Antibody-Dependent Enhancement of Porcine Reproductive and Respiratory Syndrome Virus Infection Antagonizes the Secretion of Type I Interferons in Porcine Alveolar Macrophages by Interfering with the Retinoic Acid-Inducible Gene I/Melanoma Differentiation-Associated Gene 5 Pathway via Fc Gamma Receptor I
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Virus Strain
2.2. Antibodies and Reagents
2.3. ADE Assay of PRRSV Infection in PAMs
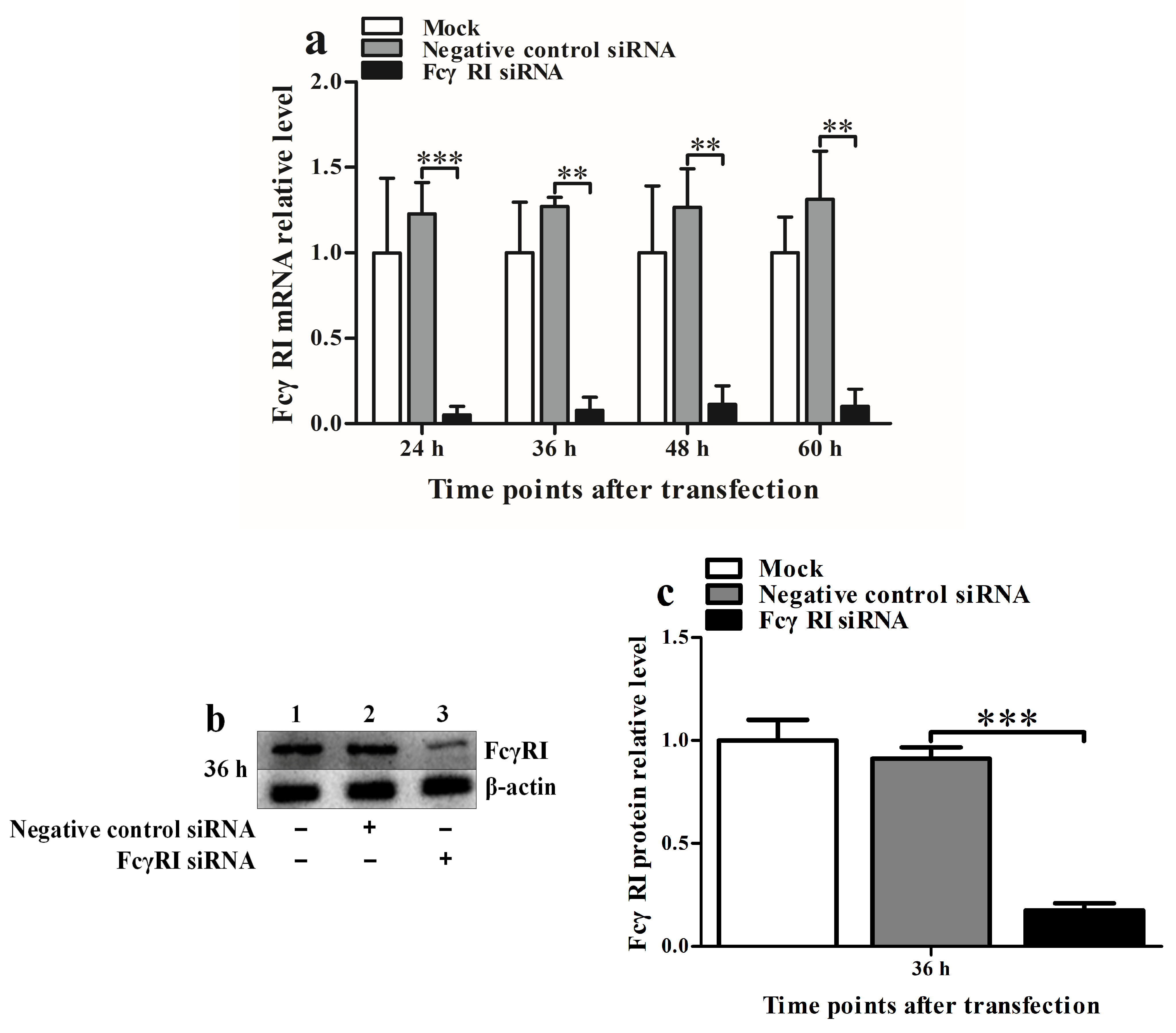
2.4. Silencing Assay of the FcγRI Gene in PAMs
2.5. Activation Assay of FcγRI in Healthy PAMs
2.6. Activation Assay of FcγRI in Poly (I: C)-Stimulated PAMs
2.7. Activation Assay of FcγRI in PRRSV-Infected PAMs
2.8. RNA Extraction, Real-Time RT-PCR, and Relative qRT-PCR
2.9. ELISA Detection of IFN-α and IFN-β Production
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. ADE Infection Phenomenon of PRRSV Is Observed in Porcine IgG Antibodies Against PRRSV in PAMs
3.2. PRRSV Induces the Production of Type I IFNs by Positively Regulating the RIG-I/MDA5 Signaling Pathway in PAMs
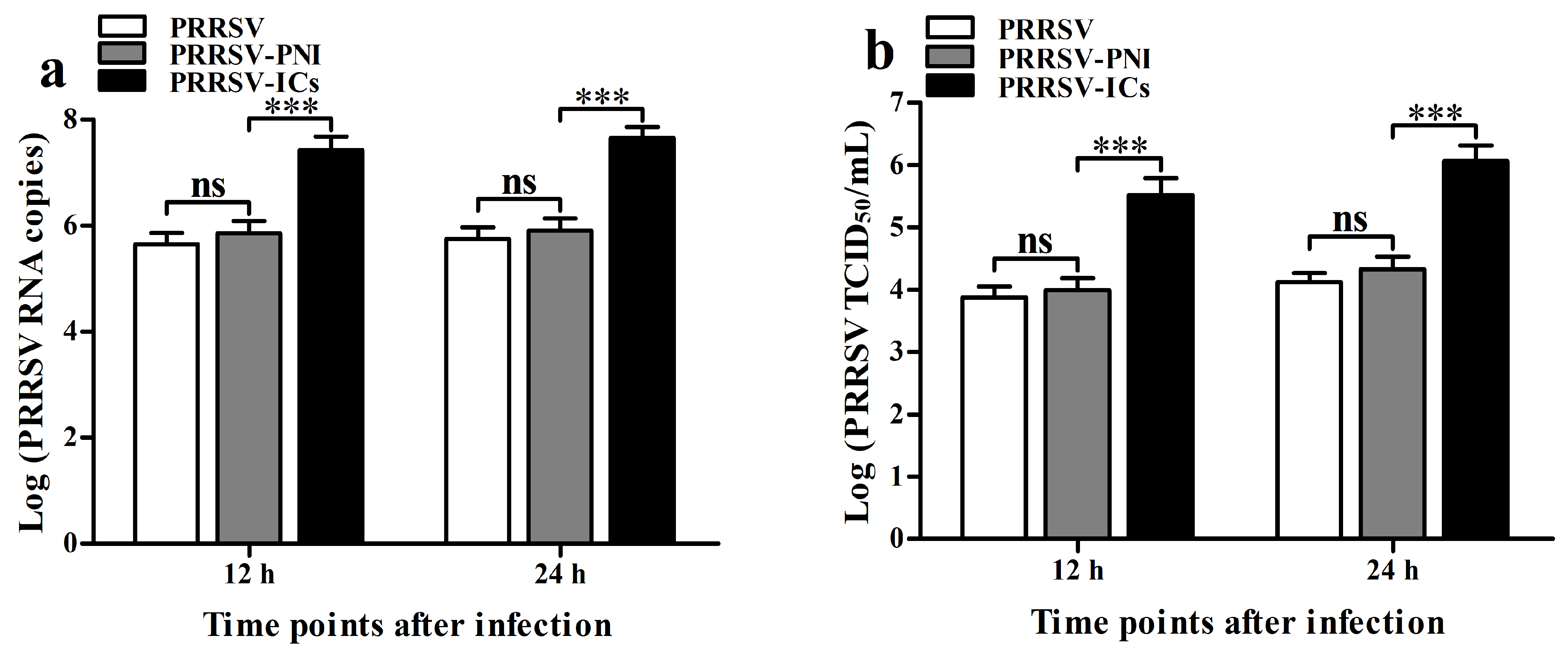
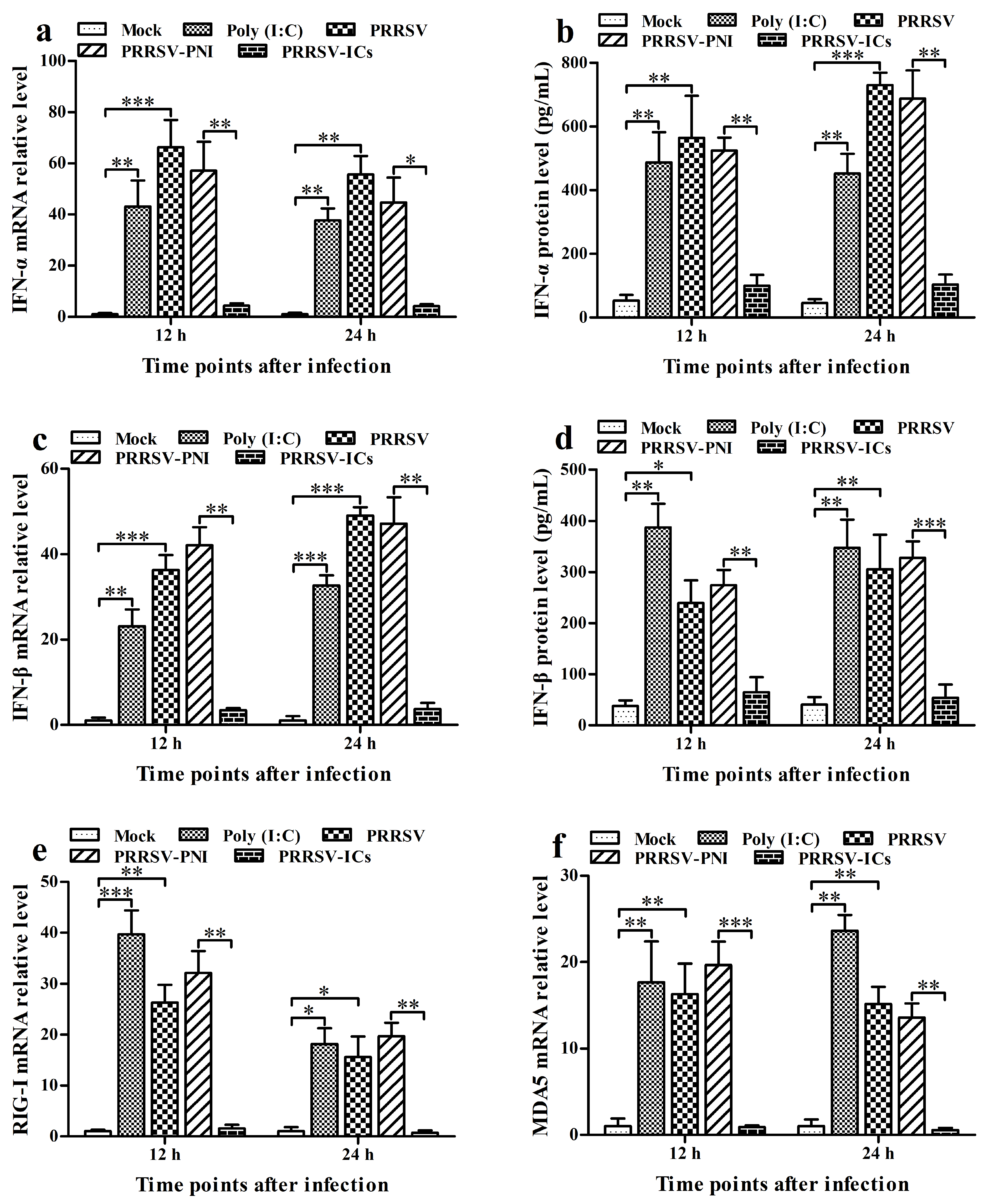
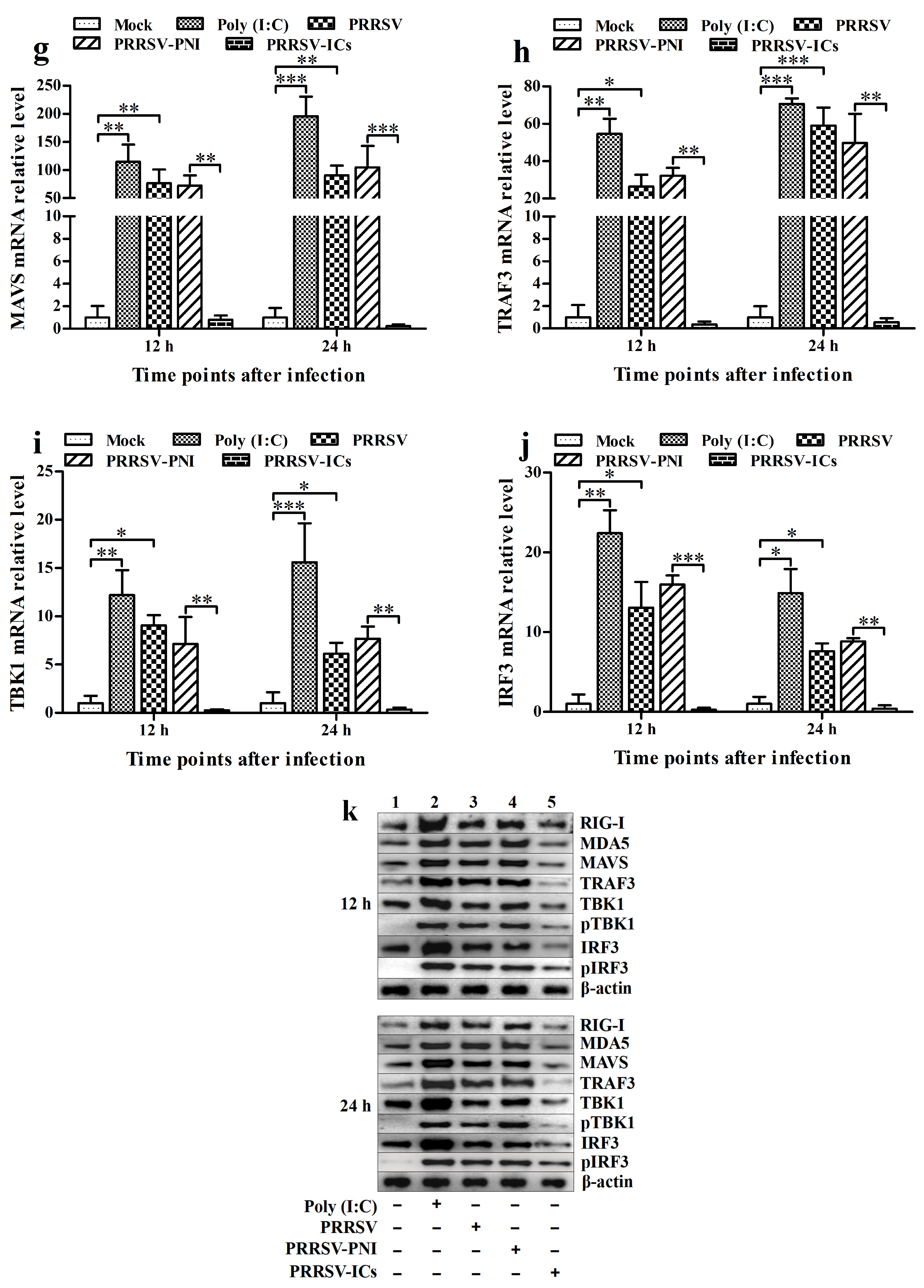
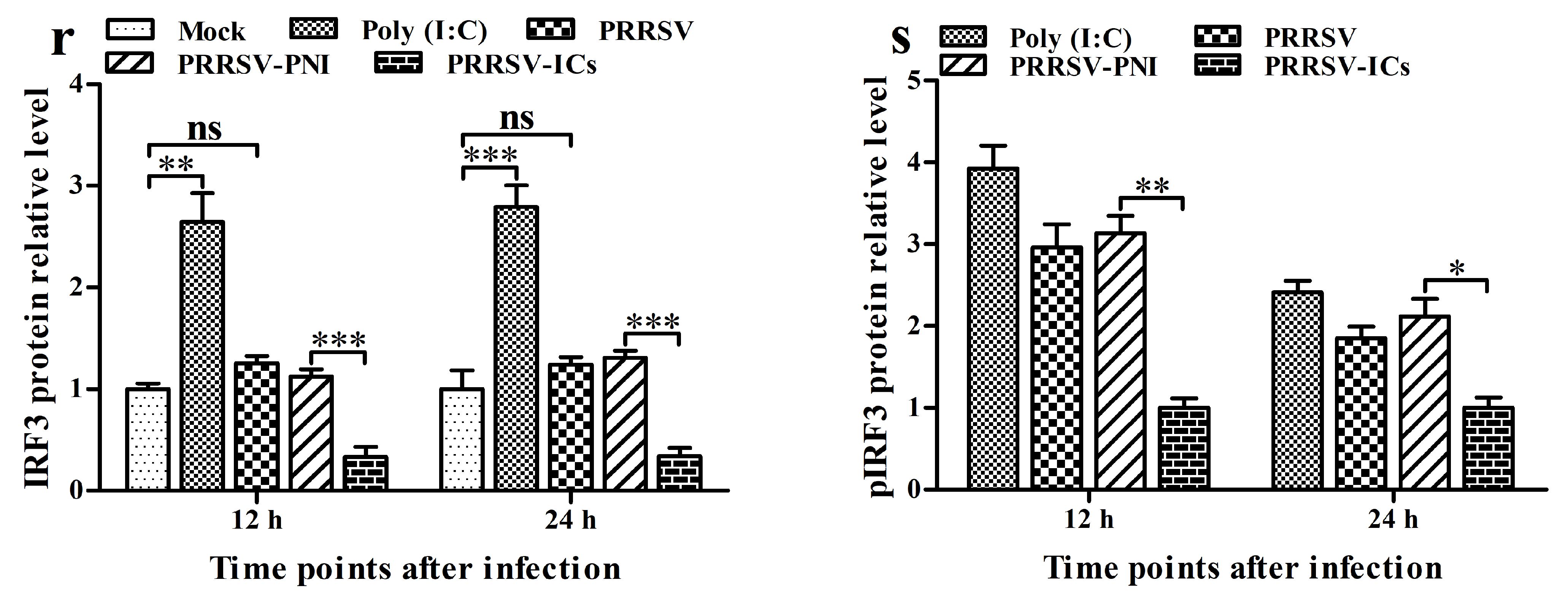
3.3. ADE Infection of PRRSV Down-Regulates the Production of Type I IFNs by Negatively Regulating the RIG-I/MDA5 Signaling Pathway in PAMs
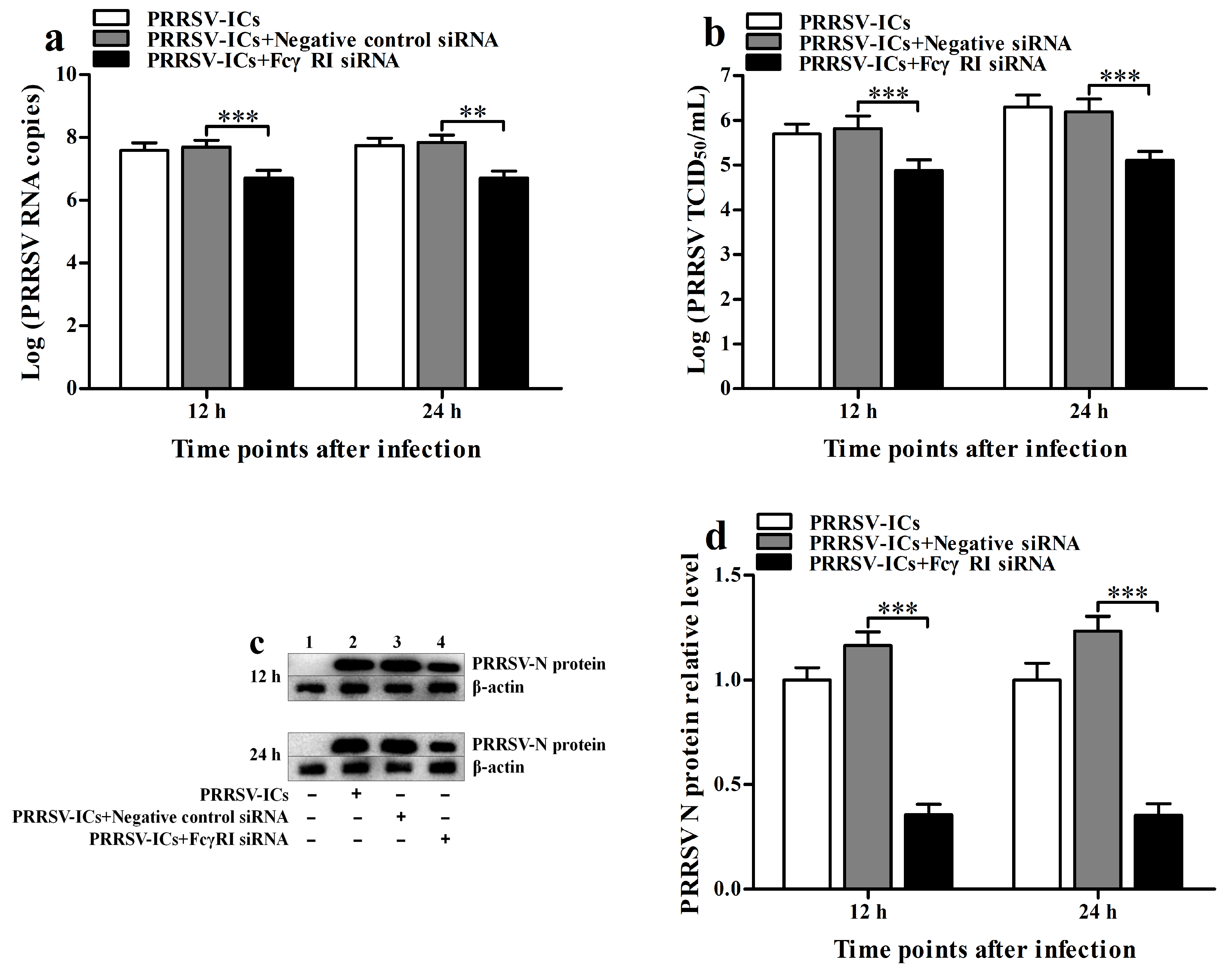
3.4. FcγRI Is Involved in ADE Infection of PRRSV in PAMs
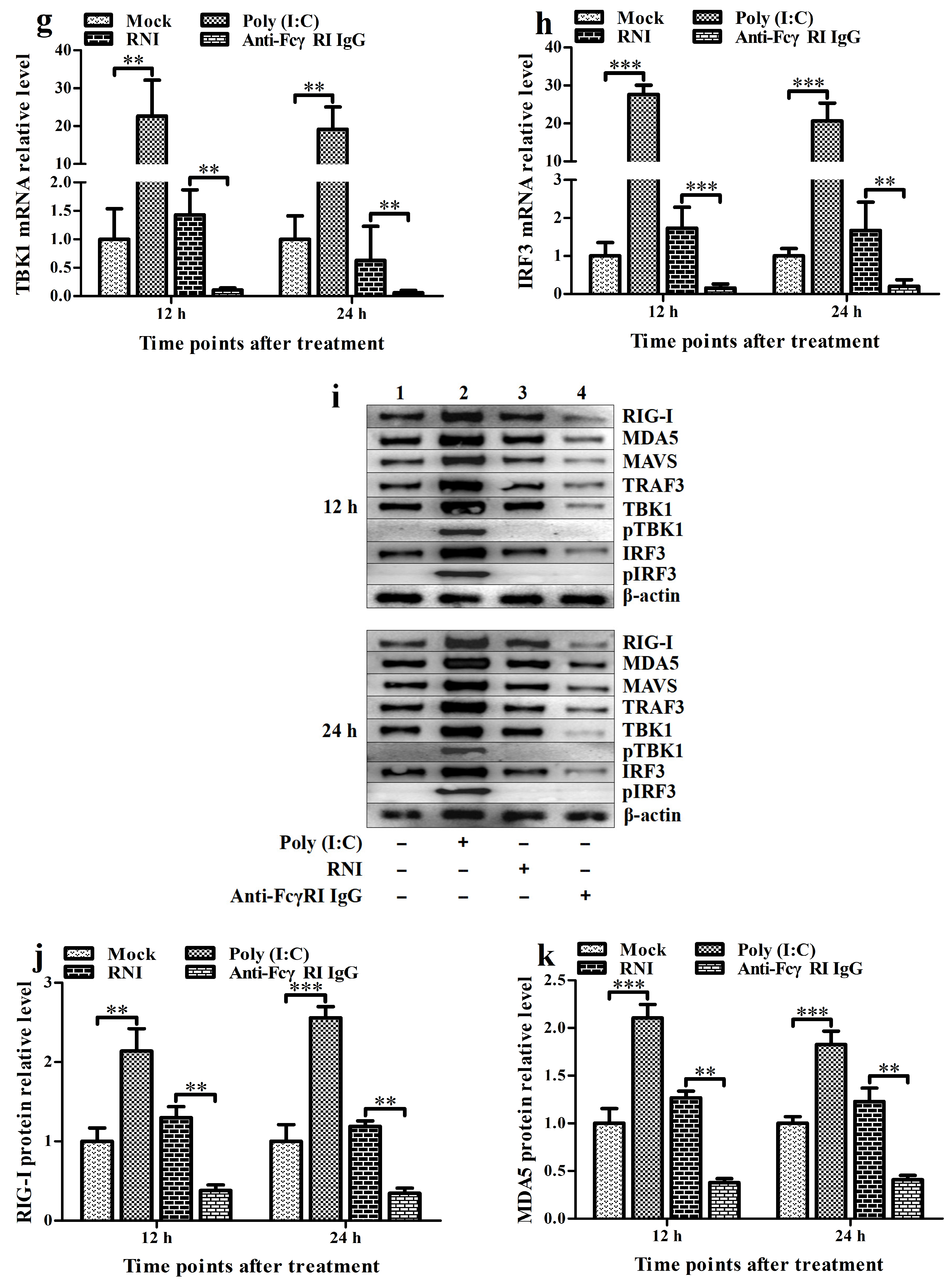
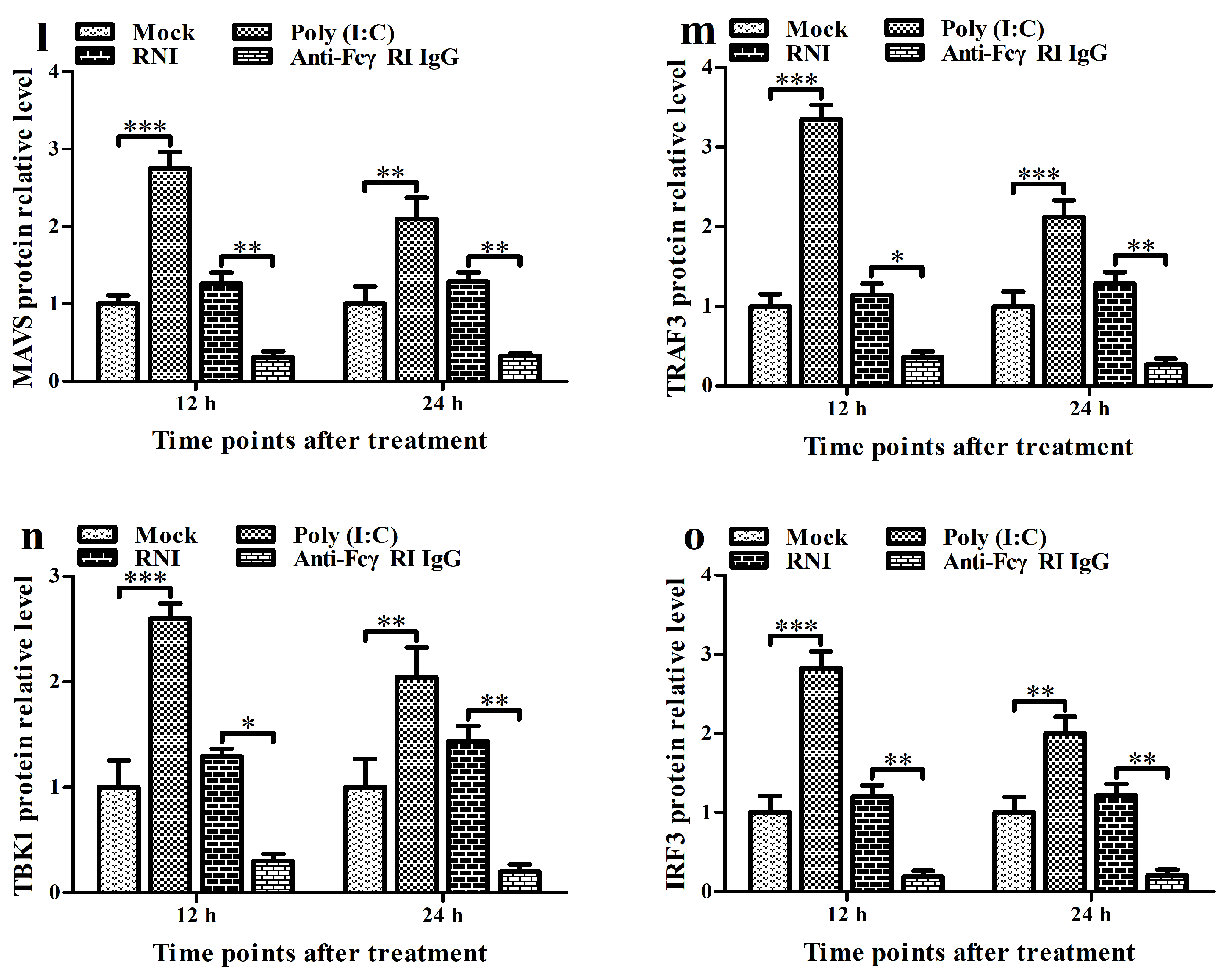
3.5. FcγRI Down-Regulates the Production of Type I IFNs by Negatively Regulating the RIG-I/MDA5 Signaling Pathway in PAMs
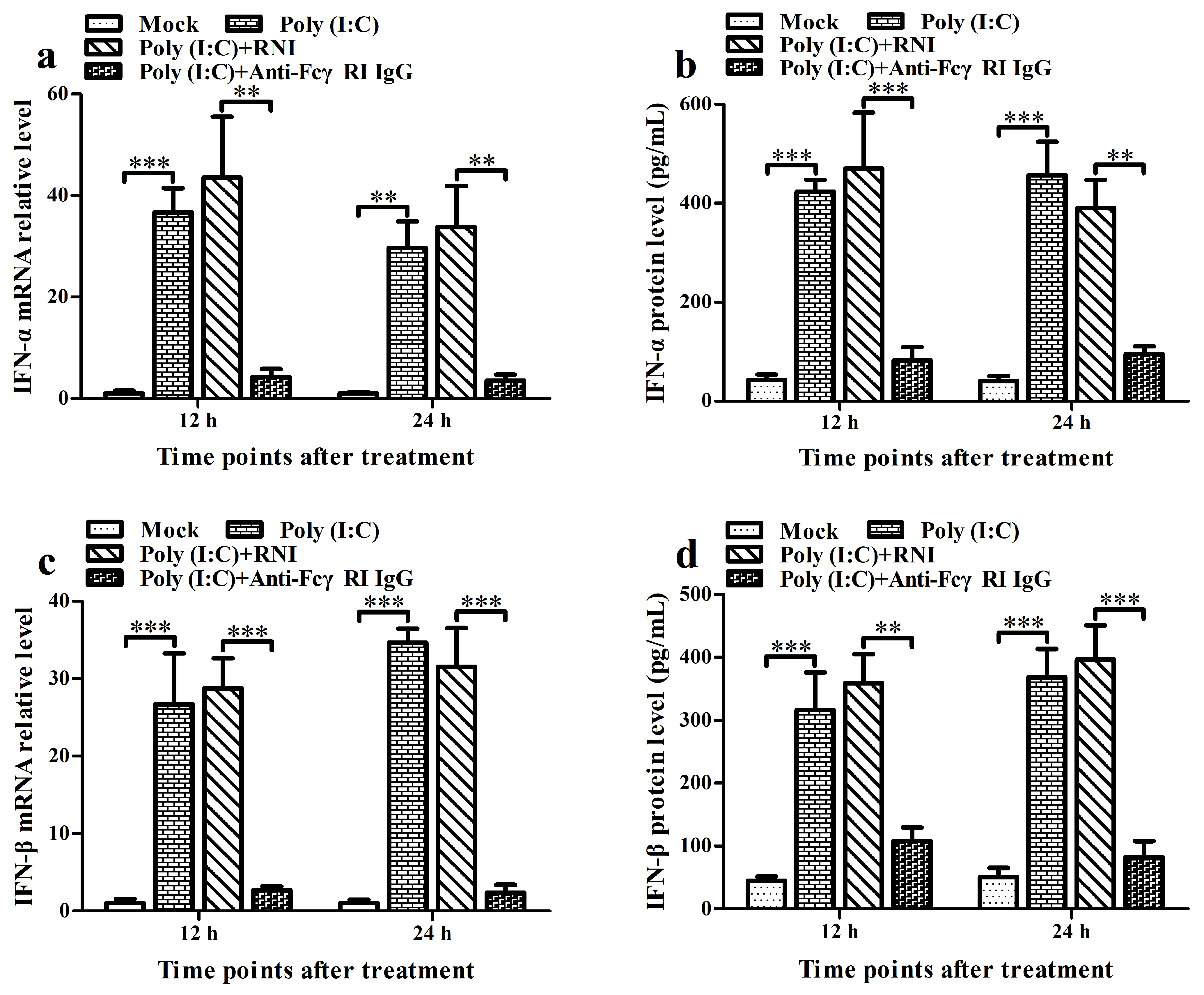
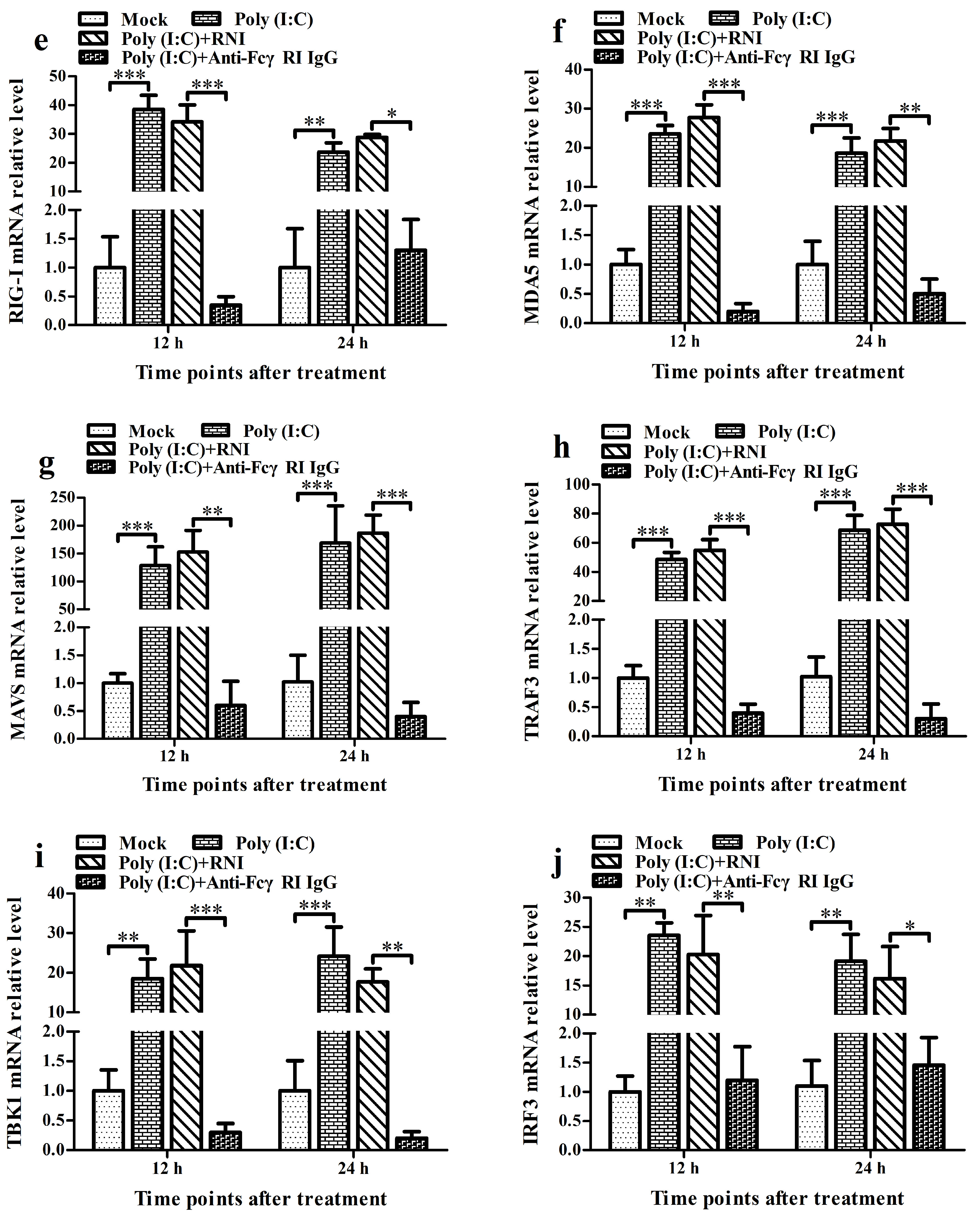
3.6. FcγRI Down-Regulates Poly (I: C)-Induced Production of Type I IFNs by Negatively Regulating Poly (I: C)-Induced Activation of the RIG-I/MDA5 Signaling Pathway in PAMs
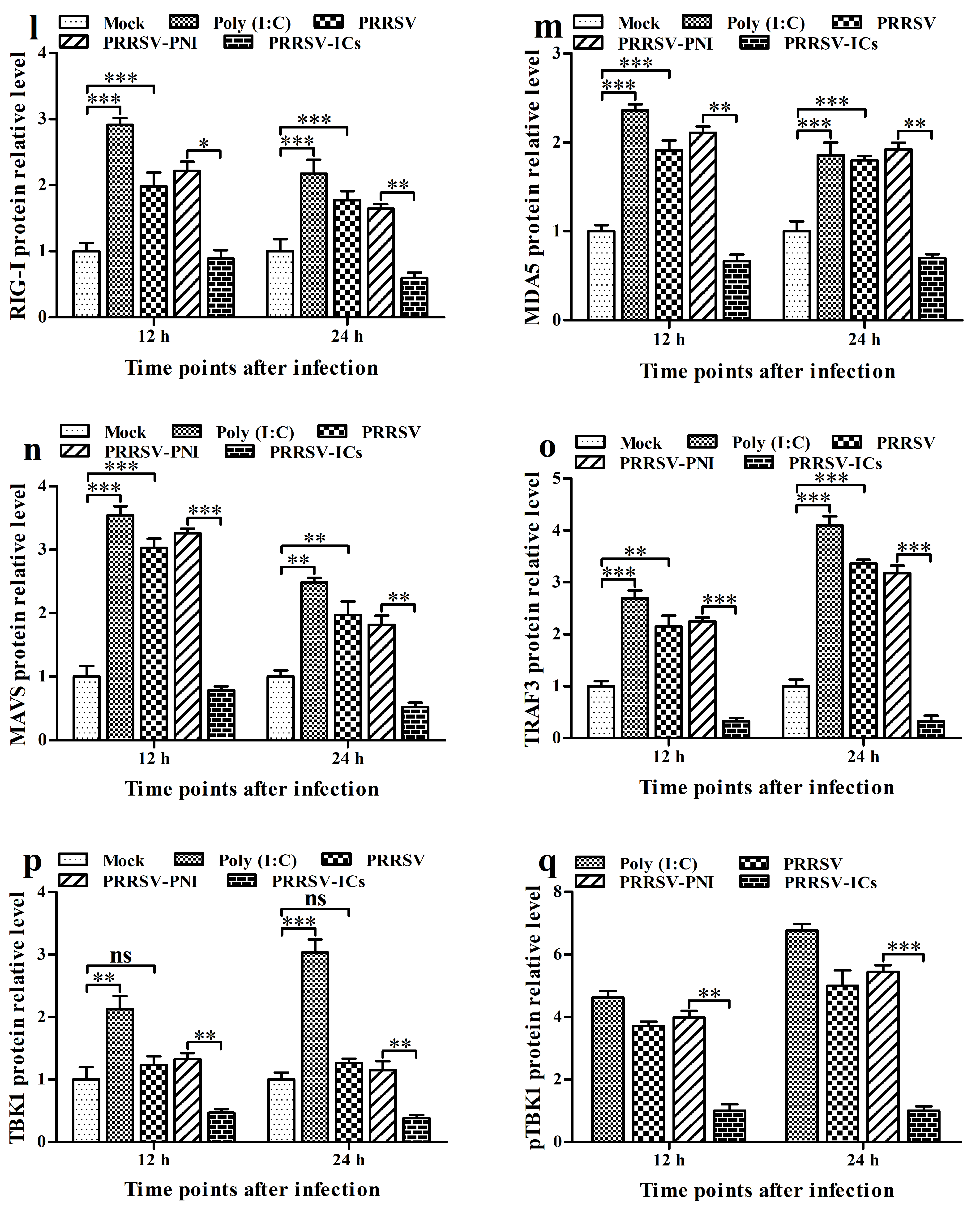
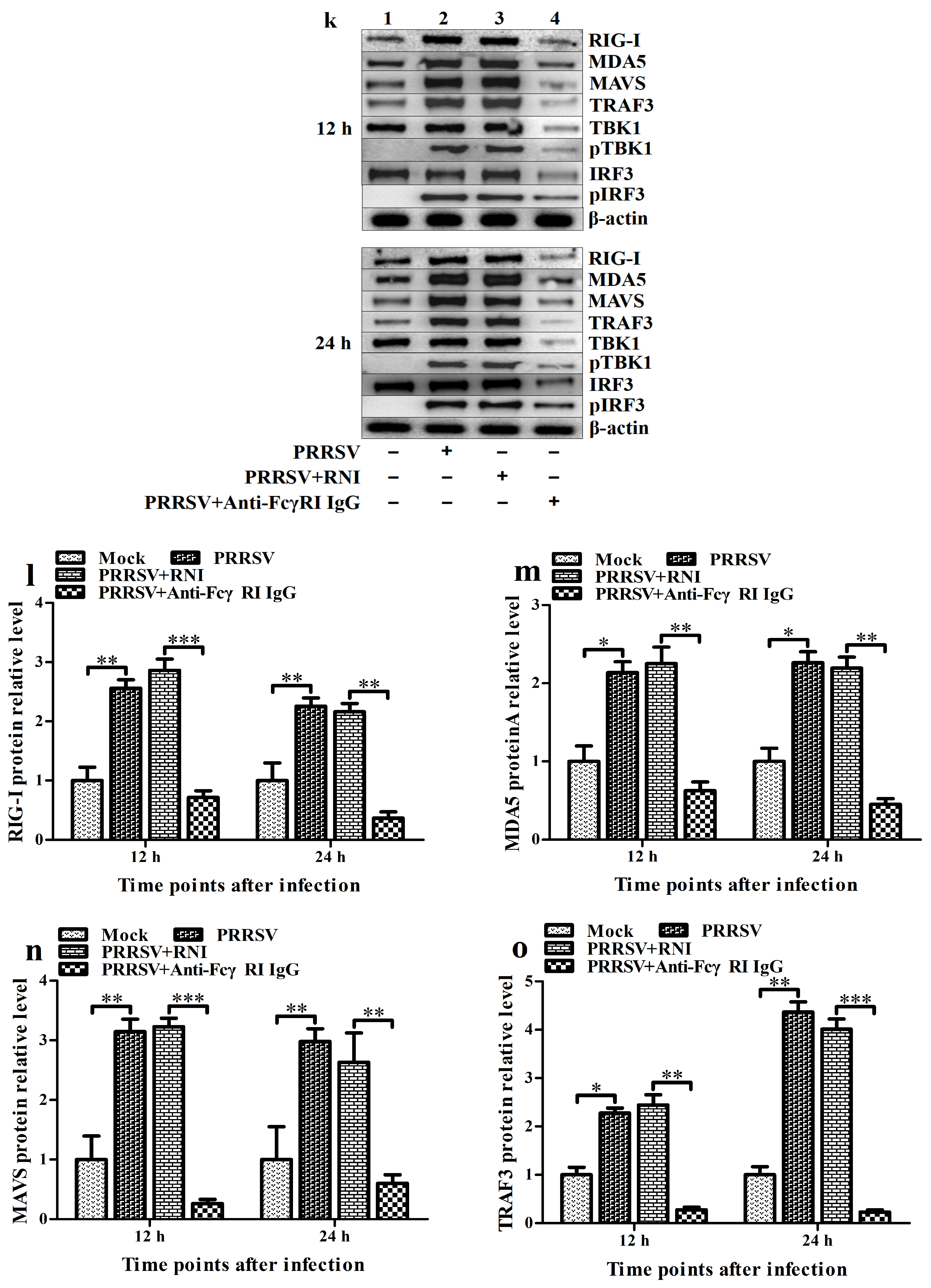
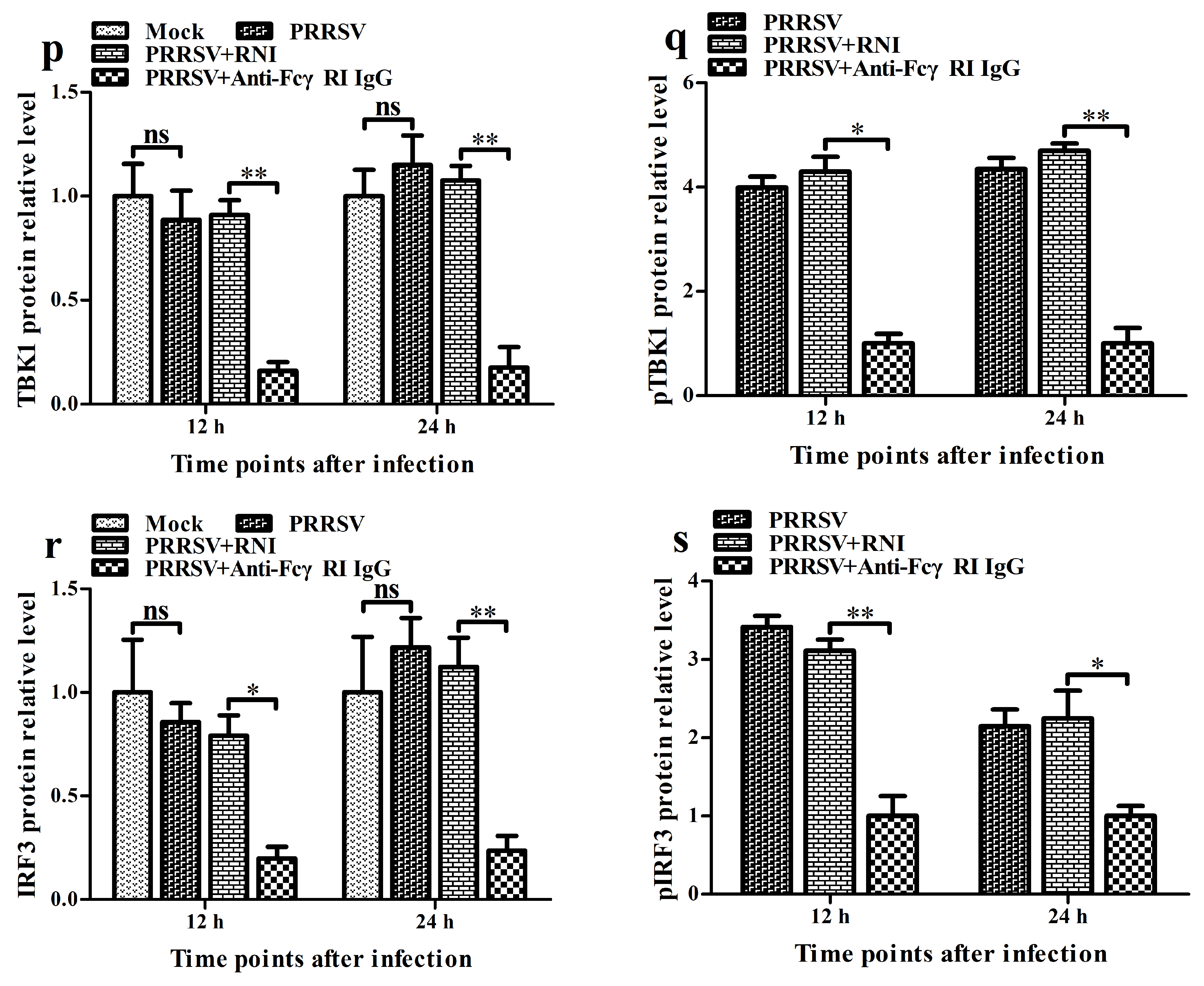
3.7. FcγRI Down-Regulates PRRSV-Induced Production of Type I IFNs by Negatively Regulating PRRSV-Induced Activation of the RIG-I/MDA5 Signaling Pathway in PAMs
