Abstract
The non-structural protein 1 (NS1) of dengue virus (DENV) plays a multifaceted role in viral pathogenesis and immune modulation. Although vaccine strategies have traditionally focused on neutralizing antibodies against the envelope (E) protein, recent evidence highlights the protective potential of anti-NS1 antibodies—particularly those that mediate Fc-dependent effector functions. These functions include antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC), which collectively bridge adaptive antibody responses with innate immune activation. However, the outcomes of anti-NS1 responses are context-dependent: certain antibody specificities confer protection, while others may contribute to immunopathology. In this review, I synthesize current evidence on the roles of anti-NS1 antibodies in modulating Fc receptor engagement, subclass-specific responses, glycosylation patterns, and their effector functions. Understanding these mechanisms is essential for guiding rational vaccine design and the development of antibody-based diagnostics and therapeutics. By integrating the findings from both innate and adaptive immunology, this review emphasizes the importance of NS1 as a multifunctional immune determinant in dengue virus infection.
1. Introduction
Dengue virus (DENV) is a positive-sense, single-stranded RNA virus that causes a wide spectrum of illnesses ranging from asymptomatic and mild dengue fever (DF) to severe dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [1]. According to the WHO, dengue incidence is rising globally, with an estimated 400 million infections annually, of which ~25% are clinically apparent [2]. No specific antiviral treatment or effective vaccine is currently available. While Dengvaxia and Qdenga are approved, their limited efficacy across serotypes—shaped by host serostatus, age, and antibody-dependent enhancement (ADE) risk—underscores the need for improved vaccines [3,4,5]. Other vaccine candidates such as Butantan TV003/TV005 are in clinical trials [4,6].
DENV, a member of the Flaviviridae family, consists of four closely related serotypes (DENV-1 to -4) sharing 60−80% nucleotide identity. Its 11 kb genome encodes a single polyprotein processed into three structural proteins (Capsid, C; pre-Membrane/Membrane, prM/M; and Envelope, E) and seven non-structural proteins (NS1-5) [7]. Structural proteins assemble the viral particle, whereas NS proteins facilitate replication and immune evasion.
DENV infects both immune (monocytes, macrophages, and dendritic cells) and non-immune cells (hepatocytes, epithelial cells, and endothelial cells) [8,9], mainly entering via receptor-mediated or Fcγ receptor (FcγR)-dependent endocytosis [7,8]. Once internalized, the viral RNA is translated and replicated in the endothelial reticulum (ER), and virions mature in the trans-Golgi network (TGN), before secretion.
Most antibodies target the E protein, although responses to other proteins are also observed [10,11,12]. While antibodies help clear virus and infected cells, some contribute to ADE where sub-neutralizing antibodies facilitate viral entry into FcR-expressing cells. Antibodies against E and prM are implicated in ADE, complicating vaccine design [10,13,14]. Because anti-NS1 antibodies do not mediate ADE, NS1 is considered a promising alternative target.
NS1 is a 45–55 kDa glycoprotein with two N-linked glycans and twelve cysteine, forming six disulfide bonds [15,16,17]. NS1 exists in monomeric, dimeric, and oligomeric forms and is found intracellularly and extracellularly [18,19,20]. After synthesis and glycosylation at N130 and N207 [21], NS1 forms dimers that associate with membranes [18,22] and participate in replication complexes in the ER [23,24]. NS1 passes through the Golgi, where glycan maturation differs by residue and host cell type [18,25]. It is then secreted as oligomeric forms and can rebind to uninfected cell membranes [26]. Circulating NS1 can reach ~50 μg/mL in dengue patients [27]. Dengue NS1 is an important target for immune responses and vaccine development, owing to its unique secretion from infected cells and absence from mature virions—features that differentiate it from conventional structural protein antigens.
Structural studies show that monomeric NS1 contains three domains: a β-roll (amino acid residue, aa 1–29), wing (aa 30–180), and β-ladder (aa 181–352), with flexible loops and glycosylation sites contributing to function and immune recognition [28,29,30].
In addition to being a key antigenic target, NS1 plays a direct role in modulating innate immune responses. NS1 can induce vascular leakage [31,32,33,34,35,36,37,38] and increase the production of pro-inflammatory cytokines, chemokines, and matrix metalloproteinases (MMPs) [39,40,41]. It can also antagonize Toll-like receptor 4 (TLR4) signaling [41,42], and inhibit complement activation [43,44,45]. These evasion strategies allow dengue virus to blunt early innate responses, facilitating viral replication and dissemination [43,45,46]. Importantly, these same pathways intersect with Fc-mediated effector functions triggered by NS1-targeting antibodies, suggesting that NS1 operates at the nexus of immune recognition and immune evasion.
