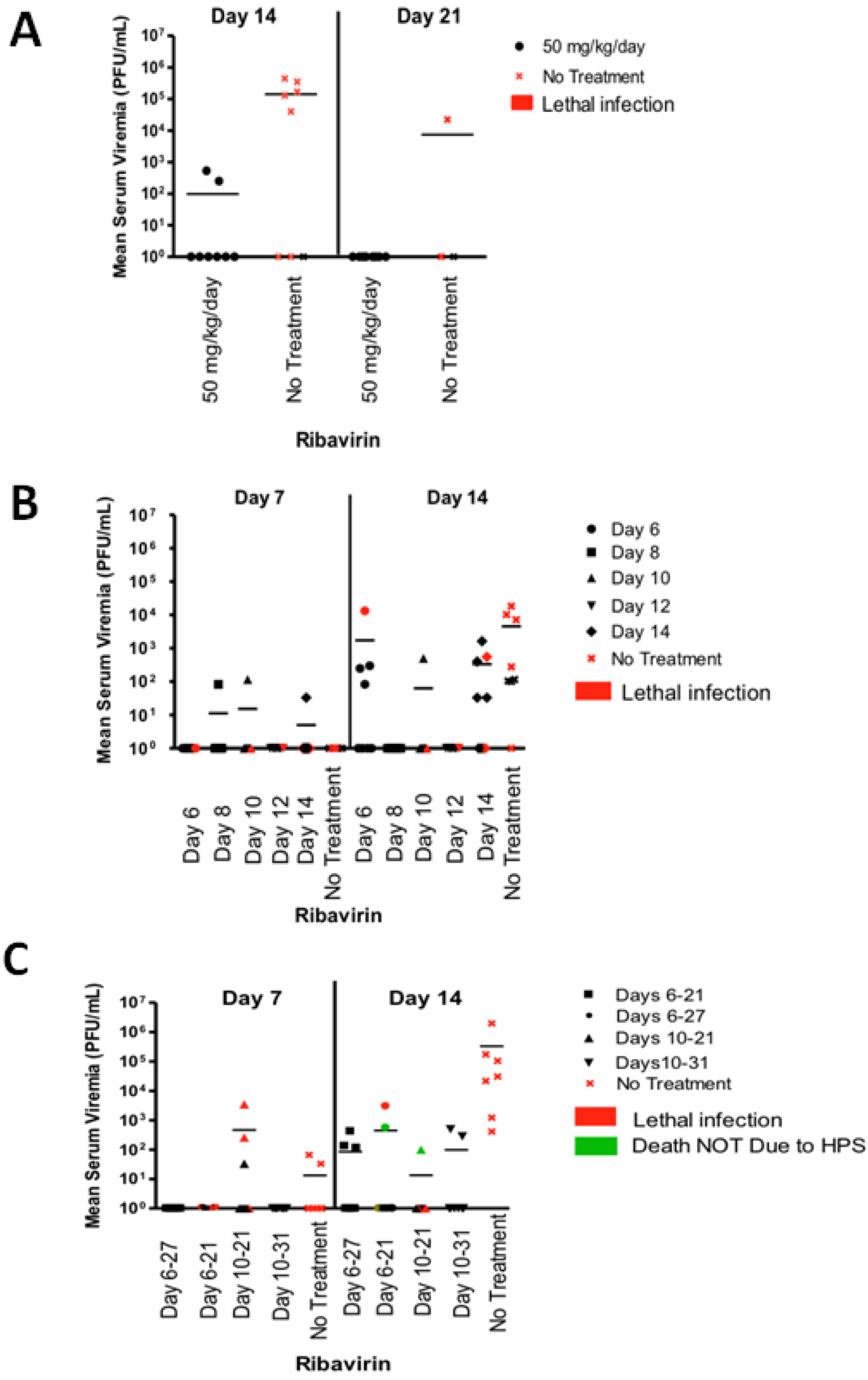
2.3. Last Effective Day Post-Exposure for Treating HPS
2.4. Confirming That Ribavirin can Be Used to Prevent HPS even When Treatment Starts 6–10 Days after a Respiratory Exposure
3. Experimental
3.1. Viruses and Cells
3.2. Virus Yield Reduction Assay
3.3. Serum Plaque Assay
3.4. Viral Spread (Comet) Assay
3.5. Intranasal Infection of Hamsters with ANDV
3.6. Post-Exposure Prophylaxis for Treating HPS
3.7. ELISA
3.8. Statistical Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jonsson, C.B.; Figueiredo, L.T.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef]
- Schmaljohn, C.; Hjelle, B. Hantaviruses: A global disease problem. Emerg. Infect. Dis. 1997, 3, 95–104. [Google Scholar] [CrossRef]
- Peters, C.J.; Simpson, G.L.; Levy, H. Spectrum of hantavirus infection: Hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu. Rev. Med. 1999, 50, 531–545. [Google Scholar] [CrossRef]
- Lee, H.W.; French, G.R.; Lee, P.W.; Baek, L.J.; Tsuchiya, K.; Foulke, R.S. Observations on natural and laboratory infection of rodents with the etiologic agent of Korean hemorrhagic fever. Am. J. Trop. Med. Hyg. 1981, 30, 477–482. [Google Scholar]
- Tsai, T.F. Hemorrhagic fever with renal syndrome: Mode of transmission to humans. Lab. Anim. Sci. 1987, 37, 428–430. [Google Scholar]
- Jonsson, C.B.; Hooper, J.; Mertz, G. Treatment of hantavirus pulmonary syndrome. Antiviral Res. 2008, 78, 162–169. [Google Scholar] [CrossRef]
- Padula, P.J.; Edelstein, A.; Miguel, S.D.; Lopez, N.M.; Rossi, C.M.; Rabinovich, R.D. Hantavirus pulmonary syndrome outbreak in Argentina: Molecular evidence for person-to-person transmission of Andes virus. Virology 1998, 241, 323–330. [Google Scholar] [CrossRef]
- Padula, P.J.; Edelstein, A.; Miguel, S.D.; Lopez, N.M.; Rossi, C.M.; Rabinovich, R.D. Epidemic outbreak of Hantavirus pulmonary syndrome in Argentina. Molecular evidence of person to person transmission of Andes virus. Medicina (B Aires) 1998, 58, 27–36. [Google Scholar]
- Enria, D.; Padula, P.; Segura, E.L.; Pini, N.; Edelstein, A.; Posse, C.R.; Weissenbacher, M.C. Hantavirus pulmonary syndrome in Argentina. Possibility of person to person transmission. Medicina (B Aires) 1996, 56, 709–711. [Google Scholar]
- Martinez, V.P.; Bellomo, C.; San Juan, J.; Pinna, D.; Forlenza, R.; Elder, M.; Padula, P.J. Person-to-person transmission of Andes virus. Emerg. Infect. Dis. 2005, 11, 1848–1853. [Google Scholar] [CrossRef]
- Wells, R.M.; Sosa Estani, S.; Yadon, Z.E.; Enria, D.; Padula, P.; Pini, N.; Gonzalez, D.V.M.; Mills, J.N.; Peters, C.J. Seroprevalence of antibodies to hantavirus in health care workers and other residents of southern Argentina. Clin. Infect. Dis. 1998, 27, 895–896. [Google Scholar] [CrossRef]
