Genome-Wide Variation Profile of the Genus Tobamovirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Tobamovirus Genomic Sequences
2.2. Tobamovirus Phylogeny
2.3. Single-Nucleotide Polymorphism and Nucleotide Diversity
2.4. Selection Analyses
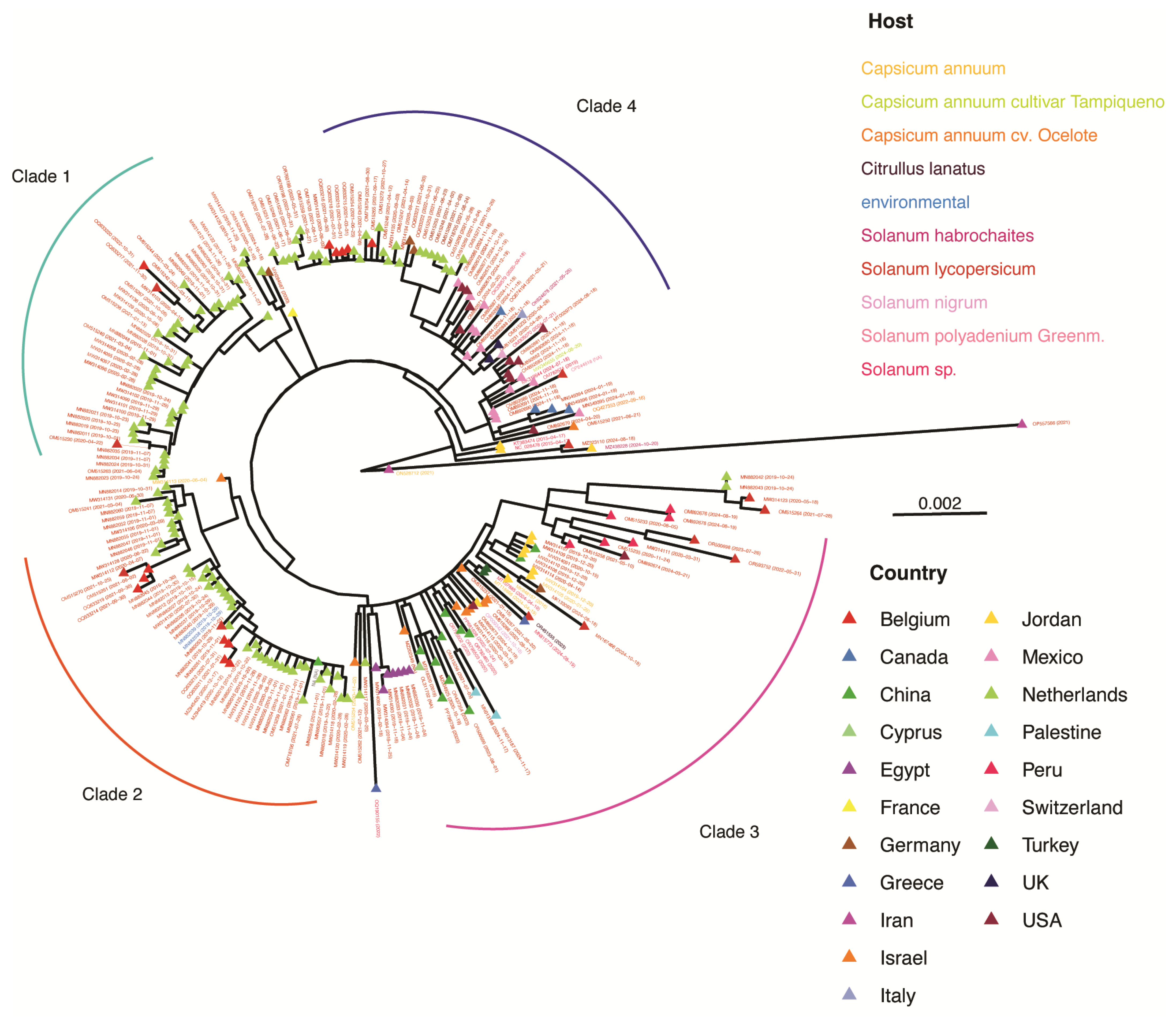
2.5. Maximum Likelihood Phylogenetic Tree for T. fructirugosum
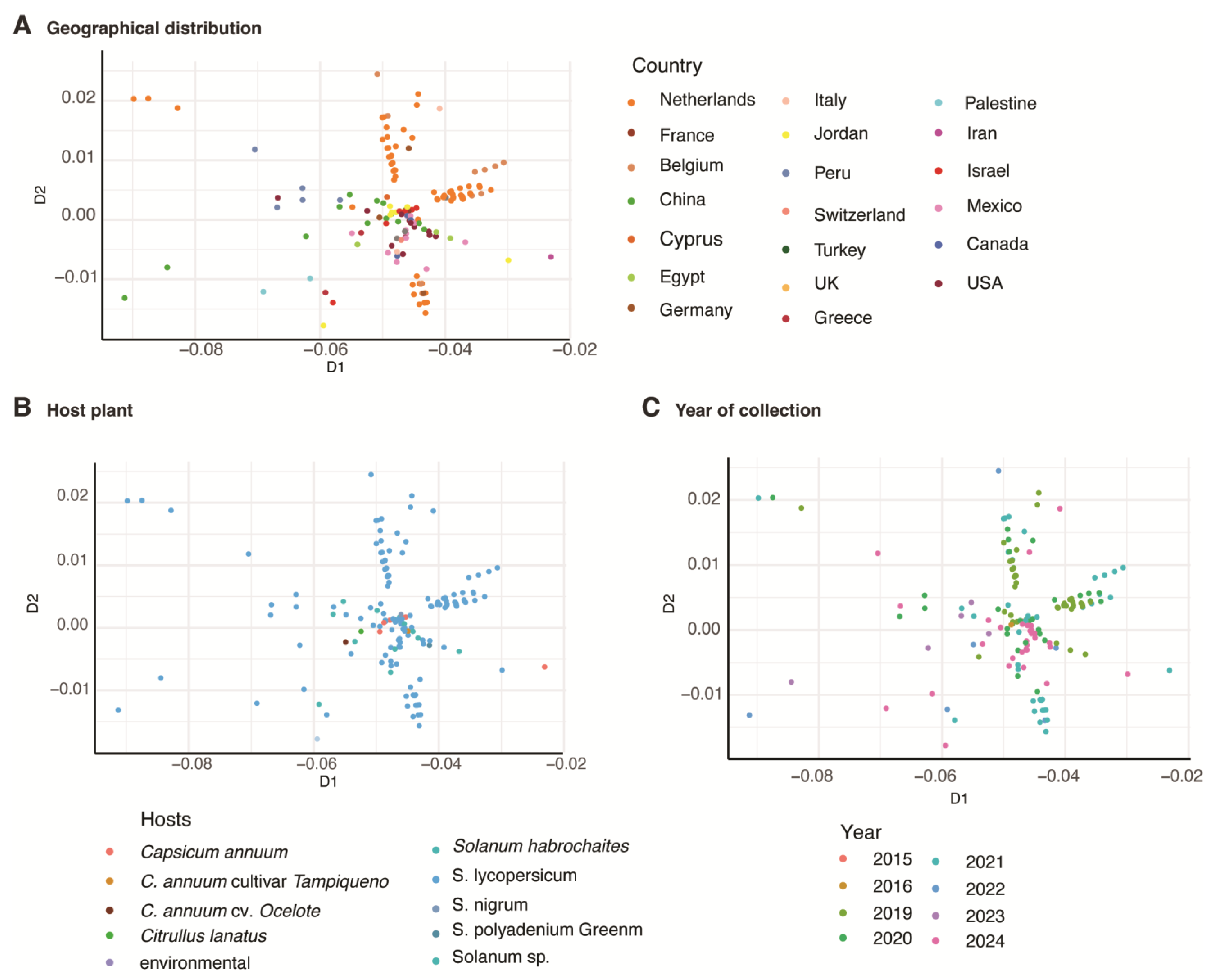
2.6. Multidimensional Scaling
3. Results
3.1. Tobamovirus Group According to the Botanical Family of Their Host
3.2. Tobamovirus Genome Diversity
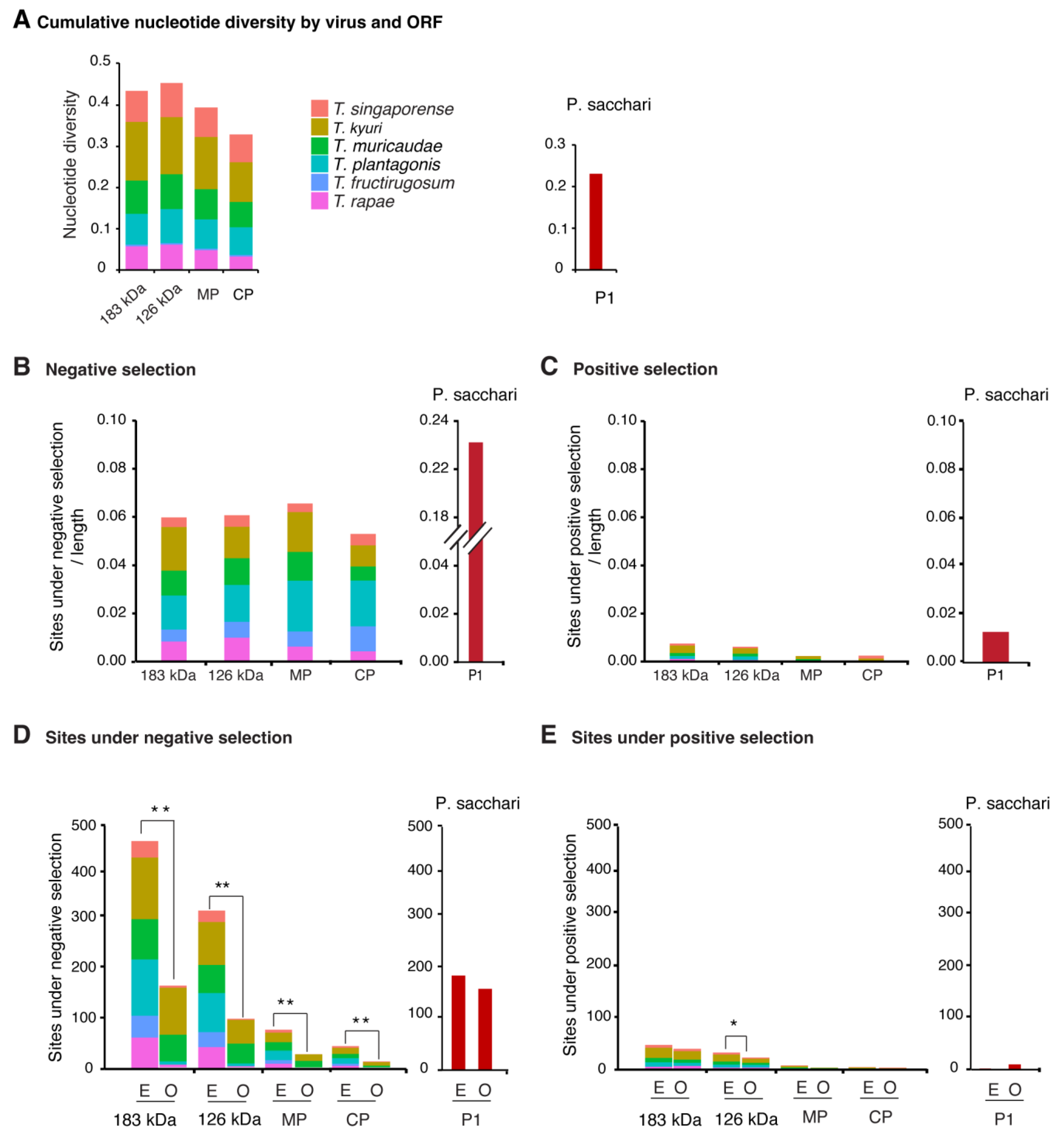
3.3. Nucleotide Diversity by Open Reading Frame
3.4. Selection Analysis by Open Reading Frame
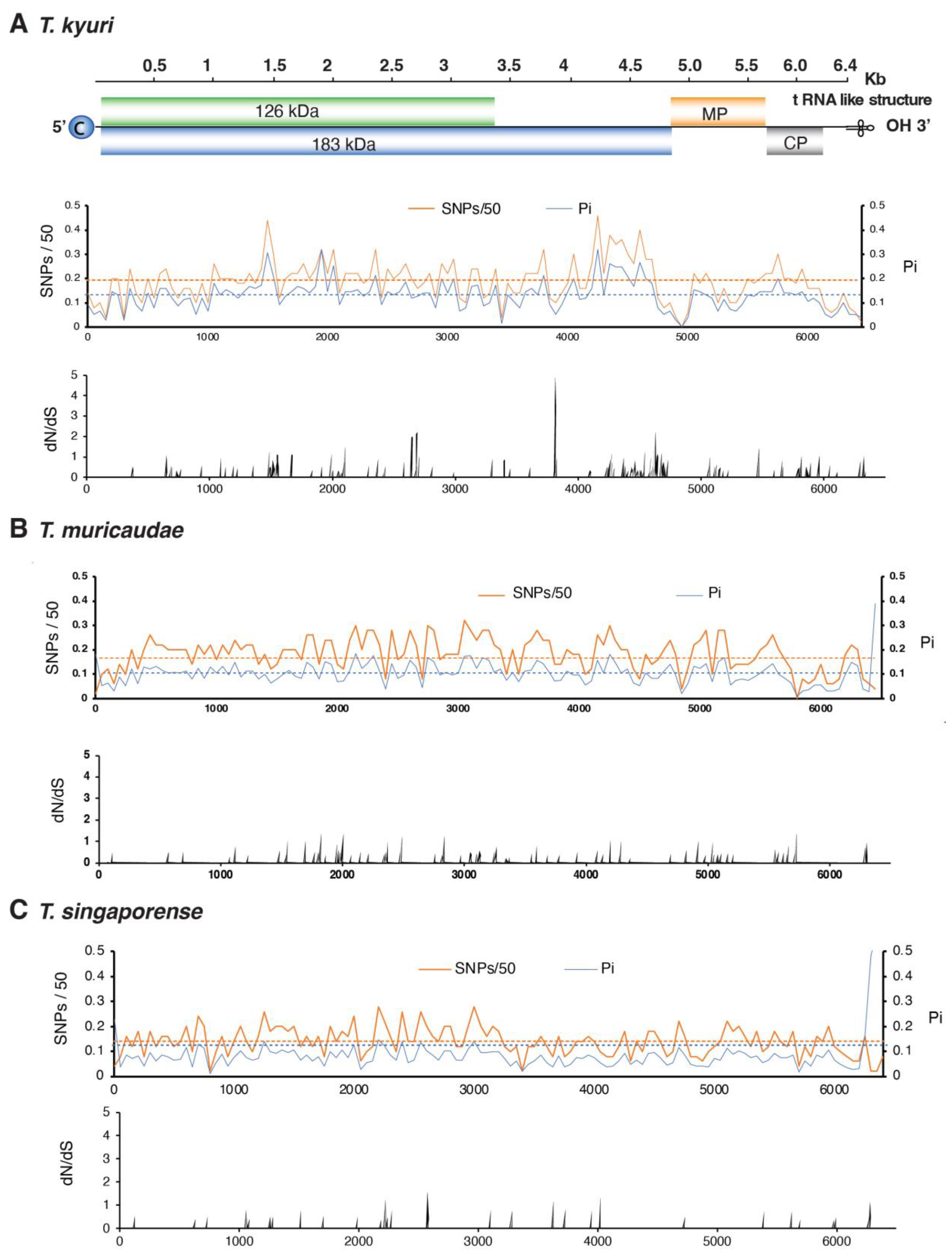
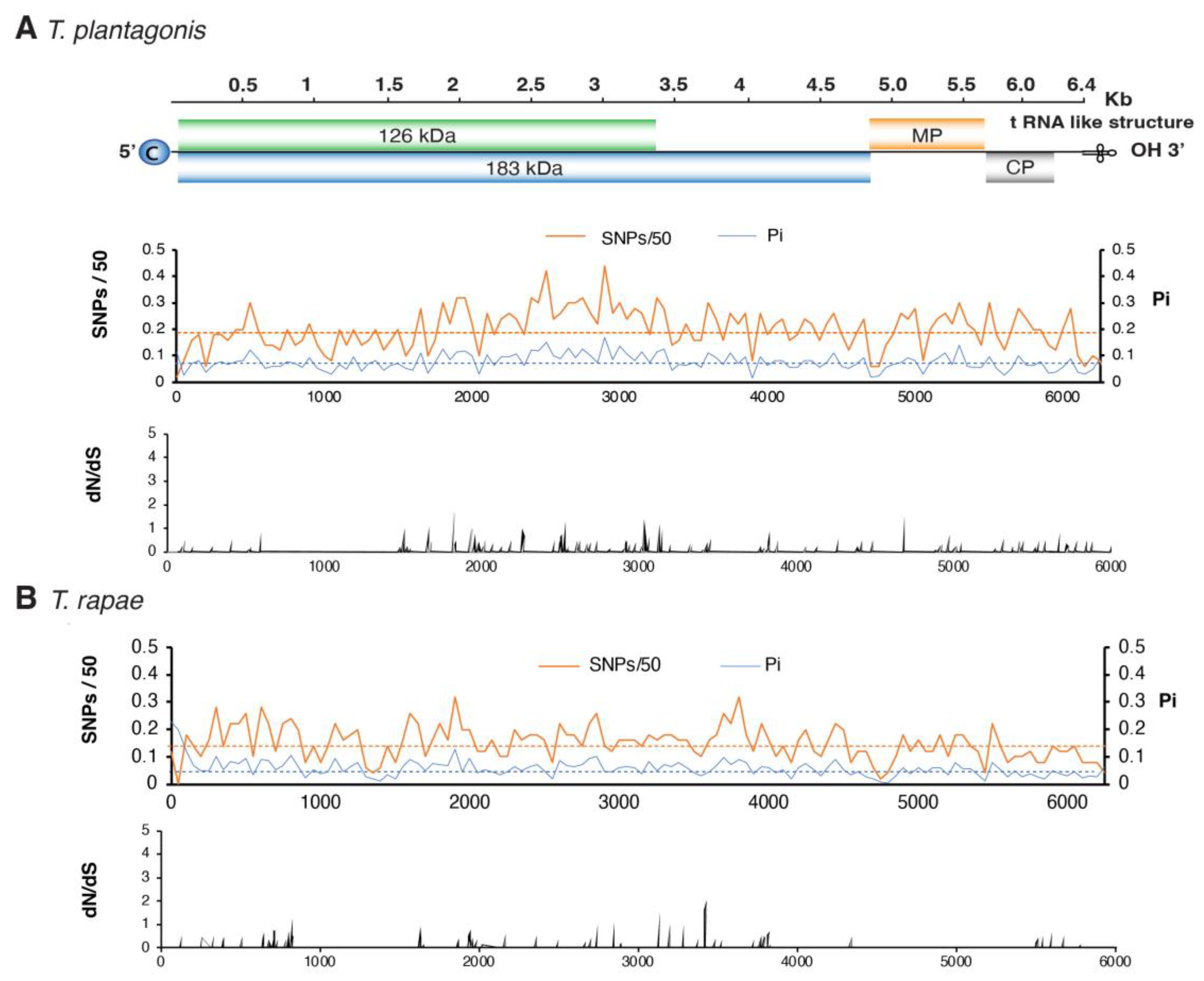
3.5. No Hypervariable Areas Were Detected in the Tobamovirus Genome
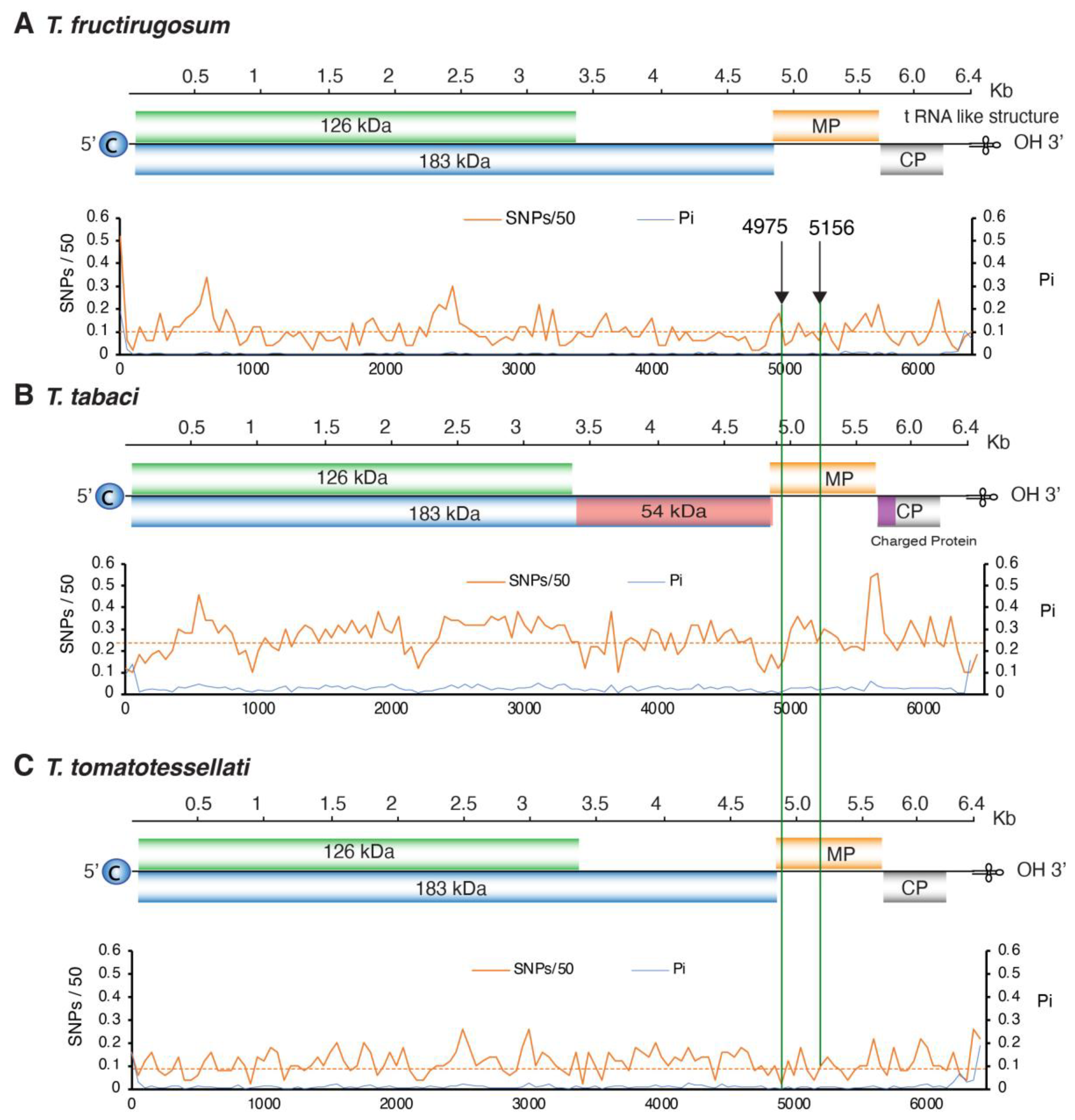
3.6. Variation in T. fructirugosum
3.7. Phylogenetic Analysis of T. fructirugosum
3.8. Geographical Distribution Correlates with Virus Variation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorokhov, Y.L.; Sheshukova, E.V.; Komarova, T.V. Tobamoviruses and their diversity. In Plant Viruses; CRC Press: Boca Raton, FL, USA, 2018; pp. 65–80. [Google Scholar]
- Spiegelman, Z.; Dinesh-Kumar, S.P. Breaking Boundaries: The Perpetual Interplay Between Tobamoviruses and Plant Immunity. Annu. Rev. Virol. 2023, 10, 455–476. [Google Scholar] [CrossRef]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A. A new Israeli Tobamovirus isolate infects tomato plants harboring Tm-22 resistance genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.W.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef]
- Carr, J.P. Engineered Resistance to Tobamoviruses. Viruses 2024, 16, 1007. [Google Scholar] [CrossRef] [PubMed]
- Dombrovsky, A.; Smith, E. Seed Transmission of Tobamoviruses: Aspects of Global Disease Distribution. In Advances in Seed Biology; InTech: London, UK, 2017. [Google Scholar]
- Darzi, E.; Smith, E.; Shargil, D.; Lachman, O.; Ganot, L.; Dombrovsky, A. The honeybee Apis mellifera contributes to Cucumber green mottle mosaic virus spread via pollination. Plant Pathol. 2018, 67, 244–251. [Google Scholar] [CrossRef]
