Abstract
Photodynamic inactivation (PDI) has been used to inactivate microorganisms through the use of photosensitizers. The inactivation of mammalian viruses and bacteriophages by photosensitization has been applied with success since the first decades of the last century. Due to the fact that mammalian viruses are known to pose a threat to public health and that bacteriophages are frequently used as models of mammalian viruses, it is important to know and understand the mechanisms and photodynamic procedures involved in their photoinactivation. The aim of this review is to (i) summarize the main approaches developed until now for the photodynamic inactivation of bacteriophages and mammalian viruses and, (ii) discuss and compare the present state of the art of mammalian viruses PDI with phage photoinactivation, with special focus on the most relevant mechanisms, molecular targets and factors affecting the viral inactivation process.
Nomenclature
| AlPcS4 | Aluminum phthalocyanine tetrasulfonate |
| AZT | Azidothymidine |
| BVDV | Bovine viral diarrhea virus |
| DMTU | Dimethylthiourea |
| EMCV | Encephalomyocarditis virus |
| HAV | Hepatitis A virus |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HIV | Human immunodeficiency virus |
| HPV | Human papillomatosis virus |
| HSV | Herpes simplex virus |
| LED | Light emitting diode |
| MB | Methylene blue |
| NM | Not mentioned |
| NQ | Not quantified |
| Pc4 | Silicon phthalocyanine |
| PDI | Photodynamic inactivation |
| PS | Photosensitizer |
| ROS | Reactive oxygen species |
| SFV | Semliki Forest virus |
| SHV | Suid herpes virus |
| SOD | Superoxide dismutase |
| SSB | Singlet strand breaks |
| Tri-Py+-Me-PF | 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin tri-iodide |
| VSV | Vesicular stomatitis virus |
| VZV | Varicella zoster virus |
| 1O2 | Singlet oxygen |
| 3O2 | Molecular oxygen |
| 1PS | Ground state photosensitizer |
| 3PS* | Triplet excited state photosensitizer |
1. Introduction
Humans are exposed to pathogenic viruses through various routes and the development of viral-induced diseases is a common occurrence.
Although the transmission of viral diseases has been reduced by the development of good water supplies and hygienic-based procedures for a whole range of human activities [1], pathogenic viruses are still the causative agents of many diseases in humans and other species. The most usual human diseases caused by viruses include the common cold (coronaviruses), influenza (influenza viruses), chickenpox (varicella zoster virus), cold sores (herpes simplex virus), gastroenteritis and diarrhoea (caliciviruses, rotaviruses and adenoviruses) [2,3]. Pathogenic viruses are also implicated in serious diseases, such as Ebola (Ebola virus), AIDS (immunodeficiency viruses), avian influenza and sudden acute respiratory syndrome (SARS) (SARS-coronavirus), and they are also an established cause of cancer (papillomavirus, hepatitis B and C viruses, Epstein–Barr virus, Kaposi’s sarcoma-associated herpes virus, human T-lymphotropic virus, and Merkel cell polyomavirus) [4].
The enhanced implication of viruses in severe infectious diseases and the increasing knowledge about the complex mechanisms of viral pathogenesis have greatly contributed to the rapid development of antiviral drugs. Consequently, the use of antivirals has largely increased in the last years and resistance to antiviral drugs is now well documented for several pathogenic viruses [5,6,7,8,9,10]. Moreover, as viruses are genetically flexible, they may mutate quickly and mutations come as no surprises, leading to the development of resistance to conventional antiviral drugs. Consequently, the emergence of antiviral drug can become a great problem, such the resistance observed for bacteria relative to antibiotics. So, alternative methods unlikely to cause resistance are required. Photodynamic inactivation (PDI) of viruses represents a promising and inexpensive potential alternative to meet that need.
The sensitivity of viruses to photodynamic procedures was reported in the 1930s [11,12] but only within the last 30 years, with the development of new active molecules, namely photosensitizers (PS), and an increment of light technologies (lasers, LED, portability, etc.), have photodynamic techniques for the inactivation of viruses received growing attention [13]. Most of the clinical applications of PDI for treatment of infections have so far been directed to viral lesions [14]. Clinical PDI was first applied to the treatment of herpes infection in the early 1970s [15], particularly for herpes genitalis. Since then, a great variety of viruses has been effectively inactivated by photodynamic treatment using in vitro conditions [16] but, considering the clinical use of viral PDI, the procedures are limited to the treatment of papillomatosis, caused by human papillomatosis virus (HPV), like laryngeal papillomatosis [17] and epidermodysplasia verruciformis [18] and, in a small scale, to the treatment of viral complications in AIDS patients [19,20]. However, considerable progress has been made in the viral photodynamic disinfection of blood products. The major threat of viral contamination in blood and blood products comes from the immunodeficiency viruses (HIV) [21], hepatitis viruses [21,22,23], cytomegalovirus [23], human parvovirus B19 [24] and human T-cell lymphotropic virus type I and type II [23]. HIV has been inactivated in vitro following a photodynamic procedure [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. The photoinactivation of hepatitis viruses in blood products has also been successfully tested against the hepatitis C virus (HCV) [37,40,41,42], hepatitis B virus (HBV) [43] and hepatitis A virus (HAV) [44]. Inactivation of cytomegalovirus [45], human parvovirus B19 [46] and human T-cell lymphotropic virus [47] in blood products was also efficiently achieved after photodynamic treatment.
The availability of a simple and quantitative assay to follow the viral photoinactivation process is important. Traditional viral quantification techniques, such as in vitro viral cultures, are time-consuming and labor-intensive processes. Molecular quantitative methods such as nucleic acid amplification procedures, including real time PCR, are rapid and sensitive but detect only viral nucleic acid and do not determine infectivity. When the virucidal properties of different photosensitizing compounds are initially evaluated, bacteriophages can be useful as surrogates of mammalian viruses. The reasons for their use are: (i) the detection methods are much simpler, faster and cheaper than those of mammalian viruses, avoiding the advanced facilities and equipment needed for propagating human pathogens; (ii) they are non-pathogenic to humans; (iii) they can be grown to higher titers than most mammalian viruses and, therefore, enhancing the sensitivity of the assay; (iv) the results of bacteriophages assays are available within several hours post-inoculation, instead of the days or weeks required by mammalian viruses infectivity-based assays; (v) they are at least as resistant as the mammalian viruses to environmental factors and to water treatment [48].
It has been shown that enveloped viruses are significantly more sensitive to photodynamic destruction than non-enveloped viruses [49,50]. As most of the bacteriophages are non-enveloped, they are more difficult to suffer photoinactivation than the enveloped viruses. In general, this property makes them good indicators to evaluate the efficiency of viral PDI. A PDI protocol that is effective to inactivate a non-enveloped phage will most likely be effective against enveloped mammalian viruses.
Several bacteriophages were used in photoinactivation studies as surrogates for mammalian viruses, e.g., MS2 [44], M13 [51,52], PM2 [53], Qβ [54,55,56], PRD1 [57], λ [58,59], φ6 [60], R17 [60], Serratia phage kappa [61], T5 [62], T3 [63], T7 [57,64] and T4-like [65,66,67,68], and the results show that they are effectively photoinactivated.
2. Antimicrobial PDI
PDI is a simple and controllable method for the inactivation of microorganisms based on the production of reactive oxygen species (ROS) (free radicals and singlet oxygen). This technology requires the combined action of oxygen, light and a photosensitizer (PS), which absorbs and uses the energy from light to produce those ROS [69]. Therefore, the photodynamic effects depend on multiple variables including: the structural features of the PS, the concentrations of PS and molecular oxygen, and the properties of the light used (e.g., wavelength, type, dose and fluence rate) [66,67,69,70,71,72]. Changes in any of these parameters will affect the rate of microbial photoinactivation [66,67,73,74].
The majority of PS used in PDI is derived from tetrapyrrolic macrocycles known as porphyrins. These chromophores and their analogs, such as chlorins and bacteriochlorins, are involved in very important biological functions, such as respiration (heme group) and photosynthesis (chlorophyll and bacteriochlorophyll (Figure 1). Based on these macrocycles, the scientific community was able to develop a number of synthetic analogs, such as meso-tetraarylporphyrins, phthalocyanines, texaphyrins, porphycenes and saphyrins, which proved to have very promising features for being used as PS (Figure 2) [16]. Also, non-tetrapyrrolic derivatives, such as the naturally occurring hypericin, or synthetic dyes like toluidine blue O, rose bengal, eosin, methylene blue (MB) and fullerenes, were considered in many PDI studies (Figure 3) [71].
In order to be efficient, photosensitizing agents used for viral PDI must bind specifically to vital viral components, such as lipid envelope (when present), the protein coat or to the nucleic acids [55].
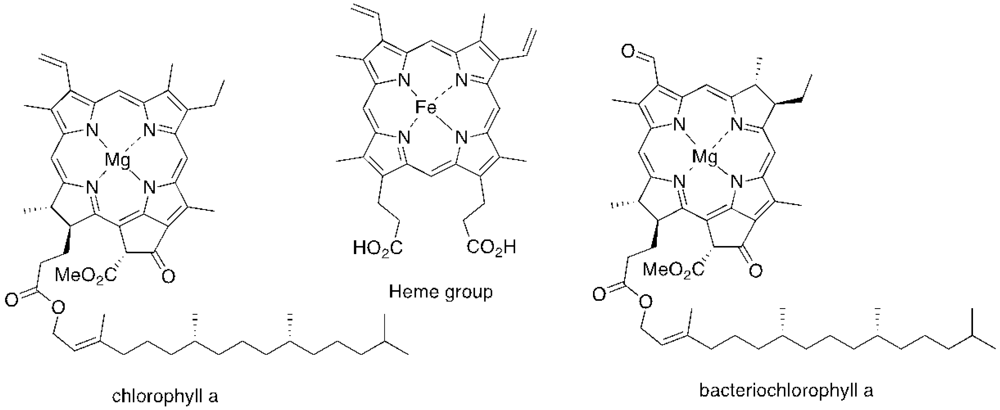
Figure 1.
Structure of some tetrapyrrolic macrocycles with natural occurrence.
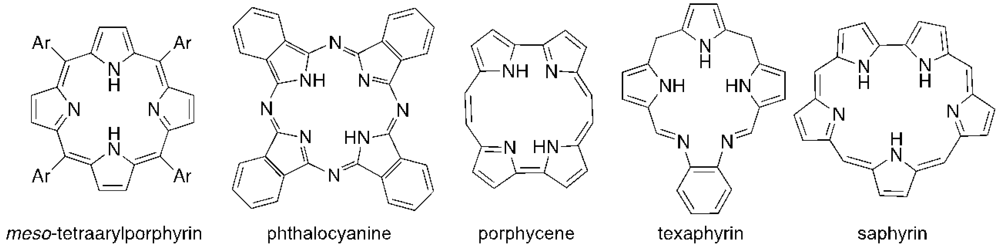
Figure 2.
Skeletons of some synthetic pyrrolic macrocycles used as photosensitizers.
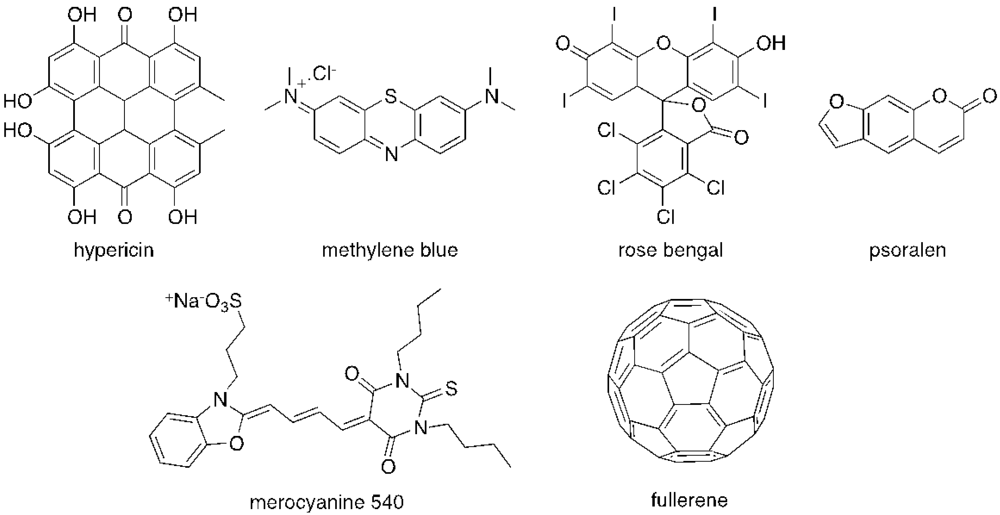
Figure 3.