- Wells, R.M.; Sosa, E.S.; Yadon, Z.E.; Enria, D.; Padula, P.; Pini, N.; Mills, J.N.; Peters, C.J.; Segura, E.L. An unusual hantavirus outbreak in southern Argentina: Person-to-person transmission? Hantavirus Pulmonary Syndrome Study Group for Patagonia. Emerg. Infect. Dis. 1997, 3, 171–174. [Google Scholar] [CrossRef]
- Toro, J.; Vega, J.D.; Khan, A.S.; Mills, J.N.; Padula, P.; Terry, W.; Yadon, Z.; Valderrama, R.; Ellis, B.A.; Pavletic, C.; et al. An outbreak of hantavirus pulmonary syndrome, Chile, 1997. Emerg. Infect. Dis. 1998, 4, 687–694. [Google Scholar] [CrossRef]
- Hooper, J.W.; Larsen, T.; Custer, D.M.; Schmaljohn, C.S. A lethal disease model for hantavirus pulmonary syndrome. Virology 2001, 289, 6–14. [Google Scholar] [CrossRef]
- Wahl-Jensen, V.; Chapman, J.; Asher, L.; Fisher, R.; Zimmerman, M.; Larsen, T.; Hooper, J.W. Temporal analysis of Andes virus and Sin Nombre virus infections of Syrian hamsters. J. Virol. 2007, 81, 7449–7462. [Google Scholar] [CrossRef]
- Campen, M.J.; Milazzo, M.L.; Fulhorst, C.F.; Obot Akata, C.J.; Koster, F. Characterization of shock in a hamster model of hantavirus infection. Virology 2006, 356, 45–49. [Google Scholar] [CrossRef]
- Safronetz, D.; Zivcec, M.; Lacasse, R.; Feldmann, F.; Rosenke, R.; Long, D.; Haddock, E.; Brining, D.; Gardner, D.; Feldmann, H.; et al. Pathogenesis and host response in Syrian hamsters following intranasal infection with Andes virus. PLoS Pathog. 2011, 7, e1002426. [Google Scholar] [CrossRef]
- Safronetz, D.; Haddock, E.; Feldmann, F.; Ebihara, H.; Feldmann, H. In vitro and in vivo activity of ribavirin against Andes virus infection. PLoS One 2011, 6, e23560. [Google Scholar]
- Safronetz, D.; Ebihara, H.; Feldmann, H.; Hooper, J.W. The Syrian hamster model of hantavirus pulmonary syndrome. Antiviral Res. 2012, 95, 282–292. [Google Scholar] [CrossRef]
- Hooper, J.W.; Ferro, A.M.; Wahl-Jensen, V. Immune serum produced by DNA vaccination protects hamsters against lethal respiratory challenge with Andes virus. J. Virol. 2008, 82, 1332–1338. [Google Scholar] [CrossRef]
- Brocato, R.; Josleyn, M.; Ballantyne, J.; Vial, P.; Hooper, J.W. DNA vaccine-generated duck polyclonal antibodies as a postexposure prophylactic to prevent hantavirus pulmonary syndrome (HPS). PLoS One 2012, 7, e35996. [Google Scholar]
- Brocato, R.L.; Josleyn, M.J.; Wahl-Jensen, V.; Schmaljohn, C.S.; Hooper, J.W. Construction and nonclinical testing of a puumala virus synthetic m gene-based DNA vaccine. Clin. Vaccine Immunol. 2013, 20, 218–226. [Google Scholar] [CrossRef]
