Abstract
The initial step in retroviral infection involves specific interactions between viral envelope proteins (Env) and specific receptors on the surface of target cells. For many years, little was known about the entry receptors for HTLV-1. During this time, however, functional domains of the HTLV-1 Env were identified by analyzing the effects of neutralizing antibodies and specific mutations in Env on HTLV-1 infectivity. More recent studies have revealed that HTLV-1 infectivity involves interactions with three different molecules: heparan sulfate proteoglycans (HSPG), the VEGF-165 receptor Neuropilin 1 (NRP-1) and glucose transporter type 1 (GLUT1). Here, we revisit previously published data on the functional domains of Env in regard to the recent knowledge acquired about this multi-receptor complex. We also discuss the similarities and differences between HTLV-1 and other deltaretroviruses in regards to receptor usage.
1. Introduction
Like other enveloped viruses, retroviruses enter target cells by fusing their membrane with the membrane of target cells. This process is initiated by interaction of the surface subunit (SU) of the virally-encoded envelope glycoprotein (Env) with host cell receptors. Data from several independent groups have determined that three cell surface proteins are involved in HTLV-1 entry: glucose transporter 1 (GLUT1), neuropilin-1 (NRP-1) and heparan sulfate proteoglycans (HSPG). This knowledge provides a new perspective on HTLV-1 tropism and associated pathologies. This also raises many questions about the molecular events that occur during entry, in particular how the HTLV-1 SU interacts with each of the receptor molecules. The impact of the receptors on the selectivity and modulation of HTLV-1 infection in vivo has been recently reviewed in detail [1,2]. In this review, we reexamine what is known from earlier studies about the functional domains of Env in light of the recent insight into the receptor complex. We will also present recent data obtained with the other members of the HTLV family and discuss their implications in terms of receptor usage.
2. The Host Cell Actors: The HTLV-1 Receptors
Here, we will only briefly summarize the evidence that identified the HTLV-1 receptor molecules and led to the proposal of a multi-receptor model for HTLV-1 entry.
2.1. Identification of the Roles of GLUT1, NRP-1 and HSPGs in HTLV-1 Entry
The identification of GLUT1 started with the observation that expression of a fragment of the HTLV-1 SU in cells prevents medium acidification [3]. Since it is known for other retroviruses that SU interacts with their receptors when coexpressed in cells, the authors hypothesized that the HTLV-1 receptor might be related to proton-dependent lactate production. Investigation of different members of the glucose transporter family led to the observation that one of these, GLUT1, was indeed able to bind the SU and promote HTLV-1-Env mediated particle entry. The same study showed that the residues D106 and Y114 of the SU were involved in GLUT1 binding [3]. A subsequent study from another group demonstrated that GLUT1 is required for HTLV-1 infection of CD4+ T cells [4].
In parallel, the role in HTLV-1 entry of another protein, Neuropilin 1 (NRP-1), was investigated. NRP-1 is a cell surface protein known to function as a co-receptor for certain heparin-binding pro-angiogenic cytokines, principally members of the vascular endothelial growth factor (VEGF) family, and for class 3 semaphorins (reviewed in [5]). It was noticed that a number of features of NRP-1 paralleled characteristics of the HTLV-1 receptor, including a high degree of conservation among vertebrate species [6], the absence of a homolog in the Drosophila genome, overexpression in transformed cells [7] and upregulation upon T-cell activation [8]. It was subsequently demonstrated that NRP-1 binds HTLV-1 SU and is required for efficient HTLV-1 entry. The same study also showed that NRP-1, GLUT1 and the HTLV-1 SU form a stable tripartite complex when coexpressed in cells [9].
The role of the third player of HTLV-1 entry, HSPGs, was the first of the three molecules identified to be important for HTLV-1 entry, through experiments showing that removal of HSPGs from cell surface abolished binding of the HTLV-1 SU as well as HTLV-1-Env mediated infection of target cells [10]. Later studies showed that HSPGs were also required for efficient HTLV-1 entry into primary T cells and dendritic cells [11,12]. The region of the SU involved in HSPG binding was characterized using the fact that, unlike HTLV-1, binding of the HTLV-2 SU to target cells does not depend on HSPGs. Analysis of various HTLV-1/HTLV-2 SU chimera demonstrated that binding to HSPGs involved the C-terminal domain of the HTLV-1 SU (amino-acids 215–313) [13].
2.2. Cooperation between the HTLV-1 Receptors
The fact that NRP-1 and GLUT1 can form a complex in the presence of HTLV-1 Env suggested that these two molecules work together to promote HTLV-1 entry. Such cooperation was also recently functionally demonstrated by data showing that inhibition of HTLV-1 entry into primary astrocytes required the blocking of the interactions with both NRP-1 and GLUT1 [14].
Previously, HSPGs and NRP-1 have been shown to cooperate while functioning as co-receptors for the pro-angiogenic factor VEGF-165. Initial binding to cells is believed to involve interactions of VEGF-165 with both NRP-1 and heparin sulfate (HS) chains, followed by interaction of VEGF-165 with its signaling receptor VEGF-R [15]. Structural and functional studies indicate that VEGF-165 directly binds to both NRP-1 and heparin, and that NRP-1 and heparin also directly bind to one another, allowing VEGF-165 dimerization and stable binding on cells [16]. The regions of VEGF-165 responsible for binding heparin and NRP-1 map to exon 7 and exon 8, respectively, with three residues in exon 8 (KPxR) critical for direct NRP-1 binding to VEGF-165 [16] (Figure 1).
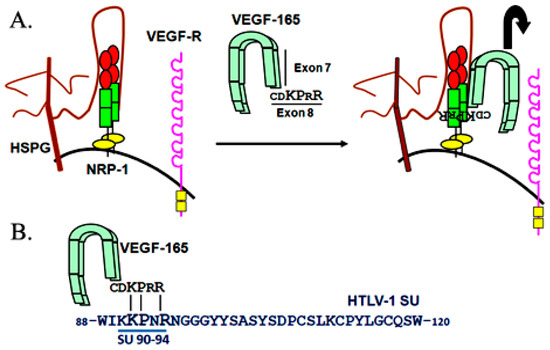
Figure 1.
