Cervical Secretions from Women After Depot Medroxyprogesterone Acetate (Depo-Provera) Administration Promote HIV Infectivity Ex Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Sample Collection
2.2. HIV Infectivity Assay
2.3. Statistical Analysis
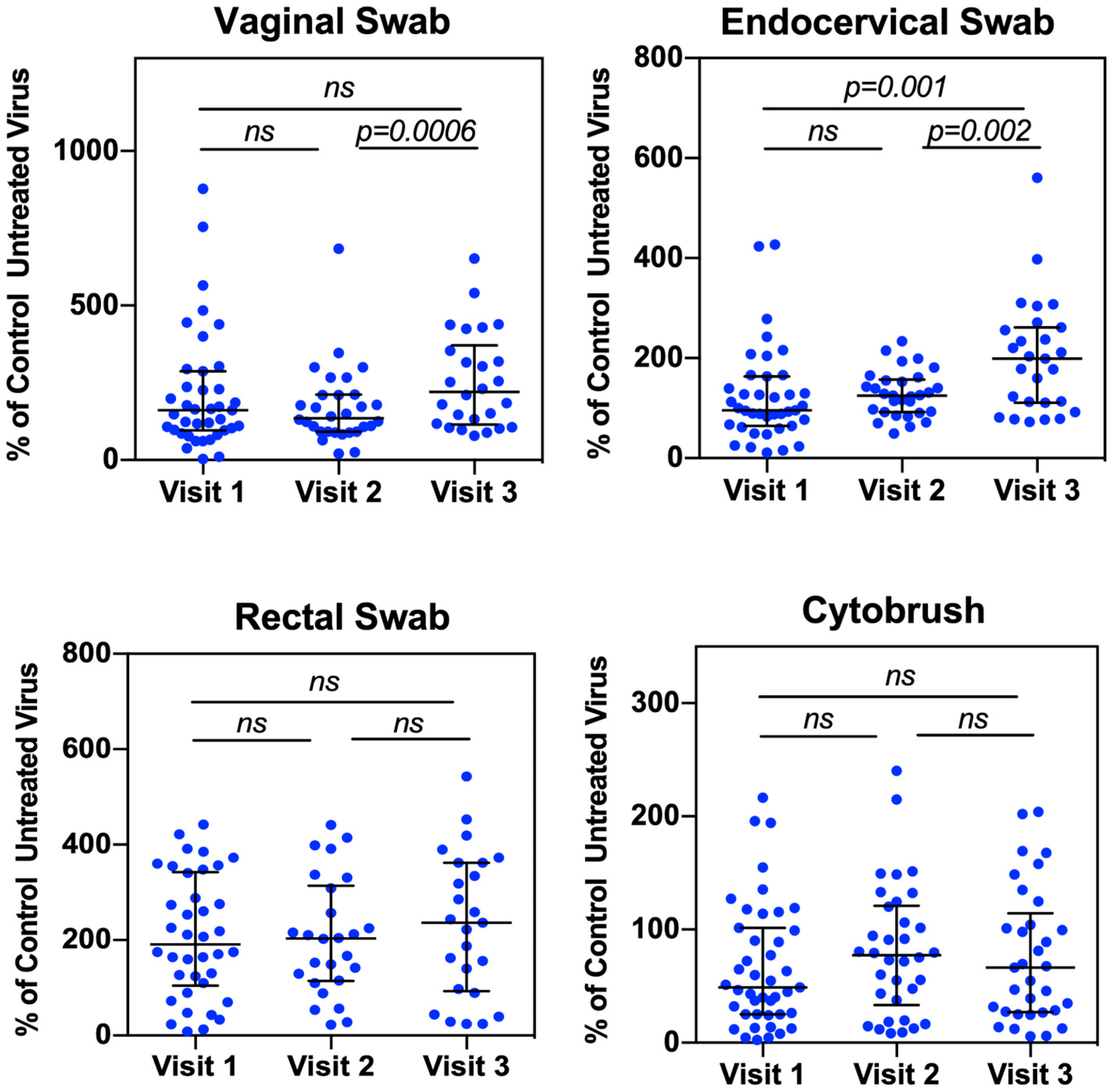
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Hel, Z.; Stringer, E.; Mestecky, J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr. Rev. 2010, 31, 79–97. [Google Scholar] [CrossRef]
- Hapgood, J.P.; Kaushic, C.; Hel, Z. Hormonal Contraception and HIV-1 Acquisition: Biological Mechanisms. Endocr. Rev. 2018, 39, 36–78. [Google Scholar] [CrossRef]
- Ahmed, K.; Baeten, J.M.; Beksinska, M.; Bekker, L.G.; Bukusi, E.A.; Donnell, D.; Gichangi, P.B.; Heller, K.B.; Hofmeyr, G.J.; Justman, J.; et al. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: A randomised, multicentre, open-label trial. Lancet 2019, 394, 303–313. [Google Scholar] [CrossRef]
- Curtis, K.M.; Hannaford, P.C.; Rodriguez, M.I.; Chipato, T.; Steyn, P.S.; Kiarie, J.N. Hormonal contraception and HIV acquisition among women: An updated systematic review. BMJ Sex. Reprod. Health 2020, 46, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Hapgood, J.P. Is the Injectable Contraceptive Depo-Medroxyprogesterone Acetate (DMPA-IM) Associated with an Increased Risk for HIV Acquisition? The Jury Is Still Out. AIDS Res. Hum. Retroviruses 2020, 36, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Gollub, E.L.; Stein, Z.; van de Wijgert, J.; Jones, H.; Ralph, L.; Padian, N. ECHO: Context and limitations. Lancet 2020, 395, e24. [Google Scholar] [CrossRef]
- Marrazzo, J.; Balkus, J.E.; Achilles, S.; Noguchi, L. ECHO: Context and limitations. Lancet 2020, 395, e23. [Google Scholar] [CrossRef] [PubMed]
- Jewell, B.L.; Smith, J.A.; Padian, N.S.; van de Wijgert, J.; Gollub, E.L.; Jones, H.E.; Ralph, L.J.; Hallett, T.B. ECHO: Context and limitations. Lancet 2020, 395, e25–e26. [Google Scholar] [CrossRef]
- Hapgood, J.P. ECHO: Context and limitations. Lancet 2020, 395, e22. [Google Scholar] [CrossRef]
- Miguel, R.D.V.; Calla, N.E.Q.; Aceves, K.M.; Lopez, F.C.D.; Cherpes, T.L. ECHO: Context and limitations. Lancet 2020, 395, e21. [Google Scholar] [CrossRef]
- Tasker, C.; Davidow, A.; Roche, N.E.; Chang, T.L. Depot medroxyprogesterone acetate administration alters immune markers for HIV preference and increases susceptibility of peripheral CD4(+) T cells to HIV infection. Immunohorizons 2017, 1, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Tasker, C.; Pizutelli, V.; Lo, Y.; Ramratnam, B.; Roche, N.E.; Chang, T.L. Depot medroxyprogesterone acetate administration increases cervical CCR5+CD4+ T cells and induces immunosuppressive milieu at the cervicovaginal mucosa. AIDS 2020, 34, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.H.; Anahtar, M.N.; Cohen, K.E.; Moodley, A.; Padavattan, N.; Ismail, N.; Bowman, B.A.; Olson, G.S.; Mabhula, A.; Leslie, A.; et al. Association between injectable progestin-only contraceptives and HIV acquisition and HIV target cell frequency in the female genital tract in South African women: A prospective cohort study. Lancet Infect. Dis. 2016, 16, 441–448. [Google Scholar] [CrossRef]
- Li, L.; Zhou, J.; Wang, W.; Huang, L.; Tu, J.; Baiamonte, L.; Stark, M.; Mills, M.; Hope, T.J.; Drobnis, E.Z.; et al. Effects of three long-acting reversible contraceptive methods on HIV target cells in the human uterine cervix and peripheral blood. Reprod. Biol. Endocrinol. 2019, 17, 26. [Google Scholar] [CrossRef]
- Roxby, A.C.; Fredricks, D.N.; Odem-Davis, K.; Asbjornsdottir, K.; Masese, L.; Fiedler, T.L.; De Rosa, S.; Jaoko, W.; Kiarie, J.N.; Overbaugh, J.; et al. Changes in Vaginal Microbiota and Immune Mediators in HIV-1-Seronegative Kenyan Women Initiating Depot Medroxyprogesterone Acetate. J. Acquir. Immune Defic. Syndr. 2016, 71, 359–366. [Google Scholar] [CrossRef]
- Michel, K.G.; Huijbregts, R.P.; Gleason, J.L.; Richter, H.E.; Hel, Z. Effect of hormonal contraception on the function of plasmacytoid dendritic cells and distribution of immune cell populations in the female reproductive tract. J. Acquir. Immune Defic. Syndr. 2015, 68, 511–518. [Google Scholar] [CrossRef]
- Smith-McCune, K.K.; Hilton, J.F.; Shanmugasundaram, U.; Critchfield, J.W.; Greenblatt, R.M.; Seidman, D.; Averbach, S.; Giudice, L.C.; Shacklett, B.L. Effects of depot-medroxyprogesterone acetate on the immune microenvironment of the human cervix and endometrium: Implications for HIV susceptibility. Mucosal Immunol. 2017, 10, 1270–1278. [Google Scholar] [CrossRef]
- Sperling, R.; Kraus, T.A.; Ding, J.; Veretennikova, A.; Lorde-Rollins, E.; Singh, T.; Lo, Y.; Quayle, A.J.; Chang, T.L. Differential profiles of immune mediators and in vitro HIV infectivity between endocervical and vaginal secretions from women with Chlamydia trachomatis infection: A pilot study. J. Reprod. Immunol. 2013, 99, 80–87. [Google Scholar] [CrossRef]
- Mishell, D.R., Jr. Pharmacokinetics of depot medroxyprogesterone acetate contraception. J. Reprod. Med. 1996, 41, 381–390. [Google Scholar]
- Jeppsson, S.; Gershagen, S.; Johansson, E.D.; Rannevik, G. Plasma levels of medroxyprogesterone acetate (MPA), sex-hormone binding globulin, gonadal steroids, gonadotrophins and prolactin in women during long-term use of depo-MPA (Depo-Provera) as a contraceptive agent. Eur. J. Endocrinol. 1982, 99, 339–343. [Google Scholar] [CrossRef]
- Bagri, P.; Anipindi, V.C.; Kaushic, C. The Role of IL-17 During Infections in the Female Reproductive Tract. Front. Immunol. 2022, 13, 861444. [Google Scholar] [CrossRef]