3.8. FcγRI Promotes the Proliferation of PRRSV in PAMs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef]
- Mesev, E.V.; LeDesma, R.A.; Ploss, A. Decoding type I and III interferon signalling during viral infection. Nat. Microbiol. 2019, 4, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Onomoto, K.; Onoguchi, K.; Yoneyama, M. Regulation of RIG-I-like receptor-mediated signaling: Interaction between host and viral factors. Cell Mol. Immunol. 2021, 18, 539–555. [Google Scholar] [CrossRef]
- Pincetic, A.; Bournazos, S.; DiLillo, D.J.; Maamary, J.; Wang, T.T.; Dahan, R.; Fiebiger, B.M.; Ravetch, J.V. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat. Immunol. 2014, 15, 707–716. [Google Scholar] [CrossRef]
- Damelang, T.; Brinkhaus, M.; van Osch, T.L.J.; Schuurman, J.; Labrijn, A.F.; Rispens, T.; Vidarsson, G. Impact of structural modifications of IgG antibodies on effector functions. Front. Immunol. 2024, 14, 1304365. [Google Scholar] [CrossRef] [PubMed]
- Frampton, S.; Smith, R.; Ferson, L.; Gibson, J.; Hollox, E.J.; Cragg, M.S.; Strefford, J.C. Fc gamma receptors: Their evolution, genomic architecture, genetic variation, and impact on human disease. Immunol. Rev. 2024, 328, 65–97. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Ravetch, J.V. Fcγ receptor pathways during active and passive immunization. Immunol. Rev. 2015, 268, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005, 310, 1510–1522. [Google Scholar] [CrossRef]
- Benadda, S.; Nugue, M.; Koumantou, D.; Bens, M.; De Luca, M.; Pellé, O.; Monteiro, R.C.; Evnouchidou, I.; Saveanu, L. Activating FcγR function depends on endosomal-signaling platforms. iScience 2023, 26, 107055. [Google Scholar] [CrossRef]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of Fcγ receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef]
- Dhodapkar, K.M.; Banerjee, D.; Connolly, J.; Kukreja, A.; Matayeva, E.; Veri, M.C.; Ravetch, J.V.; Steinman, R.M.; Dhodapkar, M.V. Selective blockade of the inhibitory Fcgamma receptor (FcgammaRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J. Exp. Med. 2007, 204, 1359–1369. [Google Scholar] [CrossRef]
- Mócsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef]
- Newling, M.; Hoepel, W.; Vogelpoel, L.T.C.; Heineke, M.H.; van Burgsteden, J.A.; Taanman-Kueter, E.W.M.; Eggink, D.; Kuijpers, T.W.; Beaumont, T.; van Egmond, M.; et al. Fc gamma receptor IIa suppresses type I and III interferon production by human myeloid immune cells. Eur. J. Immunol. 2018, 48, 1796–1809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, W.; Sun, Y.; Li, X.; Kong, L.; Xu, P.; Xia, P.; Yue, J. Activation of activating Fc gamma receptors down-regulates the levels of interferon β, interferon γ and interferon λ1 in porcine alveolar macrophages during PRRSV infection. Int. Immunopharmacol. 2020, 81, 106268. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.E.; Bournazos, S. Fc-FcγR interactions during infections: From neutralizing antibodies to antibody-dependent enhancement. Immunol. Rev. 2024, 328, 221–242. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Malik, Y.S.; Singh, R.K.; Chaicumpa, W. Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection. Front. Immunol. 2018, 9, 597. [Google Scholar] [CrossRef]
- Tao, T.; Tian, L.; Ke, J.; Zhang, C.; Li, M.; Xu, X.; Fan, J.; Tong, Y.; Fan, H. Antibody-dependent enhancement of coronaviruses. Int. J. Biol. Sci. 2025, 21, 1686–1704. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Prestwood, T.R.; Shresta, S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 2010, 7, 128–139. [Google Scholar] [CrossRef]
- Sawant, J.; Patil, A.; Kurle, S. A Review: Understanding Molecular Mechanisms of Antibody-Dependent Enhancement in Viral Infections. Vaccines 2023, 11, 1240. [Google Scholar] [CrossRef]
- Halstead, S.B.; Mahalingam, S.; Marovich, M.A.; Ubol, S.; Mosser, D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010, 10, 712–722. [Google Scholar] [CrossRef]
- Ubol, S.; Phuklia, W.; Kalayanarooj, S.; Modhiran, N. Mechanisms of immune evasion induced by a complex of dengue virus and preexisting enhancing antibodies. J. Infect. Dis. 2010, 201, 923–935. [Google Scholar] [CrossRef]