- Okada, K.; Kusakari, S.-i.; Kawaratani, M.; Negoro, J.-I.; Ohki, S.T.; Osaki, T. Tobacco mosaic virus is transmissible from tomato to tomato by pollinating bumblebees. J. Gen. Plant Pathol. 2000, 66, 71–74. [Google Scholar] [CrossRef]
- Ishibashi, K.; Ishikawa, M. Replication of tobamovirus RNA. Annu. Rev. Phytopathol. 2016, 54, 55–78. [Google Scholar] [CrossRef]
- Ahola, T.; Laakkonen, P.; Vihinen, H.; Kääriäinen, L. Critical residues of Semliki Forest virus RNA capping enzyme involved in methyltransferase and guanylyltransferase-like activities. J. Virol. 1997, 71, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Ahola, T.; den Boon, J.A.; Ahlquist, P. Helicase and capping enzyme active site mutations in brome mosaic virus protein 1a cause defects in template recruitment, negative-strand RNA synthesis, and viral RNA capping. J. Virol. 2000, 74, 8803–8811. [Google Scholar] [CrossRef]
- Fernández, A.; Laín, S.; García, J.A. RNA helicase activity of the plum pox potyvirus CI protein expressed in Escherichia coli. Mapping of an RNA binding domain. Nucleic Acids Res. 1995, 23, 1327–1332. [Google Scholar] [CrossRef]
- Goregaoker, S.P.; Culver, J.N. Oligomerization and activity of the helicase domain of the tobacco mosaic virus 126- and 183-kilodalton replicase proteins. J. Virol. 2003, 77, 3549–3556. [Google Scholar] [CrossRef] [PubMed]
- Palukaitis, P.; Akbarimotlagh, M.; Astaraki, S.; Shams-Bakhsh, M.; Yoon, J.Y. The Forgotten Tobamovirus Genes Encoding the 54 kDa Protein and the 4–6 kDa Proteins. Viruses 2024, 16, 1680. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, D.J.; Dawson, W.O. Functions of the 126- and 183-kDa proteins of tobacco mosaic virus. Virology 2000, 271, 90–98. [Google Scholar] [CrossRef]
- Chavan, R.R.; Pearson, M.N. Molecular characterisation of a novel recombinant Ribgrass mosaic virus strain FSHS. Virol. J. 2016, 13, 29. [Google Scholar] [CrossRef]
- Fraile, A.; Escriu, F.; Aranda, M.A.; Malpica, J.M.; Gibbs, A.J.; Garcia-Arenal, F. A century of tobamovirus evolution in an Australian population of Nicotiana glauca. J. Virol. 1997, 71, 8316–8320. [Google Scholar] [CrossRef]
- van de Vossenberg, B.T.L.H.; Dawood, T.; Woźny, M.; Botermans, M. First Expansion of the Public Tomato Brown Rugose Fruit Virus (ToBRFV) Nextstrain Build; Inclusion of New Genomic and Epidemiological Data. PhytoFrontiers™ 2021, 1, 359–363. [Google Scholar] [CrossRef]