- Ma, W.-T.; Yao, X.-T.; Peng, Q.; Chen, D.-K. The protective and pathogenic roles of IL-17 in viral infections: Friend or foe? Open Biol. 2019, 9, 190109. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Chege, D.; Chai, Y.; Huibner, S.; Kain, T.; Wachihi, C.; Kimani, M.; Barasa, S.; McKinnon, L.R.; Muriuki, F.K.; Kariri, A.; et al. Blunted IL17/IL22 and pro-inflammatory cytokine responses in the genital tract and blood of HIV-exposed, seronegative female sex workers in Kenya. PLoS ONE 2012, 7, e43670. [Google Scholar] [CrossRef] [PubMed]
- Mlambo, T.; Tshabalala, M.; Bandason, T.; Mhandire, K.; Mudenge, B.; Zijenah, L.S. Correlation of High Interleukin 17A and Interleukin 6 Levels with High Virus Load Among Subtype C HIV-infected, Antiretroviral Therapy-naive Zimbabwean Patients: A Cross-sectional Study. Open AIDS J. 2019, 13, 59–64. [Google Scholar] [CrossRef]
- Hoffman, J.C.; Anton, P.A.; Baldwin, G.C.; Elliott, J.; Anisman-Posner, D.; Tanner, K.; Grogan, T.; Elashoff, D.; Sugar, C.; Yang, O.O.; et al. Seminal plasma HIV-1 RNA concentration is strongly associated with altered levels of seminal plasma interferon-γ, interleukin-17, and interleukin-5. AIDS Res. Hum. Retroviruses 2014, 30, 1082–1088. [Google Scholar] [CrossRef]
- You, T.; Bi, Y.; Li, J.; Zhang, M.; Chen, X.; Zhang, K.; Li, J. IL-17 induces reactive astrocytes and up-regulation of vascular endothelial growth factor (VEGF) through JAK/STAT signaling. Sci. Rep. 2017, 7, 41779. [Google Scholar] [CrossRef]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci. Rep. 2015, 5, 16053. [Google Scholar] [CrossRef]
- Alves, J.J.P.; De Medeiros Fernandes, T.A.A.; De Araújo, J.M.G.; Cobucci, R.N.O.; Lanza, D.C.F.; Bezerra, F.L.; Andrade, V.S.; Fernandes, J.V. Th17 response in patients with cervical cancer. Oncol. Lett. 2018, 16, 6215–6227. [Google Scholar] [CrossRef]
- Mohammadi, A.; Bagherichimeh, S.; Choi, Y.; Fazel, A.; Tevlin, E.; Huibner, S.; Good, S.V.; Tharao, W.; Kaul, R. Immune parameters of HIV susceptibility in the female genital tract before and after penile-vaginal sex. Commun. Med. 2022, 2, 60. [Google Scholar] [CrossRef]
- Alkhatib, G.; Combadiere, C.; Broder, C.C.; Feng, Y.; Kennedy, P.E.; Murphy, P.M.; Berger, E.A. CC CKR5: A RANTES, MIP-1α, MIP-1β Receptor as a Fusion Cofactor for Macrophage-Tropic HIV-1. Science 1996, 272, 1955–1958. [Google Scholar] [CrossRef]