- Brown, K.S.; Safronetz, D.; Marzi, A.; Ebihara, H.; Feldmann, H. Vesicular stomatitis virus-based vaccine protects hamsters against lethal challenge with Andes virus. J. Virol. 2011, 85, 12781–12791. [Google Scholar] [CrossRef]
- Sun, Y.; Chung, D.H.; Chu, Y.K.; Jonsson, C.B.; Parker, W.B. Activity of ribavirin against Hantaan virus correlates with production of ribavirin-5'-triphosphate, not with inhibition of IMP dehydrogenase. Antimicrob. Agents Chemother. 2007, 51, 84–88. [Google Scholar] [CrossRef]
- Chung, D.H.; Sun, Y.; Parker, W.B.; Arterburn, J.B.; Bartolucci, A.; Jonsson, C.B. Ribavirin reveals a lethal threshold of allowable mutation frequency for Hantaan virus. J. Virol. 2007, 81, 11722–11729. [Google Scholar] [CrossRef]
- Severson, W.E.; Schmaljohn, C.S.; Javadian, A.; Jonsson, C.B. Ribavirin causes error catastrophe during Hantaan virus replication. J. Virol. 2003, 77, 481–488. [Google Scholar] [CrossRef]
- Jonsson, C.B.; Milligan, B.G.; Arterburn, J.B. Potential importance of error catastrophe to the development of antiviral strategies for hantaviruses. Virus Res. 2005, 107, 195–205. [Google Scholar] [CrossRef]
- Kirsi, J.J.; North, J.A.; McKernan, P.A.; Murray, B.K.; Canonico, P.G.; Huggins, J.W.; Srivastava, P.C.; Robins, R.K. Broad-spectrum antiviral activity of 2-beta-D-ribofuranosylselenazole-4-carboxamide, a new antiviral agent. Antimicrob. Agents Chemother. 1983, 24, 353–361. [Google Scholar] [CrossRef]
- Medina, R.A.; Mirowsky-Garcia, K.; Hutt, J.; Hjelle, B. Ribavirin, human convalescent plasma and anti-beta3 integrin antibody inhibit infection by Sin Nombre virus in the deer mouse model. J. Gen. Virol. 2007, 88, 493–505. [Google Scholar] [CrossRef]
- Buys, K.K.; Jung, K.H.; Smee, D.F.; Furuta, Y.; Gowen, B.B. Maporal virus as a surrogate for pathogenic New World hantaviruses and its inhibition by favipiravir. Antivir. Chem. Chemother. 2011, 21, 193–200. [Google Scholar] [CrossRef]
- Huggins, J.W.; Kim, G.R.; Brand, O.M.; McKee, K.T., Jr. Ribavirin therapy for Hantaan virus infection in suckling mice. J. Infect. Dis. 1986, 153, 489–497. [Google Scholar] [CrossRef]
- Murphy, M.E.; Kariwa, H.; Mizutani, T.; Tanabe, H.; Yoshimatsu, K.; Arikawa, J.; Takashima, I. Characterization of in vitro and in vivo antiviral activity of lactoferrin and ribavirin upon hantavirus. J. Vet. Med. Sci. 2001, 63, 637–645. [Google Scholar] [CrossRef]
- Murphy, M.E.; Kariwa, H.; Mizutani, T.; Yoshimatsu, K.; Arikawa, J.; Takashima, I. In vitro antiviral activity of lactoferrin and ribavirin upon hantavirus. Arch. Virol. 2000, 145, 1571–1582. [Google Scholar] [CrossRef]
