FMDV VP3 Induces IL-10 Expression in Porcine Macrophages via PI3K Interaction and PI3K/AKT-mTOR Pathway Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Viral Plasmids
2.2. Plasmid Transfection
2.3. RT-qPCR
2.4. Signaling Pathway Inhibition
2.5. Western Blot Analysis
2.6. ELISA for Detection
2.7. Co-IP
2.8. Immunofluorescence and Confocal Microscopy
2.9. Dual Fluorescein Reporter Gene Detection of IL-10 Promoter Activity
2.10. Data Analysis
3. Results
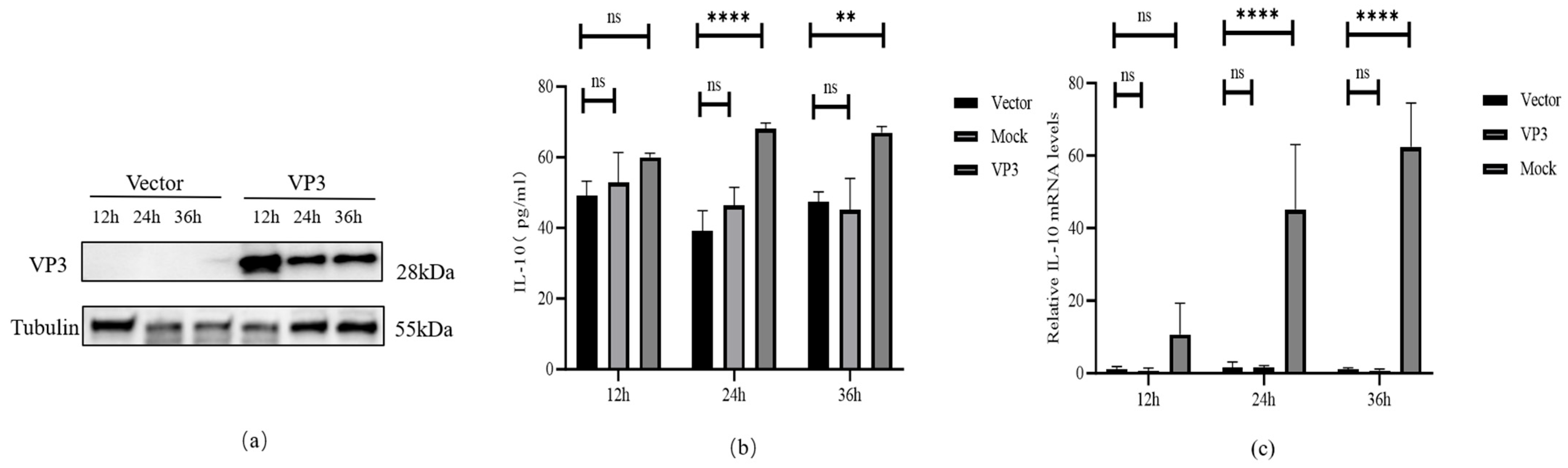
3.1. FMDV VP3 Induces High Level of IL-10 Expression in Porcine Alveolar Macrophages
3.2. FMDV VP3 Activates the PI3K/AKT-mTOR Pathway in 3D4/21 Macrophages
3.3. Inhibition of PI3K Phosphorylation Attenuates VP3-Induced IL-10 Expression in 3D4/21 Macrophages
3.4. FMDV VP3 Interacts with PI3K
3.5. FMDV VP3 Regulates Transcription Factors STAT3, ATF1, and CREB to Drive IL-10 Promoter Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Wang, J.; Liu, J.; Li, Z.; Wang, Y.; Xue, Y.; Li, X.; Cao, H.; Zheng, S.J. Engagement of soluble resistance-related calcium binding protein (sorcin) with foot-and-mouth disease virus (FMDV) VP1 inhibits type I interferon response in cells. Vet. Microbiol. 2013, 166, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Liu, T.; Qi, X.; Wang, Y.; Ren, J.; Peng, J.; Du, X.; Hu, S.; Wu, S.; Zhao, Y.; et al. A genome-wide CRISPR screening uncovers that TOB1 acts as a key host factor for FMDV infection via both IFN and EGFR mediated pathways. PLoS Pathog. 2024, 20, e1012104. [Google Scholar] [CrossRef]
- Rodriguez, L.L.; Gay, C.G. Development of vaccines toward the global control and eradication of foot-and-mouth disease. Expert. Rev. Vaccines 2011, 10, 377–387. [Google Scholar] [CrossRef]
- Sarry, M.; Romey, A.; Lefebvre, D.; Benfrid, S.; Dufour, B.; Durand, B.; Zanella, G.; De Regge, N.; Zientara, S.; Bakkali Kassimi, L.; et al. Foot and mouth disease virus: Transmission, pathogenesis, diagnosis and surveillance. Virologie 2022, 26, 355–373. [Google Scholar] [PubMed]
- Li, G.; Wubshet, A.K.; Ding, Y.; Li, Q.; Dai, J.; Wang, Y.; Hou, Q.; Chen, J.; Ma, B.; Szczotka-Bochniarz, A.; et al. Antigenicity and Immunogenicity Analysis of the E. coli Expressed FMDV Structural Proteins; VP1, VP0, VP3 of the South African Territories Type 2 Virus. Viruses 2021, 13, 1005. [Google Scholar] [CrossRef]
- Ekanayaka, P.; Lee, S.Y.; Herath, T.; Kim, J.-H.; Kim, T.-H.; Lee, H.; Chathuranga, K.; Chathuranga, W.A.G.; Park, J.-H.; Lee, J.-S. Foot-and-mouth disease virus VP1 target the MAVS to inhibit type-I interferon signaling and VP1 E83K mutation results in virus attenuation. PLoS Pathog. 2020, 16, e1009057. [Google Scholar] [CrossRef]
- Giroud, P.; Renaudineau, S.; Gudefin, L.; Calcei, A.; Menguy, T.; Rozan, C.; Mizrahi, J.; Caux, C.; Duong, V.; Valladeau-Guilemond, J. Expression of TAM-R in Human Immune Cells and Unique Regulatory Function of MerTK in IL-10 Production by Tolerogenic DC. Front. Immunol. 2020, 11, 564133. [Google Scholar] [CrossRef]