Structure of some non-tetrapyrrolic photosensitizers.
The efficiency of mammalian viruses and bacteriophages PDI has been described for porphyrin derivatives, chlorin derivatives, chlorophyll derivatives, phthalocyanine derivatives, hypericin, methylene blue, rose bengal, merocyanine 540, proflavine, and fullerene derivatives (Table 1).

Table 1.
Some PS used for mammalian viruses and bacteriophages PDI.
| Photosensitizer | Microorganism | PDI | Reference |
|---|---|---|---|
| Mammalian viruses | |||
| Hematoporphyrin derivative | HSV-1 | 7 log | [75] |
| HSV-1 | <0.8 log | [36] | |
| Uroporphyrin | Adenovirus | 7 log | [76] |
| Natural metalloporphyrin derivatives | HIV-1 | <0.8 log | [36] |
| Chlorophyll derivatives | VSV | ~6 log | [77] |
| 7-despropionate-7-hydroxypropylmesopyropheophorbide a | BVDV | ~5 log | [78] |
| Benzoporphyrin derivative monoacid ring A | HIV-1 | >4 log | [33] |
| Glycoconjugated meso-tetraarylporphyrin derivatives | HSV-1 | 6 log | [79] |
| Metallo tetrasulfonated meso-tetraarylporphyrin derivatives | HIV-1 | ≤2 log | [36] |
| Tetrasulfonated meso-tetraarylporphyrin derivatives | HIV-1 | ≤2 log | [36] |
| HAV | ~4 log | [44] | |
| meso-Tetrakis(1-methylpyridinium-4-yl)porphyrin | HAV | ~4 log | [44] |
| meso-Tetrakis(1-butylpyridinium-4-yl)porphyrin | HAV | >3.8 log | [44] |
| meso-Tetrakis(1-octylpyridinium-4-yl)porphyrin | HAV | >3.9 log | [44] |
| Cationic β-vinyl substituted meso-tetraphenylporphyrin derivatives | HSV-1 | <3 log | [80] |
| Aluminum dibenzodisulfophthalocyanine | HIV-1 | 3.7 log | [49] |
| Aluminum phthalocyanine tetrasulfonate | HIV-1 | >5 log | [49] |
| VSV | 4.2 log | [82] | |
| Adenovirus | 4 log | [76] | |
| Silicon phthalocyanine derivative | VSV | 4 log | [82] |
| Cationic phthalocyanines | HIV-1 | >5 log | [49] |
| HSV-1 | ≥5 log | [83] | |
| Hypericin | HIV-1 | NQ | [30] |
| VSV | 4-5 log | ||
| Influenza virus | NQ | ||
| Sendai virus | NQ | ||
| Methylene blue | VSV | 4.7 log | [81] |
| HSV-1 | 5 log | [84] | |
| SHV-1 | 2.5 log | [84] | |
| HCV | <2 log | [41] | |
| HIV-1 | <2 log | [41] | |
| Adenovirus | 7 log | [76] | |
| Dengue virus | 5–6.4 log | [74] | |
| Enterovirus 71 | ~8 log | [85] | |
| Vaccinia virus | 5 log | [86] | |
| Phenothiazine derivatives | VSV | >4.4 log | [60] |
| Rose bengal | Vaccinia virus | 5 log | [86] |
| HIV-1 | NQ | [30] | |
| VSV | 4–5 log | ||
| Influenza virus | NQ | ||
| Sendai virus | NQ | ||
| Adenovirus | 7 log | [76] | |
| Buckminsterfullerene | SFV | 7 log | [50] |
| Merocyanine 540 | HSV-1 | 5–6 log | [45] |
| Bacteriophages | |||
| Glycoconjugated meso-tetraarylporphyrins | T7 phage | <3 log | [64] |
| T7 phage | <3.5 log | [87] | |
| Tetrasulfonated meso-tetraarylporphyrin derivatives | MS2 phage | >3.8 log | [44] |
| meso-Tetrakis(1-methylpyridinium-4-yl)porphyrin | λ phage | <7 log | [58] |
| MS2 phage | >4.1 log | [44] | |
| T4 phage | 7 log | [66,67] | |
| T7 phage | <4 log | [88] | |
| 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin | T4 phage | 7 log | [66,67,68] |
| 5-(4-methoxicarbonylphenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin | T4 phage | 7 log | [66] |
| 5-(4-carboxyphenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin | T4 phage | 3.9 log | [66] |
| 5,10-bis(4-carboxyphenyl)-15,20-bis(1-methylpyridinium-4-yl)porphyrin | T4 phage | 1.4 log | [66] |
| 5,15-bis(4-carboxyphenyl)-10,20-bis(1-methylpyridinium-4-yl)porphyrin | T4 phage | 1.2 log | [66] |
| 5,10,15-tris(1-methylpyridinium-4-yl)-20-phenylporphyrin | T7 phage | 1.7 log | [88] |
| Methylene blue | Serratia phage kappa | >4 log | [61] |
| M13 phage | 2.2 log | [52,81] | |
| f2 phage | 5 log | [56] | |
| Qβ phage | 7–8 log | [56] | |
| Qβ phage | 7–8 log | [89] | |
| Phenothiazine derivatives | R17 phage | 4–7 log | [60] |
| Rose bengal | PRD1 phage | ~3.5 log* | [57] |
| Riboflavin | λ phage | <4 log | [59] |
| Proflavine | Serratia phage kappa | 4 log | [61] |
| T3 phage | 7–11 log | [63] | |
| Polyhydroxylated fullerene | MS2 phage | ~4 log | [90] |
| PRD1 phage | ~2.5 log* | [57] | |
*log(N/N0)
Besides this, viral PDI has also been described for phthalocyanine derivatives [81], methylene blue [53,62,91,92], toluidine blue O [53,62,93], neutral red [93], proflavine [93], azure B [53] and merocyanine 540 [45,47,94].
3. Mechanisms of Photodynamic Inactivation
The mechanisms of PDI are based on the ability of the PS to absorb energy from light and transfer that energy to molecular oxygen. In the dark, the electronic configuration of a PS exists in the so-called ground state. The absorption, by the PS, of a photon at an appropriate wavelength initially leads to the production of an unstable, electronically-excited state of the PS molecule (the lifetime of this state ranges from 10−9 to 10−6 s) [95]. The excited PS molecule can then decay to the ground state by emission of light (radiative pathway - fluorescence) or by intersystem crossing, affording the excited triplet state which has a longer lifetime (10−3 to 10 s) [95]. At this point, the PS can reach the ground state either by spin inversion followed by phosphorescence emission, or by a non-radiative process. Due to the longer lifetime of the PS triplet state, this excited state can also react in one of two ways (Figure 2): by initiating photochemical reactions that can directly generate reactive oxygen species (ROS) (type I pathway), or indirectly by energy transfer to molecular oxygen (type II pathway), leading to the formation of singlet oxygen (Figure 4). These events afford toxic species which are responsible for the irreparable oxidative damages induced to important biological targets [1,69,95,96].
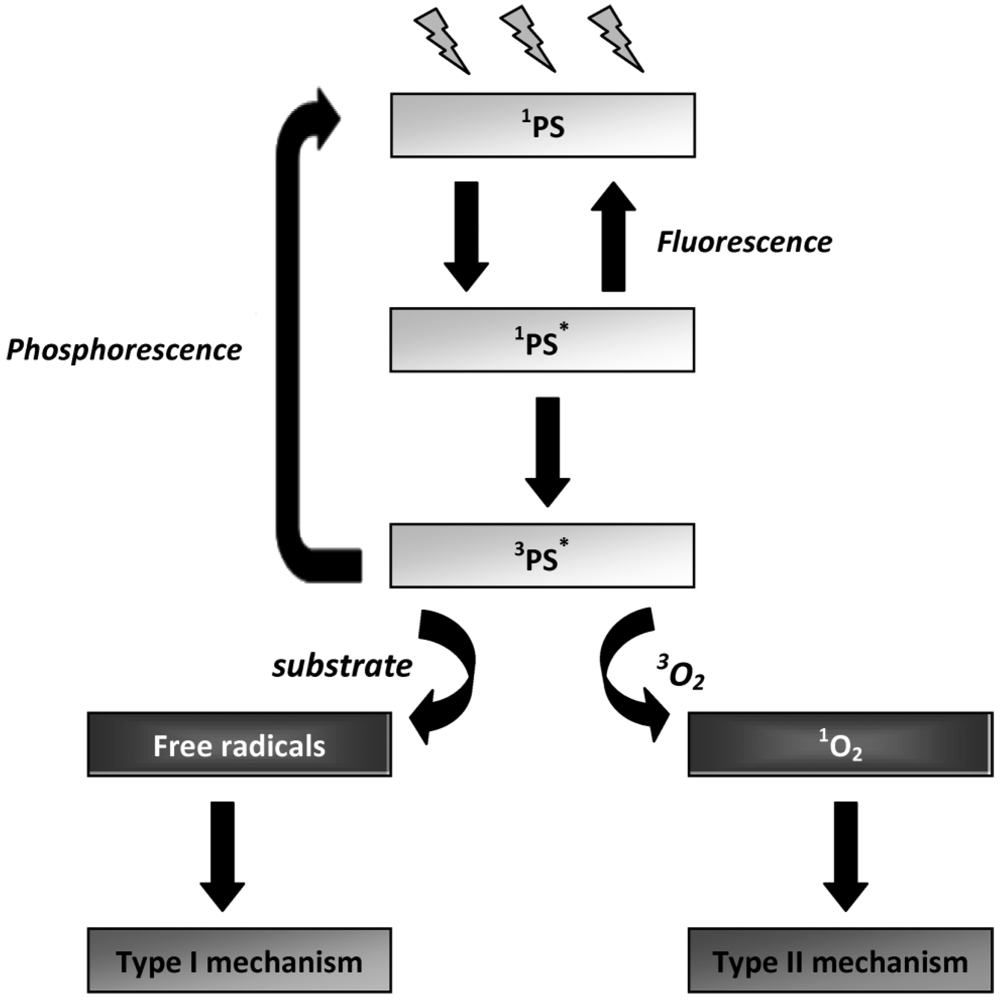
Figure 4.
Schematic representation of the photosensitization process (adapted from [97]).
3.1. Type I and Type II Mechanisms
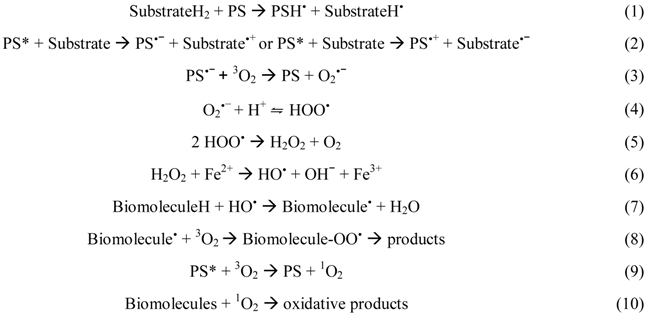
Type I mechanism involves hydrogen-atom abstraction or electron-transfer between the excited PS and a substrate, yielding free radicals [Equations (1) and (2)]. These radicals can react with oxygen to form active oxygen species, such as the superoxide radical anion [Equation (3)]. Superoxide is not particularly reactive in biological systems but, when protonated, can lead to the production of hydrogen peroxide and oxygen [Equations (4) and (5)] or highly reactive hydroxyl radicals [Equations (6)–(8)] [98]. Type II photooxidation is considerably less complex mechanistically than type I and in general there are far fewer products [99]. In this pathway, the excited triplet state PS (3PS*) can transfer the excess energy to molecular oxygen (3O2) and relax to its ground state (1PS) creating an excited singlet molecular oxygen (1O2) [Equation (9)] [69]. 1O2 is highly electrophilic and can interact with numerous enzymes, leading to the inhibition of protein synthesis and molecular alteration of DNA strands, which alters the transcription of the genetic material during its replication (mutagenic effect) and, in this way, leading to microbial death [Equation (10)] [98,100]. Like nucleic acids and proteins, unsaturated lipids are also prominent targets of 1O2 and free radical attack. Lipid peroxidation-ensuing reactions can alter surrounding proteins, nucleic acids and other molecules, in addition to the lipids themselves [98]. Therefore, it is likely that damage of different kinds caused to the viral envelope is important in the process of microbial inactivation [13].
