An HTLV-1-Infected Humanized Mouse Model Expressing HLA-A*02:01 Demonstrates Effective CTL-Mediated Suppression of HTLV-1
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Animals
2.3. Antibodies
2.4. Purification of Hematopoietic Stem Cells
2.5. Generation of Humanized Mice
2.6. HTLV-1 Infection
2.7. DNA Purification and Proviral Load Measurement
2.8. Flow Cytometric Analyses
2.9. Statistical Analysis
3. Results
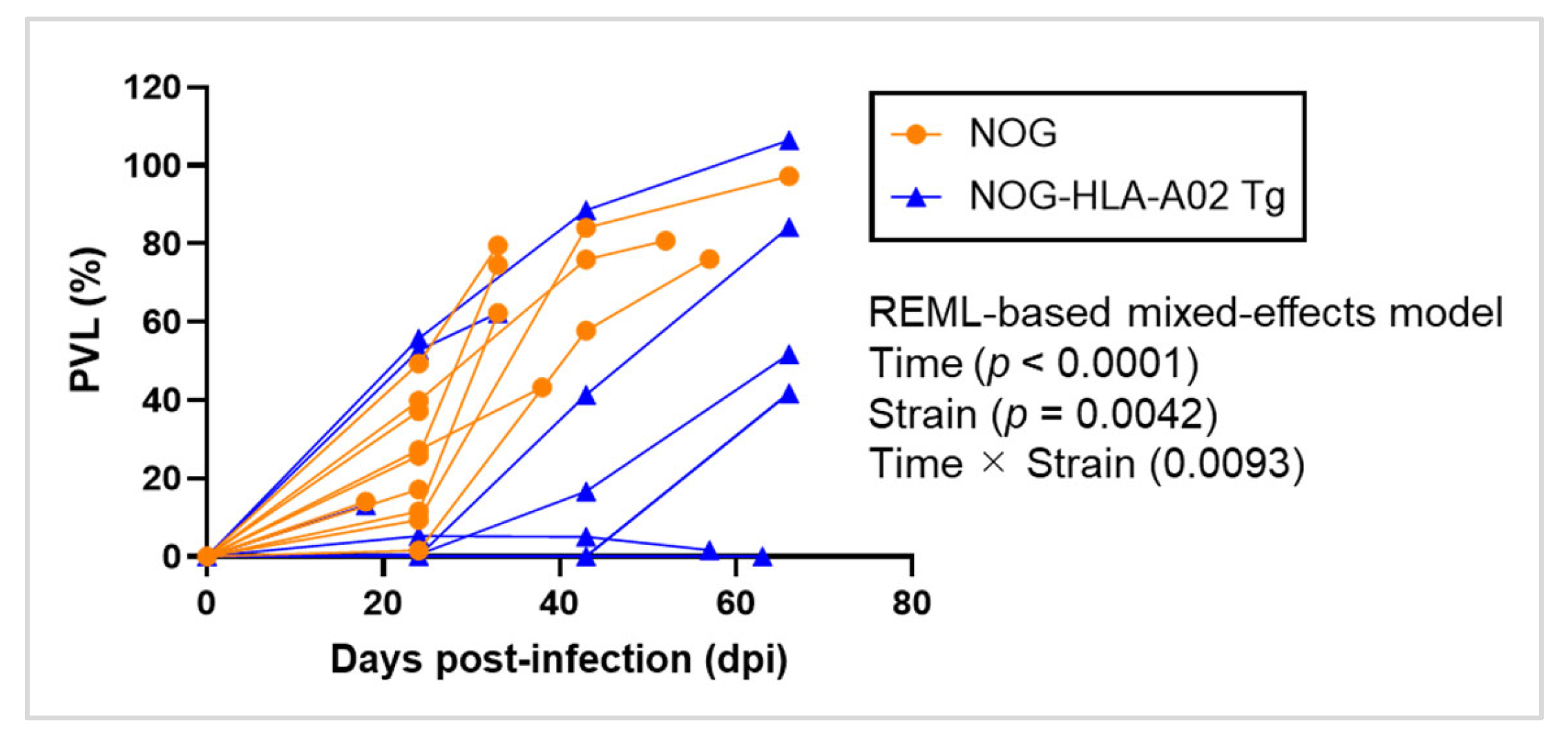
3.1. Humanized NOG-HLA-A02 Transgenic Mice May Suppress HTLV-1 Proviral Load Increase After Infection
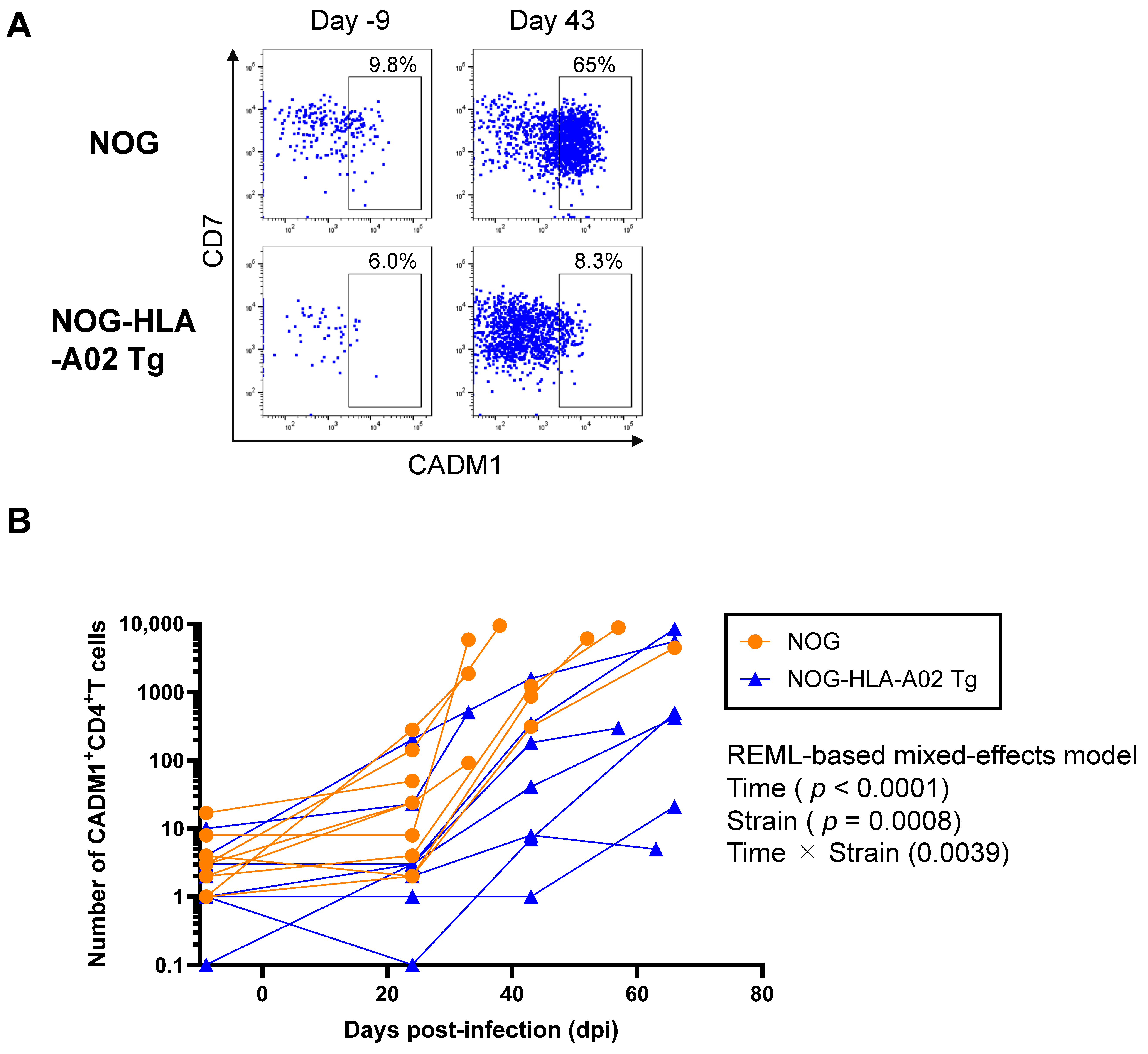
3.2. Humanized NOG-HLA-A02 Transgenic Mice Suppress the Expansion of HTLV-1-Infected CD4+ T Cells After Infection
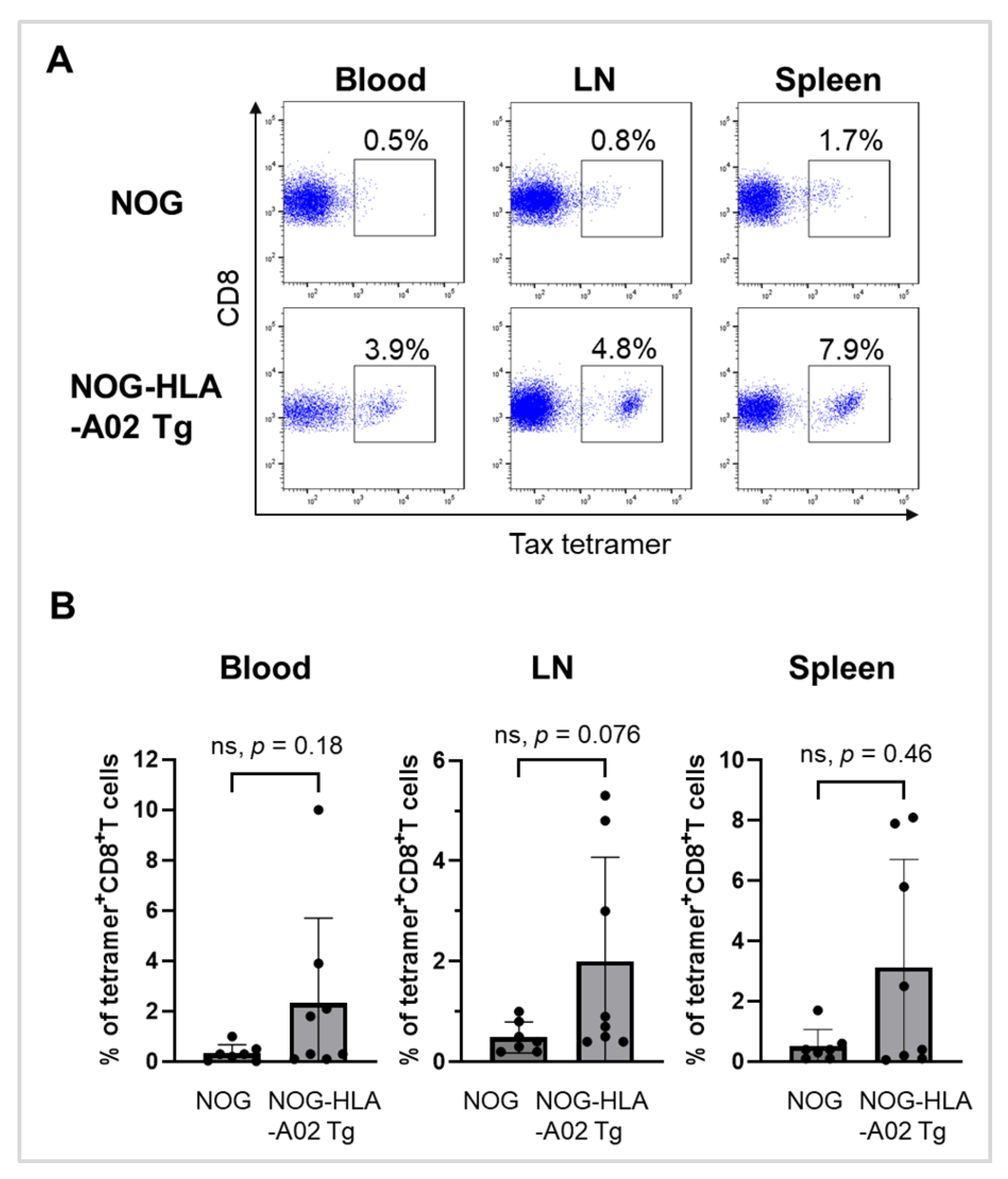
3.3. Humanized NOG-HLA-A02 Transgenic Mice Tend to Exhibit Increased Frequencies of Tax-Specific CD8+ T Cells After Infection
3.4. Prolonged Survival of Humanized NOG-HLA-A02 Transgenic Mice Following HTLV-1 Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HTLV-1 | human T-cell leukemia virus type 1 |
| ATL | adult T-cell leukemia/lymphoma |
| HAM/TSP | HTLV-1-associated myelopathy/tropical spastic paraparesis |
| CTLs | cytotoxic T lymphocytes |
| Tg | transgenic |
| PVL | proviral load |
| ACs | asymptomatic carriers |
| HSCs | hematopoietic stem cells |
| TCRs | T-cell receptors |
| MHC | major histocompatibility complex |
| MNCs | mononuclear cells |
| IBMI | intra-bone marrow injection |
| HBB | human b-globin |
| REML | restricted maximum likelihood |
| EBV | Epstein–Barr virus |
References
- Bangham, C.R.M.; Matsuoka, M. Human T-Cell Leukaemia Virus Type 1: Parasitism and Pathogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160272. [Google Scholar] [CrossRef]