(A) Schematic representation of the binding of VEGF-165 to heparan sulfate proteoglycans (HSPG)/neuropilin-1 (NRP-1) complexes prior to interaction with the VEGF receptor. The position in VEGF-165 of the exon 7-encoded sequence, which binds to HSPGs, is indicated. The primary amino acid sequence of the exon 8-encoded sequence, which directly binds to the b domain of NRP-1, is also shown. The a, b and c domains of NRP-1 are painted in red, green or yellow, respectively. (B) Homology between the exon 8-encoded domain of VEGF-165 and the amino acid 90-94 region of the HTLV-1 surface subunit (SU).
Recently, it has been shown that HSPG and NRP-1 also work together to promote HTLV-1 Env binding. Indeed, like VEGF-165, HTLV-1 SU binds via a HS-dependent manner to the same domain of NRP-1 important for binding VEGF-165 (NRP-1 b domain, see Figure 1 and Figure 2). Moreover, it was discovered that the same KPxR motif critical for direct binding of VEGF-165 exon 8 to NRP-1 was present in the SU of HTLV-1, in a region previously shown to be important for infectivity (Figure 1B and Figure 3). Further studies revealed that a pentapeptide corresponding to the region of the HTLV-1 SU encoding this motif (aa 90–94, KKPNR) directly binds to NRP-1 in vitro and was sufficient to block HTLV-1 entry into primary T or dendritic cells [17]. Thus, the HTLV-1 SU stably binds to NRP-1 through both HSPG-mediated and direct interactions by mimicking the NRP-1 ligand VEGF-165.
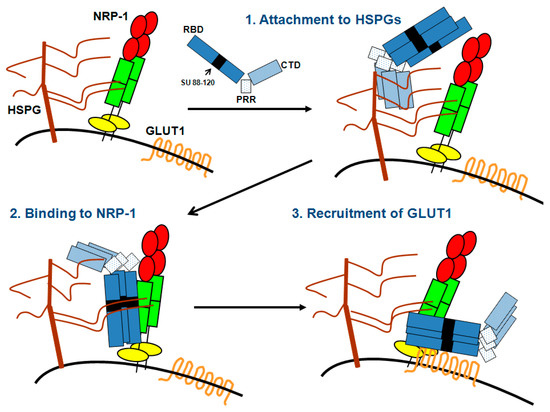
Figure 2.
A multireceptor model for HTLV-1 entry. HSPGs, NRP-1 and GLUT1 expressed on the surface of target cells work together to promote HTLV-1 entry. Step 1: The HTLV-1 SU interacts with HSPGs via its C-terminal domain (CTD), which allows the initial attachment and concentration of HTLV-1 particles at the cell surface, Step 2: HSPGs interaction with both the SU and NRP-1 as well as direct binding of the SU to the b domain of NRP-1 via the 90–94 domain allow the recruitment of NRP-1; Step 3: The stable binding of the SU to HSPGs/NRP-1 complexes triggers conformational changes within the SU allowing GLUT1 binding, notably via residues D106 and Y114 of the SU. The HTLV-1 SU is represented in blue with its three modules: the Receptor-Binding domain (RBD), the Proline-Rich Region (PRR) and the C-terminal domain (CTD). The black box within the RBD represents the 88–120 region that contains the KPxR motif and the D106/Y114 residues responsible for NRP-1 or GLUT1 binding, respectively.
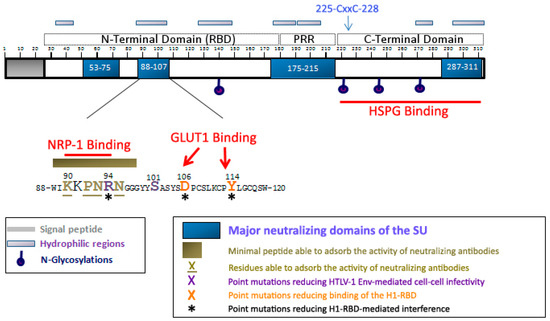
Figure 3.
Localization of the neutralizing regions and the domains and residues involved in HSPGs, NRP-1 and GLUT1 binding within the HTLV-1 SU. The 90-94 motif identified as critical for direct NRP-1 binding [17] corresponds to a minimal neutralizing epitope [43], and contains the R94 residue required for HTLV-1 particle infectivity [44]. R94, as well as D106 and Y114 that mediate binding of the H1-RBD to target cells [3] are required for H1-RBD-mediated receptor interference [36]. The C-terminal domain of the SU contains the determinants for HSPG binding [13].
2.3. A Multireceptor Model for HTLV-1 Entry
The data described above support a multi-receptor model for HTLV-1 entry that involves three phases: virus attachment, virus binding and virus/cell fusion (recently discussed in [2]) (Figure 2). Virus attachment is mediated through interaction of the SU with the HS chains, most likely on cell surface HSPG. Then, because HS chains are able to interact with NRP-1, prior binding of the SU to HSPGs increases the probability of the SU to encounter NRP-1 and allows stable binding to NRP-1, ensuring thereby the transition between virus attachment and virus binding. This binding of the SU to HSPG/NRP-1 complexes allows exposure of the GLUT1-binding domain, presumably via conformational changes. Finally, the SU binds to GLUT1, triggering the fusion process that allows entry of the HTLV-1 core in the cell cytoplasm.
The notion that HSPG/NRP-1 are important for the initial binding step is supported by previous observations that HTLV-1 SU and particle binding are dramatically reduced following removal of HS chains or upon blocking NRP-1 interactions by incubation of target cells with either VEGF-165 exon 7 or exon 8-like peptides [12,17]. Blocking interactions with HS chains and NRP-1 also decreased infection of CD4+ T cells by HTLV-1 [11]. Previous studies indicate that at least some of the HS chains are in the form of HSPGs, likely with syndecans as core proteins [13]. However, since NRP-1 itself is modified by HS on certain cell types [18], it leaves two possibilities for SU binding; the SU binds to HSPGs which interact with NRP-1 or the SU interacts directly with HS conjugated to NRP-1. A role of GLUT1 in the fusion step subsequent to the initial binding is supported by the observation that the level of GLUT1 expression on target cells correlates with the titer of HTLV-1 Env-pseudotyped viruses but not with the level of binding of the HTLV-1 viral particle or soluble full length HTLV-1 SU protein [3,13,14,19].
