Hantaviruses in the Americas and Their Role as Emerging Pathogens
Abstract
1. Introduction
2. Molecular Features and Cell Interactions
2.1. Envelope Glycoproteins
2.2. The Hantavirus Nucleocapsid Protein
3. Pathology and Diagnosis
3.1. Pathology
| HFRS | HCPS | |
| Transmission | Aerosolized wild-rodent excreta, no arthropod vector | Aerosolized wild-rodent excreta, no arthropod vector |
| Stages | Incubation period: 1–5 wk, prodrome 5–10 d, hypotensive phase 1–3 d oliguric phage 3–5 d convalescence | Incubation period: 1–5 wk, prodrome (febrile phase) 3–10 d, cardiorespiratoryphase 1–6 d diuretic phase 1–3 d convalescence |
| Primary organ of attack | Kidney, lymphoreticular system | Lungs, lymphoreticular system |
| Location of effusion | Retroperitoneal space | Thoracic bed; pulmonary interstitium |
| Pathologic hallmarks | Capillary leak. Mononuclear infiltrate. | Capillary leak. Interstitial mononuclear cell infiltrate. |
| Clinical findings | Fever, chills, nausea and vomiting, headache, lethargy, oliguria, tachycardia, facial and truncal flushing, hemorrhage, occasionally noncardiogenic pulmonary edema with tachypnea, shortness of breath, shock, death in 0.1 to 5%. | Fever, chills, nausea and vomiting, headache, lethargy, dyspnea, tachycardia, tachypnea, shortness of breath, noncardiogenic pulmonary edema, hemorrhage/petechiae (ANDV), shock, death in 10 to 50%. |
| Laboratory findings | Hypoalbuminemia, azotemia, proteinuria, hematuria, leukopenia/leukocytosis | Hypoxia, Hypoalbuminemia, thrombocytopenia, leukopenia/leukocytosis, hemoconcentration |
| Treatment | Specific: ribavirin. Nonspecific: cardioventilatory support, dialysis | Specific: none. Nonspecific: extracorporeal membrane oxygenation (ECMO), cardioventilatory support |
3.2. Adaptive Immune Responses
4. Epidemiology and Epizootiology
4.1. Epidemiology and Transmission Mechanisms from Rodents to Humans
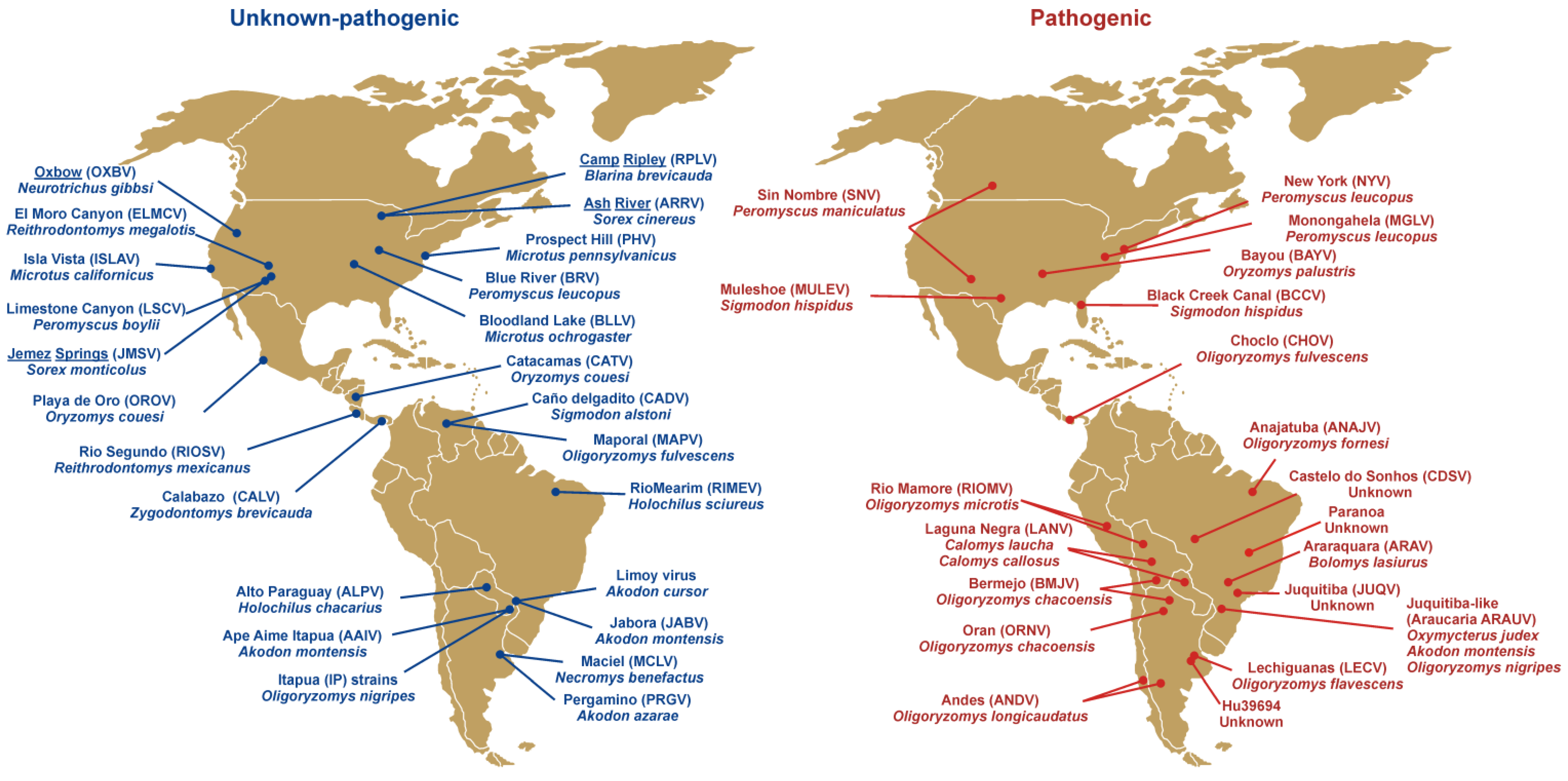
4.2. Rodent Reservoirs and Cross-species Transmission
4.3. Outbreaks
5. Concluding Remarks
Acknowledgments
References and Notes
- Holmes, E.; Drummond, A. The Evolutionary Genetics of Viral Emergence. In Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission; Childs, J.E., Mackenzie, J.S., Richt, J.A., Eds.; Springer-Verlag Berlin Heidelberg: New York, NY, USA, 2007; pp. 51–66. [Google Scholar]
- Childs, J.E.; Mackenzie, J.S.; Richt, J.A. Introduction: Conceptualizing and Partitioning the Emergence Process of Zoonotic Viruses from Wildlife to Humans. In Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission; Childs, J.E., Mackenzie, J.S., Richt, J.A., Eds.; Springer-Verlag Berlin Heidelberg: New York, NY, USA, 2007; pp. 1–31. [Google Scholar]
- Morse, S.S. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1995, 1, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E.; Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005, 11, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Holmes, E.C. The Evolutionary Genetics of Emerging Viruses. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 353–372. [Google Scholar] [CrossRef]
- Racaniello, V.R. Emerging infectious diseases. J. Clin. Invest. 2004, 113, 796–798. [Google Scholar] [CrossRef][Green Version]
- Fraser, C.; Donnelly, C.A.; Cauchemez, S.; Hanage, W.P.; Van Kerkhove, M.D.; Hollingsworth, T.D.; Griffin, J.; Baggaley, R.F.; Jenkins, H.E.; Lyons, E.J.; et al. Pandemic Potential of a Strain of Influenza A (H1N1): Early Findings. Science 2009, 324, 1557–1561. [Google Scholar] [CrossRef]
- Mills, J.N.; Childs, J.E. Ecologic studies of rodent reservoirs: Their relevance for human health. Emerg. Infect. Dis. 1998, 4, 529–537. [Google Scholar] [CrossRef]
- Zizi, M.; Heyman, P.; Vandenvelde, C. The assessment of human health risks from rodent-borne diseases by means of ecological studies of rodent reservoirs. Mil. Med. 2002, 167, 70–73. [Google Scholar]
- Hjelle, B.; Torres-Pérez, F. Rodent-Borne Viruses. In Clinical Virology Manual, 4th ed.; Specter, S., Hodinka, R., Wiedbrauk, D., Young, S., Eds.; America Society for Microbiology Press: Washington, DC, USA, 2009; pp. 641–658. [Google Scholar]
- Nichol, S.T.; Spiropoulou, C.F.; Morzunov, S.; Rollin, P.E.; Ksiazek, T.G.; Feldmann, H.; Sanchez, A.; Childs, J.; Zaki, S.; Peters, C.J. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 1993, 262, 914–917. [Google Scholar] [CrossRef]
- Childs, J.E.; Ksiazek, T.G.; Spiropoulou, C.F.; Krebs, J.W.; Morzunov, S.; Maupin, G.O.; Gage, K.L.; Rollin, P.E.; Sarisky, J.; Enscore, R.E. Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States. J. Infect. Dis. 1994, 169, 1271–1280. [Google Scholar] [CrossRef]
- Lee, H.W. Hemorrhagic fever with renal syndrome. History of Hantaan virus and epidemiological features. Scand. J. Infect. Dis. 1982, 36, 82–85. [Google Scholar]
- Lee, H.W. Hemorrhagic fever with renal syndrome in Korea. Rev. Infect. Dis. 1989, 11 (Suppl. 4), S864–S876. [Google Scholar] [CrossRef] [PubMed]
- Smadel, J.E. Epidemic hemorrhagic fever. Am. J. Public Health Nations Health 1953, 43, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, P.W. Korean hemorrhagic fever. I. Demonstration of causative antigen and antibodies. Korean J. Intern. Med. 1976, 19, 371–394. [Google Scholar]
- Lee, H.W.; Lee, P.W.; Johnson, K.M. Isolation of the etiologic agent of Korean Hemorrhagic fever. J. Infect. Dis. 1978, 137, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohn, C.S.; Hasty, S.E.; Dalrymple, J.M.; Leduc, J.W.; Lee, H.W.; von Bonsdorff, C.H.; Brummer-Korvenkontio, M.; Vaheri, A.; Tsai, T.F.; Regnery, H.L. Antigenic and genetic properties of viruses linked to hemorrhagic fever with renal syndrome. Science 1985, 227, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, R.F.; von Bonsdorff, C.H. Ribonucleoproteins of Uukuniemi virus are circular. J. Virol. 1975, 15, 386–392. [Google Scholar] [CrossRef]
- Arai, S.; Ohdachi, S.D.; Asakawa, M.; Kang, H.J.; Mocz, G.; Arikawa, J.; Okabe, N.; Yanagihara, R. Molecular phylogeny of a newfound hantavirus in the Japanese shrew mole (Urotrichus talpoides). Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 16296–16301. [Google Scholar] [CrossRef]