- Uchiyama, T.; Yodoi, J.; Sagawa, K.; Takatsuki, K.; Uchino, H. Adult T-Cell Leukemia: Clinical and Hematologic Features of 16 Cases. Blood 1977, 50, 481–492. [Google Scholar] [CrossRef]
- Gessain, A.; Vernant, J.C.; Maurs, L.; Barin, F.; Gout, O.; Calender, A.; De Thé, G. ANTIBODIES TO HUMAN T-LYMPHOTROPIC VIRUS TYPE-I IN PATIENTS WITH TROPICAL SPASTIC PARAPARESIS. Lancet 1985, 326, 407–410. [Google Scholar] [CrossRef]
- Osame, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Igata, A.; Matsumoto, M.; Tara, M. HTLV-I ASSOCIATED MYELOPATHY, A NEW CLINICAL ENTITY. Lancet 1986, 327, 1031–1032. [Google Scholar] [CrossRef]
- Iwanaga, M.; Watanabe, T.; Utsunomiya, A.; Okayama, A.; Uchimaru, K.; Koh, K.R.; Ogata, M.; Kikuchi, H.; Sagara, Y.; Uozumi, K.; et al. Human T-Cell Leukemia Virus Type I (HTLV-1) Proviral Load and Disease Progression in Asymptomatic HTLV-1 Carriers: A Nationwide Prospective Study in Japan. Blood 2010, 116, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Usuku, K.; Matsumoto, W.; Kodama, D.; Takenouchi, N.; Moritoyo, T.; Hashiguchi, S.; Ichinose, M.; Bangham, C.R.M.; Izumo, S.; et al. Analysis of HTLV-I Proviral Load in 202 HAM/TSP Patients and 243 Asymptomatic HTLV-I Carriers: High Proviral Load Strongly Predisposes to HAM/TSP. J. Neurovirol. 1998, 4, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Harhaj, E.W. Mechanisms of Oncogenesis by HTLV-1 Tax. Pathogens 2020, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.; Shida, H.; McFarlin, D.E.; Fauci, A.S.; Koenig, S. Circulating CD8+ Cytotoxic T Lymphocytes Specific for HTLV-I PX in Patients with HTLV-I Associated Neurological Disease. Nature 1990, 348, 245–248. [Google Scholar] [CrossRef]
- Akimoto, M.; Kozako, T.; Sawada, T.; Matsushita, K.; Ozaki, A.; Hamada, H.; Kawada, H.; Yoshimitsu, M.; Tokunaga, M.; Haraguchi, K.; et al. Anti-HTLV-1 Tax Antibody and Tax-Specific Cytotoxic T Lymphocyte Are Associated with a Reduction in HTLV-1 Proviral Load in Asymptomatic Carriers. J. Med. Virol. 2007, 79, 977–986. [Google Scholar] [CrossRef]