3. The Viral Actor: The HTLV-1 SU Protein
3.1. Function of Retroviral SU Proteins
Retroviral envelope glycoproteins (Env) are type-I transmembrane proteins synthesized as a precursor that is co-translationally imported into the endoplasmic reticulum, where it subsequently undergoes a number of maturation steps, including folding, oligomerization and N-glycosylations. Upon completion of these processes, the Env precursor passes through the Golgi apparatus to the trans golgi network, where it is cleaved by a cellular protease of the furin family into SU (Surface) and TM (Transmembrane) subunits [20]. SU-TM complexes organized as trimers are then transported to the surface of infected cells, where incorporation into the budding particles occurs [21].
Retrovirus entry occurs by fusion of the viral envelope either with the cell plasma membrane [22], or with endosomal membranes following internalization via a endocytic pathway [23,24,25]. In both cases, the entry process per se occurs through an initial binding step mediated by the SU and a subsequent fusion step mediated by the TM. Accordingly, the SU contains the determinants for receptor binding and the TM has the hydrophobic fusogenic peptide in its N-terminal region. During the intracellular journey of SU/TM complexes and prior to contact with the receptor, the TM is maintained by close association with the SU in a fusogenic-inactive metastable conformation in which the fusion peptide is buried. This prevents premature membrane fusion that could lead to Env inactivation and cell toxicity. The key event for TM activation is alteration of the SU/TM interactions, which allows the TM to acquire its fusogenic state. In the case of murine leukemia virus (MLV) Env, SU/TM dissociation is triggered upon binding of the SU to its receptor mCAT-1 and relies on the disruption of an intersubunit disulfite bond between cysteines in a CxxC motif in the SU and a CX6CC motif in the TM [26,27]. For human immunodeficiency virus type 1 (HIV-1) Env, TM activation requires successive contacts with two entry receptors, the primary binding receptor CD4 and the co-receptor (CXCR4 or CCR5), with the latter contact triggering SU/TM dissociation [28]. The conformational changes required for chemokine binding are also facilitated by thiol/disulfide rearrangement in the SU-gp120, which involves gp120 binding to cell surface protein disulfite isomerase [29].
For all retroviruses, final activation of the fusion process occurs through successive refolding events of the TM resulting in the projection of the N-terminal fusion peptide. This involves the formation of a six-helix coiled-coil bundle that brings the viral and target membranes in close proximity and triggers membrane fusion. Since this review is focused on the interactions between the SU subunit and the cell receptors, details of these processes will not be described here but can be found in recent reviews [22,30].
3.2. General Organization of the HTLV-1 SU
The HTLV-1 env gene encodes a 488 amino acid precursor protein, which generates a 62 kDa protein (gp62) after addition of five N-glycan chains, four of which are in the SU (Figure 3) [31]. The gp62 is cleaved at a trypsin-like proteolytic site spanning residues 309–312 into the mature SU (gp46) and TM (gp21) subunits [31]. The SU is entirely extracellular and remains linked to the virus through binding to the TM, which is embedded in the viral envelope.
No direct structural data are available for the HTLV-1 SU. However, structural domains have been predicted based on sequence homology with SU proteins of gammaretroviruses. Structural and functional studies of MLV Env revealed that the SU is organized into three structural modules: an N-terminal receptor binding domain (RBD) and a C-terminal domain (CTD) separated by a short proline-rich region (PRR) [32,33,34]. Alignment of the amino acid sequences of the HTLV-1 and Friend-MLV SU predicted that the HTLV-1 SU is similarly organized, beginning after the signal peptide (residues 1–25), with an N-terminal region (26–180), a Proline-Rich Region (181–215) and a C-terminal domain (216-312) [35,36] (Figure 3). Analysis of MLV/SU chimeras demonstrated that the N-terminal domain of the HTLV-1 SU can complement the MLV RBD and confer HTLV-1 tropism to the resulting protein, indicating that the HTLV-1 residues important for receptor interactions map within the predicted HTLV-1 RBD [36]. Later studies with a soluble form of the HTLV-1 RBD revealed that this domain is sufficient for binding to the cell surface of target cells [36]. However, binding experiments performed with the full length HTLV-1 SU further demonstrated that regions outside the RBD contain important determinants for SU binding to target cells [13]. Residues outside the RBD have also been shown to encode viral entry determinants for several different gammaretroviruses [37,38].
In gammaretroviruses, the PRR domain is believed to represent a hinge region that facilitates the conformational changes induced by receptor binding [32]. The HTLV-1 SU PRR region has been shown to complement the homologous domain of MLV SU [35], suggesting that it plays a similar role in HTLV-1 SU refolding. The CTD of HTLV-1 SU contains a typical disulfide isomerization motif (CxxC; residues 225 to 228, see Figure 3) homologous to the motif in MLV important for conformational changes required for fusion that occur after receptor binding. Mutation of C225 blocks HTLV-1 Env-mediated infection and cell-cell fusion, but dithiothreitol reverses this effect. This indicates that the thiol of this residue is responsible for the disulfite isomerization that occurs following receptor binding, thus disrupting the SU-TM bond and allowing fusion mediated by the TM [39]. The Ser residue at position 101 also controls SU/TM association [40] (Figure 3). Details of the mechanism of HTLV-1 gp41-mediated fusion have been described elsewhere [41,42].
3.3. Functional Residues of the HTLV-1 SU
One approach to identifying functional domains of retroviral envelope proteins, notably the receptor binding determinants, is to identify the epitopes of neutralizing antibodies which block Env-mediated functions. In the case of HTLV-1, analysis of the specificities of anti-HTLV-1 neutralizing antibodies identified four functional regions in the SU: two N-terminal regions located between residues 53–75 and 86–107 [43,45], a central region located between residues 175-199 [46,47] and a C-terminal one located between residues 287–311 [45] (also reviewed in [48]) (Figure 3).