- Chanda, B.; Gilliard, A.; Jaiswal, N.; Ling, K.S. Comparative Analysis of Host Range, Ability to Infect Tomato Cultivars with Tm-22 Gene, and Real-Time Reverse Transcription PCR Detection of Tomato Brown Rugose Fruit Virus. Plant Dis. 2021, 105, 3643–3652. [Google Scholar] [CrossRef]
- Zhang, S.; Griffiths, J.S.; Marchand, G.; Bernards, M.A.; Wang, A. Tomato brown rugose fruit virus: An emerging and rapidly spreading plant RNA virus that threatens tomato production worldwide. Mol. Plant Pathol. 2022, 23, 1262–1277. [Google Scholar] [CrossRef]
- Ghorbani, A. Genetic analysis of tomato brown rugose fruit virus reveals evolutionary adaptation and codon usage bias patterns. Sci. Rep. 2024, 14, 21281. [Google Scholar] [CrossRef]
- Zamfir, A.D.; Babalola, B.M.; Fraile, A.; McLeish, M.J.; García-Arenal, F. Tobamoviruses Show Broad Host Ranges and Little Genetic Diversity Among Four Habitat Types of a Heterogeneous Ecosystem. Phytopathology 2023, 113, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Maayan, Y.; Pandaranayaka, E.P.J.; Srivastava, D.A.; Lapidot, M.; Levin, I.; Dombrovsky, A.; Harel, A. Using genomic analysis to identify tomato Tm-2 resistance-breaking mutations and their underlying evolutionary path in a new and emerging tobamovirus. Arch. Virol. 2018, 163, 1863–1875. [Google Scholar] [CrossRef]
- Pagán, I.; Firth, C.; Holmes, E.C. Phylogenetic analysis reveals rapid evolutionary dynamics in the plant RNA virus genus tobamovirus. J. Mol. Evol. 2010, 71, 298–307. [Google Scholar] [CrossRef]
- Chare, E.R.; Holmes, E.C. Selection pressures in the capsid genes of plant RNA viruses reflect mode of transmission. J. Gen. Virol. 2004, 85, 3149–3157. [Google Scholar] [CrossRef]
- Adams, M.J.; Adkins, S.; Bragard, C.; Gilmer, D.; Li, D.; MacFarlane, S.A.; Wong, S.M.; Melcher, U.; Ratti, C.; Ryu, K.H.; et al. ICTV Virus Taxonomy Profile: Virgaviridae. J. Gen. Virol. 2017, 98, 1999–2000. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wan, Z.; Coarfa, C.; Drabek, R.; Chen, L.; Ostrowski, E.A.; Liu, Y.; Weinstock, G.M.; Wheeler, D.A.; Gibbs, R.A. A SNP discovery method to assess variant allele probability from next-generation resequencing data. Genome Res. 2010, 20, 273–280. [Google Scholar] [CrossRef] [PubMed]