- Wells, T.J.; Esposito, T.; Henderson, I.R.; Labzin, L.I. Mechanisms of antibody-dependent enhancement of infectious disease. Nat. Rev. Immunol. 2025, 25, 6–21. [Google Scholar] [CrossRef]
- Xu, J.; Huo, C.; Yang, Y.; Han, J.; Zhou, L.; Hu, Y.; Yang, H. Early Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Infection Induces Necroptosis in Immune Cells of Peripheral Lymphoid Organs. Viruses 2025, 17, 290. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.J.; Wu, L.L.; Zimmerman, J.J.; Hill, H.T.; Platt, K.B. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol. 1996, 9, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, W.; Sun, Y.; Kong, L.; Xu, P.; Xia, P.; Zhang, G. Antibody-Mediated Porcine Reproductive and Respiratory Syndrome Virus Infection Downregulates the Production of Interferon-α and Tumor Necrosis Factor-α in Porcine Alveolar Macrophages via Fc Gamma Receptor I and III. Viruses 2020, 12, 187. [Google Scholar] [CrossRef]
- Amona, F.M.; Pang, Y.; Gong, X.; Wang, Y.; Fang, X.; Zhang, C.; Chen, X. Mechanism of PRRSV infection and antiviral role of polyphenols. Virulence 2024, 15, 2417707. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, L.; Jiao, D.; Zheng, Z.; Chen, Z.; Jing, Y.; Li, Z.; Ma, Z.; Feng, Y.; Guo, X.; et al. Genomic similarity and antibody-dependent enhancement of immune serum potentially affect the protective efficacy of commercial MLV vaccines against NADC30-like PRRSV. Virol. Sin. 2023, 38, 813–826. [Google Scholar] [CrossRef]
- Vanhee, M.; Delputte, P.L.; Delrue, I.; Geldhof, M.; Nauwynck, H.J. Development of an experimental inactivated PRRSV vaccine that induces virus-neutralizing antibodies. Vet. Res. 2009, 40, 63. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Cornelissen, L.A.; Moormann, R.J.; Rebel, J.M.; Stockhofe-Zurwieden, N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine 2009, 27, 3704–3718. [Google Scholar] [CrossRef]