- Chung, D.H.; Strouse, J.J.; Sun, Y.; Arterburn, J.B.; Parker, W.B.; Jonsson, C.B. Synthesis and anti-Hantaan virus activity of N(1)-3-fluorophenyl-inosine. Antiviral Res. 2009, 83, 80–85. [Google Scholar] [CrossRef]
- Chung, D.H.; Kumarapperuma, S.C.; Sun, Y.; Li, Q.; Chu, Y.K.; Arterburn, J.B.; Parker, W.B.; Smith, J.; Spik, K.; Ramanathan, H.N.; et al. Synthesis of 1-beta-D-ribofuranosyl-3-ethynyl-[1,2,4]triazole and its in vitro and in vivo efficacy against Hantavirus. Antiviral Res. 2008, 79, 19–27. [Google Scholar] [CrossRef]
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D.F.; Barnard, D.L.; Gowen, B.B.; Julander, J.G.; Morrey, J.D. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009, 82, 95–102. [Google Scholar] [CrossRef]
- Safronetz, D.; Falzarano, D.; Scott, D.P.; Furuta, Y.; Feldmann, H.; Gowen, B.B. Antiviral efficacy of favipiravir against two prominent etiological agents of hantavirus pulmonary syndrome. Antimicrob. Agents Chemother. 2013, 10, 4673–4680. [Google Scholar]
- Gowen, B.B.; Wong, M.H.; Jung, K.H.; Sanders, A.B.; Mendenhall, M.; Bailey, K.W.; Furuta, Y.; Sidwell, R.W. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob. Agents Chemother. 2007, 51, 3168–3176. [Google Scholar] [CrossRef]
- Huggins, J.W.; Hsiang, C.M.; Cosgriff, T.M.; Guang, M.Y.; Smith, J.I.; Wu, Z.O.; LeDuc, J.W.; Zheng, Z.M.; Meegan, J.M.; Wang, Q.N.; et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J. Infect. Dis. 1991, 164, 1119–1127. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Zhang, T.M.; Zhang, M.V.; Zheng, Z.M.; Hu, Z.J.; Qu, C.F.; Xiang, J.M.; Huggins, J.W.; Cosgriff, T.M.; Smith, J.I. Interruption study of viremia of patients with hemorrhagic fever with renal syndrome in the febrile phase. (English). Chin. Med. J. 1991, 104, 149–153. [Google Scholar]
- Rusnak, J.M.; Byrne, W.R.; Chung, K.N.; Gibbs, P.H.; Kim, T.T.; Boudreau, E.F.; Cosgriff, T.; Pittman, P.; Kim, K.Y.; Erlichman, M.S.; et al. Experience with intravenous ribavirin in the treatment of hemorrhagic fever with renal syndrome in Korea. Antiviral Res. 2009, 81, 68–76. [Google Scholar] [CrossRef]
- Chapman, L.E.; Ellis, B.A.; Koster, F.T.; Sotir, M.; Ksiazek, T.G.; Mertz, G.J.; Rollin, P.E.; Baum, K.F.; Pavia, A.T.; Christenson, J.C.; et al. Discriminators between hantavirus-infected and -uninfected persons enrolled in a trial of intravenous ribavirin for presumptive hantavirus pulmonary syndrome. Clin. Infect. Dis. 2002, 34, 293–304. [Google Scholar] [CrossRef]
- Mertz, G.J.; Miedzinski, L.; Goade, D.; Pavia, A.T.; Hjelle, B.; Hansbarger, C.O.; Levy, H.; Koster, F.T.; Baum, K.; Lindemulder, A.; et al. Placebo-controlled, double-blind trial of intravenous ribavirin for the treatment of hantavirus cardiopulmonary syndrome in North America. Clin. Infect. Dis. 2004, 39, 1307–1313. [Google Scholar] [CrossRef]