- Fu, S.; Li, J.; You, J.; Liu, S.; Dong, Q.; Fu, Y.; Luo, R.; Sun, Y.; Tian, X.; Liu, W.; et al. Baicalin attenuates PD-1/PD-L1 axis-induced immunosuppression in piglets challenged with Glaesserella parasuis by inhibiting the PI3K/Akt/mTOR and RAS/MEK/ERK signalling pathways. Vet. Res. 2024, 55, 95. [Google Scholar] [CrossRef]
- Sabat, R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010, 21, 315–324. [Google Scholar] [CrossRef]
- Cerqueira, C.; Manfroi, B.; Fillatreau, S. IL-10-producing regulatory B cells and plasmocytes: Molecular mechanisms and disease relevance. Semin. Immunol. 2019, 44, 101323. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, S.; Zhang, Y.; Chen, M.; Lv, Y. Porcine circovirus type 2 increases interleukin-1beta and interleukin-10 production via the MyD88-NF-kappa B signaling pathway in porcine alveolar macrophages in vitro. J. Vet. Sci. 2017, 18, 183–191. [Google Scholar] [CrossRef]
- Song, S.; Bi, J.; Wang, D.; Fang, L.; Zhang, L.; Li, F.; Chen, H.; Xiao, S. Porcine reproductive and respiratory syndrome virus infection activates IL-10 production through NF-kappaB and p38 MAPK pathways in porcine alveolar macrophages. Dev. Comp. Immunol. 2013, 39, 265–272. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Zhang, Y.; Zhu, X.; Ren, S.; Guo, L.; Liu, X.; Sun, W.; Chen, Z.; Cong, X.; et al. The integrity of PRRSV nucleocapsid protein is necessary for up-regulation of optimal interleukin-10 through NF-kappaB and p38 MAPK pathways in porcine alveolar macrophages. Microb. Pathog. 2017, 109, 319–324. [Google Scholar] [CrossRef]
- Sei, J.J.; Waters, R.A.; Kenney, M.; Barlow, J.W.; Golde, W.T. Effect of Foot-and-Mouth Disease Virus Infection on the Frequency, Phenotype and Function of Circulating Dendritic Cells in Cattle. PLoS ONE 2016, 11, e152192. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Y.; Zhang, Z.; Li, Y. Interleukin-10-Mediated Lymphopenia Caused by Acute Infection with Foot-and-Mouth Disease Virus in Mice. Viruses 2021, 13, 2358. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, F.; Zhao, S.; Zhang, Z.; Zhang, H.; Bai, L.; Zhang, Z.; Li, Y. IL-10 Promotes CXCL13 Expression in Macrophages Following Foot-and-Mouth Disease Virus Infection. Int. J. Mol. Sci. 2023, 24, 6322. [Google Scholar] [CrossRef] [PubMed]
- Díaz-San Segundo, F.; Rodríguez-Calvo, T.; de Avila, A.; Sevilla, N. Immunosuppression during acute infection with foot-and-mouth disease virus in swine is mediated by IL-10. PLoS ONE 2009, 4, e5659. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; He, Q.; Zhang, Y.; Li, Y.; Zhang, Z. Mechanisms of Interleukin-10-Mediated Immunosuppression in Viral Infections. Pathogens 2025, 14, 989. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, P.; Lee, B.H.; Weerawardhana, A.; Chathuranga, K.; Park, J.-H.; Lee, J.-S. Inhibition of MAVS Aggregation-Mediated Type-I Interferon Signaling by Foot-and-Mouth Disease Virus VP3. Viruses 2021, 13, 1776. [Google Scholar] [CrossRef]