- LaTourrette, K.; Holste, N.M.; Garcia-Ruiz, H. Polerovirus genomic variation. Virus Evol. 2021, 7, veab102. [Google Scholar] [CrossRef]
- Pitman, T.; Posis, K.; Tian, T.; Belanger, C.; Roy, A.; Falk, B. First report of watermelon green mottle mosaic virus in North America. Plant Dis. 2019, 103, 3288. [Google Scholar] [CrossRef]
- Rambaut, A. Fig Tree: Tree Figure Drawing Tool, Version 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2016. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 10 January 2025).
- Nigam, D.; LaTourrette, K.; Souza, P.F.N.; Garcia-Ruiz, H. Genome-Wide Variation in Potyviruses. Front. Plant Sci. 2019, 10, 1439. [Google Scholar] [CrossRef]
- Hazra, A. Using the confidence interval confidently. J. Thorac. Dis. 2017, 9, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Nigam, D.; Garcia-Ruiz, H. Variation Profile of the Orthotospovirus Genome. Pathogens 2020, 9, 521. [Google Scholar] [CrossRef]
- Korunes, K.L.; Samuk, K. Pixy: Unbiased estimation of nucleotide diversity and divergence in the presence of missing data. Mol. Ecol. Resour. 2021, 21, 1359–1368. [Google Scholar] [CrossRef]
- Delport, W.; Poon, A.F.; Frost, S.D.; Kosakovsky Pond, S.L. Datamonkey 2010: A suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 2010, 26, 2455–2457. [Google Scholar] [CrossRef]
- Murrell, B.; Wertheim, J.O.; Moola, S.; Weighill, T.; Scheffler, K.; Kosakovsky Pond, S.L. Detecting individual sites subject to episodic diversifying selection. PLoS Genet. 2012, 8, e1002764. [Google Scholar] [CrossRef] [PubMed]
- Kryazhimskiy, S.; Plotkin, J.B. The population genetics of dN/dS. PLoS Genet. 2008, 4, e1000304. [Google Scholar] [CrossRef]
- Mugal, C.F.; Wolf, J.B.W.; Kaj, I. Why Time Matters: Codon Evolution and the Temporal Dynamics of dN/dS. Mol. Biol. Evol. 2013, 31, 212–231. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, J.B. Multidimensional Scaling; Bell Laboratories: Murray Hill, NJ, USA, 1978. [Google Scholar]
- Braidwood, L.; Quito-Avila, D.F.; Cabanas, D.; Bressan, A.; Wangai, A.; Baulcombe, D.C. Maize chlorotic mottle virus exhibits low divergence between differentiated regional sub-populations. Sci. Rep. 2018, 8, 1173. [Google Scholar] [CrossRef]