- Arai, S.; Song, J.W.; Sumibcay, L.; Bennett, S.N.; Nerurkar, V.R.; Parmenter, C.; Cook, J.A.; Yates, T.L.; Yanagihara, R. Hantavirus in northern short-tailed shrew, United States. Emerg. Infect. Dis. 2007, 13, 1420–1423. [Google Scholar] [CrossRef]
- Schmaljohn, C.S.; Schmaljohn, A.L.; Dalrymple, J.M. Hantaan virus M RNA: Coding strategy, nucleotide sequence, and gene order. Virology 1987, 157, 31–39. [Google Scholar] [CrossRef]
- Lober, C.; Anheier, B.; Lindow, S.; Klenk, H.D.; Feldmann, H. The Hantaan virus glycoprotein precursor is cleaved at the conserved pentapeptide WAASA. Virology 2001, 289, 224–229. [Google Scholar] [CrossRef]
- Pensiero, M.N.; Hay, J. The Hantaan virus M-segment glycoproteins G1 and G2 can be expressed independently. J. Virol. 1992, 66, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Antic, D.; Wright, K.E.; Kang, C.Y. Maturation of Hantaan virus glycoproteins G1 and G2. Virology 1992, 189, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Ruusala, A.; Persson, R.; Schmaljohn, C.S.; Pettersson, R.F. Coexpression of the membrane glycoproteins G1 and G2 of Hantaan virus is required for targeting to the Golgi complex. Virology 1992, 186, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Elliott, R.M. Golgi localization of Hantaan virus glycoproteins requires coexpression of G1 and G2. Virology 2002, 300, 31–38. [Google Scholar] [CrossRef]
- Spiropoulou, C.F.; Goldsmith, C.S.; Shoemaker, T.R.; Peters, C.J.; Compans, R.W. Sin Nombre virus glycoprotein trafficking. Virology 2003, 308, 48–63. [Google Scholar] [CrossRef]
- Gavrilovskaya, I.N.; Brown, E.J.; Ginsberg, M.H.; Mackow, E.R. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta(3) integrins. J. Virol. 1999, 73, 3951–3959. [Google Scholar] [CrossRef]
- Krautkramer, E.; Zeier, M. Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J. Virol. 2008, 82, 4257–4264. [Google Scholar] [CrossRef]
- Choi, Y.; Kwon, Y.C.; Kim, S.I.; Park, J.M.; Lee, K.H.; Ahn, B.Y. A hantavirus causing hemorrhagic fever with renal syndrome requires gC1qR/p32 for efficient cell binding and infection. Virology 2008, 381, 178–183. [Google Scholar] [CrossRef]
- Hepojoki, J.; Strandin, T.; Vaheri, A.; Lankinen, H. Interactions and oligomerization of hantavirus glycoproteins. J. Virol. 2010, 84, 227–242. [Google Scholar] [CrossRef]
- Tischler, N.D.; Gonzalez, A.; Perez-Acle, T.; Rosemblatt, M.; Valenzuela, P.D.T. Hantavirus Gc glycoprotein: Evidence for a class II fusion protein. J. Gen. Virol. 2005, 86, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Tischler, N.D.; Rosemblatt, M.; Valenzuela, P.D. Characterization of cross-reactive and serotype-specific epitopes on the nucleocapsid proteins of hantaviruses. Virus Res. 2008, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.; Gerding, M.; Koeman, J.P.; Roholl, P.J.; van Amerongen, G.; Jordans, H.G.; Niesters, H.G.; Osterhaus, A.D. A macaque model for hantavirus infection. J. Infect. Dis. 1995, 172, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Sen, A.; Mackow, E.R. Degrons at the C terminus of the pathogenic but not the nonpathogenic hantavirus G1 tail direct proteasomal degradation. J. Virol. 2007, 81, 4323–4330. [Google Scholar] [CrossRef]
- Geimonen, E.; Fernandez, I.; Gavrilovskaya, I.N.; Mackow, E.R. Tyrosine residues direct the ubiquitination and degradation of the NY-1 hantavirus G1 cytoplasmic tail. J. Virol. 2003, 77, 10760–10768. [Google Scholar] [CrossRef]
- Wang, H.; Strandin, T.; Hepojoki, J.; Lankinen, H.; Vaheri, A. Degradation and aggresome formation of the Gn tail of the apathogenic Tula hantavirus. J. Gen. Virol. 2009, 90, 2995–3001. [Google Scholar] [CrossRef]
- Geimonen, E.; Neff, S.; Raymond, T.; Kocer, S.S.; Gavrilovskaya, I.N.; Mackow, E.R. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 13837–13842. [Google Scholar] [CrossRef]
- Kraus, A.A.; Raftery, M.J.; Giese, T.; Ulrich, R.; Zawatzky, R.; Hippenstiel, S.; Suttorp, N.; Kruger, D.H.; Schonrich, G. Differential antiviral response of endothelial cells after infection with pathogenic and nonpathogenic hantaviruses. J. Virol. 2004, 78, 6143–6150. [Google Scholar] [CrossRef]
- Spiropoulou, C.F.; Albarino, C.G.; Ksiazek, T.G.; Rollin, P.E. Andes and Prospect Hill hantaviruses differ in early induction of interferon although both can downregulate interferon signaling. J. Virol. 2007, 81, 2769–2776. [Google Scholar] [CrossRef]
- Handke, W.; Oelschlegel, R.; Franke, R.; Kruger, D.H.; Rang, A. Hantaan virus triggers TLR3-dependent innate immune responses. J. Immunol. 2009, 182, 2849–2858. [Google Scholar] [CrossRef]
- Prescott, J.; Ye, C.Y.; Sen, G.; Hjelle, B. Induction of innate immune response genes by Sin Nombre hantavirus does not require viral replication. J. Virol. 2005, 79, 15007–15015. [Google Scholar] [CrossRef] [PubMed]
- Alff, P.J.; Gavrilovskaya, I.N.; Gorbunova, E.; Endriss, K.; Chong, Y.S.; Geimonen, E.; Sen, N.; Reich, N.C.; Mackow, E.R. The pathogenic NY-1 hantavirus G1 cytoplasmic tail inhibits RIG-I- and TBK-1-directed interferon responses. J. Virol. 2006, 80, 9676–9686. [Google Scholar] [CrossRef]
- Jaaskelainen, K.M.; Kaukinen, P.; Minskaya, E.S.; Plyusnina, A.; Vapalahti, O.; Elliott, R.M.; Weber, F.; Vaheri, A.; Plyusnin, A. Tula and Puumala hantavirus NSs ORFs are functional and the products inhibit activation of the interferon-beta promoter. J. Med. Virol. 2007, 79, 1527–1536. [Google Scholar] [CrossRef]
- Gavrilovskaya, I.N.; Peresleni, T.; Geimonen, E.; Mackow, E.R. Pathogenic hantaviruses selectively inhibit beta(3) integrin directed endothelial cell migration. Arch. Virol. 2002, 147, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovskaya, I.N.; Gorbunova, E.E.; Mackow, N.A.; Mackow, E.R. Hantaviruses Direct Endothelial Cell Permeability by Sensitizing Cells to the Vascular Permeability Factor, VEGF, while Angiopoietin-1 and Sphingosine 1-Phosphate inhibit Hantavirus-directed Permeability. J. Virol. 2008, 82, 5797–5806. [Google Scholar] [CrossRef]
- Severson, W.; Partin, L.; Schmaljohn, C.S.; Johnsson, C.B. Characterization of the hantaan nucleocapsid protein-ribonucleic acid interaction. J. Biol. Chem. 1999, 274, 33732–33739. [Google Scholar] [CrossRef]
- Mir, M.A.; Panganiban, A.T. Trimeric hantavirus nucleocapsid protein binds specifically to the viral RNA panhandle. J. Virol. 2004, 78, 8281–8288. [Google Scholar] [CrossRef] [PubMed]