- Chapman, L.E.; Mertz, G.J.; Peters, C.J.; Jolson, H.M.; Khan, A.S.; Ksiazek, T.G.; Koster, F.T.; Baum, K.F.; Rollin, P.E.; Pavia, A.T.; et al. Intravenous ribavirin for hantavirus pulmonary syndrome: Safety and tolerance during 1 year of open-label experience. Ribavirin Study Group. Antivir. Ther. 1999, 4, 211–219. [Google Scholar]
- Borio, L.; Inglesby, T.; Peters, C.J.; Schmaljohn, A.L.; Hughes, J.M.; Jahrling, P.B.; Ksiazek, T.; Johnson, K.M.; Meyerhoff, A.; O’Toole, T.; et al. Hemorrhagic fever viruses as biological weapons: Medical and public health management. JAMA 2002, 287, 2391–2405. [Google Scholar] [CrossRef]
- Fernandez, H.; Banks, G.; Smith, R. Ribavirin: A clinical overview. Eur. J. Epidemiol. 1986, 2, 1–14. [Google Scholar] [CrossRef]
- Fisher-Hoch, S.P.; Khan, J.A.; Rehman, S.; Mirza, S.; Khurshid, M.; McCormick, J.B. Crimean Congo-haemorrhagic fever treated with oral ribavirin. Lancet 1995, 346, 472–475. [Google Scholar] [CrossRef]
- Pergam, S.A.; Schmidt, D.W.; Nofchissey, R.A.; Hunt, W.C.; Harford, A.H.; Goade, D.E. Potential renal sequelae in survivors of hantavirus cardiopulmonary syndrome. Am. J. Trop. Med. Hyg. 2009, 80, 279–285. [Google Scholar]
- Hooper, J.W.; Kamrud, K.I.; Elgh, F.; Custer, D.; Schmaljohn, C.S. DNA vaccination with hantavirus M segment elicits neutralizing antibodies and protects against seoul virus infection. Virology 1999, 255, 269–278. [Google Scholar] [CrossRef]
- Snell, N.J. Ribavirin—Current status of a broad spectrum antiviral agent. Expert Opin. Pharmacother. 2001, 2, 1317–1324. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ogg, M.; Jonsson, C.B.; Camp, J.V.; Hooper, J.W. Ribavirin Protects Syrian Hamsters against Lethal Hantavirus Pulmonary Syndrome — After Intranasal Exposure to Andes Virus. Viruses 2013, 5, 2704-2720. https://doi.org/10.3390/v5112704
Ogg M, Jonsson CB, Camp JV, Hooper JW. Ribavirin Protects Syrian Hamsters against Lethal Hantavirus Pulmonary Syndrome — After Intranasal Exposure to Andes Virus. Viruses. 2013; 5(11):2704-2720. https://doi.org/10.3390/v5112704
Chicago/Turabian StyleOgg, Monica, Colleen B. Jonsson, Jeremy V. Camp, and Jay W. Hooper. 2013. "Ribavirin Protects Syrian Hamsters against Lethal Hantavirus Pulmonary Syndrome — After Intranasal Exposure to Andes Virus" Viruses 5, no. 11: 2704-2720. https://doi.org/10.3390/v5112704
APA StyleOgg, M., Jonsson, C. B., Camp, J. V., & Hooper, J. W. (2013). Ribavirin Protects Syrian Hamsters against Lethal Hantavirus Pulmonary Syndrome — After Intranasal Exposure to Andes Virus. Viruses, 5(11), 2704-2720. https://doi.org/10.3390/v5112704