The 86–107 region was initially mapped from antibodies raised against HTLV-1 peptides corresponding to regions of the SU predicted to be hydrophilic. Adsorption of the neutralizing activities using a set of shorter peptides identified six residues (KKPNRN) at position 90 to 95 as the minimal neutralizing epitope in this domain [43] (Figure 3). Previous analysis of mutants carrying single amino acid changes in HTLV-1 SU identified two residues in this region critical for cell-cell transmission of the virus: one (R94) which maps in the minimal neutralizing epitope and another in the larger (S101) [44]. The central 175–199 region overlaps with the PRR, which has been shown for gammaretroviruses to mediate the SU refolding events that occur following binding to the receptor, resulting in activation of fusion by the TM. The observation that the MLV TM can become fusogenic when present on a chimeric molecule with the HTLV RBD and PRR indicates that the HTLV PRR also functions to transmit the signal from the RBD to the CTD [36].
3.4. Relationships between the Neutralizing Domains of the SU and the Residues Involved in Receptor Binding
Of the four neutralizing regions of HTLV-1 SU described above, the two N-terminal regions spanning residues 53–75 and 86–107 are located within the predicted HTLV-1 RBD. The second of these contains residues that were recently shown to be involved in interactions with NRP-1 and GLUT1 (Figure 1 and Figure 3). Located within the minimal neutralizing SU 90–98 epitope are residues that, as described above, mediate direct binding of the SU to NRP-1: a pentapeptide corresponding to residues 90–94 was shown to block both the binding to, and infection of, cells by HTLV-1, and to be capable of directly binding to NRP-1 [17].The arginine residue within this motif is the same residue (R94) shown in early studies to be important for cell-cell transmission of the virus [44]. The 86–107 region also encodes one of the two residues shown to be involved in GLUT1 binding (D106); the other residue, Y114, is very close to this region [3]. The importance of these three residues for receptor interactions is also supported by observations that expression of an SU fragment corresponding to the H1-RBD (aa 1–215) in target cells reduced the titer of HTLV-1 Env-pseudotyped viruses, presumably by receptor interference, but that H1-RBD carrying a mutation in R94, D106, or Y114 did not [36].
HTLV-1 is a member of the deltaretrovirus family that includes the closely related primate T-lymphotropic viruses (PTLVs, see below) and the more distantly related bovine leukemia virus (BLV). A structural model for the SU of bovine leukemia virus, which shares 36% identity in amino acid sequence with the HTLV-1 SU, was previously generated. This model predicted that the aa 97–106 region of the BLV SU, which is the homologous to the aa 92–103 region of the HTLV-1 SU, is exposed at the surface of the globular structure of the protein [49]. The BLV SU 97–106 region was also described earlier as a neutralizing epitope [50]. However, the KPxR motif involved in NRP-1 binding, which is conserved in all the PTLV SU sequences (see below), is not found in this region of BLV, in agreement with early reports that HTLV-1 and BLV use different receptors [51].
The C-terminal neutralizing region, aa 287–311, also includes a region previously reported to interact with a component of the proposed HTLV-1 receptor complex. Studies with chimera generated between the HTLV-1 SU and the SU of HTLV-2, which does not efficiently bind HSPG, indicate that the residues involved in binding HSPGs map to the CTD (aa 215–313). It would be of interest to investigate whether deletion in the 287–311 domain reduces HSPG binding, which would explain the neutralizing activity of antibodies directed to this part of the SU.
3.5. Conformational Changes in the HTLV-1 SU
As mentioned above, attachment to HSPGs is mediated by the C-terminal region of the SU while NRP-1 and GLUT1 binding occur via the N-terminal region. This distance between the HSPGs and NRP-1/GLUT1 interacting domains are compatible with successive binding events. In contrast, the interacting domains for NRP-1 (aa 90–94) or GLUT1 (D106, Y114) are only separated by 16 residues (Figure 3). This is consistent with the model we proposed, in which the stable SU binding to HSPG/NRP-1 complexes triggers conformational changes allowing the subsequent recruitment of GLUT1. In this line, it has been reported that the level of cell surface GLUT1 does not correlate with the level of binding of the HTLV-1 viral particles or with full-length HTLV-1 SU [13,19], but does correlate with the level of binding of the N-terminal H1-RBD fragment (aa 25–215) [3]. This suggests that the isolated H1-RBD (N-terminal domain) may have conformational flexibility which allows it to bind to GLUT1 independently of conformational changes triggered in the full length SU by NRP-1 binding (Figure 3). Since SU are organized as trimers, NRP-1 and GLUT1 could also simultaneously bind to distinct monomer within SU trimers, as reported for the HIV-1 SU [52]. This could explain the fact that tripartite SU/NRP-1/GLUT1 complexes can be detected in cells [9].
4. Comparison between HTLV-1 and the other PTLVs
HTLV-1 is one of a group of deltaretroviruses that infect old world primates and humans, referred to as primate T-lymphotropic viruses (PTLVs). The viruses in this group that infect humans (HTLVs) are believed to have originated from interspecies transmission of simian T-lymphotropic viruses (STLVs). To date, the PTLV group includes three human viruses for which a simian counterpart is known (HTLV-1/ STLV-1, HTLV-2/ STLV-2, and HTLV-3/ STLV-3) as well as two viruses (HTLV-4 and STLV-5) for which no counterpart has yet been identified [53,54,55].