- Kaukinen, P.; Vaheri, A.; Plyusnin, A. Hantavirus nucleocapsid protein: A multifunctional molecule with both housekeeping and ambassadorial duties. Arch. Virol. 2005, 150, 1693–1713. [Google Scholar] [CrossRef]
- Ramanathan, H.N.; Chung, D.H.; Plane, S.J.; Sztul, E.; Chu, Y.K.; Guttieri, M.C.; McDowell, M.; Ali, G.; Jonsson, C.B. Dynein-dependent transport of the hantaan virus nucleocapsid protein to the endoplasmic reticulum-Golgi intermediate compartment. J. Virol. 2007, 81, 8634–8647. [Google Scholar] [CrossRef]
- Ravkov, E.V.; Nichol, S.T.; Peters, C.J.; Compans, R.W. Role of actin microfilaments in Black Creek Canal virus morphogenesis. J. Virol. 1998, 72, 2865–2870. [Google Scholar] [CrossRef]
- Kaukinen, P.; Vaheri, A.; Plyusnin, A. Non-covalent interaction between nucleocapsid protein of Tula hantavirus and small ubiquitin-related modifier-1, SUMO-1. Virus Res. 2003, 92, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Frias-Staheli, N.; Garcia-Sastre, A.; Schmaljohn, C.S. Hantaan virus nucleocapsid protein binds to importin alpha proteins and inhibits tumor necrosis factor alpha-induced activation of nuclear factor kappa B. J. Virol. 2009, 83, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Panganiban, A.T. A protein that replaces the entire cellular eIF4F complex. EMBO J. 2008, 27, 3129–3139. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Panganiban, A.T. Characterization of the RNA chaperone activity of hantavirus nucleocapsid protein. J. Virol. 2006, 80, 6276–6285. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, A.T.; Mir, M.A. Bunyavirus N: eIF4F surrogate and cap-guardian. Cell Cycle 2009, 8, 1332–1337. [Google Scholar] [CrossRef]
- Ramanathan, H.N.; Jonsson, C.B. New and Old World hantaviruses differentially utilize host cytoskeletal components during their life cycles. Virology 2008, 374, 138–150. [Google Scholar] [CrossRef]
- Buranda, T.; Wu, Y.; Perez, D.; Jett, S.D.; Bondu-Hawkins, V.; Ye, C.; Edwards, B.; Hall, P.; Larson, R.S.; Lopez, G.P.; Sklar, L.A.; Hjelle, B. Recognition of DAF and alpha(v)beta(3) by inactivated Hantaviruses, towards the development of HTS flow cytometry assays. Anal. Biochem. 2010, 402, 151–160. [Google Scholar] [CrossRef]
- Hjelle, B. Pathogenesis of hantavirus infections. In UpToDate; Rose, B.D., Ed.; UpToDate: Wellesley, MA, USA, 2008. [Google Scholar]
- Duchin, J.S.; Koster, F.T.; Peters, C.J.; Simpson, G.L.; Tempest, B.; Zaki, S.R.; Ksiazek, T.G.; Rollin, P.E.; Nichol, S.; Umland, E.T.; Moolenaar, R.L.; Reef, S.E.; Nolte, K.B.; Gallaher, M.M.; Butler, J.C.; Breiman, R.F.; Group, f.T.H.S. Hantavirus pulmonary syndrome: A clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 1994, 330, 949–955. [Google Scholar] [CrossRef]
- Nolte, K.B.; Feddersen, R.M.; Foucar, K.; Zaki, S.R.; Koster, F.T.; Madar, D.; Merlin, T.L.; McFeeley, P.J.; Umland, E.T.; Zumwalt, R.E. Hantavirus pulmonary syndrome in the United States: A pathological description of a disease caused by a new agent. Hum. Pathol. 1995, 26, 110–120. [Google Scholar] [CrossRef]
- Mori, M.; Rothman, A.L.; Kurane, I.; Montoya, J.M.; Nolte, K.B.; Norman, J.E.; Waite, D.C.; Koster, F.T.; Ennis, F.A. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J. Infect. Dis. 1999, 179, 295–302. [Google Scholar] [CrossRef]
- Campen, M.J.; Milazzo, M.L.; Fulhorst, C.F.; Obot Akata, C.J.; Koster, F. Characterization of shock in a hamster model of hantavirus infection. Virology 2006, 356, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Raftery, M.J.; Kraus, A.A.; Ulrich, R.; Kruger, D.H.; Schonrich, G. Hantavirus infection of dendritic cells. J. Virol. 2002, 76, 10724–10733. [Google Scholar] [CrossRef] [PubMed]
- Markotic, A.; Hensley, L.; Daddario, K.; Spik, K.; Anderson, K.; Schmaljohn, C. Pathogenic hantaviruses elicit different immunoreactions in THP-1 cells and primary monocytes and induce differentiation of human monocytes to dendritic-like cells. Coll. Antropol. 2007, 31, 1159–1167. [Google Scholar] [PubMed]
- Mackow, E.R.; Gavrilovskaya, I.N. Hantavirus regulation of endothelial cell functions. Thromb. Haemost. 2009, 102, 1030–1041. [Google Scholar]
- Gavrilovskaya, I.N.; Gorbunova, E.E.; Mackow, E.R. Pathogenic Hantaviruses Direct the Adherence of Quiescent Platelets to Infected Endothelial Cells. J. Virol. 2010, 84, 4832–4839. [Google Scholar] [CrossRef]
- Milazzo, M.L.; Eyzaguirre, E.J.; Molina, C.P.; Fulhorst, C.F. Maporal viral infection in the Syrian golden hamster: A model of hantavirus pulmonary syndrome. J. Infect. Dis. 2002, 186, 1390–1395. [Google Scholar] [CrossRef]
- Hooper, J.W.; Larsen, T.; Custer, D.M.; Schmaljohn, C.S. A lethal disease model for hantavirus pulmonary syndrome. Virology 2001, 289, 6–14. [Google Scholar] [CrossRef]
- Botten, J.; Mirowsky, K.; Kusewitt, D.; Bharadwaj, M.; Yee, J.; Ricci, R.; Feddersen, R.M.; Hjelle, B. Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus). Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 10578–10583. [Google Scholar] [CrossRef]
- Lundkvist, A.; Cheng, Y.; Sjolander, K.B.; Niklasson, B.; Vaheri, A.; Plyusnin, A. Cell culture adaptation of Puumala hantavirus changes the infectivity for its natural reservoir, Clethrionomys glareolus, and leads to accumulation of mutants with altered genomic RNA S segment. J. Virol. 1997, 71, 9515–9523. [Google Scholar] [CrossRef]
- Mertz, G.J.; Hjelle, B.; Crowley, M.; Iwamoto, G.; Tomicic, V.; Vial, P.A. Diagnosis and treatment of new world hantavirus infections. Curr. Opin. Infect. Dis. 2006, 19, 437–442. [Google Scholar] [CrossRef]
- Wahl-Jensen, V.; Chapman, J.; Asher, L.; Fisher, R.; Zimmerman, M.; Larsen, T.; Hooper, J.W. Temporal Analysis of Andes Virus and Sin Nombre Virus Infection of Syrian Hamsters. J Virol. 2007, 81, 7449–7462. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, M.; Nofchissey, R.; Goade, D.; Koster, F.; Hjelle, B. Humoral immune responses in the hantavirus cardiopulmonary syndrome. J. Infect. Dis. 2000, 182, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Manigold, T.; Mori, A.; Graumann, R.; Llop, E.; Simon, V.; Ferres, M.; Valdivieso, F.; Castillo, C.; Hjelle, B.; Vial, P. Highly differentiated, resting gn-specific memory CD8 T cells persist years after infection by andes hantavirus. PLoS Pathog. 2010, 6, e1000779. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.R.; Greer, P.W.; Coffield, L.M.; Goldsmith, C.S.; Nolte, K.B.; Foucar, K.; Feddersen, R.M.; Zumwalt, R.E.; Miller, G.L.; Khan, A.S. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 1995, 146, 552–579. [Google Scholar] [PubMed]