Both type I and type II mechanisms can occur simultaneously or exclusively, and the ratio between these processes depends on the PS used and on the concentrations of substrate and oxygen [95]. The competition between organic substrates and molecular oxygen for the 3PS* determines whether the reaction pathway is type I or type II and the predominant mechanism can be changed during the course of the PDI process [101].
3.2. Evaluation of the Specific Involvement of Type I and Type II Mechanisms
An important goal in the investigation of viral PDI is to identify the type of mechanism involved (type I or type II) in the presence of a selected PS [102]. The simple detection of a reactive species does not necessarily explain the mechanism by which a specific PS induces the toxic effect. It is generally easier to draw a negative conclusion, i.e., if singlet oxygen is absent, it cannot be the reactive species responsible for the photodynamic effect [103]. The simplest approach for determining whether singlet oxygen (type II mechanism) or free radicals (type I mechanism) is involved in the photodynamic process is to study the inhibitory effects of various scavengers, i.e., compounds that can intercept these ROS at high rates and in a putatively selective manner [99,101,104].
3.2.1. Type I Mechanism Scavengers
A first line of defence against ROS is, of course, the protection against their formation. However, the interception of the damaging species once formed, to prevent it from further deleterious reactions, is also a deactivation strategy of defence. In general, free radical scavengers neutralize the radical species by donating one of their own electrons. The quenching agents themselves are not particularly toxic before and after the electron donation [105].
Three different types of quenching are possible, which include the transfer of the radical character with the formation of a reactive scavenger-derived radical; trapping of free radicals with the formation of a stable or inert free radical trap; and molecules which mimic quenching enzyme activities. In general, scavenger molecules either prevent free radicals from being formed or remove them before they can damage vital molecular components [105].
Several free radical scavengers have been used to evaluate the specific involvement of type I mechanism during mammalian viruses and bacteriophages PDI with different PS (Table 2).

Table 2.
Free radical scavengers used in mammalian viruses and bacteriophages PDI.
| PS | Scavenger | Microorganism | Scavenger protection | Reference |
|---|---|---|---|---|
| Mammalian viruses | ||||
| Aluminum phthalocyanine tetrasulfonate | Reduced glutathione | VSV | Little/no effect | [106] |
| Polyhydroxylated fullerene | Glutathione (2.0 mM) | SFV | no effect | [50] |
| Hydroquinone (2.0 mM) | SFV | no effect | [50] | |
| Merocyanine 540 | Glutathione (10 and 30 mmol L−1) | HSV-1 | 30-50% | [45] |
| Methylene blue | Mannitol (100 mM) | HSV-1 | 24% | [84] |
| Bacteriophages | ||||
| 5,10,15-(4-β- D-glucosylphenyl)-20-phenylporphyrin | DMTU (0.1–5.0 mM) | T7 phage | 44% | [64] |
| 5,10.15,20-Tetrakis(4-β- D-glucosylphenyl) porphyrin | DMTU (0.1–5.0 mM) | T7 phage | 79% | [64] |
| 5,10,15-(4-β- D-galactosylphenyl)-20-(pentafluorophenyl)-porphyrin | DMTU (0.1–5.0 mM) | T7 phage | 89% | [87] |
| 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4- yl)porphyrin | D-mannitol (100 mM) | T4 phage | 20% | [107] |
| L-cysteine (100 mM) | T4 phage | 9% | [107] | |
| 5,10,15,20-tetrakis(1-methylpyridinium-4-yl)porphyrin | D-mannitol (100 mM) | T4 phage | no effect | [107] |
| Proflavine | L-cysteine (0.025 M) | T3 phage | 75–80% | [63] |
| Polyhydroxylated fullerene | SOD | MS2 phage | no effect | [90] |
3.2.1.1. Free Radicals in PDI of Mammalian Viruses
Free radical species had, in general, little or no effect on the photoinactivation of the studied mammalian viruses (Table 2). In fact, it can be observed that the rate of inactivation of HSV [45,84,106], influenza virus [108], Semliki Forest virus (SFV) and VSV [50] in the presence of different PS and scavengers like glutathione, D-mannitol, glycerol, superoxide dismutase (SOD), catalase and hydroquinone was not significantly affected. Although this data suggest that free radicals are not major players in the viral inactivation process, the participation of type I reaction pathways cannot be ruled out, as was shown by the considerable level of protection afforded by glutathione and cysteamine when merocyanine 540 was used as PS for inactivation of HSV-1 [45].
3.2.1.2. Free Radicals in PDI of Bacteriophages
The photoinactivation rate of some bacteriophages can be reduced in the presence of free radical scavengers, suggesting a contribution of radical species in the inactivation process (Table 2). In particular, it was reported that the inhibition of T7 phage photoinactivation in the presence of glycoconjugated meso-tetraarylporphyrins varied according to the structure of the PS and the concentration of dimethylthiourea (DMTU) [64,87]. In fact, T7 phage PDI by meso-tetrakis(4-β-D-glucosylphenyl)porphyrin [64] and 5,10,15-(4-β-D-galactosylphenyl)-20-(pentafluorophenyl)porphyrin [87] seemed to be mainly mediated by free radical species, as revealed by the protection effect of free radical scavenger DMTU, contrary to T7 phage photosensitization by 5,10,15-(4-β-D-glucosylphenyl)-20-phenylporphyrin, which revealed a significantly smaller contribution from type I mechanism. The highest inhibition was reached at about 1.0 mM of DMTU; further increase in scavenger concentration did not decrease the slope of photoinduced inactivation of phages. However, in spite of inhibiting the efficacy of the PS, DMTU did not completely inhibit T7 phage PDI [64,87]. Similar results were reported for T3 phage in the presence of L-cysteine as the scavenger and proflavine as the PS. However, the photoinactivation rate of MS2 by a polydroxylated fullerene was not affected by the presence of SOD, suggesting a negligible contribution of radical species, such as the superoxide radical anion [90]. T4-like phage PDI was also little or not affected by the presence of free radical scavengers L-cysteine and D-mannitol in the presence of porphyrin derivatives, leading to the conclusion that free radical species are not major participants in phage PDI [107].
3.2.2. Type II Mechanism Quenchers
In general, the action of chemical singlet oxygen quenchers involves the reaction of singlet oxygen with the quenching agent, producing an oxidized product. Another possibility is the deactivation of singlet oxygen to ground state (3O2) by physical quenching, achieved by either energy or charge transfer, without consumption of oxygen or product formation [101,109]. Residues of histidine, tryptophan and tyrosine in proteins are considered to be major natural quenchers of singlet oxygen [110].
Several singlet oxygen quenchers have been used to evaluate the specific involvement of type II mechanism during viral PDI with different PS (Table 3).

Table 3.
Singlet oxygen quenchers used on mammalian viruses and bacteriophage PDI.
| PS | Quencher | Microorganism | Quencher protection | Reference |
|---|---|---|---|---|
| Mammalian viruses | ||||
| Aluminum phthalocyanine tetrasulfonate | Sodium azide | VSV | significant effect | [106] |
| Rose bengal | β-carotene | Influenza virus | Significant effect | [108] |
| Hypericin | Sodium azide | HIV | Significant effect | [111] |
| Methylene blue | Imidazole (5.0 and 10 mM) | HSV-1 | 55–75% | [84] |
| Bacteriophages | ||||
| 5,10,15-(4-β- D-galactosylphenyl)-20-(pentafluorophenyl)porphyrin | Sodium azide (0.1–5.0 mM) | T7 phage | 38% | [87] |
| 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin | Sodium azide (100 mM) | T4 phage | 80% | [107] |
| L-histidine (50 mM) | T4 phage | 74% | ||
| meso-tetrakis(1-methylpyridinium-4-yl)porphyrin | Sodium azide (100 mM) | T4 phage | 90% | [107] |
| L-histidine (100 mM) | T4 phage | 78% | ||
| 5,10,15,20-Tetrakis(4-β- D-glucosylphenyl)porphyrin | 1,3-diphenylisobenzofuran (0.1-5.0 mM) | T7 phage | 42% | [64] |
| 5,10,15-(4-β- D-glucosylphenyl)-20-phenylporphyrin | 1,3-diphenylisobenzofuran (0.1-5.0 mM) | T7 phage | 74% | [64] |
| Polyhydroxylated fullerene | β-carotene | T7 phage | 69% | [57] |
| β-carotene (26 μM) | MS2 phage | 50–60% | [90] | |
| Rose bengal | Sodium azide (3.5–35 mM) | M13 phage | 31% | [52] |
3.2.2.1. Singlet Oxygen in PDI of Mammalian Viruses
Singlet oxygen seems to be the most important mediator of virucidal activity (Table 3) on mammalian viruses. The rate of viral photoinactivation is significantly inhibited by oxygen removal or by addition of singlet oxygen quenchers, such as β-carotene, imidazole, L-histidine or sodium azide [45,84,106,107,108]. Hypericin may induce photochemical alterations on HIV major capsid protein p24, which are inhibited by sodium azide, suggesting that the damage results from singlet oxygen [111]. When merocyanine 540 [45], phthalocyanine derivatives [106] or rose bengal [108] were used as PS, the results suggest that 1O2 is the main cytotoxic species involved in VSV photoinactivation, while type I reactants such as hydroxyl radicals are less important.
3.2.2.2. Singlet Oxygen in PDI of Bacteriophages
Considering the PDI of bacteriophages in the presence of singlet oxygen quenchers, the results (Table 3) suggest that, in most of the studied cases, singlet oxygen is an important mediator of the toxic effect induced by PDI. However, the participation of free radicals cannot be ruled out. For instance, the inactivation of M13 bacteriophage by MB was inhibited from 1.72 log to 0.54 log by sodium azide in a quencher dose-dependent mode, up to a concentration of 3.5 mM. However, photoinactivation occurred even in the presence of sodium azide, suggesting that both type I and type II mechanisms may be involved in the M13 photoinactivation process. In the presence of quencher concentrations ranging from 3.5 to 35 mM, a sodium azide protective effect was not observed, as evidenced by increasing rates of M13 phage photoinactivation, reaching a plateau thereafter [52]. Also, the effect of singlet oxygen quenchers and of hydrogen peroxide indicated singlet oxygen as the main factor responsible for the loss of biological activity of bacteriophage M13 by rose bengal [51].