- Kozako, T.; Arima, N.; Toji, S.; Masamoto, I.; Akimoto, M.; Hamada, H.; Che, X.-F.; Fujiwara, H.; Matsushita, K.; Tokunaga, M.; et al. Reduced Frequency, Diversity, and Function of Human T Cell Leukemia Virus Type 1-Specific CD8+ T Cell in Adult T Cell Leukemia Patients. J. Immunol. 2006, 177, 5718–5726. [Google Scholar] [CrossRef]
- Takamori, A.; Hasegawa, A.; Utsunomiya, A.; Maeda, Y.; Yamano, Y.; Masuda, M.; Shimizu, Y.; Tamai, Y.; Sasada, A.; Zeng, N.; et al. Functional Impairment of Tax-Specific but Not Cytomegalovirus-Specific CD8+ T Lymphocytes in a Minor Population of Asymptomatic Human T-Cell Leukemia Virus Type 1-Carriers. Retrovirology 2011, 8, 100. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kubota, R.; Tara, M.; Izumo, S.; Osame, M. Existence of Escape Mutant in HTLV-I Tax during the Development of Adult T-Cell Leukemia. Blood 2001, 97, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Koiwa, T.; Hamano-Usami, A.; Ishida, T.; Okayama, A.; Yamaguchi, K.; Kamihira, S.; Watanabe, T. 5′-Long Terminal Repeat-Selective CpG Methylation of Latent Human T-Cell Leukemia Virus Type 1 Provirus In Vitro and In Vivo. J. Virol. 2002, 76, 9389–9397. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Maeda, M.; Morikawa, S.; Taniguchi, Y.; Yasunaga, J.I.; Nosaka, K.; Tanaka, Y.; Matsuoka, M. Genetic and Epigenetic Inactivation of TAX Gene in Adult T-Cell Leukemia Cells. Int. J. Cancer 2004, 109, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ishii, N.; Ine, S.; Ikeda, S.; Fujimura, T.; Ndhlovu, L.C.; Soroosh, P.; Tada, K.; Harigae, H.; Kameoka, J.; et al. Regulatory T Cell-like Activity of Foxp3+ Adult T Cell Leukemia Cells. Int. Immunol. 2006, 18, 269–277. [Google Scholar] [CrossRef]