- Rubio, L.; Galipienso, L.; Ferriol, I. Detection of plant viruses and disease management: Relevance of genetic diversity and evolution. Front. Plant Sci. 2020, 11, 1092. [Google Scholar] [CrossRef]
- Bhatt, S.; Katzourakis, A.; Pybus, O.G. Detecting natural selection in RNA virus populations using sequence summary statistics. Infect. Genet. Evol. 2010, 10, 421–430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Z.; Nielsen, R.; Goldman, N.; Pedersen, A.-M.K. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 2000, 155, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Gojobori, T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986, 3, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Jewehan, A.; Kiemo, F.W.; Salem, N.; Tóth, Z.; Salamon, P.; Szabó, Z. Isolation and molecular characterization of a tomato brown rugose fruit virus mutant breaking the tobamovirus resistance found in wild Solanum species. Arch. Virol. 2022, 167, 1559–1563. [Google Scholar] [CrossRef] [PubMed]
- Zisi, Z.; Ghijselings, L.; Vogel, E.; Vos, C.; Matthijnssens, J. Single amino acid change in tomato brown rugose fruit virus breaks virus-specific resistance in new resistant tomato cultivar. Front. Plant Sci. 2024, 15, 1382862. [Google Scholar] [CrossRef]
- Gibbs, A. Evolution and origins of tobamoviruses. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999, 354, 593–602. [Google Scholar] [CrossRef]
- Fraile, A.; García-Arenal, F. Tobamoviruses as models for the study of virus evolution. Adv. Virus Res. 2018, 102, 89–117. [Google Scholar]
- Yan, Z.Y.; Ma, H.Y.; Wang, L.; Tettey, C.; Zhao, M.S.; Geng, C.; Tian, Y.P.; Li, X.D. Identification of genetic determinants of tomato brown rugose fruit virus that enable infection of plants harbouring the Tm-22 resistance gene. Mol. Plant Pathol. 2021, 22, 1347–1357. [Google Scholar] [CrossRef]
- Hak, H.; Raanan, H.; Schwarz, S.; Sherman, Y.; Dinesh-Kumar, S.P.; Spiegelman, Z. Activation of Tm-22 resistance is mediated by a conserved cysteine essential for tobacco mosaic virus movement. Mol. Plant Pathol. 2023, 24, 838–848. [Google Scholar] [CrossRef]
- Caruso, A.G.; Bertacca, S.; Parrella, G.; Rizzo, R.; Davino, S.; Panno, S. Tomato brown rugose fruit virus: A pathogen that is changing the tomato production worldwide. Ann. Appl. Biol. 2022, 181, 258–274. [Google Scholar] [CrossRef]