The efficiency of 5,10,15-(4-β-D-galactosylphenyl)-20-(pentafluorophenyl)porphyrin to photoinactivate T7 phage decreased in 38% in the presence of sodium azide [87]. This result, and the ones obtained in the presence of DMTU (Table 2), proved that for this PS, both mechanisms play a role in T7 phage photoinactivation, with type I being the predominant one. Similar results were obtained by Gábor et al. [64] in the presence of glycoconjugated meso-tetraarylporphyrin derivatives as PS and using 1,3-diphenylisobenzofuran as the singlet oxygen quencher. When T7 phage was phototreated with 5,10,15,20-tetrakis(4-β-D-glucosylphenyl)porphyrin, the rate of inactivation decreased 42% in the presence of 1,3-diphenylisobenzofuran. When 5,10,15-(4-β-D-glucosylphenyl)-20-phenylporphyrin was used, the rate of protection substantially increased (74%). It can then be concluded that the type of PDI mechanism depends on the PS structure, with the symmetric derivative exerting its toxic effect mainly via the generation of free radicals, whether the asymmetric derivative proceeds mainly by singlet production [64]. However, in the study of Egyeki et al. [87] using the same asymmetric 5,10,15-(4-β-D-galactosylphenyl)-20-(pentafluorophenyl)porphyrin as PS, and the same phage, the toxic effect occurred mainly via free radical generation. Besides this, the contribution of type I and type II processes was PS concentration-dependent and the sum of the photoinactivation rate measured in the presence of scavengers was smaller than the one measured without the scavengers. This result may imply a synergism between singlet oxygen and hydroxyl radical-mediated damages or it can also be supposed that the efficiency of neither scavenger is 100% [64,87].
A recent study showed that irradiation of polyhydroxylated fullerene suspensions (40 μM) in the presence of β-carotene reduced the photoinactivation rate of PRD1 and T7 phages, demonstrating singlet oxygen involvement [57]. Also, when the T4-like phage was irradiated in the presence of porphyrin derivatives and singlet oxygen quenchers sodium azide and L-histidine, the rate of phage inactivation was considerably reduced, suggesting that singlet oxygen may be an important mediator of the virucidal activity of these PS [107]. However, from the data obtained, other inactivation mechanisms cannot be excluded [57,107].
Although some data about the importance of the type I and II mechanisms in PDI of bacteriophages are discrepant, in general, it seems that the type II pathway is more important than the type I mechanism in phage PDI. On the other hand, there are only a few studies focusing on the simultaneous effect of singlet oxygen and free radicals scavengers under the same protocol of viral PDI [64,84,87,90,106,107].
5. Resistance to PDI and Recovery of Viability
The development of increasing numbers of antiviral agents over the past decades, in the same way as with antibiotics, has provided the clinician with therapeutic options previously unavailable. With the increasing utilization of antiviral drugs, however, has come an enhanced appreciation of the development of antiviral resistance [1,7,137,138,139,140]. Drug resistance is costly to the health service, to the patient who fails to gain maximum therapeutic benefit, and for the community in which resistant viruses may be spread [9].
There is now an urgent need for the development of novel, convenient and inexpensive measures for combating antimicrobial-untreatable infections and limiting the development of additional antimicrobial resistant microorganisms. Photodynamic technology may provide one approach to meet this need, both in terms of therapy and in terms of sterilization, by a mechanism that is markedly different from that typical of most antimicrobials [1,141,142].
As mentioned before, photosensitization involves the generation of singlet oxygen and free radical species, which cause molecular damage. Whether microorganisms could develop resistance to these active oxygen species is still questionable [143] and, consequently, the development of microbial resistance to photosensitization is still under debate. Until now, the development of microbial resistance to PDI is not known and is thought very improbable to be developed. In general, the development of resistance to PDI by microbial strains should be considered as an unlikely event since this process is typically multi-target, with ROS causing damage to many microbial components, which is at a variance with the mechanism of action of most antimicrobial drugs [139,144,145]. In contrast to most common antimicrobials, the number of molecular alterations required to ensure survival would be too great and the microorganism would require multi-site mutations to become highly resistant, an event with significantly lower probability than single-site mutations, which is often sufficient for conferring resistance to small-molecule inhibitors [42,146]. This particular property of antimicrobial PDI is important regarding the repeated treatment of chronic and/or recurrent infections [139].
Antimicrobial PDI, when compared to standard treatments which may require application for several weeks to achieve an effective killing of the microorganism, shortly after initiation of light exposure, exhibits serious and irreversible damage of microorganisms [66,68]. This damage does not allow the creation or operation of any kind of anti-drug or mutagenic mechanism. Antimicrobial PDI is therefore very effective and, up until now, no photosensitization-resistant mutants have been found [68].
5.1. Resistance of Mammalian Viruses and Recovery of Viability after Photosensitization
Data from North et al. [33] show that HIV azidothymidine (AZT)-resistant strains were as susceptible as the AZT-sensitive ones to photosensitization with a benzoporphyrin derivative. This finding comes as no surprise since the mechanisms of action of AZT (inhibition of reverse transcription) and light-activated benzoporphyrin derivative are different. Thus, mutations in the virus that occur at the reverse transcriptase level will not affect photodynamic destruction [33].
Studies focusing on the possible development of viral resistance are extremely scarce and little is known about the recovery of viral viability after consecutive photodynamic treatments.
5.2. Bacteriophage Resistance and Viability Recovery after Photosensitization
Concerning bacteriophages, there is only one study focusing on the possible development of viral resistance after photosensitization [68]. After 10 consecutive cycles of photodynamic treatment, a T4-like phage, in the presence of the tricationic porphyrin 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin (Tri-Py+-Me-PF) at 5.0 μM under white light irradiation, exhibited no changes in the rate of photoinactivation during the course of the experiments, meaning that no resistance was observed. If phage resistance would occur, important reductions on phage photoinactivation efficiency would be detected between experiments. Besides that, T4-like phage did not recover its viability after exposure to Tri-Py+-Me-PF during 120 min of irradiation [68]. In a preliminary study by Perdrau and Todd [12], all attempts at reactivating the inactivated Staphylococcus phage by MB were unsuccessful.
6. Factors Affecting Viral PDI
6.1. Effect of the Number of Charges, Symmetry, Size of Meso Substituent Groups and Photosensitizer Concentration
It has been shown that the location and binding site of the PS, which is highly dependent on the structure and intramolecular charge distribution, is an important factor in microbial PDI [143,147].
In terms of molecular structure, molecular charge is important in determining antimicrobial activity. Positively charged PS are generally more efficient and can act at lower concentrations than neutral and anionic PS molecules [144]. The positive charges on the PS molecule appear to promote a tight electrostatic interaction between the positively charged PS and the negatively charged sites at the viral capsids and envelopes, orientating the PS toward sites which are critical for the stability and metabolism of a particular microorganism [44,147,148]. This kind of association increases the efficiency of the photoinactivation process.
Cationic PS photodamage can be induced in nucleic acid or viral outer structures by PS binding or by PS localized in its vicinity [136]. For instance, it is more likely that positively charged PS will be effective in causing nucleic acid damage than will neutral or anionic congeners, which mainly act against the outer side of the microorganism [149].
The symmetry and the size of the chain of meso substituent groups also affect the photodynamic effect. PS with opposite charged groups are more symmetrical than PS with adjacent charged groups. The adjacent positive charges in the PS macrocycle should result in a molecular distortion due to electrostatic repulsion [150]. The toxicity of a PS can be modulated by the introduction of selected substituents on the macrocycle periphery. In this way, the physicochemical properties of a synthetic PS can be manipulated in order to enhance its interactions with the structural features of the viruses, such as viral capsids, and to minimize the interactions with plasma membranes or mammalian cell membranes [44].
The amphiphilic nature of a PS is another important feature affecting PDI efficiency and can be modulated by the introduction of adequate functionalities in the macrocycle periphery, such as different numbers of positive charges, an asymmetrical charge distribution, or introduction of aromatic hydrocarbon side chains [16,151].
PS concentration is also an important parameter that must be taken into account since viral PDI was shown to be strongly influenced by PS concentration. Increasing the PS concentration reduces the time needed to achieve complete viral inactivation, thus increasing the efficiency of a particular PDI protocol [66].
6.1.1. Mammalian Viruses PDI
Complete inactivation of VSV (4.2 log) can be obtained by treating it with 1.0 μM of the anionic phthalocyanine derivative AlPcS4 and 5 min illumination with red light. For the neutral phthalocyanine derivative (Pc4), complete inactivation (4 log) was achieved using a much lower amount of PS (4.5 nM) in combination with 10 min illumination [82]. The inactivation of VSV in PBS showed a linear relationship with illumination time [82]. Inactivation of the fusion activity of VSV, influenza and Sendai viruses was reached with nanomolar concentrations of hypericin and rose bengal and was absolutely dependent upon light and increased with increasing time of illumination [30]. HAV in PBS or plasma was completely inactivated within 10 min (>3.7 log) by the cationic symmetric porphyrin meso-tetrakis(1-methylpyridinium-4-yl)porphyrin. In contrast, inactivation of HAV to 3.6 log with the anionic symmetric porphyrin meso-tetrakis(4-sulfonatephenyl)porphyrin required 90 min [44]. The rate and extent of inactivation appeared to vary with the nature of the meso substituent groups [44]. HIV and VSV lost infectivity upon illumination with hypericin and rose bengal in a concentration-dependent manner [30].
6.1.2. Bacteriophage PDI
MS2 phage inactivation has been observed with neutral porphyrin derivatives. However, this required higher irradiation periods (30 min) than for the cationic ones (1 min) [44]. Neutral glycosylated substituted porphyrins can also significantly photoinactivate the T7 phage [64,87]. The T4-like phage PDI was achieved by exposing the phage in the presence of six cationic porphyrins at different concentrations (0.5, 1.0 and 5.0 μM) to white light for 270 min. The results showed that phage photoinactivation varied according with the PS concentration, with higher concentrations being the most efficient ones [66]. The T4-like phage PDI also varied with the number of porphyrin charges, with tri- and tetracationic porphyrin derivatives being more effective in viral inactivation that the dicationic ones, which inactivated the phage below the limit of detection. Tetra- and tricationic porphyrin derivatives (meso-tetrakis(1-methylpyridinium-4-yl)porphyrin and 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin, respectively) lead to complete T4-like phage inactivation (~7 log) after 270 min of irradiation with 40 W m−2 [66]. This tetracationic porphyrin showed similar results in another study (7 log of reduction) for lambda phage inactivation, when irradiated with light of 658 nm [58]. Increasing porphyrin concentration at a fixed light dose leads to increased viral inactivation [58]. A concentration-dependent effect was also detected with a porphyrin derivative [87], but over 2.0 μM of PS the process was saturated. A further increase in porphyrin concentration did not lead to a higher inactivation rate of T7 phage. Aggregation and/or photobleaching of PS are likely explanations [87]. Cationic meso-tetrakis(1-alkylpyridinium-4-yl)porphyrin derivatives with different alkyl substituent groups were tested for MS2 phage inactivation but, with the exception of 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin, showed toxicity even in the absence of light [44].
In a study conducted by Gábor et al. [64], the porphyrin derivative with symmetrical glycosylated groups was found to be twice as effective as the asymmetrical one on the inactivation process of T7 phage. According to Costa and colleagues [66], the rate of T4-like phage inactivation was also dependent on the lipophilic character of the meso-substituent groups. The presence of a lipophilic aryl group in one of the meso positions of the porphyrin core appears to have an important role in phage inactivation, affecting the rate and efficiency of T4-like phage [66]. Casteel et al. [44] have also observed differences in the photoinactivation rate of MS2 phage when they used PS with different alkyl substituent groups and concluded that the rate and extent of inactivation appeared to vary with the nature of the meso substituent groups.
6.2. Effect of Different Light Sources and Fluence Rate on Antimicrobial PDT
PDT requires a source of light to activate the PS by exposing it to visible or near-visible light at a specific wavelength [152]. The light source for PDT must also exhibit suitable spectral characteristics coinciding preferentially with the maximum absorption wavelength range of the PS, applied in order to generate enough ROS to produce an efficient toxic effect [153].
In parallel with the advances in chemistry (related with the discovery and synthesis of new and more efficient PS) there has also been much activity in developing new light sources, better suited for the photosensitization process. Briefly, these include user-friendly lasers frequently based on solid state laser diodes, as well as inexpensive light emitting diodes (LED) and filtered broad-band lamps [154].
PS activation has been achieved via a variety of light sources, such as arc plasma discharge lamps, metal halogen lamps, slide projector illumination assemblies, and a variety of lasers. For treatment of larger areas, non-coherent light sources, such as tungsten filament, quartz halogen, xenon arc, metal halide, and phosphor-coated sodium lamps, are in use. Recently, non-laser light sources, such as LED, have also been applied in PDT. These light sources are much less expensive and small, lightweight and highly flexible, its lifetime can reach up to one hundred thousands hours, and can be manufactured to wavelengths that activate commercially available PS [152,155,156,157,158,159].