4.1. Difference between HTLV-1 and HTLV-2
Among the PTLV group, nearly all of the studies performed to date have examined the in vivo tropism and receptor usage of HTLV-1 and/or HTLV-2. The nucleotide sequence homology of the genomes of these two viruses is approximately 60% [56] and their SU proteins share 65% identity on an amino acid level [57] (see Figure 4). The notion that these two viruses share the same receptor is based on early observations that target cells first infected with HTLV-1 became resistant to HTLV-2 infection [51]. However, the in vivo tropism of HTLV-1 and HTLV-2 is not identical. HTLV-1 is found primarily in CD4+ T cells, while HTLV-2 is found primarily in CD8+ T cells [58]. Moreover, the ability of these viruses to transform primary T cells in vitro parallel the in vivo observations: HTLV-1 preferentially transforms CD4+ T cells and HTLV-2 preferentially transforms CD8+ T cells [58]. Studies using HTLV-1/HTLV-2 recombinant viruses have mapped this difference to the region of the genome encoding Env: HTLV-2 viruses carrying the HTLV-1 Env preferentially transform CD4+ cells, while HTLV-1 viruses with HTLV-2 Env preferentially transform CD8+ T cells [59]. While this difference could be due to steps in the viral life cycle after entry, one explanation for the distinct T-cell tropism of HTLV-1 and HTLV-2 could be differences in the receptors the viruses use to enter T cells. Both primary CD4+ and CD8+ T cells up-regulate NRP-1 upon activation [60], consistent with previous reports that HTLV-1 and HTLV-2 bind to activated, but not naïve, T cells [61,62]. Moreover, reducing levels of NRP-1 has been shown to reduce the titer of both HTLV-1 and HTLV-2 Env-pseudotyped viruses [9]. In contrast, major differences in respect to HSPG and GLUT1 usage have been reported. Removal of HSPGs have been shown to dramatically reduce binding of the HTLV-1, but not HTLV-2, full-length SU to target cells [13]. It was also observed that activated primary CD4+ or CD8+ T lymphocytes have a reciprocal phenotype in regards to the cell surface expression of HSPG and GLUT1, with a high HSPG/low GLUT1 phenotype for activated CD4+ T cells and a low HSPG/high GLUT1 for activated CD8+ T cells. Moreover, an increase in HTLV-1 but not HTLV-2 particle internalization was observed upon overexpression of HSPG in CD8+ T cells while transfection of GLUT1 in a CD4+ T cell line only enhanced the internalization of the HTLV-2 particle [13]. Hence, at least in T cells, HTLV-1 and HTLV-2 do not appear to use the exact same receptor complex, with HTLV-2 being more dependent on the level of GLUT1 and HTLV-1 on HSPGs. However, HTLV-1 and HTLV-2 appear to both interact with NRP-1 and GLUT1, explaining why they belong to the same receptor interference group [51].
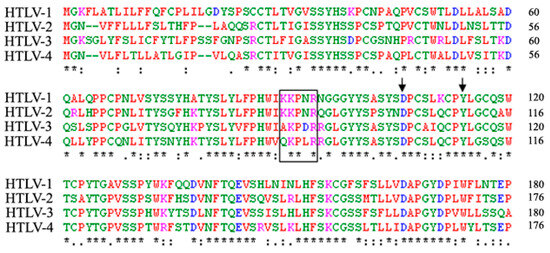
Figure 4.
Alignment of the amino acid sequence of the HTLV-1 RBD with the corresponding regions of the HTLV-2, HTLV-3 and HTLV-4 SU. The KKPNR motif of HTLV-1 that mediates direct binding to NRP-1 is boxed and the D106 and Y114 residues that mediate binding to GLUT1 are indicated by arrows. The alignment was performed with the Clustal W program using the following accession numbers: HTLV-1: Genbank AAC82582; HTLV-2: GenBank M10060; HTLV-3: GenBank EU649782 and HTVL-4: NCBI NC_011800. An asterisk indicates identical residues, a colon indicates conserved substitutions and a period indicates semiconserved substitutions.
The three KPxR residues of the HTLV-1 SU that mediate direct binding to NRP-1 (K91, P92 and R94) and the two residues involved in GLUT1 binding (D106 and Y114) are conserved between the HTLV-1 and HTLV-2 SU [17,63] (Figure 4). Moreover, R90 of HTLV-2, which is the equivalent of R94 of HTLV-1, has been shown to be critical for HTLV-2 infectivity [57]. These findings are fully consistent with the notion that HTLV-1 and HTLV-2 both use NRP-1 and GLUT1 during entry.
4.2. Receptor Usage of Other PTLV
HTLV-1 and its simian counterpart STLV-1 are highly related viruses which can share up to 98% nucleotide sequence homology [64]. The entire SU sequences of HTLV-1 and STLV-1 are highly conserved and notably, the aforementioned residues K91, P92, D106 and Y114 are identical between a number of HTLV-1 and STLV-1 isolates [65]. This strongly suggests that like HTLV-1, STLV-1 uses HSPG, NRP-1 and GLUT1 as entry receptors. High homology between HTLV-2 and STLV-2 isolates [66] may similarly indicate that the human and simian type 2 also share the same entry mechanism.
The HTLV-3 genome has been sequenced [67] and the primary amino acid sequences of the HTLV-1, HTLV-2 and HTLV-3 Env have been compared [63]. Interestingly, although the KKPNR sequence is not strictly conserved in the HTLV-3 SU, the three residues in the KPxR motif shown to be critical for binding to NRP-1 are present in HTLV-3. Moreover, the D106 and Y114 residues important for GLUT1 binding are also strictly conserved between the HTLV-1, HTLV-2 and HTLV-3 sequences (Figure 4). The only functional studies with the HTLV-3 Env reported to date revealed that, unlike HTLV-1 and HTLV-2, the HTLV-3 SU can bind to primary resting CD4+ T cells that do not express detectable levels of HSPG, NRP-1 or GLUT1 [63]. Blocking interactions with either HSPGs or NRP-1 reduced the level of binding of HTLV-3 SU and the titer of HTLV-3 Env-pseudotyped viruses, but the effect of blocking these interactions was less dramatic than for HTLV-1. It was also reported that, as for HTLV-1, the level of GLUT1 correlates with the titer of HTLV-3 Env-pseudotyped virus but not with the level of binding of the HTLV-3 SU [63]. These finding suggest that as for HTLV-1, HSPG and NRP-1 may participate in the initial binding step and GLUT1 in the final fusion step of the HTLV-3 entry process. However, other molecules expressed by resting T cells appear to be also involved, that presumably can substitute for HSPG and/or NRP-1 during the initial stages of binding.
The nucleotide sequence of the env gene is not yet available for STLV-5 but the sequence for one HTLV-4 isolate has been reported (accession NC_011800.1). Examination of this sequence reveals that both the KPxR motif and D106 and Y114 of the HTLV-1 SU are conserved in the amino acid sequence of the HTLV-4 SU (Figure 4), suggesting that HTLV-4 may also use NRP-1 and GLUT1 as entry receptors.