- Jenison, S.; Hjelle, B.; Simpson, S.; Hallin, G.; Feddersen, R.; Koster, F. Hantavirus pulmonary syndrome: Clinical, diagnostic, and virologic aspects. Semin. Respir. Infect. 1995, 10, 259–269. [Google Scholar] [PubMed]
- Koster, F.; Foucar, K.; Hjelle, B.; Scott, A.; Chong, Y.Y.; Larson, R.; McCabe, M. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am. J. Clin. Pathol. 2001, 116, 665–672. [Google Scholar] [CrossRef]
- Ennis, F.A.; Cruz, J.; Spiropoulou, C.F.; Waite, D.; Peters, C.J.; Nichol, S.T.; Kariwa, H.; Koster, F.T. Hantavirus pulmonary syndrome: CD8(+) and CD4(+) cytotoxic T lymphocytes to epitopes on Sin Nombre virus nucleocapsid protein isolated during acute illness. Virology 1997, 238, 380–390. [Google Scholar] [CrossRef]
- Kilpatrick, E.D.; Terajima, M.; Koster, F.T.; Catalina, M.D.; Cruz, J.; Ennis, F.A. Role of specific CD8(+) T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J. Immunol. 2004, 172, 3297–3304. [Google Scholar] [CrossRef]
- Terajima, M.; Hayasaka, D.; Maeda, K.; Ennis, F.A. Immunopathogenesis of hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome: Do CD8(+) T cells trigger capillary leakage in viral hemorrhagic fevers? Immunol. Lett. 2007, 113, 117–120. [Google Scholar] [CrossRef]
- Linderholm, M.; Ahlm, C.; Settergren, B.; Waage, A.; Tarnvik, A. Elevated plasma levels of tumor necrosis factor (TNF)-alpha, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 1996, 173, 38–43. [Google Scholar] [CrossRef]
- Temonen, M.; Mustonen, J.; Helin, H.; Pasternack, A.; Vaheri, A.; Holthofer, H. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: An immunohistochemical study. Clin. Immunol. Immunopathol. 1996, 78, 47–55. [Google Scholar] [CrossRef]
- Hjelle, B.; Spiropoulou, C.F.; Torrez-Martinez, N.; Morzunov, S.; Peters, C.J.; Nichol, S.T. Detection of Muerto Canyon virus RNA in peripheral blood mononuclear cells from patients with hantavirus pulmonary syndrome. J. Infect. Dis. 1994, 170, 1013–1017. [Google Scholar] [CrossRef]
- Terajima, M.; Hendershot, J.D., III; Kariwa, H.; Koster, F.T.; Hjelle, B.; Goade, D.; DeFronzo, M.C.; Ennis, F.A. High levels of viremia in patients with the Hantavirus pulmonary syndrome. J. Infect. Dis. 1999, 180, 2030–2034. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Yang, S.; Koster, F.; Ye, C.; Stidley, C.; Hjelle, B. Sin Nombre viral RNA load in patients with hantavirus cardiopulmonary syndrome. J. Infect. Dis. 2006, 194, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Dietl, C.A.; Wernly, J.A.; Pett, S.B.; Yassin, S.F.; Sterling, J.P.; Dragan, R.; Milligan, K.; Crowley, M.R. Extracorporeal membrane oxygenation support improves survival of patients with severe Hantavirus cardiopulmonary syndrome. J. Thorac. Cardiovasc. Surg. 2008, 135, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Case Information: Hantavirus Pulmonary Syndrome Case Count and Descriptive Statistics. Available online: http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/caseinfo.htm (accessed on 12 November 2010).
- Vincent, M.J.; Quiroz, E.; Gracia, F.; Sanchez, A.J.; Ksiazek, T.G.; Kitsutani, P.T.; Ruedas, L.A.; Tinnin, D.S.; Caceres, L.; Garcia, A.; Rollin, P.E.; Mills, J.N.; Peters, C.J.; Nichol, S.T. Hantavirus pulmonary syndrome in Panama: Identification of novel Hantaviruses and their likely reservoirs. Virology 2000, 277, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Armien, A.G.; Armien, B.; Koster, F.; Pascale, J.M.; Avila, M.; Gonzalez, P.; de la Cruz, M.; Zaldivar, Y.; Mendoza, Y.; Gracia, F.; Hjelle, B.; Lee, S.J.; Yates, T.L.; Salazar-Bravo, J. Hantavirus infection and habitat associations among rodent populations in agroecosystems of Panama: Implications for human disease risk. Am. J. Trop. Med. Hyg. 2009, 81, 59–66. [Google Scholar] [CrossRef]
- Suzan, G.; Ceballos, G.; Mills, J.; Ksiazek, T.G.; Yates, T. Serologic evidence of hantavirus infection in sigmodontine rodents in Mexico. J. Wildl. Dis. 2001, 37, 391–393. [Google Scholar] [CrossRef]
- Mantooth, S.J.; Milazzo, M.L.; Bradley, R.D.; Hice, C.L.; Ceballos, G.; Tesh, R.B.; Fulhorst, C.F. Geographical distribution of rodent-associated hantaviruses in Texas. J. Vector Ecol. 2001, 26, 7–14. [Google Scholar]
- Castro-Arellano, I.; Suzan, G.; Leon, R.F.; Jimenez, R.M.; Lacher, T.E., Jr. Survey for antibody to hantaviruses in Tamaulipas, Mexico. J. Wildl. Dis. 2009, 45, 207–212. [Google Scholar] [CrossRef]
- Hjelle, B.; Anderson, B.; Torrez-Martinez, N.; Song, W.; Gannon, W.L.; Yates, T.L. Prevalence and geographic genetic variation of hantaviruses of New World harvest mice (Reithrodontomys): Identification of a divergent genotype from a Costa Rican Reithrodontomys mexicanus. Virology 1995, 207, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Vado-Solís, I.; Pérez-Osorio, C.; Lara-Lara, J.; Ruiz-Piña, H.A.; Cárdenas-Marrufo, M.; Milazzo, M.L.; Fulhorst, C.F.; Zavala-Velázquez, J. Evidencia serológica de infección por Hantavirus en población humana del estado de Yucatán, México. Rev. Biomed. 2003, 14, 221–225. [Google Scholar] [CrossRef]
- Rivas, Y.J.; Moros, Z.; Moron, D.; Uzcategui, M.G.; Duran, Z.; Pujol, F.H.; Liprandi, F.; Ludert, J.E. The seroprevalences of anti-hantavirus IgG antibodies among selected Venezuelan populations. Ann. Trop. Med. Parasitol. 2003, 97, 61–67. [Google Scholar] [CrossRef]
- Ramos, M.; Mola, C.L.d.; Salmon, G.; Guevara, C.; Pacheco, V.; Vasquez, A.; Kochel, T.; Albujar, C.; Rollin, P.; Comer, J.A.; Montgomery, J. Evidence of occupational exposure to Hantavirus and other vector-borne viral pathogens among mammalogist and other field workers in Peru. In 58th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Washington, DC, USA, 18–22 November 2009. [Google Scholar]
- Chilean Ministry of Health. Boletín Epidemiológico de Hantavirus Situación al 14 octubre de 2010. Available online: http://epi.minsal.cl/epi/html/bolets/reportes/Hantavirus/Hantavirus.pdf (accessed on 12 November 2010).