- Nakajima, S.; Okuma, K. Mouse Models for HTLV-1 Infection and Adult T Cell Leukemia. Int. J. Mol. Sci. 2023, 24, 11737. [Google Scholar] [CrossRef]
- Shultz, L.D.; Brehm, M.A.; Victor Garcia-Martinez, J.; Greiner, D.L. Humanized Mice for Immune System Investigation: Progress, Promise and Challenges. Nat. Rev. Immunol. 2012, 12, 786. [Google Scholar] [CrossRef]
- Mori, A.; Murata, S.; Tashiro, N.; Tadokoro, T.; Okamoto, S.; Otsuka, R.; Wada, H.; Murata, T.; Takahashi, T.; Seino, K.I.; et al. Establishment of Human Leukocyte Antigen-Mismatched Immune Responses after Transplantation of Human Liver Bud in Humanized Mouse Models. Cells 2021, 10, 476. [Google Scholar] [CrossRef]
- Jaiswal, S.; Pearson, T.; Friberg, H.; Shultz, L.D.; Greiner, D.L.; Rothman, A.L.; Mathew, A. Dengue Virus Infection and Virus-Specific HLA-A2 Restricted Immune Responses in Humanized NOD-Scid IL2rγnull Mice. PLoS ONE 2009, 4, e7251. [Google Scholar] [CrossRef]
- Shultz, L.D.; Saito, Y.; Najima, Y.; Tanaka, S.; Ochi, T.; Tomizawa, M.; Doi, T.; Sone, A.; Suzuki, N.; Fujiwara, H.; et al. Generation of Functional Human T-Cell Subsets with HLA-Restricted Immune Responses in HLA Class I Expressing NOD/SCID/IL2rγnull Humanized Mice. Proc. Natl. Acad. Sci. USA 2010, 107, 13022–13027. [Google Scholar] [CrossRef]
- Yao, J.; Tanaka, M.; Takenouchi, N.; Ren, Y.; Lee, S.-I.; Fujisawa, J.-I. Induction of APOBEC3B Cytidine Deaminase in HTLV-1-Infected Humanized Mice. Exp. Ther. Med. 2019, 17, 3701. [Google Scholar] [CrossRef]