5. Conclusions
The functional domains of the HTLV-1 SU were extensively studied long before the identification of the cellular receptors. These earlier studies on Env and recent work on the receptors now converge to identify the N-terminal domain (NRP-1 and GLUT1 binding) and the C-terminal domain (HSPG binding) of the HTLV-1 SU as critical determinants for HTLV-1 entry. Analysis of the primary sequence of other members of the PTLV family predicts that some members may also use NRP-1 and GLUT1 as entry receptors, but may differ from HTLV-1 in HSPG usage. Further studies are clearly needed to better define the receptor complex used by each PTLV member and understand the impact of virus/receptor interactions on the in vivo tropism and infection by HTLV-1 and the other PTLVs.
Acknowledgments
The authors would like to thank Cent pour sang la Vie, Institut National du cancer (INCA), Association pour la Recherche sur le cancer (ARC), Ligue Nationale contre le Cancer, Fondation pour la Recherche Médicale and the Fondation de France for the financial supports of their works. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Ilinskaya, A.; Heidecker, G.; Jones, K. Interaction between the HTLV-1 envelope and cellular proteins: impact on virus infection and restriction. Future Med. Chem. 1651, 2, 1651–1668. [Google Scholar] [CrossRef] [PubMed]
- Ghez, D.; Lepelletier, Y.; Jones, K.S.; Pique, C.; Hermine, O. Current concepts regarding the HTLV-1 receptor complex. Retrovirology 2010, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Kim, F.J.; Kinet, S.; Taylor, N.; Sitbon, M.; Battini, J.L. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 2003, 115, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Agrawal, L.; VanHorn-Ali, Z.; Alkhatib, G. Infection of CD4+ T lymphocytes by the human T cell leukemia virus type 1 is mediated by the glucose transporter GLUT-1: Evidence using antibodies specific to the receptor's large extracellular domain. Virology 2006, 349, 184–196. [Google Scholar] [CrossRef]
- Pellet-Many, C.; Frankel, P.; Jia, H.; Zachary, I. Neuropilins: Structure, function and role in disease. Biochem. J. 2008, 411, 211–226. [Google Scholar] [CrossRef]
- He, Z.; Tessier-Lavigne, M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 1997, 90, 739–751. [Google Scholar] [CrossRef]
- Bagri, A.; Tessier-Lavigne, M.; Watts, R.J. Neuropilins in tumor biology. Clin. Cancer Res. 2009, 15, 1860–1864. [Google Scholar] [CrossRef]
- Tordjman, R.; Lepelletier, Y.; Lemarchandel, V.; Cambot, M.; Gaulard, P.; Hermine, O.; Romeo, P.H. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 2002, 3, 477–482. [Google Scholar] [CrossRef]
- Ghez, D.; Lepelletier, Y.; Lambert, S.; Fourneau, J.M.; Blot, V.; Janvier, S.; Arnulf, B.; van Endert, P.M.; Heveker, N.; Pique, C.; et al. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 2006, 80, 6844–6854. [Google Scholar] [CrossRef]
- Pinon, J.D.; Klasse, P.J.; Jassal, S.R.; Welson, S.; Weber, J.; Brighty, D.W.; Sattentau, Q.J. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J. Virol. 2003, 77, 9922–9930. [Google Scholar] [CrossRef]
- Jones, K.S.; Petrow-Sadowski, C.; Huang, Y.K.; Bertolette, D.C.; Ruscetti, F.W. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat. Med. 2008, 14, 429–436. [Google Scholar] [CrossRef]
- Jones, K.S.; Petrow-Sadowski, C.; Bertolette, D.C.; Huang, Y.; Ruscetti, F.W. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 2005, 79, 12692–12702. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Fugo, K.; Petrow-Sadowski, C.; Huang, Y.; Bertolette, D.C.; Lisinski, I.; Cushman, S.W.; Jacobson, S.; Ruscetti, F.W. Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 use different receptor complexes to enter T cells. J. Virol. 2006, 80, 8291–8302. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Alkhatib, B.; Cornetta, K.; Alkhatib, G. Alternate receptor usage of neuropilin-1 and glucose transporter protein 1 by the human T cell leukemia virus type 1. Virology 2009, 396, 203–212. [Google Scholar] [CrossRef]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef]
- Vander Kooi, C.W.; Jusino, M.A.; Perman, B.; Neau, D.B.; Bellamy, H.D.; Leahy, D.J. Structural basis for ligand and heparin binding to neuropilin B domains. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Bouttier, M.; Vassy, R.; Seigneuret, M.; Petrow-Sadowski, C.; Janvier, S.; Heveker, N.; Ruscetti, F.W.; Perret, G.; Jones, K.S.; et al. HTLV-1 uses HSPG and neuropilin 1 for entry by molecular mimicry of VEGF165. Blood 2009, 6, 6. [Google Scholar] [CrossRef]
- Shintani, Y.; Takashima, S.; Asano, Y.; Kato, H.; Liao, Y.; Yamazaki, S.; Tsukamoto, O.; Seguchi, O.; Yamamoto, H.; Fukushima, T.; et al. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. EMBO J. 2006, 25, 3045–3055. [Google Scholar] [CrossRef]
- Takenouchi, N.; Jones, K.S.; Lisinski, I.; Fugo, K.; Yao, K.; Cushman, S.W.; Ruscetti, F.W.; Jacobson, S. GLUT1 is not the primary binding receptor but is associated with cell-to-cell transmission of human T-cell leukemia virus type 1. J. Virol. 2007, 81, 1506–1510. [Google Scholar] [CrossRef]
- Moulard, M.; Decroly, E. Maturation of HIV envelope glycoprotein precursors by cellular endoproteases. Biochim. Biophys. Acta 2000, 1469, 121–132. [Google Scholar] [CrossRef]
- Waheed, A.A.; Freed, E.O. Lipids and membrane microdomains in HIV-1 replication. Virus Res. 2009, 143, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Melikyan, G.B. Common principles and intermediates of viral protein-mediated fusion: The HIV-1 paradigm. Retrovirology 2008, 5, 111. [Google Scholar] [CrossRef]