- Bellomo, C.; Nudelman, J.; Kwaszka, R.; Vazquez, G.; Cantoni, G.; Weinzettel, B.; Larrieu, E.; Padula, P. Expansión geográfica del síndrome pulmonar por hantavirus en la Argentina: Informe del caso más austral. Medicina (B Aires) 2009, 69, 647–650. [Google Scholar] [PubMed]
- Figueiredo, L.T.; Moreli, M.L.; de-Sousa, R.L.; Borges, A.A.; de-Figueiredo, G.G.; Machado, A.M.; Bisordi, I.; Nagasse-Sugahara, T.K.; Suzuki, A.; Pereira, L.E.; de-Souza, R.P.; de-Souza, L.T.; Braconi, C.T.; Harsi, C.M.; de-Andrade-Zanotto, P.M. Hantavirus pulmonary syndrome, central plateau, southeastern, and southern Brazil. Emerg. Infect. Dis. 2009, 15, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Armien, B.; Pascale, J.M.; Bayard, V.; Munoz, C.; Mosca, I.; Guerrero, G.; Armien, A.; Quiroz, E.; Castillo, Z.; Zaldivar, Y.; Gracia, F.; Hjelle, B.; Koster, F. High seroprevalence of hantavirus infection on the Azuero peninsula of Panama. Am. J. Trop. Med. Hyg. 2004, 70, 682–687. [Google Scholar] [CrossRef]
- Sotomayor, V.; Olea, A.M.; Labrana, M. Diagnosis and treatment of cardiopulmonary hantavirus syndrome. Chile-2007. Rev. Chil. Infectol. 2009, 26, 68–84. [Google Scholar]
- Ferrer, J.F.; Galligan, D.; Esteban, E.; Rey, V.; Murua, A.; Gutierrez, S.; Gonzalez, L.; Thakuri, M.; Feldman, L.; Poiesz, B.; Jonsson, C. Hantavirus infection in people inhabiting a highly endemic region of the Gran Chaco territory, Paraguay: Association with Trypanosoma cruzi infection, epidemiological features and haematological characteristics. Ann. Trop. Med. Parasitol. 2003, 97, 269–280. [Google Scholar] [CrossRef]
- Ferrer, J.F.; Jonsson, C.B.; Esteban, E.; Galligan, D.; Basombrio, M.A.; Peralta-Ramos, M.; Bharadwaj, M.; Torrez-Martinez, N.; Callahan, J.; Segovia, A.; Hjelle, B. High prevalence of hantavirus infection in Indian communities of the Paraguayan and Argentinean Gran Chaco. Am. J. Trop. Med. Hyg. 1998, 59, 438–444. [Google Scholar] [CrossRef]
- Torres-Pérez, F.; Navarrete-Droguett, J.; Aldunate, R.; Yates, T.L.; Mertz, G.J.; Vial, P.A.; Ferres, M.; Marquet, P.A.; Palma, R.E. Peridomestic small mammals associated with confirmed cases of human hantavirus disease on southcentral Chile. Am. J. Trop. Med. Hyg. 2004, 70, 305–309. [Google Scholar] [CrossRef]
- Frey, M.T.; Vial, P.C.; Castillo, C.H.; Godoy, P.M.; Hjelle, B.; Ferres, M.G. Hantavirus prevalence in the IX Region of Chile. Emerg. Infect. Dis. 2003, 9, 827–832. [Google Scholar]
- Pini, N.; Levis, S.; Calderon, G.; Ramirez, J.; Bravo, D.; Lozano, E.; Ripoll, C.; St Jeor, S.; Ksiazek, T.G.; Barquez, R.M.; Enria, D. Hantavirus infection in humans and rodents, northwestern Argentina. Emerg. Infect. Dis. 2003, 9, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Limongi, J.E.; da Costa, F.C.; Pinto, R.M.; de Oliveira, R.C.; Bragagnolo, C.; Lemos, E.R.; de Paula, M.B.; Pajuaba Neto, A.A.; Ferreira, M.S. Cross-sectional Survey of Hantavirus Infection, Brazil. Emerg. Infect. Dis. 2009, 15, 1981–1983. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.M.; Lindsey, A.E.; Hjelle, B.; Dominguez, D.; Brown, J.; Goade, D.; Jonsson, C.B. Prevalence of antibodies to Sin Nombre virus in humans living in rural areas of southern New Mexico and western Texas. Virus Res. 2001, 74, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Douglass, R.J.; Semmens, W.J.; Matlock-Cooley, S.J.; Kuenzi, A.J. Deer mouse movements in peridomestic and sylvan settings in relation to Sin Nombre virus antibody prevalence. J. Wildl. Dis. 2006, 42, 813–818. [Google Scholar] [CrossRef]
- Kuenzi, A.J.; Douglass, R.J.; White, D.; Bond, C.W.; Mills, J.N. Antibody to Sin Nombre virus in rodents associated with peridomestic habitats in west central Montana. Am. J. Trop. Med. Hyg. 2001, 64, 137–146. [Google Scholar] [CrossRef]
- Botten, J.; Mirowsky, K.; Ye, C.Y.; Gottlieb, K.; Saavedra, M.; Ponce, L.; Hjelle, B. Shedding and intracage transmission of Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus) model. J. Virol. 2002, 76, 7587–7594. [Google Scholar] [CrossRef]
- Padula, P.; Figueroa, R.; Navarrete, M.; Pizarro, E.; Cadiz, R.; Bellomo, C.; Jofre, C.; Zaror, L.; Rodriguez, E.; Murua, R. Transmission study of Andes hantavirus infection in wild sigmodontine rodents. J. Virol. 2004, 78, 11972–11979. [Google Scholar] [CrossRef]
- Yates, T.L.; Mills, J.N.; Parmenter, C.A.; Ksiazek, T.G.; Parmenter, R.R.; Vande Castle, J.R.; Calisher, C.H.; Nichol, S.T.; Abbott, K.D.; Young, J.C.; Morrison, M.L.; Beaty, B.J.; Dunnum, J.L.; Baker, R.J.; Salazar-Bravo, J.; Peters, C.J. The ecology and evolutionary history of an emergent disease: Hantavirus pulmonary syndrome. Bioscience 2002, 52, 989–998. [Google Scholar] [CrossRef]
- Parmenter, R.R.; Brunt, J.W.; Moore, D.I.; Ernest, S. The Hantavirus epidemic in the Southwest: Rodent population dynamics and the implications for transmission of Hantavirus-associated Adult Respiratory Distress Syndrome HARDS) in the Four Corners Region. Report to the Federal Centers for Disease Control and Prevention; Publication No. 41; Sevilleta Long-Term Ecological Research Program (LTER): Atlanta, GA, USA, July 1993; pp. 1–45. [Google Scholar]
- Suzan, G.; Armien, A.; Mills, J.N.; Marce, E.; Ceballos, G.; Avila, M.; Salazar-Bravo, J.; Ruedas, L.; Armien, B.; Yates, T.L. Epidemiological considerations of rodent community composition in fragmented landscapes in Panama. J. Mammal. 2008, 89, 684–690. [Google Scholar] [CrossRef]
- Suzan, G.; Marce, E.; Giermakowski, J.T.; Armien, B.; Pascale, J.; Mills, J.; Ceballos, G.; Gomez, A.; Aguirre, A.A.; Salazar-Bravo, J.; Armien, A.; Parmenter, R.; Yates, T. The effect of habitat fragmentation and species diversity loss on hantavirus prevalence in panama. Ann. N. Y. Acad. Sci. 2008, 1149, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Goodin, D.G.; Koch, D.E.; Owen, R.D.; Chu, Y.K.; Hutchinson, J.M.S.; Jonsson, C.B. Land cover associated with hantavirus presence in Paraguay. Global Ecol. Biogeogr. 2006, 15, 519–527. [Google Scholar] [CrossRef]
- Hjelle, B.; Glass, G.E. Outbreak of hantavirus infection in the four corners region of the United States in the wake of the 1997–1998 El Nino-southern oscillation. J. Infect. Dis. 2000, 181, 1569–1573. [Google Scholar] [CrossRef]