- Nan, Y.; Wu, C.; Gu, G.; Sun, W.; Zhang, Y.J.; Zhou, E.M. Improved Vaccine against PRRSV: Current Progress and Future Perspective. Front. Microbiol. 2017, 8, 1635. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Zhao, X.; Yuan, M.; Zhang, K.; Dai, J.; Guan, X.; Qiu, H.J.; Li, Y. Antibody-Dependent Enhancement: ″Evil″ Antibodies Favorable for Viral Infections. Viruses 2022, 14, 1739. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Wang, R.; Qiao, S.; Wan, B.; Wang, Y.; Liu, M.; Shi, X.; Guo, J.; Zhang, G. Antibody-dependent enhancement of PRRSV infection down-modulates TNF-α and IFN-β transcription in macrophages. Vet. Immunol. Immunopathol. 2013, 156, 128–134. [Google Scholar] [CrossRef]
- Shi, P.; Su, Y.; Li, Y.; Zhang, L.; Lu, D.; Li, R.; Zhang, L.; Huang, J. The alternatively spliced porcine FcγRI regulated PRRSV-ADE infection and proinflammatory cytokine production. Dev. Comp. Immunol. 2019, 90, 186–198. [Google Scholar] [CrossRef]
- Wan, B.; Chen, X.; Li, Y.; Pang, M.; Chen, H.; Nie, X.; Pan, Y.; Qiao, S.; Bao, D. Porcine FcγRIIb mediated PRRSV ADE infection through inhibiting IFN-β by cytoplasmic inhibitory signal transduction. Int. J. Biol. Macromol. 2019, 138, 198–206. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Q.; Guo, X.K.; Yu, Z.B.; Xu, A.T.; Tang, J.; Feng, W.H. Porcine reproductive and respiratory syndrome virus nonstructural protein 4 antagonizes beta interferon expression by targeting the NF-κB essential modulator. J. Virol. 2014, 88, 10934–10945. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Chen, W.; Wang, B.; He, S.; Fan, H.; Liu, D. Non-infectious immune complexes downregulate the production of interferons and tumor necrosis factor-α in primary porcine alveolar macrophages in vitro. Front. Vet. Sci. 2024, 11, 1420466. [Google Scholar] [CrossRef]
- Nan, Y.; Wang, R.; Shen, M.; Faaberg, K.S.; Samal, S.K.; Zhang, Y.J. Induction of type I interferons by a novel porcine reproductive and respiratory syndrome virus isolate. Virology 2012, 432, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Calzada-Nova, G.; Schnitzlein, W.M.; Husmann, R.J.; Zuckermann, F.A. North American porcine reproductive and respiratory syndrome viruses inhibit type I interferon production by plasmacytoid dendritic cells. J. Virol. 2011, 85, 2703–2713. [Google Scholar] [CrossRef]