- Chang, T.L.; Gordon, C.J.; Roscic-Mrkic, B.; Power, C.; Proudfoot, A.E.; Moore, J.P.; Trkola, A. Interaction of the CC-chemokine RANTES with glycosaminoglycans activates a p44/p42 mitogen-activated protein kinase-dependent signaling pathway and enhances human immunodeficiency virus type 1 infectivity. J. Virol. 2002, 76, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, J.; Su, S.; Wang, Q.; Jiang, S.; Lu, L.; Chen, Y.-H. Enhancement of endocytic uptake of HIV-1 virions into CD4-negative epithelial cells by HIV-1 gp41 via its interaction with POB1. Cell. Mol. Immunol. 2017, 14, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Gunst, J.D.; Goonetilleke, N.; Rasmussen, T.A.; Søgaard, O.S. Immunomodulation with IL-7 and IL-15 in HIV-1 infection. J. Virus Erad. 2023, 9, 100347. [Google Scholar] [CrossRef] [PubMed]
- Chahroudi, A.; Silvestri, G. Interleukin-7 in HIV pathogenesis and therapy. Eur. Cytokine Netw. 2010, 21, 202–207. [Google Scholar] [CrossRef]
- Vandergeeten, C.; Fromentin, R.; DaFonseca, S.; Lawani, M.B.; Sereti, I.; Lederman, M.M.; Ramgopal, M.; Routy, J.-P.; Sékaly, R.-P.; Chomont, N. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 2013, 121, 4321–4329. [Google Scholar] [CrossRef]
- Managlia, E.Z.; Landay, A.; Al-Harthi, L. Interleukin-7 induces HIV replication in primary naive T cells through a nuclear factor of activated T cell (NFAT)-dependent pathway. Virology 2006, 350, 443–452. [Google Scholar] [CrossRef]
- Coiras, M.; Bermejo, M.; Descours, B.; Mateos, E.; García-Pérez, J.; López-Huertas, M.R.; Lederman, M.M.; Benkirane, M.; Alcamí, J. IL-7 Induces SAMHD1 Phosphorylation in CD4+ T Lymphocytes, Improving Early Steps of HIV-1 Life Cycle. Cell Rep. 2016, 14, 2100–2107. [Google Scholar] [CrossRef]
- Introini, A.; Vanpouille, C.; Lisco, A.; Grivel, J.C.; Margolis, L. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathog. 2013, 9, e1003148. [Google Scholar] [CrossRef]
- An, P.; Nelson, G.W.; Wang, L.; Donfield, S.; Goedert, J.J.; Phair, J.; Vlahov, D.; Buchbinder, S.; Farrar, W.L.; Modi, W.; et al. Modulating influence on HIV/AIDS by interacting RANTES gene variants. Proc. Natl. Acad. Sci. USA 2002, 99, 10002–10007. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Marinho, R.L.; Dos Santos, P.A.S.; Dos Santos, C.S.; Ribeiro, L.R.; Rodrigues, Y.C.; Lima, K.V.B.; Lima, L. The Association between CCL5/RANTES SNPs and Susceptibility to HIV-1 Infection: A Meta-Analysis. Viruses 2023, 15, 1958. [Google Scholar] [CrossRef] [PubMed]
- Mauck, C.K.; Callahan, M.M.; Baker, J.; Arbogast, K.; Veazey, R.; Stock, R.; Pan, Z.; Morrison, C.S.; Chen-Mok, M.; Archer, D.F.; et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception 1999, 60, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.; Denny, L.; Pollack, A.E.; Wright, T.C. Prevalence of visible disruption of cervical epithelium and cervical ectopy in African women using Depo-Provera. Contraception 1999, 59, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Petta, C.A.; Faundes, A.; Dunson, T.R.; Ramos, M.; DeLucio, M.; Faundes, D.; Bahamondes, L. Timing of onset of contraceptive effectiveness in Depo-Provera users: Part I. Changes in cervical mucus. Fertil. Steril. 1998, 69, 252–257. [Google Scholar] [CrossRef]