- Klein, S.; Calisher, C. Emergence and Persistence of Hantaviruses. In Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission; Childs, J.E., Mackenzie, J.S., Richt, J.A., Eds.; Springer-Verlag Berlin Heidelberg: New York, NY, USA, 2007; pp. 217–252. [Google Scholar]
- Kallio, E.R.; Klingstrom, J.; Gustafsson, E.; Manni, T.; Vaheri, A.; Henttonen, H.; Vapalahti, O.; Lundkvist, A. Prolonged survival of Puumala hantavirus outside the host: Evidence for indirect transmission via the environment. J. Gen. Virol. 2006, 87, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Douron, E.; Moriniere, B.; Matheron, S.; Girard, P.M.; Gonzalez, J.P.; Hirsch, F.; McCormick, J.B. HFRS after a wild rodent bite in the Haute-Savoie—and risk of exposure to Hantaan-like virus in a Paris laboratory. Lancet 1984, 1, 676–677. [Google Scholar] [CrossRef]
- Schultze, D.; Lundkvist, A.; Blauenstein, U.; Heyman, P. Tula virus infection associated with fever and exanthema after a wild rodent bite. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 304–306. [Google Scholar] [CrossRef]
- St Jeor, S.C. Three-week incubation period for hantavirus infection. Pediatr. Infect. Dis. J. 2004, 23, 974–975. [Google Scholar]
- Fulhorst, C.F.; Milazzo, M.L.; Armstrong, L.R.; Childs, J.E.; Rollin, P.E.; Khabbaz, R.; Peters, C.J.; Ksiazek, T.G. Hantavirus and arenavirus antibodies in persons with occupational rodent exposure, North America. Emerg. Infect. Dis. 2007, 13, 532–538. [Google Scholar] [CrossRef]
- Torres-Pérez, F.; Wilson, L.; Collinge, S.K.; Harmon, H.; Ray, C.; Medina, R.A.; Hjelle, B. Sin nombre virus infection in field workers, colorado, USA. Emerg. Infect. Dis. 2010, 16, 308–310. [Google Scholar] [CrossRef]
- Kelt, D.A.; Van Vuren, D.H.; Hafner, M.S.; Danielson, B.J.; Kelly, M.J. Threat of hantavirus pulmonary syndrome to field biologists working with small mammals. Emerg. Infect. Dis. 2007, 13, 1285–1287. [Google Scholar] [CrossRef]
- Ferres, M.; Vial, P.; Marco, C.; Yanez, L.; Godoy, P.; Castillo, C.; Hjelle, B.; Delgado, I.; Lee, S.J.; Mertz, G.J. Prospective evaluation of household contacts of persons with hantavirus cardiopulmonary syndrome in Chile. J. Infect. Dis. 2007, 195, 1563–1571. [Google Scholar] [CrossRef]
- Lazaro, M.E.; Cantoni, G.E.; Calanni, L.M.; Resa, A.J.; Herrero, E.R.; Iacono, M.A.; Enria, D.A.; Cappa, S.M.G. Clusters of hantavirus infection, southern Argentina. Emerg. Infect. Dis. 2007, 13, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Padula, P.J.; Edelstein, A.; Miguel, S.D.L.; Lopez, N.M.; Rossi, C.M.; Rabinovich, R.D. Hantavirus Pulmonary Syndrome outbreak in Argentina: Molecular evidence for person-to-person transmission of Andes virus. Virology 1998, 241, 323–330. [Google Scholar] [CrossRef]
- Wells, R.M.; Estani, S.S.; Yadon, Z.E.; Enria, D.; Padula, P.; Pini, N.; Della Valle, M.G.; Mills, J.N.; Peters, C.J. Seroprevalence of antibodies to hantavirus in health care workers and other residents of southern Argentina. Clin. Infect. Dis. 1998, 27, 895–896. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.M.; Estani, S.S.; Yadon, Z.E.; Enria, D.; Padula, P.; Pini, N.; Mills, J.N.; Peters, C.J.; Segura, E.L.; Guthmann, N.; Arguelo, E.; Klein, F.; Levy, R.; Nagel, C.; Calfin, R.; deRosas, F.; Lazaro, M.; Rosales, H.; Sandoval, P. An unusual hantavirus outbreak in southern Argentina: Person-to-person transmission? Emerg. Infect. Dis. 1997, 3, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.P.; Bellomo, C.; San Juan, J.; Pinna, D.; Forlenza, R.; Elder, M.; Padula, P.J. Person-to-person transmission of Andes virus. Emerg. Infect. Dis. 2005, 11, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Marsac, D.; Stefas, E.; Ferrer, P.; Tischler, N.D.; Pino, K.; Ramdohr, P.; Vial, P.; Valenzuela, P.D.; Ferres, M.; Veas, F.; Lopez-Lastra, M. Andes Virus Antigens are shed in urine of patients with acute Hantavirus Cardiopulmonary Syndrome. J. Virol. 2009, 83, 5046–5055. [Google Scholar] [CrossRef]
- Pettersson, L.; Klingstrom, J.; Hardestam, J.; Lundkvist, A.; Ahlm, C.; Evander, M. Hantavirus RNA in saliva from patients with hemorrhagic Fever with renal syndrome. Emerg. Infect. Dis. 2008, 14, 406–411. [Google Scholar] [CrossRef]
- Kang, H.J.; Bennett, S.N.; Dizney, L.; Sumibcay, L.; Arai, S.; Ruedas, L.A.; Song, J.W.; Yanagihara, R. Host switch during evolution of a genetically distinct hantavirus in the American shrew mole (Neurotrichus gibbsii). Virology 2009, 388, 8–14. [Google Scholar] [CrossRef]
- Arai, S.; Bennett, S.N.; Sumibcay, L.; Cook, J.A.; Song, J.W.; Hope, A.; Parmenter, C.; Nerurkar, V.R.; Yates, T.L.; Yanagihara, R. Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am. J. Trop. Med. Hyg. 2008, 78, 348–351. [Google Scholar] [CrossRef]
- Plyusnin, A.; Vapalahti, O.; Vaheri, A. Hantaviruses: Genome structure, expression and evolution. J. Gen. Virol. 1996, 77, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.K.; Owen, R.D.; Sanchez-Hernandez, C.; Romero-Almaraz, M.D.; Jonsson, C.B. Genetic characterization and phylogeny of a hantavirus from Western Mexico. Virus Res. 2007, 131, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.L.; Cajimat, M.N.B.; Hanson, J.D.; Bradley, R.D.; Quintana, M.; Sherman, C.; Velasquez, R.T.; Fulhorst, C.F. Catacamas virus, a hantaviral species naturally associated with Oryzomys couesi (Coues’oryzomys) in Honduras. Am. J. Trop. Med. Hyg. 2006, 75, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Fulhorst, C.F.; Cajimat, M.N.B.; Utrera, A.; Milazzo, M.L.; Duno, G.M. Maporal virus, a hantavirus associated with the fulvous pygmy rice rat (Oligoryzomys fulvescens) in western Venezuela. Virus Res. 2004, 104, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.A.; Torrez-Martinez, N.; Neill, S.U.; Moore, G.M.; Hicks, B.N.; Pichuantes, S.; Nguyen, A.; Bharadwaj, M.; Hjelle, B. Cocirculation of multiple hantaviruses in Texas, with characterization of the small (S) genome of a previously undescribed virus of cotton rats (Sigmodon hispidus). Am. J. Trop. Med. Hyg. 1996, 55, 672–679. [Google Scholar] [CrossRef]
- Rollin, P.E.; Ksiazek, T.G.; Elliott, L.H.; Ravkov, E.V.; Martin, M.L.; Morzunov, S.; Livingstone, W.; Monroe, M.; Glass, G.; Ruo, S. Isolation of Black Creek Canal virus, a new hantavirus from Sigmodon hispidus in Florida. J. Med. Virol. 1995, 46, 35–39. [Google Scholar] [CrossRef]