- Hiyoshi, M.; Takahashi, N.; Eltalkhawy, Y.M.; Noyori, O.; Lotfi, S.; Panaampon, J.; Okada, S.; Tanaka, Y.; Ueno, T.; Fujisawa, J.I.; et al. M-Sec Induced by HTLV-1 Mediates an Efficient Viral Transmission. PLoS Pathog. 2021, 17, e1010126. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Ge, S.; Symbatyan, G.; Rosol, M.S.; Olch, A.J.; Crooks, G.M. Effects of Sublethal Irradiation on Patterns of Engraftment after Murine Bone Marrow Transplantation. Biol. Blood Marrow Transplant. 2011, 17, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Katano, I.; ITO, R.; Eto, T.; Aiso, S.; Ito, M. Immunodeficient NOD-Scid IL-2Rγnull Mice Do Not Display T and B Cell Leakiness. Exp. Anim. 2011, 60, 181–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tezuka, K.; Xun, R.; Tei, M.; Ueno, T.; Tanaka, M.; Takenouchi, N.; Fujisawa, J.I. An Animal Model of Adult T-Cell Leukemia: Humanized Mice with HTLV-1–Specific Immunity. Blood 2014, 123, 346–355. [Google Scholar] [CrossRef]
- Manivannan, K.; Rowan, A.G.; Tanaka, Y.; Taylor, G.P.; Bangham, C.R.M. CADM1/TSLC1 Identifies HTLV-1-Infected Cells and Determines Their Susceptibility to CTL-Mediated Lysis. PLoS Pathog. 2016, 12, e1005560. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nakano, K.; Watanabe, E.; Ishigaki, T.; Ohno, N.; Yuji, K.; Oyaizu, N.; Asanuma, S.; Yamagishi, M.; Yamochi, T.; et al. CADM1 Expression and Stepwise Downregulation of CD7 Are Closely Associated with Clonal Expansion of HTLV-I-Infected Cells in Adult T-Cell Leukemia/Lymphoma. Clin. Cancer Res. 2014, 20, 2851–2861. [Google Scholar] [CrossRef]
- Cook, L.B.M.; Melamed, A.; Demontis, M.A.; Laydon, D.J.; Fox, J.M.; Tosswill, J.H.C.; Freitas, D.; Price, A.D.; Medcalf, J.F.; Martin, F.; et al. Rapid Dissemination of Human T-Lymphotropic Virus Type 1 during Primary Infection in Transplant Recipients. Retrovirology 2016, 13, 3. [Google Scholar] [CrossRef]
- Lan, P.; Tonomura, N.; Shimizu, A.; Wang, S.; Yang, Y.G. Reconstitution of a Functional Human Immune System in Immunodeficient Mice through Combined Human Fetal Thymus/Liver and CD34+ Cell Transplantation. Blood 2006, 108, 487–492. [Google Scholar] [CrossRef]