- LaTourrette, K.; Holste, N.M.; Rodriguez-Peña, R.; Leme, R.A.; Garcia-Ruiz, H. Genome-Wide Variation in Betacoronaviruses. J. Virol. 2021, 95, e0049621. [Google Scholar] [CrossRef]
- Gibbs, A.J.; Wood, J.; Garcia-Arenal, F.; Ohshima, K.; Armstrong, J.S. Tobamoviruses have probably co-diverged with their eudicotyledonous hosts for at least 110 million years. Virus Evol. 2015, 1, vev019. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Yang, Y.; Sun, X.; Jiang, S.; Hong, H.; Zhu, X.; Liu, Y. Genetic Variability and Molecular Evolution of Tomato Mosaic Virus Populations in Three Northern China Provinces. Viruses 2023, 15, 1617. [Google Scholar] [CrossRef] [PubMed]
- Abrahamian, P.; Cai, W.; Nunziata, S.O.; Ling, K.S.; Jaiswal, N.; Mavrodieva, V.A.; Rivera, Y.; Nakhla, M.K. Comparative Analysis of Tomato Brown Rugose Fruit Virus Isolates Shows Limited Genetic Diversity. Viruses 2022, 14, 2816. [Google Scholar] [CrossRef]
- Esmaeilzadeh, F.; Santosa, A.I.; Çelik, A.; Koolivand, D. Revealing an Iranian Isolate of Tomato Brown Rugose Fruit Virus: Complete Genome Analysis and Mechanical Transmission. Microorganisms 2023, 11, 2434. [Google Scholar] [CrossRef] [PubMed]
- LaTourrette, K.; Garcia-Ruiz, H. Determinants of virus variation, evolution, and host adaptation. Pathogens 2022, 11, 1039. [Google Scholar] [CrossRef]
- Ju, H.-K.; Kim, I.-H.; Hu, W.-X.; Kim, B.; Choi, G.-W.; Kim, J.; Lim, Y.P.; Domier, L.L.; Hammond, J.; Lim, H.-S. A single nucleotide change in the overlapping MP and CP reading frames results in differences in symptoms caused by two isolates of Youcai mosaic virus. Arch. Virol. 2019, 164, 1553–1565. [Google Scholar] [CrossRef]
- Gomaa, A.E.; El Mounadi, K.; Parperides, E.; Garcia-Ruiz, H. Cell Fractionation and the Identification of Host Proteins Involved in Plant–Virus Interactions. Pathogens 2024, 13, 53. [Google Scholar] [CrossRef]
- Jewehan, A.; Salem, N.; Tóth, Z.; Salamon, P.; Szabó, Z. Screening of Solanum (sections Lycopersicon and Juglandifolia) germplasm for reactions to the tomato brown rugose fruit virus (ToBRFV). J. Plant Dis. Prot. 2022, 129, 117–123. [Google Scholar] [CrossRef]
- Garcia-Ruiz, H.; Diaz, A.; Ahlquist, P. Intermolecular RNA recombination occurs at different frequencies in alternate forms of brome mosaic virus RNA replication compartments. Viruses 2018, 10, 131. [Google Scholar] [CrossRef]