- Johnson, A.M.; Bowen, M.D.; Ksiazek, T.G.; Williams, R.J.; Bryan, R.T.; Mills, J.N.; Peters, C.J.; Nichol, S.T. Laguna Negra virus associated with HPS in western Paraguay and Bolivia. Virology 1997, 238, 115–127. [Google Scholar] [CrossRef]
- Carroll, D.S.; Mills, J.N.; Montgomery, J.M.; Bausch, D.G.; Blair, I.J.; Burans, J.P.; Felices, V.; Gianella, A.; Iihoshi, N.; Nichol, S.T.; Olson, J.G.; Rogers, D.S.; Salazar, M.; Ksiazek, T.G. Hantavirus pulmonary syndrome in central Bolivia: Relationships between reservoir hosts, habitats, and viral genotypes. Am. J. Trop. Med. Hyg. 2005, 72, 42–46. [Google Scholar] [CrossRef]
- Maes, P.; Klempa, B.; Clement, J.; Matthijnssens, J.; Gajdusek, D.C.; Kruger, D.H.; Van Ranst, M. A proposal for new criteria for the classification of hantaviruses, based on S and M segment protein sequences. Infect. Genet. Evol. 2009, 9, 813–820. [Google Scholar] [CrossRef]
- Plyusnin, A. Genetics of hantaviruses: Implications to taxonomy. Arch. Virol. 2002, 147, 665–682. [Google Scholar] [CrossRef]
- Lloyd-Smith, J.O.; George, D.; Pepin, K.M.; Pitzer, V.E.; Pulliam, J.R.; Dobson, A.P.; Hudson, P.J.; Grenfell, B.T. Epidemic dynamics at the human-animal interface. Science 2009, 326, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Hjelle, B.; Yates, T. Modeling hantavirus maintenance and transmission in rodent communities. Curr. Top. Microbiol. Immunol. 2001, 256, 77–90. [Google Scholar]
- Schmidt-Chanasit, J.; Essbauer, S.; Petraityte, R.; Yoshimatsu, K.; Tackmann, K.; Conraths, F.J.; Sasnauskas, K.; Arikawa, J.; Thomas, A.; Pfeffer, M.; Scharninghausen, J.J.; Splettstoesser, W.; Wenk, M.; Heckel, G.; Ulrich, R.G. Extensive host sharing of central European Tula virus. J. Virol. 2010, 84, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Delfraro, A.; Tome, L.; D’Elia, G.; Clara, M.; Achaval, F.; Russi, J.C.; Rodonz, J.R. Juquitiba-like hantavirus from 2 nonrelated rodent species, Uruguay. Emerg. Infect. Dis. 2008, 14, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, M.; Klempa, B.; Auste, B.; Bemmann, M.; Schmidt-Chanasit, J.; Buchner, T.; Groschup, M.H.; Meier, M.; Balkema-Buschmann, A.; Zoller, H.; Kruger, D.H.; Ulrich, R.G. Dobrava-belgrade virus spillover infections, Germany. Emerg. Infect. Dis. 2009, 15, 2017–2020. [Google Scholar] [CrossRef]
- Weidmann, M.; Schmidt, P.; Vackova, A.; Krivanec, K.; Munclinger, P.; Hufert, F.T. Identification of genetic evidence for Dobrava virus spillover in rodents by nested reverse transcription (RT)-PCR and TaqMan RT-PCR. J. Clin. Microbiol. 2005, 43, 808–812. [Google Scholar] [CrossRef]
- Vapalahti, O.; Lundkvist, A.; Fedorov, V.; Conroy, C.J.; Hirvonen, S.; Plyusnina, A.; Nemirov, K.; Fredga, K.; Cook, J.A.; Niemimaa, J.; Kaikusalo, A.; Henttonen, H.; Vaheri, A.; Plyusnin, A. Isolation and characterization of a hantavirus from Lemmus sibiricus: Evidence for host switch during hantavirus evolution. J. Virol. 1999, 73, 5586–5592. [Google Scholar] [CrossRef]
- Medina, R.A.; Torres-Pérez, F.; Galeno, H.; Navarrete, M.; Vial, P.A.; Palma, R.E.; Ferres, M.; Cook, J.A.; Hjelle, B. Ecology, genetic diversity, and phylogeographic structure of Andes virus in humans and rodents in Chile. J. Virol. 2009, 83, 2446–2459. [Google Scholar] [CrossRef]
- Toro, J.; Vega, J.D.; Khan, A.S.; Mills, J.N.; Padula, P.; Terry, W.; Yadon, Z.; Valderrama, R.; Ellis, B.A.; Pavletic, C.; Cerda, R.; Zaki, S.; Wun-Ju, S.; Meyer, R.; Tapia, M.; Mansilla, C.; Baro, M.; Vergara, J.A.; Concha, M.; Calderon, G.; Enria, D.; Peters, C.J.; Ksiazek, T.G. An outbreak of hantavirus pulmonary syndrome, Chile, 1997. Emerg. Infect. Dis. 1998, 4, 687–694. [Google Scholar] [CrossRef]
- McIntyre, N.E.; Chu, Y.K.; Owen, R.D.; Abuzeineh, A.; De La Sancha, N.; Dick, C.W.; Holsomback, T.; Nisbett, R.A.; Jonsson, C. A longitudinal study of Bayou virus, hosts, and habitat. Am. J. Trop. Med. Hyg. 2005, 73, 1043–1049. [Google Scholar] [CrossRef]
- Holsomback, T.S.; McIntyre, N.E.; Nisbett, R.A.; Strauss, R.E.; Chu, Y.-K.; Abuzeineh, A.A.; Sancha, N.d.l.; Dick, C.W.; Jonsson, C.B.; Morris, B.E.L. Bayou virus detected in non-oryzomyine rodent hosts: An assessment of habitat composition, reservoir community structure, and marsh rice rat social dynamics. J. Vector Ecol. 2009, 34, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Torrez-Martinez, N.; Bharadwaj, M.; Goade, D.; Delury, J.; Moran, P.; Hicks, B.; Nix, B.; Davis, J.L.; Hjelle, B. Bayou virus-associated hantavirus pulmonary syndrome in eastern Texas: Identification of the rice rat, Oryzomys palustris, as reservoir host. Emerg. Infect. Dis. 1998, 4, 105–111. [Google Scholar] [CrossRef]
- Allen, L.J.; Wesley, C.L.; Owen, R.D.; Goodin, D.G.; Koch, D.; Jonsson, C.B.; Chu, Y.K.; Shawn Hutchinson, J.M.; Paige, R.L. A habitat-based model for the spread of hantavirus between reservoir and spillover species. J. Theor. Biol. 2009, 260, 510–522. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, V.R.; Villarreal, L.P. An introduction to the evolutionary ecology of viruses. In Viral Ecology; Hurst, C.J., Ed.; Academic Press: San Diego, USA, 2000; pp. 125–208. [Google Scholar]
- Schmaljohn, C.; Hjelle, B. Hantaviruses: A global disease problem. Emerg. Infect. Dis. 1997, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bayard, V.; Kitsutani, P.T.; Barria, E.O.; Ruedas, L.A.; Tinnin, D.S.; Munoz, C.; de Mosca, I.B.; Guerrero, G.; Kant, R.; Garcia, A.; Caceres, L.; Gracia, F.G.; Quiroz, E.; Castillo, Z.C.; Armien, B.; Libel, M.; Mills, J.N.; Khan, A.S.; Nichol, S.T.; Rollin, P.E.; Ksiazek, T.G.; Peters, C.J. Outbreak of hantavirus pulmonary syndrome, Los Santos, Panama, 1999–2000. Emerg. Infect. Dis. 2004, 10, 1635–1642. [Google Scholar] [CrossRef]
- Bayard, V.; Ortega, E.; Garcia, A.; Caceres, L.; Castillo, Z.; Quiroz, E.; Armien, B.; Gracia, F.; Serrano, J.; Guerrero, G.; Kant, R.; Pinifla, E.; Bravo, L.; Munoz, C.; de Mosca, I.B.; Rodriguez, A.; Campos, C.; Diaz, M.A.; Munoz, B.; Crespo, F.; Villalaz, I.; Rios, P.; Morales, E.; Sitton, J.M.T.; Reneau-Vernon, L.; Libel, M.; Castellanos, L.; Ruedas, L.; Tinnin, D.; Yates, T. Hantavirus pulmonary syndrome—Panama, 1999–2000. MMWR 2000, 49, 205–207, Reprinted J. Am. Med. Assoc. 2000, 283, 2232–2233. [Google Scholar]
- Williams, R.J.; Bryan, R.T.; Mills, J.N.; Palma, R.E.; Vera, I.; De Velasquez, F.; Baez, E.; Schmidt, W.E.; Figueroa, R.E.; Peters, C.J.; Zaki, S.R.; Khan, A.S.; Ksiazek, T.G. An outbreak of hantavirus pulmonary syndrome in western Paraguay. Am. J. Trop. Med. Hyg. 1997, 57, 274–282. [Google Scholar] [CrossRef]