At first glance, the available literature on fluence rate effects for PDT seems contradictory. Some studies indicate less damage at low fluence rate, others indicate more killing at lower, compared to higher, fluence rates for the same total fluence and some indicate no influence of fluence rate at all [152,157,158]. A reduction in the fluence rate lowers the rate of oxygen consumption, thereby extending the radius over which singlet oxygen may be formed and consequently increasing the phototoxic effect [159]. Qin et al. [160] showed that an increase in the fluence rate increases microbial damage, although, it seems to have an upper limit of photons to observe this effect. Since each PS molecule can only absorb one photon at a time, when the number of light photons bypasses the number of PS molecules, the PS will no longer be able to absorb the photons “in excess” and the rate of PDI will not increase. In fact, if the number of photons is higher than this limit, the antimicrobial effect will decrease because the dye in suspension will not absorb all the excess light [160]. Schindl et al. [161] referred that the biological effect of light depends on the fluence, irrespective of the time over which this dose is delivered. Maclean et al. [162] also indicate that the inactivating light may be applied at high irradiance over a short time or at lower irradiance over a longer time. A numerical model, assuming that the rate of photodynamic damage occurring at time t is proportional to the fluence rate at that time and the local concentrations of PS and oxygen can be established. However, according to this model, relatively low fluence rates can be nearly as effective as high fluence rate sources if applied over the same period of time [163].
There is also a direct correlation between the phototoxic effect and the PS concentration and light fluence. With a lowering of the PS concentration, more light has to be applied to achieve identical effects, and vice versa. Lower doses of PS require higher activating light fluences, and higher fluence requires a longer duration of light application [96].
6.2.1. Effect of Light on Mammalian Viruses PDI
The effects of dengue virus inactivation were increased with the increase of MB concentration, the enhancement of power density of the light source and the extension of illumination time, as well as the decrease of illumination distance. This enabled the narrow bandwidth light system to kill or inactivate the enveloped virus at much greater distance in much shorter time [74]. VSV in the presence of MB was rapidly inactivated by red (provided by LED incident light at 272 W cm−2) or green-yellow light (provided by low-pressure sodium lamps at a fluence rate of 165 W cm−2) but slower by white light (provided by a bank of fluorescent tubes at a fluence rate of 42 W cm−2) [46], showing that higher power densities produce a high rate of viral inactivation than low fluence rates. Wagner et al. [164] also showed that red light of 9 W m−2, given at a total dose of 1.8 × 104 and 3.2 × 104 J m-2, inactivated MB-treated VSV by 6 and ≥7 log, respectively. VSV inactivation was linearly dependent on the fluence rate of red light illumination [165].
6.2.2. Effect of Light on Bacteriophage PDI
In terms of what is known about phage PDI, only one study focusing on the effect of different light sources and power densities [67] exists. In this study, cationic porphyrin derivatives (meso-tetrakis(1-methylpyridinium-4-yl)porphyrin and 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin), when irradiated with different sources of light (fluorescent PAR lamps, sun light and halogen lamp) with fluence rates ranging from 40 W m−2 to 1690 W m−2, efficiently photoinactivated non-enveloped phages. All light sources tested lead to reductions of about 7 log for the somatic T4-like phage. However, the rate and the extent of inactivation were dependent on the light source, namely when low fluence rates were used (40 W m−2) and on the energy dose, being considerably more effective when light was delivered at a lower fluence rate. However, depending on the light source used, different irradiation periods were required to inactivate T4-like phage to the limits of detection. The results also showed that the efficacy of T4-like phage inactivation, using the same fluence rate, was dependent on the light source used, in particular when the light is delivered at a low fluence rate. M13 phage was phototreated with 5.0 μM MB and was inactivated in an irradiation dose-dependent manner [52]. Kastury and Platz [58] showed that increasing the concentration of a PS at a fixed light dose leads to increased viral inactivation as does an increase in the total light exposure at a fixed PS concentration. The inactivation rate of T1 bacteriophage increased with increasing fluence rate, indicating that the distance of the sample from the light source is a variable which must be controlled [73]. At higher PS concentrations, the inactivation rate reaches a maximum and then decreases, because the filtering effect of the dye decreases the effective fluence rate [73]. In a simple model purposed by Lee et al. [56], the phage survival ratio can also be considered as a decreasing exponential fraction of the light fluence (assuming that the fluence is uniform throughout the system).
7. Conclusion
The efficiency of different types of PS in viral PDI has been proved for different types of mammalian viruses and bacteriophages, whether they are enveloped or non-enveloped, for either DNA or RNA viruses. Even though enveloped viruses are more easily inactivated than non-enveloped ones, several studies confirm that non-enveloped mammalian viruses and phages can be efficiently inactivated by PDI. The type of viral nucleic acid has not been described as an important factor affecting viral photoinactivation but, as far as it is known, no studies specifically focus on the photoinactivation behaviour of DNA and RNA viruses. However, RNA phage MS2 was highly susceptible to photoinactivation when compared with DNA phages under the same conditions of photosensitization.
The type of mechanisms involved in the process of viral photosensitization was already elucidated and singlet oxygen and free radical species were identified as important contributors for an effective viral PDI. However, the contribution of singlet oxygen seems to be more pronounced in mammalian viruses and bacteriophage PDI. There are, however, few studies simultaneously comparing the contribution of both types of mechanisms (type I and type II) involved in viral PDI. The primary targets for the photoinactivation of viruses, whether treating mammalian viruses or phages, are the outer structures. Although there are several studies about the specific effects of PDI on viral proteins, for different types of mammalian viruses and phages, there are no studies concerning the specific effects of PDI on viral lipids. However, it has been clearly shown that enveloped viruses are more easily inactivated than their non-enveloped counterparts, which imply that the lipids present on viral envelopes are important targets of viral PDI.
PS are effective in inactivating the phages to the limits of detection in a way that they do not recover viability, avoiding the development of viral resistance. Nothing is known yet for the particular case of mammalian viruses but, as the viral targets are the same for mammalian viruses and phages, it is also expected that no resistance will be developed in the case of mammalian viruses. Besides that, antiviral PDI is equally effective whether the mammalian virus is sensitive or resistant to conventional antiviral agents. Taking into account all these advantages, PDI for viral inactivation can be regarded as a promising alternative therapy to conventional antiviral treatments, namely for the disinfection of blood and blood products, preventing viral contamination and for the treatment of wound and burn infections. Viral PDI has a fast mode of action and has also the additional benefits of being more economical and an environmental friendly technology, which might be successfully used also in the environmental field for wastewater, drinking water and fish-farming water disinfection.
Different PS concentrations and different light sources and fluence rates were tested, showing that they are important PDI parameters that must inevitably be taken into account when a viral photosensitization protocol has to be elaborated. The inactivation of mammalian viruses and phages can be attained at micromolar-level PS concentrations and different light sources are equally effective, depending on the final dose at which the viruses are exposed to. Besides that, PS can also be modulated by the addition of different meso substituent groups and positive charges in order to facilitate their interactions with the viruses, making them more efficient for mammalian viruses and phage PDI.
The similarity of the results obtained for mammalian viruses and bacteriophages show that they exhibit a similar behaviour when submitted to viral photoinactivation techniques: (i) the PS used for viral PDI were equally effective in the photoinactivation of mammalian viruses and bacteriophages; (ii) the mechanism of mammalian viruses and bacteriophage photosensitization involves the production of singlet oxygen (type II mechanism) with a slight contribution of free radical species (type I mechanism); (iii) singlet oxygen and free radicals were shown to affect viral nucleic acids and also the proteins and lipids present in the mammalian viruses and bacteriophage outer surfaces, with the latter being considerably more affected by PDI; and (iv) the rate and extent of mammalian viruses and phage PDI is also affected by the same factors, like the PS concentration and number of positive charges, the nature and position of meso substituent groups, the fluence rate and energy dose. Consequently, it is important to persist in the development of more PDI phage studies to clarify some aspects of viral PDI, such as influence of viral nucleic acid type (DNA or RNA) in the photoinactivation efficiency and the possibility of viral resistance development and viability recovery after photosensitization. It will also be important to study the synergistic effect between viral PDI and antiviral classical methodologies using bacteriophages as models of mammalian virus’ photoinactivation.
Acknowledgments
Thanks are due to the University of Aveiro, Fundação para a Ciência e a Tecnologia (FCT) and FEDER for funding the QOPNA unit (project PEst-C/QUI/UI0062/2011) and to Centre for Environmental and Marine Studies (CESAM) for funding the Microbiology Research Group. Liliana Costa is also grateful to FCT for her grant (SFRH/BD/39906/2007).
Conflict of Interest
The authors declare no conflict of interest.