- Du, T.; Nan, Y.; Xiao, S.; Zhao, Q.; Zhou, E.M. Antiviral Strategies against PRRSV Infection. Trends Microbiol. 2017, 25, 968–979. [Google Scholar] [CrossRef]
- Gimeno, M.; Darwich, L.; Diaz, I.; de la Torre, E.; Pujols, J.; Martín, M.; Inumaru, S.; Cano, E.; Domingo, M.; Montoya, M.; et al. Cytokine profiles and phenotype regulation of antigen presenting cells by genotype-I porcine reproductive and respiratory syndrome virus isolates. Vet. Res. 2011, 42, 9. [Google Scholar] [CrossRef]
- Lee, S.M.; Schommer, S.K.; Kleiboeker, S.B. Porcine reproductive and respiratory syndrome virus field isolates differ in in vitro interferon phenotypes. Vet. Immunol. Immunopathol. 2004, 102, 217–231. [Google Scholar] [CrossRef]
- Weesendorp, E.; Morgan, S.; Stockhofe-Zurwieden, N.; Popma-De Graaf, D.J.; Graham, S.P.; Rebel, J.M. Comparative analysis of immune responses following experimental infection of pigs with European porcine reproductive and respiratory syndrome virus strains of differing virulence. Vet. Microbiol. 2013, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ji, P.; Zhang, M.; Wang, X.; Li, N.; Wang, C.; Xiao, S.; Mu, Y.; Zhao, Q.; Du, T.; et al. GP5 expression in Marc-145 cells inhibits porcine reproductive and respiratory syndrome virus infection by inducing beta interferon activity. Vet. Microbiol. 2014, 174, 409–418. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, X.; Nelson, E.; Christopher-Hennings, J.; Wang, X. Porcine reproductive and respiratory syndrome virus activates the transcription of interferon alpha/beta (IFN-α/β) in monocyte-derived dendritic cells (Mo-DC). Vet. Microbiol. 2012, 159, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Lidbury, B.A.; Mahalingam, S. Specific ablation of antiviral gene expression in macrophages by antibody-dependent enhancement of Ross River virus infection. J. Virol. 2000, 74, 8376–8381. [Google Scholar] [CrossRef]
- Mahalingam, S.; Lidbury, B.A. Suppression of lipopolysaccharide-induced antiviral transcription factor (STAT-1 and NF-kappa B) complexes by antibody-dependent enhancement of macrophage infection by Ross River virus. Proc. Natl. Acad. Sci. USA 2002, 99, 13819–13824. [Google Scholar] [CrossRef]