| Species Name | Common Name | ICTV Abbreviation | No. of Accessions 1 | Reference Genome 2 | Length (nt) | 95% Length | Accessions (≥95%) 3 |
|---|---|---|---|---|---|---|---|
| T. maculacapsici | Bell pepper mottle virus | BPMV | 5 | NC 009642.1 | 6375 | 6056 | 4 |
| T. brugmansiae | Brugmansia mild mottle virus | BrMMV | 2 | NC 010944.1 | 6381 | 6062 | 2 |
| T. cacti | Cactus mild mottle virus | CMMoV | 5 | NC 011803.1 | 6449 | 6127 | 3 |
| NA 4 | Cactus tobamovirus 1 | 2 | MW938767.1 | 6458 | 6135 | 2 | |
| NA 4 | Cactus tobamovirus 2 | 2 | MW938766.1 | 6368 | 6050 | 2 | |
| NA 4 | Chili pepper mild mottle virus | 14 | MN164455.1 | 6383 | 6064 | 2 | |
| T. clitoriae | Clitoria yellow mottle virus | CliYMV | 2 | NC 016519.1 | 6514 | 6188 | 2 |
| T. maculafructi | Cucumber fruit mottle mosaic virus | CFMMV | 19 | NC 002633.1 | 6562 | 6234 | 4 |
| T. viridimaculae | Cucumber green mottle mosaic virus | CGMMV | 484 | NC 001801.1 | 6424 | 6103 | 185 |
| T. cucumeris | Cucumber mottle virus | CMoV | 2 | NC 008614.1 | 6485 | 6161 | 2 |
| T. frangipani | Frangipani mosaic virus | FrMV | 9 | NC 014546.1 | 6643 | 6311 | 4 |
| T. fortpiercense | Hibiscus latent Fort Pierce virus | HLFPV | 26 | NC 025381.1 | 6431 | 6109 | 10 |
| T. singaporense | Hibiscus latent Singapore virus | HLSV | 9 | NC 008310.2 | 6485 | 6161 | 6 |
| NA 4 | Hoya chlorotic spot virus | 2 | NC 034509.1 | 6386 | 6067 | 2 | |
| NA 4 | Hoya necrotic spot virus | 4 | LC807720.1 | 6425 | 6104 | 3 | |
| T. kyuri | Kyuri green mottle mosaic virus | KGMMV | 51 | NC 003610.1 | 6514 | 6188 | 4 |
| T. maracujae | Maracuja mosaic virus | MarMV | 2 | NC 008716.1 | 6794 | 6454 | 2 |
| T. obudae | Obuda pepper virus | ObPV | 4 | NC 003852.1 | 6507 | 6182 | 4 |
| T. odontoglossi | Odontoglossum ringspot virus | ORSV | 241 | NC 001728.1 | 6618 | 6287 | 13 |
| NA 4 | Opuntia virus 2 | 19 | NC 040685.2 | 6453 | 6130 | 5 | |
| T. paprikae | Paprika mild mottle virus | PaMMV | 19 | NC 004106.1 | 6524 | 6198 | 8 |
| T. passiflorae | Passion fruit mosaic virus | PFMV | 2 | NC 015552.1 | 6791 | 6451 | 2 |
| T. capsici | Pepper mild mottle virus | PMMoV | 662 | NC 003630.1 | 6357 | 6039 | 77 |
| NA 4 | Piper chlorosis virus | 3 | ON924221.1 | 6237 | 5925 | 2 | |