References and Notes
- Jori, G.; Brown, S.B. Photosensitized inactivation of microorganisms. Photochem. Photobio. Sci. 2004, 3, 403–405. [Google Scholar] [CrossRef]
- van Der Poel, W.H.; Vinjé, J.; van Der Heide, R.; Herrera, M.I.; Vivo, A.; Koopmans, M.P. Norwalk-like calicivirus genes in farm animals. Emerg. Infect. Dis. 2000, 6, 36–41. [Google Scholar]
- Blerkom, L.V.L. Role of viruses in human evolution. Yearbk. Phys. Anthropol. 2009, 46, 14–46. [Google Scholar]
- Pulitzer, M.P.; Amin, B.D.; Busam, K.J. Merkel cell carcinoma: Review. Adv. Anat. Pathol. 2009, 16, 135–44. [Google Scholar] [CrossRef]
- Sullivan, V.; Biron, K.K.; Talarico, C.; Stanat, S.C.; Davis, M.; Pozzi, L.M.; Coen, D.M. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob. Agents Ch. 1993, 37, 19–25. [Google Scholar]
- Smee, D.F.; Barnett, B.B.; Sidwell, R.W.; Reist, E.J.; Holy, A. Antiviral activities of nucleosides and nucleotides against wild-type and drug-resistant strains of murine cytomegalovirus. Antivir. Res. 1995, 26, 1–9. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Whitley, R.J. Antiviral resistance: Mechanisms, clinical significance, and future implications. J. Antimicrob. Chemother. 1996, 37, 403–421. [Google Scholar] [CrossRef]
- Jabs, D.A.; Enger, C.; Forman, M.; Dunn, J.P. for The cytomegalovirus retinitis and viral resistance study group. Incidence of foscarnet resistance and cidofovir resistance in patients treated for cytomegalovirus retinitis. Antimicrob. Agents Chemother. 1998, 42, 2240–2244. [Google Scholar]
- Pillay, D.; Zambon, M. Antiviral drug resistance. Br. Med. J. 1998, 317, 660–662. [Google Scholar] [CrossRef]
- Smee, D.F.; Sidwell, R.W.; Kefauver, D.; Bray, M.; Huggins, J.W. Characterization of wild-type and cidofovir-resistant strains of camelpox, cowpox, monkeypox, and vaccinia viruses. Antimicrob. Agents Ch. 2002, 46, 1329–1335. [Google Scholar] [CrossRef]
- Schultz, E.W.; Krueger, A.P. Inactivation of Staphylococcus bacteriophage by methylene blue. P. Soc. Exp. Biol. Med. 1928, 26, 100–101. [Google Scholar]
- Perdrau, J.R.; Todd, C. The photodynamic action of methylene blue on certain viruses. Proc. Roy. Soc. Lond. B Biol. Sci. 1933, 112, 288–298. [Google Scholar] [CrossRef]
- Käsermann, F.; Kempf, C. Buckminsterfullerene and photodynamic inactivation of viruses. Rev. Med. Virol. 1998, 8, 143–151. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photoch. Photobio. Sci. 2004, 5, 436–450. [Google Scholar] [CrossRef]
- Felber, T.D.; Smith, E.B.; Knox, J.M.; Wallis, C.; Melnick, J.L. Photodynamic inactivation of herpes simplex: Report of a clinical trial. J. Am. Med. Assoc. 1973, 92, 223–289. [Google Scholar]
- Almeida, A.; Cunha, A.; Faustino, M.A.F.; Tomé, A.C.; Neves, M.G.P.M.S. Porphyrins as antimicrobial photosensitizing agents. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 83–160. [Google Scholar]
- Mullooly, V.M.; Abramson, A.L.; Shikowitz, M.J. Dihemato-porphyrin ether-induced photosensitivity in laryngeal papilloma patients. Laser. Surg. Med. 1990, 10, 349–356. [Google Scholar] [CrossRef]
- Karrer, S.; Szeimies, R.M.; Abels, C.; Wlotzke, U.; Stolz, W.; Landthaler, M. Epidermodysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br. J. Dermatol. 1999, 140, 935–938. [Google Scholar] [CrossRef]
- Lavie, G.; Mazur, Y.; Lavie, D.; Meruelo, D. The chemical and biological properties of hypericin—A compound with a broad spectrum of biological activities. Med. Res. Rev. 1995, 15, 111–119. [Google Scholar] [CrossRef]
- Smetana, Z.; Malik, Z.; Orenstein, A.; Mendelson, E.; Ben-Hur, E. Treatment of viral infections with 5-aminolevulinic acid and light. Laser. Surg. Med. 1997, 21, 351–358. [Google Scholar] [CrossRef]
- Sloand, E.M.; Pitt, E.; Klein, H.G. Safety of the blood supply. J. Am. Med. Assoc. 1995, 274, 1368–1373. [Google Scholar]
- Mannucci, P.M. Outbreak of hepatitis A among Italian patients with haemophilia. Lancet 1992, 339, 819. [Google Scholar] [CrossRef]
- Klein, H.G. Oxygen carriers and transfusion medicine. Artif. Cell. Blood Substit. Biotechnol. 1994, 22, 123–135. [Google Scholar] [CrossRef]
- Azzi, A.; Fanci, R.; Ciappi, S.; Zakrzewska, K.; Bosi, A. Human parvovirus B19 infection in bone marrow transplantation patients. Am. J. Hematol. 1993, 44, 207–209. [Google Scholar] [CrossRef]
- Asanaka, M.; Kurimura, T.; Toya, H.; Ogaki, J. Anti-HIV activity of protoporphyrin. AIDS 1989, 3, 403–404. [Google Scholar] [CrossRef]
- Dixon, D.W.; Marzilli, L.G.; Schinazi, R.F. Porphyrins as agents against the human immunodeficiency virus. Ann. N. Y. Acad. Sci. 1990, 616, 511–513. [Google Scholar] [CrossRef]
- Lambrecht, B.; Mohr, H.; Knuver-Hopf, J.; Schmitt, H. Photoinactivation of viruses in human fresh plasma by phenothiazine dyes in combination with visible light. Vox Sang. 1991, 60, 207–213. [Google Scholar] [CrossRef]
- Levere, R.D.; Gong, Y.F.; Kappas, A.; Bucher, D.J.; Wormser, G.; Abraham, N.G. Heme inhibits human immunodeficiency virus 1 replication in cell cultures and enhances the antiviral effect of zidovudine. Proc. Natl. Acad. Sci. U. S. A. 1991, 88, 1756–1759. [Google Scholar]
- Matthews, J.L.; Sogandares-Bernal, F.; Judy, M.; Gulliya, K.; Newman, J.; Chanh, T.; Marengo-Rowe, A.J. Inactivation of viruses with photoactive compounds. Blood Cell. 1992, 18, 75–88. [Google Scholar]
- Lenard, J.; Rabson, A.; Vanderoef, R. Photodynamic inactivation of infectivity of humam immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: Inhibition of fusion and syncytia formation. Proc. Natl. Acad. Sci. U. S. A. 1993, 90, 158–162. [Google Scholar]
- Neurath, A.R.; Strick, N.; Jiang, S. Rapid prescreening for antiviral agents against HIV-1 based on their inhibitory activity in site-directed immunoassays—Approaches applicable to epidemic HIV-1 strains. Antivir. Chem. Chemother. 1993, 4, 207–214. [Google Scholar]
- Debnath, A.K.; Jiang, S.; Strick, N.; Lin, K.; Haberfield, P.; Neurath, A.R. 3-Dimensional structure-activity analysis of a series of porphyrin derivatives withanti-HIV-1 activity targeted to the v3 loop of the gp120 envelope glycoprotein of the human-immunodeficiency-virus type 1. J. Med. Chem. 1994, 37, 1099–1108. [Google Scholar] [CrossRef]
- North, J.; Coombs, R.; Levy, J. Photodynamic inactivation of free and cell-associated HIV-1 using the photosensitizer, benzoporphyrin derivative. J. Acquir. Immune Defic. Syndr. 1994, 7, 891–898. [Google Scholar]
- Bachmann, B.K-H.J.B.; Lambrecht, B.; Mohr, H. Target structures for HIV-1 inactivation by methylene blue and light. J. Med. Virol. 1995, 47, 172–178. [Google Scholar] [CrossRef]
- Song, R.; Witvrouw, M.; Schols, D.; Robert, A.; Balzarini, J.; De Clercq, E.; Bernadou, J.; Meunier, B. Anti-HIV activities of anionic metalloporphyrins and related compounds. Antivir. Chem. Chemother. 1997, 8, 85–97. [Google Scholar]
- Vzorov, A.N.; Dixon, D.W.; Trommel, J.S.; Marzilli, L.G.; Compans, R.W. Inactivation of human immunodeficiency virus type 1 by porphyrins. Antimicrob. Agents Ch. 2002, 46, 3917–3925. [Google Scholar] [CrossRef]
- Vanyur, R.; Heberger, K.; Jakus, J. Prediction of anti-HIV-1 activity of a series of tetrapyrrole molecules. J. Chem. Inform. Comput. Sci. 2003, 43, 1829–1836. [Google Scholar] [CrossRef]
- Dairou, J.; Vever-Bizet, C.; D. Brault, D. Interaction of sulfonated anionic porphyrins with HIV glycoprotein gp120: photodamages revealed by inhibition of antibody binding to V3 and C5 domains. Antivir. Res. 2004, 61, 37–47. [Google Scholar]
- Marchesan, S.; Da Ros, T.; Spalluto, G.; Balzarini, J.; Prato, M. Anti-HIV properties of cationic fullerene derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 3615–3618. [Google Scholar]
- North, J.; Freeman, S.; Overbaugh, J.; Levy, J.; Lansman, R. Photodynamic inactivation of retrovirus by benzoporphyrin derivative: A feline leukemia virus model. Transfusion 1992, 32, 121–128. [Google Scholar]
- Müller-Breitkreutz, K.; Mohr, H. Hepatitis C and human immunodeficiency virus RNA degradation by methylene blue/light treatment of human plasma. J. Med. Virol. 1998, 56, 239–245. [Google Scholar] [CrossRef]
- Cheng, Y.; Tsou, L.K.; Cai, J.; Aya, T.; Dutschman, G.E.; Gullen, E.A.; Grill, S.P.; Chen, A.P-C.; Lindenbach, B.D.; Hamilton, A.D.; Cheng, Y-C. A novel class of meso-tetrakis-porphyrin derivatives exhibits potent activities against hepatitis C virus genotype 1b replicons in vitro. Antimicrob. Agents Ch. 2010, 54, 197–206. [Google Scholar]
- Lin, L.; Hu, J. Inhibition of hepadnavirus reverse transcriptase RNA interaction by porphyrin compounds. J. Virol. 2008, 82, 2305–2312. [Google Scholar] [CrossRef]
- Casteel, B.M.J.; Jayaraj, K.; Avram, G.; Bail, L.M.; Sobsey, M.D. Photoinactivation of hepatitis A virus by synthetic porphyrins. Photochem. Photobiol. 2004, 80, 294–300. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Gaffney, D.K.; Wang, T.P.; Sieber, F. Merocyanine 540 sensitized photoinactivation of enveloped viruses in blood products: Site and mechanism of phototoxicity. Blood 1992, 80, 277–285. [Google Scholar]
- Mohr, H.; Bachmann, B.; Klein-Struckmeier, A.; Lambrecht, B. Virus inactivation of blood products by phenothiazine dyes and light. Photochem. Photobiol. 1997, 65, 441–445. [Google Scholar] [CrossRef]
- Sieber, F.; O’Brien, J.M.; Krueger, G.J.; Schober, S.L.; Burns, W.H.; Sharkis, S.J.; Sensenbrenner, L.L. Antiviral activity of merocyanine 540. Photochem. Photobiol. 1987, 46, 707–711. [Google Scholar] [CrossRef]
- Leclerc, H.; Edberg, S.; Pierzo, V.; Delattre, J.M. Bacteriophages as indicators of enteric viruses and public health risk in groundwaters. A review. J. Appl. Microbiol. 2000, 88, 5–21. [Google Scholar]
- Rywkin, S.; Ben-Hur, E.; Malik, Z.; Prince, A.M.; Li, Y.S.; Kenney, M.E.; Oleinick, N.L.; Horowitz, B. New phthalocynanines for photodynamic virus inactivation in red blood cell concentrates. Photochem. Photobiol. 1994, 60, 165–170. [Google Scholar] [CrossRef]
- Käsermann, F.; Kempf, C. Photodynamic inactivation of enveloped viruses by buckminsterfullerene. Antivir. Res. 1997, 34, 65–70. [Google Scholar]
- DiMascio, P.; Wefers, H.; Do-Thi, H-P.; Lafleur, M.V.M.; Sies, H. Singlet molecular oxygen causes loss of biological activity in plasmid and bacteriophage DNA and induces single strand breaks. Biochim. Biophys. Acta 1989, 1007, 151–157. [Google Scholar]
- Abe, H.; Ikebuchi, K.; Wagner, S.J.; Kuwabara, M.; Kamo, N.; Sekiguchi, S. Potential involvement of both type I and type II mechanisms in M13 virus inactivation by methylene blue photosensitization. Photochem. Photobiol. 1997, 66, 204–208. [Google Scholar] [CrossRef]
- Specht, K.G. The role of DNA damage in PM2 viral inactivation by methylene blue photosensitization. Photochem. Photobiol. 1994, 59, 506–514. [Google Scholar] [CrossRef]
- Schneider, J.E.; Philips, J.R.; Pye, Q.; Maidt, M.L.; Price, S.; Floyd, R.A. Methylene blue and rose bengal photoinactivation of RNA bacteriophages: Comparative studies of 8-oxoguanine formation in isolated RNA. Arch. Biochem. Biophys. 1993, 301, 91–97. [Google Scholar] [CrossRef]