- Lee, M.F.; Voon, G.Z.; Lim, H.X.; Chua, M.L.; Poh, C.L. Innate and adaptive immune evasion by dengue virus. Front. Cell Infect. Microbiol. 2022, 12, 1004608. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Kalayanarooj, S.; Ubol, S. Subversion of innate defenses by the interplay between DENV and pre-existing enhancing antibodies: TLRs signaling collapse. PLoS Negl. Trop. Dis. 2010, 4, e924. [Google Scholar] [CrossRef]
- Luo, R.; Fang, L.; Jin, H.; Jiang, Y.; Wang, D.; Chen, H.; Xiao, S. Antiviral activity of type I and type III interferons against porcine reproductive and respiratory syndrome virus (PRRSV). Antivir. Res. 2011, 91, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Noel, G.J.; Salgame, P.; Mosser, D.M. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J. Exp. Med. 1998, 188, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.S.; Mosser, D.M. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J. Immunol. 2001, 166, 6861–6868. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.R.; Zhang, S.L.; Tan, H.C.; Chan, Y.K.; Chow, A.; Lim, A.P.; Vasudevan, S.G.; Hanson, B.J.; Ooi, E.E. Ligation of Fc gamma receptor IIB inhibits antibody-dependent enhancement of dengue virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 12479–12484. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Li, W.; Feng, X.; Han, F.; Zhang, Y.; Chen, J.; Liu, D.; Xia, P. Activating Fc Gamma Receptors and Viral Receptors Are Required for Antibody-Dependent Enhancement of Porcine Reproductive and Respiratory Syndrome Virus Infection. Vet. Sci. 2022, 9, 470. [Google Scholar] [CrossRef]

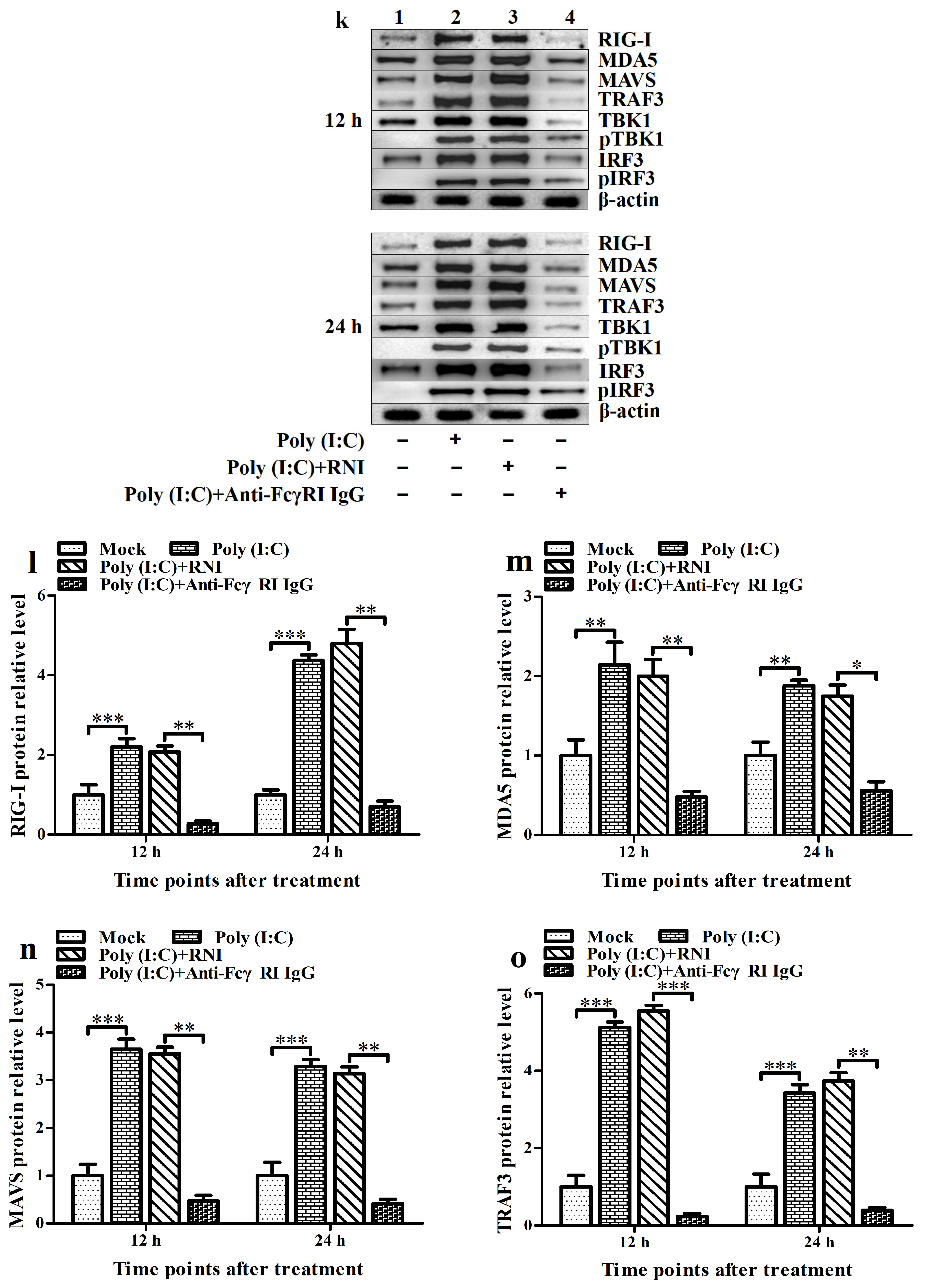
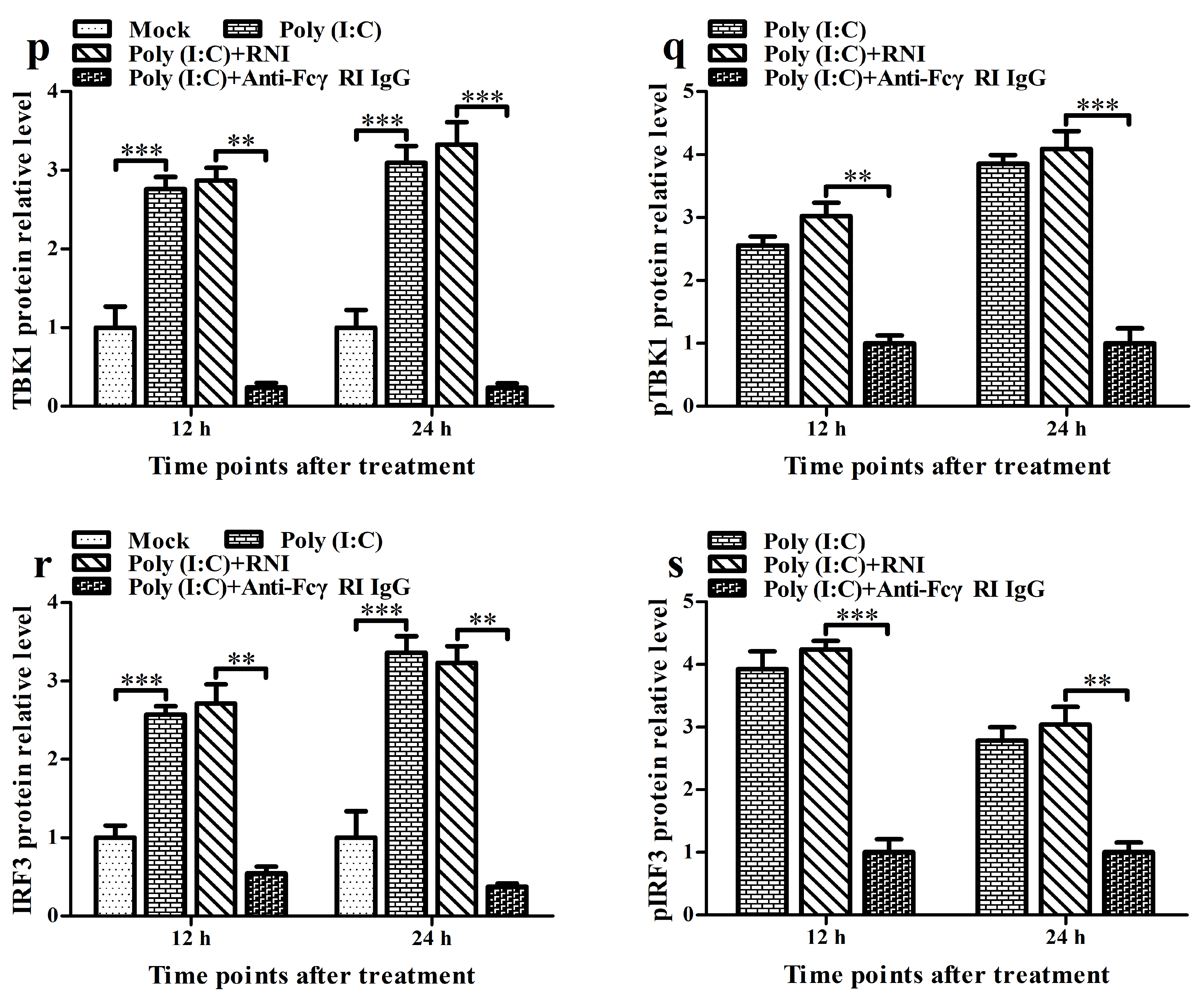
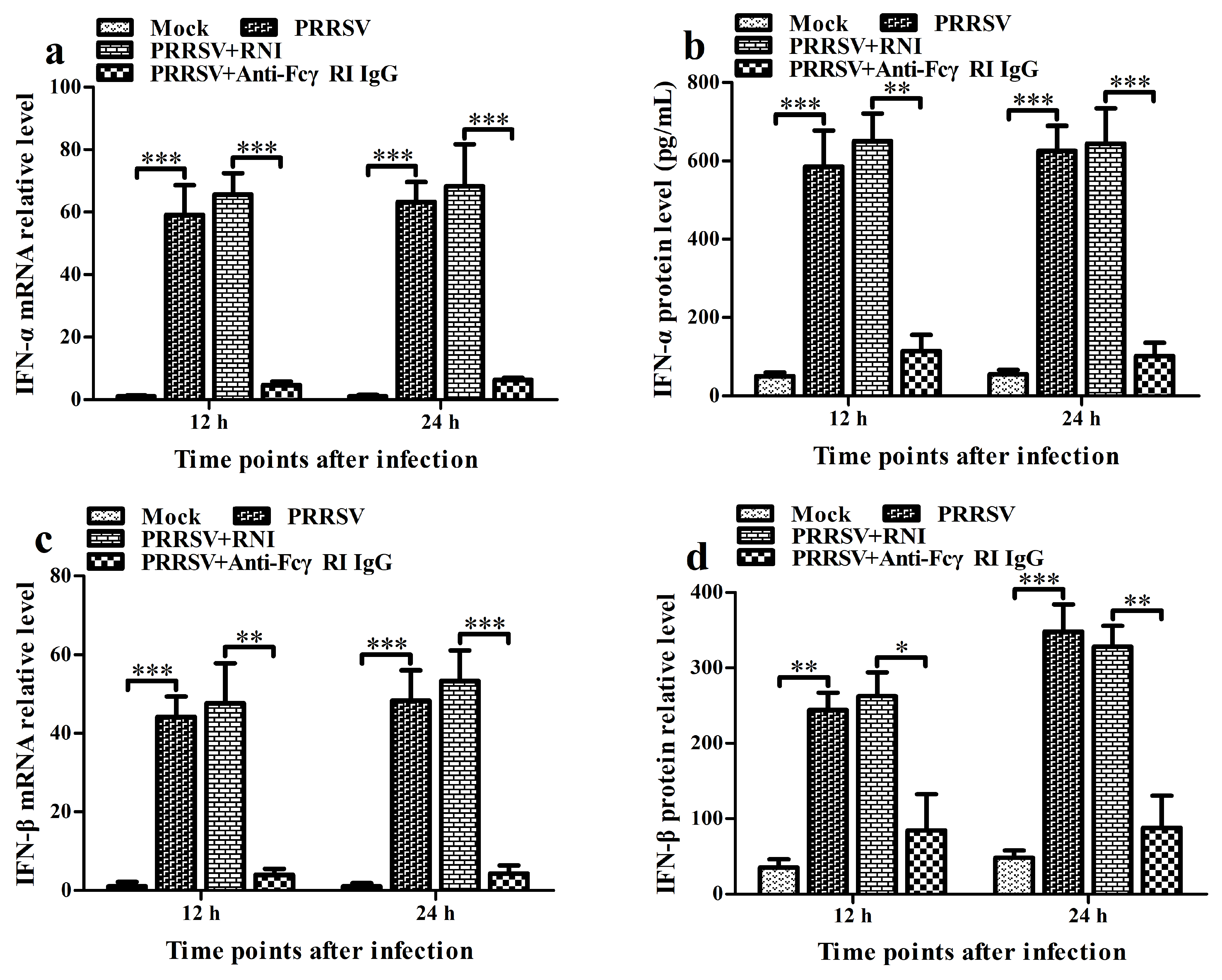
| Name # | Sequence (5′–3′) |
|---|---|
| IFN-α F IFN-α R | GGATCAGCAGCTCAGGG GAGGGTGAGTCTGTGGAAGTA |
| IFN-β F IFN-β R | CAACAAAGGAGCAGCAAT TGGAGCATCTCGTGGATA |
| RIG-I F RIG-I R | AGCACCTCATACTTACAGCCC CCTTCCCCTTTCGTCCTTGT |
| MDA5 F MDA5 R | TGACGAATGCCATCACACCA TTGGCTTGCTTTTTGGCTCC |
| MAVS F MAVS R | AGTGTCCCGTGTCTACCAGA GGACAGGCATGGGGTAACTT |