- Mendes, W.S.; da Silva, A.A.M.; Aragao, L.F.C.; Aragao, N.J.L.; Raposo, M.D.; Elkhoury, M.R.; Suzuky, A.; Ferreira, I.B.; de Sousa, L.T.; Pannuti, C.S. Hantavirus infection in Anajatuba, Maranhao, Brazil. Emerg. Infect. Dis. 2004, 10, 1496–1498. [Google Scholar] [CrossRef]
- Glass, G.E.; Shields, T.; Cai, B.; Yates, T.L.; Parmenter, R. Persistently highest risk areas for hantavirus pulmonary syndrome: Potential sites for refugia. Ecol. Appl. 2007, 17, 129–139. [Google Scholar] [CrossRef]
- Glass, G.E.; Yates, T.L.; Fine, J.B.; Shields, T.M.; Kendall, J.B.; Hope, A.G.; Parmenter, C.A.; Peters, C.J.; Ksiazek, T.G.; Li, C.S.; Patz, J.A.; Mills, J.N. Satellite imagery characterizes local animal reservoir populations of Sin Nombre virus in the southwestern United States. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 16817–16822. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Glass, G.E.; Keesing, F. Spatial epidemiology: An emerging (or re-emerging) discipline. Trends Ecol. Evol. 2005, 20, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Carbajo, A.E.; Pardinas, U.F.J. Spatial distribution model of a Hantavirus reservoir, the long-tailed colilargo. J. Mammal. 2007, 88, 1555–1568. [Google Scholar] [CrossRef]
- Mills, J.N.; Amman, B.R.; Glass, G.E. Ecology of Hantaviruses and Their Hosts in North America. Vector Borne Zoonotic Dis. 2009, 10, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.N. Regulation of rodent-borne viruses in the natural host: Implications for human disease. Arch. Virol. Suppl. 2005, 45–57. [Google Scholar]
- Adler, F.R.; Pearce-Duvet, J.M.C.; Dearing, M.D. How host population dynamics translate into time-lagged prevalence: An investigation of Sin Nombre virus in deer mice. Bull. Math. Biol. 2008, 70, 236–252. [Google Scholar] [CrossRef]
- Clay, C.A.; Lehmer, E.M.; Previtali, A.; St. Jeor, S.; Dearing, M.D. Contact heterogeneity in deer mice: Implications for Sin Nombre virus transmission. Proc. R. Soc. B. 2009, 276, 1305–1312. [Google Scholar] [CrossRef]
- Dizney, L.J.; Ruedas, L.A. Increased host species diversity and decreased prevalence of Sin Nombre virus. Emerg. Infect. Dis. 2009, 15, 1012–1018. [Google Scholar] [CrossRef]
- Suzan, G.; Marce, E.; Giermakowski, J.T.; Mills, J.N.; Ceballos, G.; Ostfeld, R.S.; Armien, B.; Pascale, J.M.; Yates, T.L. Experimental Evidence for Reduced Rodent Diversity Causing Increased Hantavirus Prevalence. PLoS ONE 2009, 4, e5461. [Google Scholar] [CrossRef]
- Langlois, J.P.; Fahrig, L.; Merriam, G.; Artsob, H. Landscape structure influences continental distribution of hantavirus in deer mice. Landscape Ecol. 2001, 16, 255–266. [Google Scholar] [CrossRef]
- Goodin, D.G.; Paige, R.; Owen, R.D.; Ghimire, K.; Koch, D.E.; Chu, Y.-K.; Jonsson, C.B. Microhabitat characteristics of Akodon montensis, a reservoir for hantavirus, and hantaviral seroprevalence in an Atlantic forest site in eastern Paraguay. J. Vector Ecol. 2009, 34, 104–113. [Google Scholar] [CrossRef]
- Torres-Pérez, F.; Palma, R.E.; Hjelle, B.; Ferres, M.; Cook, J.A. Andes virus infections in the rodent reservoir and in humans vary across contrasting landscapes in Chile. Infect. Genet. Evol. 2010, 10, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Klempa, B. Hantaviruses and climate change. Clin Microbiol Infect 2009, 15, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Vercauteren, J.; Verstraeten, W.W.; Ducoffre, G.; Barrios, J.M.; Vandamme, A.M.; Maes, P.; Van Ranst, M. Relating increasing hantavirus incidences to the changing climate: The mast connection. Int. J. Health. Geogr. 2009, 8, 1. [Google Scholar] [CrossRef]
- Engelthaler, D.M.; Mosley, D.G.; Cheek, J.E.; Levy, C.E.; Komatsu, K.K.; Ettestad, P.; Davis, T.; Tanda, D.T.; Miller, L.; Frampton, J.W.; Porter, R.; Bryan, R.T. Climatic and environmental patterns associated with hantavirus pulmonary syndrome, Four Corners region, United States. Emerg. Infect. Dis. 1999, 5, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Jaksic, F.M.; Lima, M. Myths and facts on ratadas: Bamboo blooms, rainfall peaks and rodent outbreaks in South America. Austral Ecol. 2003, 28, 237–251. [Google Scholar] [CrossRef]
- Buceta, J.; Escudero, C.; de la Rubia, F.J.; Lindenberg, K. Outbreaks of Hantavirus induced by seasonality. Phys. Rev. E 2004, 69, 8. [Google Scholar] [CrossRef]
- Weiss, R.A.; McMichael, A.J. Social and environmental risk factors in the emergence of infectious diseases. Nat. Med. 2004, 10, S70–S76. [Google Scholar] [CrossRef]
- Sauvage, F.; Langlais, M.; Pontier, D. Predicting the emergence of human hantavirus disease using a combination of viral dynamics and rodent demographic patterns. Epidemiol. Infect. 2007, 135, 46–56. [Google Scholar] [CrossRef]
| Virus strain | Acronym | Geographic Distribution | Host | Pathology | Case-fatality ratio (%) | |
| New World | Sin Nombre | SNV | North America | Peromyscus maniculatus | HCPS | –35 |
| Choclo | CHOV | Panamá | Oligoryzomys fulvescens | HCPS | ~21 | |
| Andes | ANDV | Argentina, Chile | Oligoryzomys longicaudatus | HCPS | 35 | |
| Laguna Negra | LANV | Argentina, Bolivia, Paraguay, | Calomys laucha, C. callosus | HCPS | 5–15 | |
| Old World | Hantaan | HTNV | China, Korea, Russia | Apodemus agrarius | HFRS | 5–0 |
| Seoul | SEOV | Worldwide | Rattus rattus, R. norvegicus | HFRS | 1–2 | |
| Puumala | PUUV | Scandinavia, western Europe, Russia | Myodes glareolus | HFRS/NE | 0.1–0.4 | |
| Dobrava-Belgrade | DOBV | Balkans | Apodemus flavicollis | HFRS | 5–10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hjelle, B.; Torres-Pérez, F. Hantaviruses in the Americas and Their Role as Emerging Pathogens. Viruses 2010, 2, 2559-2586. https://doi.org/10.3390/v2122559
Hjelle B, Torres-Pérez F. Hantaviruses in the Americas and Their Role as Emerging Pathogens. Viruses. 2010; 2(12):2559-2586. https://doi.org/10.3390/v2122559
Chicago/Turabian StyleHjelle, Brian, and Fernando Torres-Pérez. 2010. "Hantaviruses in the Americas and Their Role as Emerging Pathogens" Viruses 2, no. 12: 2559-2586. https://doi.org/10.3390/v2122559
APA StyleHjelle, B., & Torres-Pérez, F. (2010). Hantaviruses in the Americas and Their Role as Emerging Pathogens. Viruses, 2(12), 2559-2586. https://doi.org/10.3390/v2122559