- Permanyer, M.; Ballana, E.; Este, J.A. Endocytosis of HIV: Anything goes. Trends Microbiol. 2010, 18, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J.; Narayan, S.; Dornadula, G.; Miller, M.D.; Young, J.A. Low pH is required for avian sarcoma and leukosis virus Env-dependent viral penetration into the cytosol and not for viral uncoating. J. Virol. 2004, 78, 10433–10441. [Google Scholar] [CrossRef]
- Ross, S.R.; Schofield, J.J.; Farr, C.J.; Bucan, M. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 12386–12390. [Google Scholar] [CrossRef] [PubMed]
- Wallin, M.; Ekstrom, M.; Garoff, H. Isomerization of the intersubunit disulphide-bond in Env controls retrovirus fusion. EMBO J. 2004, 23, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Pinter, A.; Kopelman, R.; Li, Z.; Kayman, S.C.; Sanders, D.A. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J. Virol. 1997, 71, 8073–8077. [Google Scholar] [CrossRef]
- Ray, N.; Doms, R.W. HIV-1 coreceptors and their inhibitors. Curr. Top. Microbiol. Immunol. 2006, 303, 97–120. [Google Scholar]
- Markovic, I.; Stantchev, T.S.; Fields, K.H.; Tiffany, L.J.; Tomic, M.; Weiss, C.D.; Broder, C.C.; Strebel, K.; Clouse, K.A. Thiol/disulfide exchange is a prerequisite for CXCR4-tropic HIV-1 envelope-mediated T-cell fusion during viral entry. Blood 2004, 103, 1586–1594. [Google Scholar] [CrossRef]
- Weissenhorn, W.; Hinz, A.; Gaudin, Y. Virus membrane fusion. FEBS Lett. 2007, 581, 2150–2155. [Google Scholar] [CrossRef]
- Pique, C.; Pham, D.; Tursz, T.; Dokhelar, M.C. Human T-cell leukemia virus type I envelope protein maturation process: requirements for syncytium formation. J. Virol. 1992, 66, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Lavillette, D.; Maurice, M.; Roche, C.; Russell, S.J.; Sitbon, M.; Cosset, F.L. A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes. J. Virol. 1998, 72, 9955–9965. [Google Scholar] [CrossRef] [PubMed]
- Fass, D.; Davey, R.A.; Hamson, C.A.; Kim, P.S.; Cunningham, J.M.; Berger, J.M. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science 1997, 277, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Battini, J.L.; Danos, O.; Heard, J.M. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J. Virol. 1995, 69, 713–719. [Google Scholar] [CrossRef]
- Kim, F.J.; Seiliez, I.; Denesvre, C.; Lavillette, D.; Cosset, F.L.; Sitbon, M. Definition of an amino-terminal domain of the human T-cell leukemia virus type 1 envelope surface unit that extends the fusogenic range of an ecotropic murine leukemia virus. J. Biol. Chem. 2000, 275, 23417–23420. [Google Scholar] [CrossRef]
- Kim, F.J.; Manel, N.; Garrido, E.N.; Valle, C.; Sitbon, M.; Battini, J.L. HTLV-1 and -2 envelope SU subdomains and critical determinants in receptor binding. Retrovirology 2004, 1, 41. [Google Scholar] [CrossRef]
- Argaw, T.; Figueroa, M.; Salomon, D.R.; Wilson, C.A. Identification of residues outside of the receptor binding domain that influence the infectivity and tropism of porcine endogenous retrovirus. J. Virol. 2008, 82, 7483–7491. [Google Scholar] [CrossRef]
- Rey, M.A.; Prasad, R.; Tailor, C.S. The C domain in the surface envelope glycoprotein of subgroup C feline leukemia virus is a second receptor-binding domain. Virology 2008, 370, 273–284. [Google Scholar] [CrossRef]
- Li, K.; Zhang, S.; Kronqvist, M.; Wallin, M.; Ekstrom, M.; Derse, D.; Garoff, H. Intersubunit disulfide isomerization controls membrane fusion of human T-cell leukemia virus Env. J. Virol. 2008, 82, 7135–7143. [Google Scholar] [CrossRef]
- Delamarre, L.; Pique, C.; Pham, D.; Tursz, T.; Dokhelar, M.C. Identification of functional regions in the human T-cell leukemia virus type I SU glycoprotein. J. Virol. 1994, 68, 3544–3549. [Google Scholar] [CrossRef]
- Lamb, D.; Mirsaliotis, A.; Kelly, S.M.; Brighty, D.W. Basic residues are critical to the activity of peptide inhibitors of human T cell leukemia virus type 1 entry. J. Biol. Chem. 2009, 284, 6575–6584. [Google Scholar] [CrossRef] [PubMed]
- Mirsaliotis, A.; Nurkiyanova, K.; Lamb, D.; Woof, J.M.; Brighty, D.W. Conformation-specific antibodies targeting the trimer-of-hairpins motif of the human T-cell leukemia virus type 1 transmembrane glycoprotein recognize the viral envelope but fail to neutralize viral entry. J. Virol. 2007, 81, 6019–6031. [Google Scholar] [CrossRef] [PubMed]
- Palker, T.J.; Riggs, E.R.; Spragion, D.E.; Muir, A.J.; Scearce, R.M.; Randall, R.R.; McAdams, M.W.; McKnight, A.; Clapham, P.R.; Weiss, R.A.; et al. Mapping of homologous, amino-terminal neutralizing regions of human T-cell lymphotropic virus type I and II gp46 envelope glycoproteins. J. Virol. 1992, 66, 5879–5889. [Google Scholar] [CrossRef]
- Delamarre, L.; Rosenberg, A.R.; Pique, C.; Pham, D.; Dokhelar, M.C. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J. Virol. 1997, 71, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Desgranges, C.; Souche, S.; Vernant, J.C.; Smadja, D.; Vahlne, A.; Horal, P. Identification of novel neutralization-inducing regions of the human T cell lymphotropic virus type I envelope glycoproteins with human HTLV-I-seropositive sera. AIDS Res. Hum. Retrovir. 1994, 10, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Baba, E.; Nakamura, M.; Tanaka, Y.; Kuroki, M.; Itoyama, Y.; Nakano, S.; Niho, Y. Multiple neutralizing B-cell epitopes of human T-cell leukemia virus type 1 (HTLV-1) identified by human monoclonal antibodies. A basis for the design of an HTLV-1 peptide vaccine. J. Immunol. 1993, 151, 1013–1024. [Google Scholar] [CrossRef]