- Li, D.; Wei, J.; Yang, F.; Liu, H.-N.; Zhu, Z.-X.; Cao, W.-J.; Li, S.; Liu, X.-T.; Zheng, H.-X.; Shu, H.-B. Foot-and-mouth disease virus structural protein VP3 degrades Janus kinase 1 to inhibit IFN-gamma signal transduction pathways. Cell Cycle 2016, 15, 850–860. [Google Scholar] [CrossRef]
- Mao, R.; Zhu, Z.; Yang, F.; Sun, D.; Zhou, X.; Cao, W.; Qin, X.; Dang, W.; Liu, H.; Tian, H.; et al. Picornavirus VP3 protein induces autophagy through the TP53-BAD-BAX axis to promote viral replication. Autophagy 2024, 20, 1928–1947. [Google Scholar] [CrossRef]
- Du, Y.; Bi, J.; Liu, J.; Liu, X.; Wu, X.; Jiang, P.; Yoo, D.; Zhang, Y.; Wu, J.; Wan, R.; et al. 3Cpro of foot-and-mouth disease virus antagonizes the interferon signaling pathway by blocking STAT1/STAT2 nuclear translocation. J. Virol. 2014, 88, 4908–4920. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Saemann, M.D. The PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic applications. Ann. Rheum. Dis. 2008, 67, i70–i74. [Google Scholar] [CrossRef] [PubMed]
- Cheekatla, S.S.; Aggarwal, A.; Naik, S. mTOR signaling pathway regulates the IL-12/IL-10 axis in Leishmania donovani infection. Med. Microbiol. Immun. 2012, 201, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Schifferle, R.E.; Cuesta, N.; Vogel, S.N.; Katz, J.; Michalek, S.M. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J. Immunol. 2003, 171, 717–725. [Google Scholar] [CrossRef]
| Gene Product | Sense Primer (5′ to 3′) | Antisense Primer (5′ to 3′) |
|---|---|---|
| IL-10 | CGTGGAGGAGGTGAGAGAGTG | TTAGTAGAGTCGTCATCCTGGAAG |
| STAT3 | ACAGGATCCTTGACGAGCAC | CTCTCTGAGCCTGTTCCT |
| CREB | CATGGAATCTGGAGCAGACAA | CTGGGCTAATGTGGCAATCT |
| C-FOS | GGCAAGGTGGAACAGTTGTC | CGCTTGGAGTGTGTCAGTCA |
| ATF1 | TTGTGCCCAGCAACCAAGTGG | CACGGTCTGTGCAGGGAAAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li, Y.; Guo, Z.; Zhang, Y.; Luo, L.; He, C.; Xia, Q.; Zhang, J.; Zhang, Z.; Li, Y. FMDV VP3 Induces IL-10 Expression in Porcine Macrophages via PI3K Interaction and PI3K/AKT-mTOR Pathway Activation. Viruses 2026, 18, 66. https://doi.org/10.3390/v18010066
Li Y, Guo Z, Zhang Y, Luo L, He C, Xia Q, Zhang J, Zhang Z, Li Y. FMDV VP3 Induces IL-10 Expression in Porcine Macrophages via PI3K Interaction and PI3K/AKT-mTOR Pathway Activation. Viruses. 2026; 18(1):66. https://doi.org/10.3390/v18010066
Chicago/Turabian StyleLi, Yuling, Zijing Guo, Yan Zhang, Li Luo, Chunsai He, Qiqi Xia, Jingyuan Zhang, Zhidong Zhang, and Yanmin Li. 2026. "FMDV VP3 Induces IL-10 Expression in Porcine Macrophages via PI3K Interaction and PI3K/AKT-mTOR Pathway Activation" Viruses 18, no. 1: 66. https://doi.org/10.3390/v18010066
APA StyleLi, Y., Guo, Z., Zhang, Y., Luo, L., He, C., Xia, Q., Zhang, J., Zhang, Z., & Li, Y. (2026). FMDV VP3 Induces IL-10 Expression in Porcine Macrophages via PI3K Interaction and PI3K/AKT-mTOR Pathway Activation. Viruses, 18(1), 66. https://doi.org/10.3390/v18010066