- Yang, L.; Hao, Y.; Hu, J.; Kelly, D.; Li, H.; Brown, S.; Tasker, C.; Roche, N.E.; Chang, T.L.; Pei, Z. Differential effects of depot medroxyprogesterone acetate administration on vaginal microbiome in Hispanic White and Black women. Emerg. Microbes Infect. 2019, 8, 197–210. [Google Scholar] [CrossRef]
- Balle, C.; Happel, A.U.; Heffron, R.; Jaspan, H.B. Contraceptive effects on the cervicovaginal microbiome: Recent evidence including randomized trials. Am. J. Reprod. Immunol. 2023, 90, e13785. [Google Scholar] [CrossRef]
- Marx, P.A.; Spira, A.I.; Gettie, A.; Dailey, P.J.; Veazey, R.S.; Lackner, A.A.; Mahoney, C.J.; Miller, C.J.; Claypool, L.E.; Ho, D.D.; et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 1996, 2, 1084–1089. [Google Scholar] [CrossRef]
- Delgado-Diaz, D.J.; Tyssen, D.; Hayward, J.A.; Gugasyan, R.; Hearps, A.C.; Tachedjian, G. Distinct Immune Responses Elicited From Cervicovaginal Epithelial Cells by Lactic Acid and Short Chain Fatty Acids Associated With Optimal and Non-optimal Vaginal Microbiota. Front. Cell Infect. Microbiol. 2019, 9, 446. [Google Scholar] [CrossRef]
- Shukair, S.A.; Allen, S.A.; Cianci, G.C.; Stieh, D.J.; Anderson, M.R.; Baig, S.M.; Gioia, C.J.; Spongberg, E.J.; Kauffman, S.M.; McRaven, M.D.; et al. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013, 6, 427–434. [Google Scholar] [CrossRef]
- Mall, A.S.; Habte, H.; Mthembu, Y.; Peacocke, J.; de Beer, C. Mucus and Mucins: Do they have a role in the inhibition of the human immunodeficiency virus? Virol. J. 2017, 14, 192. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasker, C.; Roche, N.E.; Lo, Y.; Chang, T.L. Cervical Secretions from Women After Depot Medroxyprogesterone Acetate (Depo-Provera) Administration Promote HIV Infectivity Ex Vivo. Viruses 2025, 17, 1283. https://doi.org/10.3390/v17091283
Tasker C, Roche NE, Lo Y, Chang TL. Cervical Secretions from Women After Depot Medroxyprogesterone Acetate (Depo-Provera) Administration Promote HIV Infectivity Ex Vivo. Viruses. 2025; 17(9):1283. https://doi.org/10.3390/v17091283
Chicago/Turabian StyleTasker, Carley, Natalie E. Roche, Yungtai Lo, and Theresa L. Chang. 2025. "Cervical Secretions from Women After Depot Medroxyprogesterone Acetate (Depo-Provera) Administration Promote HIV Infectivity Ex Vivo" Viruses 17, no. 9: 1283. https://doi.org/10.3390/v17091283
APA StyleTasker, C., Roche, N. E., Lo, Y., & Chang, T. L. (2025). Cervical Secretions from Women After Depot Medroxyprogesterone Acetate (Depo-Provera) Administration Promote HIV Infectivity Ex Vivo. Viruses, 17(9), 1283. https://doi.org/10.3390/v17091283