| T. plumeriae | Plumeria mosaic virus | PluMV | 4 | NC 026816.1 | 6688 | 6354 | 4 |
| T. muricaudae | Rattail cactus necrosis-associated virus | RCNaV | 22 | NC 016442.1 | 6506 | 6181 | 5 |
| T. rehmanniae | Rehmannia mosaic virus | RheMV | 75 | NC 009041.1 | 6395 | 6075 | 8 |
| T. plantagonis | Ribgrass mosaic virus | RMV | 41 | NC 002792.2 | 6311 | 5995 | 11 |
| T. streptocarpi | Streptocarpus flower break virus | SFBV | 6 | NC 008365.1 | 6279 | 5965 | 4 |
| T. mititessellati | Tobacco mild green mosaic virus | TMGMV | 398 | NC 001556.1 | 6355 | 6037 | 36 |
| T. tabaci | Tobacco mosaic virus | TMV | 653 | NC 001367.1 | 6395 | 6075 | 94 |
| T. fructirugosum | Tomato brown rugose fruit virus | TBRFV | 451 | NC 028478.1 | 6393 | 6073 | 232 |
| T. tomatotessellati | Tomato mosaic virus | ToMV | 372 | NC 002692.1 | 6383 | 6064 | 86 |
| T. maculatessellati | Tomato mottle mosaic virus | ToMMV | 62 | NC 022230.1 | 6398 | 6078 | 26 |
| T. tropici | Tropical soda apple mosaic virus | TSAMV | 13 | NC 030229.1 | 6350 | 6033 | 11 |
| T. rapae | Turnip vein clearing virus | TVCV | 29 | NC 001873.1 | 6311 | 5995 | 10 |
| T. wasabi | Wasabi mottle virus | WMoV | 6 | NC 003355.1 | 6298 | 5983 | 6 |
| NA 4 | Watermelon green mottle mosaic virus | WGMMV | 6 | MH837097.1 | 6482 | 6158 | 6 |
| T. anthocercis | Yellow tailflower mild mottle virus | YTMMV | 97 | NC 022801.1 | 6379 | 6060 | 4 |
| T. youcai | Youcai mosaic virus | YoMV | 129 | NC 004422.1 | 6303 | 5988 | 30 |
| T. cucurbitae | Zucchini green mottle mosaic virus | ZGMMV | 10 | NC 003878.1 | 6513 | 6187 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomaa, A.E.; Garcia-Ruiz, H. Genome-Wide Variation Profile of the Genus Tobamovirus. Viruses 2025, 17, 1284. https://doi.org/10.3390/v17091284
Gomaa AE, Garcia-Ruiz H. Genome-Wide Variation Profile of the Genus Tobamovirus. Viruses. 2025; 17(9):1284. https://doi.org/10.3390/v17091284
Chicago/Turabian StyleGomaa, Amany E., and Hernan Garcia-Ruiz. 2025. "Genome-Wide Variation Profile of the Genus Tobamovirus" Viruses 17, no. 9: 1284. https://doi.org/10.3390/v17091284
APA StyleGomaa, A. E., & Garcia-Ruiz, H. (2025). Genome-Wide Variation Profile of the Genus Tobamovirus. Viruses, 17(9), 1284. https://doi.org/10.3390/v17091284