- Jockush, S.; Lee, D.; Turro, N.J.; Leonard, E.F. Photoinduced inactivation of viruses: Adsorption of methylene blue, thionine and thiopyronine on Qβ bacteriophage. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 7446–7451. [Google Scholar]
- Lee, D.; Foux, M.; Leonard, E.F. The effects of methylene blue and oxygen concentration on the photoinactivation of Qβ bacteriophage. Photochem. Photobiol. 1997, 65, 161–165. [Google Scholar] [CrossRef]
- Hotze, E.M.; Badireddy, A.R.; Chellam, S.; Wiesner, M.R. Mechanisms of bacteriophage inactivation via singlet oxygen generation in UV illuminated fullerol suspensions. Environ. Sci. Tech. 2009, 43, 6639–6645. [Google Scholar] [CrossRef]
- Kasturi, C.; Platz, M.S. Inactivation of lambda phage with 658 nm light using a DNA binding porphyrin sensitizer. Photochem. Photobiol. 1992, 56, 427–429. [Google Scholar] [CrossRef]
- Martin, C.B.; Wilfong, E.; Ruane, P.; Goodrich, R.; Platz, M. An action spectrum of the riboflavin-photosensitized inactivation of lambda phage. Photochem. Photobiol. 2005, 81, 474–480. [Google Scholar] [CrossRef]
- Wagner, S.J.; Skripchenkol, A.; Robinenel, D.; Foley, J.W.; Cincotta, L. Factors affecting virus photoinactivation by a series of phenothiazine dyes. Photochem. Photobiol. 1998, 67, 343–349. [Google Scholar] [CrossRef]
- Brendel, M. Different photodynamic action of proflavine and methylene blue on bacteriophage. I. Host cell reactivation of Serratiaphage kappa. Mol. Gen. Genet. 1970, 108, 303–311. [Google Scholar] [CrossRef]
- Yamamoto, N. Photodynamic inactivation of bacteriophage and its inhibition. J. Bacteriol. 1957, 6, 510–521. [Google Scholar]
- Witmer, H.; Fraser, D. Photodynamic action of proflavine on coliphage T3 II. Protection by L-cysteine. J. Virol. 1971, 7, 319–322. [Google Scholar]
- Gábor, F.; Szolnoki, J.; Tóth, K.; Fekete, A.; Maillard, P.; Csík, G. Photoinduced inactivation of T7 phage sensitized by symmetrically and asymmetrically substituted tetraphenyl porphyrin: comparison of efficiency and mechanism of action. Photochem. Photobiol. 2001, 73, 304–311. [Google Scholar] [CrossRef]
- Kadish, L.L.; Fisher, D.B.; Pardee, A.B. Photodynamic inactivation of free and vegetative bacteriophage T4. Biochim. Biophys. Acta 1967, 138, 57–65. [Google Scholar]
- Costa, L.; Alves, E.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Almeida, A. Sewage bacteriophage photoinactivation by cationic porphyrins: a study of charge effect. Photochem. Photobio. Sci. 2008, 7, 415–422. [Google Scholar]
- Costa, L.; Carvalho, C.M.B.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Sewage bacteriophage inactivation by cationic porphyrins: influence of light parameters. Photochem. Photobio. Sci. 2010, 9, 1126–1133. [Google Scholar]
- Costa, L.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Evaluation of resistance development and viability recovery by a non-enveloped virus after repeated cycles of aPDT. Antivir. Res. 2011, 91, 278–282. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar]
- Capella, M.A.M.; Capella, L.S. A light in multidrug resistance: Photodynamic treatment of multidrug-resistant tumors. J. Biomed. Sci. 2003, 10, 361–366. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Prates, R.A.; da Silva, E.G.; Yomada, A.M., Jr.; Suzuki, L.C.; Paula, C.R.; Ribeiro, M.S. Light parameters influence cell viability in antifungal photodynamic therapy in a fluence and rate fluence dependent manner. Laser Phys. 2009, 19, 1038–1044. [Google Scholar]
- Welsh, J.N.; Adams, M.H. Photodynamic inactivation of bacteriophage. J. Bacteriol. 1954, 1, 122–127. [Google Scholar]
- Huang, Q.; Fu, W-L.; Chen, B.; Huang, J-F.; Zhang, X.; Xue, Q. Inactivation of dengue virus by methylene blue/narrow bandwidth light system. J. Photochem. Photobiol. B Biol. 2004, 77, 39–43. [Google Scholar]
- Schnipper, L.E.; Lewin, A.A.; Swartz, M.; Crumpacker, C.S. Mechanisms of photodynamic inactivation of herpes simplex viruses; comparison between methylene blue, light plus electricity, and hematoporphyrin plus light. J. Clin. Investig. 1980, 65, 432–438. [Google Scholar] [CrossRef]
- Schagen, F.H.E.; Moor, A.C.E.; Cheong, S.C.; Cramer, S.J.; van Ormondt, H.; van der Eb, A.J.; Dubbelman, T.M.A.R.; Hoeben, R.C. Photodynamic treatment of adenoviral vectors with visible light: An easy and convenient method for viral inactivation. Gene Ther. 1999, 6, 873–881. [Google Scholar]
- Lim, D-S.; Ko, S-H.; Kim, S-J.; Park, Y-J.; Park, J-H.; Lee, W-Y. Photoinactivation of vesicular stomatitis virus by a photodynamic agent, chlorophyll derivatives from silkworm excreta. J. Photochem. Photobiol. B Biol. 2002, 67, 149–156. [Google Scholar] [CrossRef]
- Sagristá, M.L.; Postigo, F.; De Madariaga, M.A.; Pinto, R.M.; Caballero, S.; Bosch, A.; Vallés, M.A.; Mora, M. Photodynamic inactivation of viruses by immobilized chlorine-containing liposomes. J. Porphyrin Phthalocyanines. 2009, 13, 578–588. [Google Scholar] [CrossRef]
- Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Mendonça, A.F.; Pegado, I.N.; Duarte, R.; Valdeira, M.L. Synthesis of glycoporphyrin derivatives and their antiviral activity against herpes simplex virus types 1 and 2. Bioorg. Med. Chem. 2005, 13, 3878–3888. [Google Scholar]
- Silva, E.M.P.; Giuntini, F.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.S.; Santana-Marques, M.G.; Ferrer-Correia, A.J.; Cavaleiro, J.A.S.; et al. Synthesis of cationic β-vinyl substituted meso-tetraphenylporphyrins and their in vitro activity against herpes simplex virus type 1. Bioorg. Med. Chem. Lett. 2005, 15, 3333–3337. [Google Scholar]
- Abe, H.; Wagner, S.J. Analysis of viral DNA, protein and envelope damage after methylene blue, phthalocyanine derivative or merocyanine 540 photosensitization. Photochem. Photobiol. 1995, 61, 402–409. [Google Scholar] [CrossRef]
- Moor, A.C.E.; Wagenaars-van Gompel, A.E.; Brand, A.; Dubbelman, T.M.A.R.; Van Steveninck, J. Primary targets for photoinactivation of vesicular stomatitis virus by AlPcS4 or Pc4 and red light. Photochem. Photobiol. 1997, 65, 465–470. [Google Scholar] [CrossRef]
- Smetana, Z.; Ben-Hur, E.; Mendelson, E.; Salzberg, S.; Wagner, P.; Malik, Z. Herpes simplex virus proteins are damaged following photodynamic inactivation with phthalocyanines. J. Photochem. Photobiol. B Biol. 1998, 44, 77–83. [Google Scholar] [CrossRef]
- Müller-Breitkreutz, K.; Mohr, H.; Briviba, K.; Sies, H. Inactivation of viruses by chemically and photochemically generated singlet molecular oxygen. J. Photochem. Photobiol. B Biol. 1995, 30, 63–70. [Google Scholar] [CrossRef]
- Wong, T-W.; Huang, H-J.; Wang, Y-F. ; Lee, Y-P. ; Huang, C-C. ; Yu, C-K. Methylene blue-mediated photodynamic inactivation as a novel disinfectant of enterovirus 71. J. Antimicrob. Chemother. 2010, 65, 2176–2182. [Google Scholar] [CrossRef]
- Turner, G.S.; Kaplan, C. Photoinactivation of vaccinia virus with rose bengal. J. Gen. Virol. 1968, 3, 433–443. [Google Scholar] [CrossRef]
- Egyeki, M.; Turóczy, G.; Majer, Zs.; Tóth, K.; Fekete, A.; Maillard, Ph.; Csík, G. Photosensitized inactivation of T7 phage as surrogate of non-enveloped DNA viruses: Efficiency and mechanism of action. Biochim. Biophys. Acta 2003, 1624, 115–124. [Google Scholar] [CrossRef]
- Zupán, K.; Egyeki, M.; Tóth, K.; Fekete, A.; Herényi, L.; Módos, K.; Csík, G. Comparison of the efficiency and the specificity of DNA-bound and free cationic porphyrin in photodynamic virus inactivation. J. Photochem. Photobiol. B Biol. 2008, 90, 105–112. [Google Scholar] [CrossRef]
- Schneider, J.E., Jr.; Tabatabale, T.; Maidt, L.; Smith, R.H.; Nguyen, X.; Pye, Q.; Floyd, R.A. Potential mechanisms of photodynamic inactivation of virus by methylene blue I. RNA-protein crosslinks and other oxidative lesions in Qβ bacteriophage. Photochem. Photobiol. 1998, 67, 350–357. [Google Scholar] [CrossRef]
- Badireddy, A.R.; Hotze, E.M.; Chellam, S.; Alvarez, P.J.J.; Wiesner, M.R. Inactivation of bacteriophages via photosensitization of fullerol nanoparticles. Environ. Sci. Tech. 2007, 41, 6627–6632. [Google Scholar] [CrossRef]
- Marotti, J.; Aranha, A.C.C.; Eduardo, C.D.P.; Ribeiro, M.S. Photodynamic therapy can be effective as a treatment for herpes simplex labialis. Photomed. Laser Surg. 2009, 27, 357–363. [Google Scholar] [CrossRef]
- Floyd, R.A.; Schneider, J.E.; Dittmer, D.P. Methylene blue photoinactivation of RNA viruses. Antivir. Res. 2004, 61, 141–151. [Google Scholar]
- Wallis, C.; Melnick, J.L. Photodynamic inactivation of animal viruses: A review. Photochem. Photobiol. 1965, 4, 159–170. [Google Scholar] [CrossRef]
- Lytle, C.D.; Budacz, A.P.; Keville, E.; Miller, S.A.; Prodouz, K.N. Differential inactivation of surrogate viruses with merocyanine 540. Photochem. Photobiol. 1991, 54, 489–493. [Google Scholar] [CrossRef]
- Via, L.D.; Magno, S.M. Photochemotherapy in the treatment of cancer. Curr. Med. Chem. 2001, 8, 1405–1418. [Google Scholar]
- Schmidt-Erfurth, U.; Hasan, T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 195–214. [Google Scholar] [CrossRef]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Bonnett, R. Chemical Aspects of Photodynamic Therapy; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Girotti, A.W. Photosensitized oxidation of membrane lipids: Reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B Biol. 2001, 63, 103–113. [Google Scholar] [CrossRef]
- Calin, M.A.; Parasca, S.V. Light sources for photodynamic inactivation of bacteria. Laser Med. Sci. 2009, 24, 453–460. [Google Scholar] [CrossRef]
- Min, D.B.; Boff, J.M. Chemistry and reaction of singlet oxygen in foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef]
- Maisch, T.; Bosl, C.; Szeimies, R.M.; Lehn, N.; Abels, C. Photodynamic effects of novel XF porphyrin derivativeson prokaryotic and eukaryotic cells. Antimicrob. Agents Ch. 2005, 49, 1542–1552. [Google Scholar]
- Ochsner, M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J. Photochem. Photobiol. B Biol. 1997, 39, 1–18. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Identification of quenchers of photoexcited states as novel agents for skin photoprotection. J. Pharmacol. Exp. Therapeut. 2005, 312, 482–491. [Google Scholar]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar]
- Rywkin, S.; Lenny, L.; Goldstein, J.; Geacintov, N.E.; Margolis-Nunno, H.; Horowitz, B. Importance of type I and type II mechanisms in the photodynamic inactivation of viruses in blood with aluminum phthalocyanine derivatives. Photochem. Photobiol. 1992, 56, 463–469. [Google Scholar] [CrossRef]
- Costa, L.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Almeida, A. Involvement of type I and type II mechanisms on the photoinactivation of non-enveloped DNA and RNA bacteriophages. Environ. Sci. Tech. 2012. submitted for publication. [Google Scholar]