- Melkus, M.W.; Estes, J.D.; Padgett-Thomas, A.; Gatlin, J.; Denton, P.W.; Othieno, F.A.; Wege, A.K.; Haase, A.T.; Garcia, J.V. Humanized Mice Mount Specific Adaptive and Innate Immune Responses to EBV and TSST-1. Nat. Med. 2006, 12, 1316–1322. [Google Scholar] [CrossRef]
- Brainard, D.M.; Seung, E.; Frahm, N.; Cariappa, A.; Bailey, C.C.; Hart, W.K.; Shin, H.-S.; Brooks, S.F.; Knight, H.L.; Eichbaum, Q.; et al. Induction of Robust Cellular and Humoral Virus-Specific Adaptive Immune Responses in Human Immunodeficiency Virus-Infected Humanized BLT Mice. J. Virol. 2009, 83, 7305–7321. [Google Scholar] [CrossRef]
- Strowig, T.; Gurer, C.; Ploss, A.; Liu, Y.F.; Arrey, F.; Sashihara, J.; Koo, G.; Rice, C.M.; Young, J.W.; Chadburn, A.; et al. Priming of Protective T Cell Responses against Virus-Induced Tumors in Mice with Human Immune System Components. J. Exp. Med. 2009, 206, 1423. [Google Scholar] [CrossRef] [PubMed]
- Hanon, E.; Hall, S.; Taylor, G.P.; Saito, M.; Davis, R.; Tanaka, Y.; Usuku, K.; Osame, M.; Weber, J.N.; Bangham, C.R.M.; et al. Abundant Tax Protein Expression in CD4+ T Cells Infected with Human T-Cell Lymphotropic Virus Type I (HTLV-I) Is Prevented by Cytotoxic T Lymphocytes. Blood 2000, 95, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, M.; Yasunaga, J.I.; Iwami, S.; Nakaoka, S.; Koizumi, Y.; Shimura, K.; Matsuoka, M. Sporadic on/off Switching of HTLV-1 Tax Expression Is Crucial to Maintain the Whole Population of Virus-Induced Leukemic Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1269–E1278. [Google Scholar] [CrossRef] [PubMed]
- Billerbeck, E.; Horwitz, J.A.; Labitt, R.N.; Donovan, B.M.; Vega, K.; Budell, W.C.; Koo, G.C.; Rice, C.M.; Ploss, A. Characterization of Human Antiviral Adaptive Immune Responses during Hepatotropic Virus Infection in HLA-Transgenic Human Immune System Mice. J. Immunol. 2013, 191, 1753. [Google Scholar] [CrossRef]