- Kuroki, M.; Nakamura, M.; Itoyama, Y.; Tanaka, Y.; Shiraki, H.; Baba, E.; Esaki, T.; Tatsumoto, T.; Nagafuchi, S.; Nakano, S.; et al. Identification of new epitopes recognized by human monoclonal antibodies with neutralizing and antibody-dependent cellular cytotoxicity activities specific for human T cell leukemia virus type 1. J. Immunol. 1992, 149, 940–948. [Google Scholar] [CrossRef]
- Le Blanc, I.; Grange, M.P.; Delamarre, L.; Rosenberg, A.R.; Blot, V.; Pique, C.; Dokhelar, M.C. HTLV-1 structural proteins. Virus Res. 2001, 78, 5–16. [Google Scholar] [CrossRef]
- Johnston, E.R.; Albritton, L.M.; Radke, K. Envelope proteins containing single amino acid substitutions support a structural model of the receptor-binding domain of bovine leukemia virus surface protein. J. Virol. 2002, 76, 10861–10872. [Google Scholar] [CrossRef]
- Bruck, C.; Mathot, S.; Portetelle, D.; Berte, C.; Franssen, J.D.; Herion, P.; Burny, A. Monoclonal antibodies define eight independent antigenic regions on the bovine leukemia virus (BLV) envelope glycoprotein gp51. Virology 1982, 122, 342–352. [Google Scholar] [CrossRef]
- Sommerfelt, M.A.; Weiss, R.A. Receptor interference groups of 20 retroviruses plating on human cells. Virology 1990, 176, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Salzwedel, K.; Berger, E.A. Cooperative subunit interactions within the oligomeric envelope glycoprotein of HIV-1: functional complementation of specific defects in gp120 and gp41. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 12794–12799. [Google Scholar] [CrossRef] [PubMed]
- Mahieux, R.; Gessain, A. The human HTLV-3 and HTLV-4 retroviruses: New members of the HTLV family. Pathol. Biol. (Paris) 2009, 57, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Liegeois, F.; Lafay, B.; Switzer, W.M.; Locatelli, S.; Mpoudi-Ngole, E.; Loul, S.; Heneine, W.; Delaporte, E.; Peeters, M. Identification and molecular characterization of new STLV-1 and STLV-3 strains in wild-caught nonhuman primates in Cameroon. Virology 2008, 371, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Calattini, S.; Chevalier, S.A.; Duprez, R.; Bassot, S.; Froment, A.; Mahieux, R.; Gessain, A. Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology 2005, 2, 30. [Google Scholar] [CrossRef]
- Shimotohno, K.; Takahashi, Y.; Shimizu, N.; Gojobori, T.; Golde, D.W.; Chen, I.S.; Miwa, M.; Sugimura, T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc. Natl. Acad. Sci. U. S. A. 1985, 82, 3101–3105. [Google Scholar] [CrossRef]
- Rosenberg, A.R.; Delamarre, L.; Preira, A.; Dokhelar, M.C. Analysis of functional conservation in the surface and transmembrane glycoprotein subunits of human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2. J. Virol. 1998, 72, 7609–7614. [Google Scholar] [CrossRef]
- Feuer, G.; Green, P.L. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene 2005, 24, 5996–6004. [Google Scholar] [CrossRef]
- Xie, L.; Green, P.L. Envelope is a major viral determinant of the distinct in vitro cellular transformation tropism of human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2. J. Virol. 2005, 79, 14536–14545. [Google Scholar] [CrossRef]
- Milpied, P.; Renand, A.; Bruneau, J.; Mendes-da-Cruz, D.A.; Jacquelin, S.; Asnafi, V.; Rubio, M.T.; MacIntyre, E.; Lepelletier, Y.; Hermine, O. Neuropilin-1 is not a marker of human Foxp3+ Treg. Eur. J. Immunol. 2009, 39, 1466–1471. [Google Scholar] [CrossRef]
- Nath, M.D.; Ruscetti, F.W.; Petrow-Sadowski, C.; Jones, K.S. Regulation of the cell-surface expression of an HTLV-I binding protein in human T cells during immune activation. Blood 2003, 101, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Kinet, S.; Battini, J.L.; Kim, F.J.; Taylor, N.; Sitbon, M. The HTLV receptor is an early T-cell activation marker whose expression requires de novo protein synthesis. Blood 2003, 101, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Huang, Y.K.; Chevalier, S.A.; Afonso, P.V.; Petrow-Sadowski, C.; Bertolette, D.C.; Gessain, A.; Ruscetti, F.W.; Mahieux, R. The receptor complex associated with human T-cell lymphotropic virus type 3 (HTLV-3) Env-mediated binding and entry is distinct from, but overlaps with, the receptor complexes of HTLV-1 and HTLV-2. J. Virol. 2009, 83, 5244–5255. [Google Scholar] [CrossRef] [PubMed]
- Nerrienet, E.; Meertens, L.; Kfutwah, A.; Foupouapouognigni, Y.; Gessain, A. Molecular epidemiology of simian T-lymphotropic virus (STLV) in wild-caught monkeys and apes from Cameroon: a new STLV-1, related to human T-lymphotropic virus subtype F, in a Cercocebus agilis. J. Gen. Virol. 2001, 82, 2973–2977. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.J.; Lavanya, M.; Gessain, A.; Gallego, S.; Battini, J.L.; Sitbon, M.; Courgnaud, V. Intrahost variations in the envelope receptor-binding domain (RBD) of HTLV-1 and STLV-1 primary isolates. Retrovirology 2006, 3, 29. [Google Scholar] [CrossRef]
- Vandamme, A.M.; Salemi, M.; Van Brussel, M.; Liu, H.F.; Van Laethem, K.; Van Ranst, M.; Michels, L.; Desmyter, J.; Goubau, P. African origin of human T-lymphotropic virus type 2 (HTLV-2) supported by a potential new HTLV-2d subtype in Congolese Bambuti Efe Pygmies. J. Virol. 1998, 72, 4327–4340. [Google Scholar] [CrossRef]
- Calattini, S.; Chevalier, S.A.; Duprez, R.; Afonso, P.; Froment, A.; Gessain, A.; Mahieux, R. Human T-cell lymphotropic virus type 3: complete nucleotide sequence and characterization of the human tax3 protein. J. Virol. 2006, 80, 9876–9888. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).