- Lenard, J.; Vanderoef, R. Photoinactivation of influenza virus fusion and infectivity by rose bengal. Photochem. Photobiol. 1993, 58, 527–531. [Google Scholar] [CrossRef]
- Bisby, R.H.; Morgan, C.G.; Hamblett, I.; Gorman, A.A. 1999. Quenching of singlet oxygen by trolox c, ascorbate, and amino acids: effects on pH and temperature. J. Phys. Chem. A 1999, 103, 7454–7459. [Google Scholar]
- Baker, A.; Kanofsky, J.R. Quenching of singlet oxygen bybiomolecules from Ll210 leukemia cells. Photochem. Photobiol. 1992, 55, 523–528. [Google Scholar] [CrossRef]
- Degar, S.; Prince, A.M.; Pascual, D.; Lavie, G.; Levin, B.; Mazur, Y.; Lavie, D.; Ehrlich, L.S.; Carter, C.; Meruelo, D. Inactivation of the human immunodeficiency virus by hypericin: Evidence for photochemical alterations of p24 and a block in uncoating. AIDS Res. Hum. Retrovir. 1992, 8, 1929–1936. [Google Scholar] [CrossRef]
- Wainwright, M. Local treatment of viral disease using photodynamic therapy. Int. J. Antimicrob. Agents 2003, 21, 510–520. [Google Scholar] [CrossRef]
- Garcia, G.; Sarrazy, V.; Sol, V.; Morvan, C.L.; Granet, R.; Alves, S.; Krausz, P. DNA photocleavage by porphyrin–polyamine conjugates. Bioorg. Med. Chem. 2009, 17, 767–776. [Google Scholar]
- Miranda, M.A. Photosensitization by drugs. Pure Appl. Chem. 2001, 73, 481–486. [Google Scholar] [CrossRef]
- Wainwright, M. The use of methylene blue derivatives in blood product disinfection. Int. J. Antimicrob. Agents 2000, 16, 381–394. [Google Scholar] [CrossRef]
- McBride, T.J.; Schneider, J.E.; Floyd, R.E.; Loeb, L.A. Mutations induced by methylene blue plus light in single stranded M13mp2. Proc. Natl. Acad. Sci. U. S. A. 1992, 89, 6866–6870. [Google Scholar]
- OhUigin, C.; McConnell, D.J.; Kelly, J.M.; van der Putten, W.J.M. Methylene blue photosensitised strand cleavage of DNA: Effects of dye binding and oxygen. Nucleic Acids Res. 1987, 15, 7411–7427. [Google Scholar] [CrossRef]
- Mettath, S.; Munson, B.R.; Pandey, R.K. DNA interaction and photocleavage properties of porphyrins containing cationic substituents at the peripheral position. Bioconjugate Chem. 1999, 10, 94–102. [Google Scholar] [CrossRef]
- Kubát, P.; Lang, K.; Anzenbacher, P., Jr.; Jursíkova, K.; Král, V.; Ehrenberg, B. Interaction of novel cationic meso-tetraphenylporphyrins in the ground and excited states with DNA and nucleotides. J. Chem. Soc., Perkin Trans. 1 2000, 1, 933–941. [Google Scholar]
- Caminos, D.A.; Durantini, E.N. Interaction and photodynamic activity of cationic porphyrin derivativesbearing different patterns of charge distribution with GMP and DNA. J. Photochem. Photobiol. A: Chem. 2008, 198, 274–281. [Google Scholar] [CrossRef]
- Müller-Breitkreutz, K.; Mohr, H. Infection cycle of herpes viruses after photodynamic treatment with methylene blue and light. Transfusions Medizin 1997, 34, 37–42. [Google Scholar]
- Schneider, J.E., Jr.; Pye, Q.; Floyd, R.A. Qβ bacteriophage photoinactivated by methylene blue plus light involves inactivation of its genomic RNA. Photochem. Photobiol. 1999, 70, 902–909. [Google Scholar] [CrossRef]
- Smetana, Z.; Mendelson, E.; Manor, J.; Van Lier, J.E.; Ben-Hur, E.; Salzberg, S.; Malik, Z. Photodynamic inactivation of herpes simplex viruses with phthalocyanine derivatives. J. Photochem. Photobiol. B: Biol. 1994, 22, 37–43. [Google Scholar] [CrossRef]
- Ben-Hur, E.; Horowitz, B. Virus inactivation in blood. AIDS 1996, 11, 1183–1190. [Google Scholar] [CrossRef]
- Girotti, A.W. Photodynamic action of protoporphyrin IX on human erythrocytes: Cross-linking of membrane proteins. Biochem. Biophys. Res. Comm. 1976, 72, 1367–1374. [Google Scholar] [CrossRef]
- Verweij, H.; van Steveninck, J. Model studies on photodynamic cross-linking. Photochem. Photobiol. 1982, 35, 265–267. [Google Scholar] [CrossRef]
- Macdonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyrin Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Comm. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Afonso, S.G.; Enriquez, S.R.; Batlle, C.A.M. The photodynamic and non photodynamic actions of porphyrins. Braz. J. Med. Biol. Res. 1999, 32, 255–266. [Google Scholar]
- Jori, G.; Galiazzo, G.; Tamburro, A.M.; Scoffone, E. Dye-sensitized photooxidation as a tool for determining the degree of exposure of amino acid residues in proteins. J. Biol. Chem. 1970, 245, 3375–3383. [Google Scholar]
- Malik, Z.; Ladan, H.; Nitzan, Y.; Smetana, Z. Antimicrobial and antiviral activity of porphyrin photosensitization. Proc. SPIE 1993, 2078, 305–312. [Google Scholar]
- Malik, Z.; Smetana, Z.; Mendelson, E.; Wagner, P.; Salzberg, S.; Ben-Hur, E. Alteration in herpes simplex virus proteins following photodynamic treatment with phthalocyanines. Photochem. Photobiol. 1996, 63, 59S. [Google Scholar]
- Yip, L.; Hudson, J.B.; Gruszecka-Kowalik, E.; Zalkow, L.H.; Neil Towers, G.H.N. Antiviral activity of a derivative of the photosensitive compound hypericin. Phytomedicine 1996, 2, 185–190. [Google Scholar]
- Sieber, F.; Krueger, G.J.; O’Brien, J.M.; Schober, S.L.; Sensenbrenner, L.L.; Sharkis, S.J. Inactivation of Friend erythroleukemia virus and Friend virus-transformed cells by merocyanine 540-mediated photosensitization. Blood 1989, 73, 345–350. [Google Scholar]
- Melki, R.; Gaudin, Y.; Blondel, D. Interaction between tubulin and the viral matrix protein of vesicular stomatitis virus: possible implications in the viral cytopathic effect. Virology 1994, 202, 339–347. [Google Scholar] [CrossRef]
- Zupán, K.; Herényi, L.; Tóth, K.; Majer, Z.; Csík, G. Binding of cationic porphyrin to isolated and encapsidated viral DNA analyzed by comprehensive spectroscopic methods. Biochemistry 2004, 43, 9151–9159. [Google Scholar]
- Yoshikawa, T.T. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J. Am. Geriatr. Soc. 2002, 50, S226–S229. [Google Scholar] [CrossRef]
- Malik, Z.; Gozhansky, S.; Nitzan, Y. Effects of photoactivated HPD on bacteria and antibiotic resistance. Microbios Lett. 1982, 21, 103–112. [Google Scholar]
- Maisch, T.; Szeimies, R-M.; Jori, G.; Abels, C. Antibacterial photodynamic therapy in dermatology. Photochem. Photobio. Sci. 2004, 3, 907–917. [Google Scholar] [CrossRef]
- Pillay, D. Emergence and control of resistance to antiviral drugs in resistance in herpes viruses, hepatitis B virus, and HIV. Commun. Dis. Public Health 1998, 1, 5–13. [Google Scholar]
- Reddi, E.; Ceccon, M.; Valduga, G.; Jori, G.; Bommer, J.C.; Elisei, F.; Latterini, L.; Mazzucato, U. Photophysical properties and antibacterial activity of meso-substituted cationic porphyrins. Photochem. Photobiol. 2002, 75, 462–470. [Google Scholar] [CrossRef]
- Jori, G.; Coppellotti, O. Inactivation of pathogenic microorganisms by photodynamic techniques: Mechanistic aspects and perspective applications. Anti-Infect. Agents Med. Chem. 2007, 6, 119–131. [Google Scholar]
- Minnock, A.; Vernon, D.I.; Schofield, J.; Griffiths, J.; Parish, J.H.; Brown, S.B. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob. Agents Ch. 2000, 44, 522–527. [Google Scholar] [CrossRef]
- Demidova, T.; Hamblin, M. Effects of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Ch. 2005, 6, 2329–2335. [Google Scholar] [CrossRef]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Laser. Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef]
- Wainwright, M. Photoantimicrobials—So what’s stopping us? Photodiagn. Photodyn. 2009, 6, 167–169. [Google Scholar] [CrossRef]
- Merchat, M.; Bertolini, G.; Giacomini, P.; Villanueva, A.; Jori, G. Meso-substituted cationic porphyrins as efficient photosensitizers of Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. 1996, 32, 153–157. [Google Scholar] [CrossRef]
- Dowd, S.E.; Pillai, S.D.; Wang, S.; Corapcioglu, M.Y. Delineating the specific influence of virus isoelectric point and size on virus adsorption and transport through sandy soils. Appl. Environ. Microbiol. 1998, 64, 405–410. [Google Scholar]
- Wainwright, W. Photoantimicrobials—A PACT against resistance and infection. Drugs Future 2004, 29, 85–93. [Google Scholar] [CrossRef]
- Kessel, D.; Raymund, L.; Vicente, M.G.H. Localization and photodynamic efficacy of two cationic porphyrins varying in charge distribution. Photochem. Photobiol. 2003, 78, 431–435. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Buccafurni, L.; Battini, V.; Zazzaron, S.; Barbieri, P.; Orlandi, V. Antibacterial activity of tetraaryl-porphyrin photosensitizers: An in vivo study on Gram negative and Gram positive bacteria. J. Photochem. Photobiol. B Biol. 2006, 85, 28–38. [Google Scholar] [CrossRef]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. Crit. Rev. Oral Biol. Med. 2007, 8, 694–707. [Google Scholar]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Brancaleon, L.; Moseley, H. Laser and non-laser light sources for photodynamic therapy. Laser Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef]
- Veenhuizen, R.B.; Stewart, F.A. The importance of fluence rate in photodynamic therapy: Is there a parallel with ionizing radiation dose-rate effects? Radiother. Oncol. 1995, 37, 131–135. [Google Scholar] [CrossRef]
- Allison, R.R.; Mota, H.C.; Sibata, C.H. Clinical PD/PDT in North America: an historical review. Photodiagn. Photodyn. 2004, 1, 263–277. [Google Scholar] [CrossRef]
- Juzeniene, A.; Juzena, P.; Ma, L-W.; Iani, V.; Moan, J. Effectiveness of different light sources for 5-aminolevulinic acid photodynamic therapy. Laser Med. Sci. 2004, 19, 139–149. [Google Scholar] [CrossRef]
- Kübler, A.C. Photodynamic therapy. Med. Laser Appl. 2005, 20, 37–45. [Google Scholar] [CrossRef]
- Lukšiene, Z. New approach to inactivation of harmful and pathogenic microorganisms by photosensitization. Food Tech. Biotechnol. 2005, 43, 411–418. [Google Scholar]
- Qin, Y.; Luan, X.; Bi, L.; He, G.; Bai, X.; Zhou, C.; Zhang, Z. Toluidine blue-mediated photoinactivation of periodontal pathogens from supragingival plaques. Laser Med. Sci. 2008, 23, 49–54. [Google Scholar] [CrossRef]
- Schindl, A.; Rosado-Sholosser, B.; Trautinger, F. Reciprocity regulation in photobiology: An overview (in German). Hautarzt 2001, 52, 779–785. [Google Scholar] [CrossRef]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G.A. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J. Photochem. Photobiol. B Biol. 2008, 92, 180–184. [Google Scholar] [CrossRef]
- Langmack, K.; Mehta, R.; Twyman, P.; Norris, P. Topical photodynamic therapy at low fluence rates—theory and practice. J. Photochem. Photobiol. B Biol. 2001, 60, 37–43. [Google Scholar] [CrossRef]
- Wagner, S.J.; Storry, J.R.; Mallory, D.A.; Stromberg, R.R.; Benade, L.E.; Friedman, L.I. Red cell alterations associated with virucidal methylene blue phototreatment. Transfusion 1993, 33, 30–36. [Google Scholar]
- Wagner, S.J. Virus inactivation in blood components by photoactive phenothiazine dyes. Transfus. Med. Rev. 2002, 16, 61–66. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).