| TRAF3 F TRAF3 R | GCCAGGTCCCAATGATCACA GGGGCATTGACACACTCTGA |
| TBK1 F TBK1 R | TTTAGATGGGGATGCCAGCG CCATCGTATCCCCTTTCGCA |
| IRF3 F IRF3 R | ATCGAAGGAAGCAGACGCTC AACCTTGACCATCACCAGCC |
| FcγRI F FcγRI R | TGAAACAAAGTTGCTCCCA GCTGCGCTTGATGACCT |
| β-actin F β-actin R | CGGGACATCAAGGAGAAGC CTCGTTGCCGATGGTGATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, A.; Chen, W.; Feng, X.; Wang, B.; He, S.; Fan, H. Antibody-Dependent Enhancement of Porcine Reproductive and Respiratory Syndrome Virus Infection Antagonizes the Secretion of Type I Interferons in Porcine Alveolar Macrophages by Interfering with the Retinoic Acid-Inducible Gene I/Melanoma Differentiation-Associated Gene 5 Pathway via Fc Gamma Receptor I. Viruses 2025, 17, 1277. https://doi.org/10.3390/v17091277
Zhang L, Wang A, Chen W, Feng X, Wang B, He S, Fan H. Antibody-Dependent Enhancement of Porcine Reproductive and Respiratory Syndrome Virus Infection Antagonizes the Secretion of Type I Interferons in Porcine Alveolar Macrophages by Interfering with the Retinoic Acid-Inducible Gene I/Melanoma Differentiation-Associated Gene 5 Pathway via Fc Gamma Receptor I. Viruses. 2025; 17(9):1277. https://doi.org/10.3390/v17091277
Chicago/Turabian StyleZhang, Liujun, Aiyang Wang, Weizhen Chen, Xing Feng, Bo Wang, Shaojun He, and Hongjie Fan. 2025. "Antibody-Dependent Enhancement of Porcine Reproductive and Respiratory Syndrome Virus Infection Antagonizes the Secretion of Type I Interferons in Porcine Alveolar Macrophages by Interfering with the Retinoic Acid-Inducible Gene I/Melanoma Differentiation-Associated Gene 5 Pathway via Fc Gamma Receptor I" Viruses 17, no. 9: 1277. https://doi.org/10.3390/v17091277
APA StyleZhang, L., Wang, A., Chen, W., Feng, X., Wang, B., He, S., & Fan, H. (2025). Antibody-Dependent Enhancement of Porcine Reproductive and Respiratory Syndrome Virus Infection Antagonizes the Secretion of Type I Interferons in Porcine Alveolar Macrophages by Interfering with the Retinoic Acid-Inducible Gene I/Melanoma Differentiation-Associated Gene 5 Pathway via Fc Gamma Receptor I. Viruses, 17(9), 1277. https://doi.org/10.3390/v17091277