- Majji, S.; Wijayalath, W.; Shashikumar, S.; Pow-Sang, L.; Villasante, E.; Brumeanu, T.D.; Casares, S. Differential Effect of HLA Class-I versus Class-II Transgenes on Human T and B Cell Reconstitution and Function in NRG Mice. Sci. Rep. 2016, 6, 28093. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, S.; Goto, M.; Lee, S.-I.; Odaka, T.; Hino, M.; Tezuka, K.; Takenouchi, N.; Ueno, T.; Admadiani, F.R.; Takahashi, R.; et al. An HTLV-1-Infected Humanized Mouse Model Expressing HLA-A*02:01 Demonstrates Effective CTL-Mediated Suppression of HTLV-1. Viruses 2025, 17, 1249. https://doi.org/10.3390/v17091249
Nakajima S, Goto M, Lee S-I, Odaka T, Hino M, Tezuka K, Takenouchi N, Ueno T, Admadiani FR, Takahashi R, et al. An HTLV-1-Infected Humanized Mouse Model Expressing HLA-A*02:01 Demonstrates Effective CTL-Mediated Suppression of HTLV-1. Viruses. 2025; 17(9):1249. https://doi.org/10.3390/v17091249
Chicago/Turabian StyleNakajima, Shinsuke, Motohito Goto, Sung-Il Lee, Tokifumi Odaka, Masaki Hino, Kenta Tezuka, Norihiro Takenouchi, Takaharu Ueno, Fhahira Rizkhika Admadiani, Riichi Takahashi, and et al. 2025. "An HTLV-1-Infected Humanized Mouse Model Expressing HLA-A*02:01 Demonstrates Effective CTL-Mediated Suppression of HTLV-1" Viruses 17, no. 9: 1249. https://doi.org/10.3390/v17091249
APA StyleNakajima, S., Goto, M., Lee, S.-I., Odaka, T., Hino, M., Tezuka, K., Takenouchi, N., Ueno, T., Admadiani, F. R., Takahashi, R., Hamaguchi, I., Takahashi, T., Ito, M., & Okuma, K. (2025). An HTLV-1-Infected Humanized Mouse Model Expressing HLA-A*02:01 Demonstrates Effective CTL-Mediated Suppression of HTLV-1. Viruses, 17(9), 1249. https://doi.org/10.3390/v17091249