While vaccine development has traditionally focused on E protein-targeting neutralizing antibodies, growing evidence highlights the importance of Fc-mediated effector functions—particularly those triggered by anti-NS1 antibodies—in shaping dengue immunity. These antibodies bridge adaptive and innate responses by engaging Fcγ receptors on NK cells and monocytes or activating complement.
Despite increasing interest, how NS1-targeting antibodies exert these Fc-dependent effects remains underexplored. This review synthesizes evidence from in vitro studies, murine models, and clinical cohorts to map the functional landscape of anti-NS1 antibodies, highlighting their implications for rational vaccine and therapeutic development.
2. Roles of Anti-NS1 Antibodies
Anti-NS1 antibodies can mediate both protective and pathogenic effects, depending on their epitope specificity, IgG subclass, and Fc effector properties. Extracellular NS1 is highly immunogenic, and growing evidence indicates that both NS1-based vaccination and passive transfer of anti-NS1 antibodies confer protection in flavivirus-infected mice [12,32,34,47,48,49,50,51,52,53,54,55].
In DENV mouse models, anti-NS1 antibodies have been shown to inhibit vascular leakage [32,34,40] and trigger complement-dependent cytotoxicity (CDC) via Fc-dependent mechanisms [53,56]. Consistent with these findings, human studies show that children who later developed mild dengue fever (DF) exhibited greater Fc effector functions against NS1 [57]. Additionally, individuals with subclinical secondary dengue had higher levels of anti-NS1 IgG, marked by a stronger binding affinity and enhanced NK cell activation, compared to symptomatic cases [58]. These findings suggest that functionally potent anti-NS1 antibodies, especially those capable of engaging Fcγ receptors and mediating ADCC, may contribute to protective immunity.
Clinical data further support this potential. The tetravalent live-attenuated vaccine TAK-003 elicits robust NS1-specific IgG responses, which have been shown to block NS1-induced endothelial hyperpermeability in vitro, highlighting the translational relevance of anti-NS1 immunity in humans [59].
However, anti-NS1 antibodies can also mediate pathogenic processes. In human studies, anti-NS1 antibodies can amplify complement activation, which is correlated with disease severity [60]. Moreover, NS1 can induce autoantibodies that cross-react with platelet and endothelial antigens via molecular mimicry [61,62,63,64,65]. These autoantibodies can contribute to vascular leakage and thrombocytopenia in mouse models [66]. Notably, some studies show that NS1 vaccination or passive antibody transfer fails to protect mice challenged with highly virulent, non-mouse-adapted DENV2 strains, raising concerns about strain-specific limitations [67].
While mouse models offer important mechanistic insights, they may not fully recapitulate human disease as these models are usually interferon receptor-deficient strains (e.g., AG129, Ifnar–/–) which may alter Fc effector responses. Differences in Fcγ receptor expression, complement regulation, and Fc glycosylation between species can affect antibody function and disease outcomes. Therefore, the findings from murine models should be interpreted cautiously and validated in human cohorts or controlled human infection models (CHIMs).
Clinically, elevated plasma levels of NS1-specific antibodies have been associated with severe dengue and secondary infections, suggesting a potential correlation between anti-NS1 responses and disease severity [11,68,69]. However, other cohort studies reported that higher anti-NS1 antibody levels correlated with lower circulating NS1 antigens, but not consistently with clinical protection [70]. These findings underscore that antibody quality—particularly Fc functionality and epitope specificity—rather than quantity alone—determines clinical outcomes. This reinforces the need for integrated serological and functional profiling to fully understand the dual roles of anti-NS1 antibodies.
Whether anti-NS1 antibodies act protectively or pathogenically appears to depend on multiple factors, including epitope specificity, subclass, and binding geometry. Protective responses are typically associated with antibodies targeting membrane-distal or surface-accessible epitopes, which can promote FcγR-mediated clearance through ADCC or phagocytosis [48,51,71]. In contrast, antibodies that form large immune complexes or bind near endothelial-binding domains may enhance immune deposition and inflammation, contributing to pathology [60,62,64,69]. The nature of the antibody response—homotypic or heterotypic—especially during secondary infections, may further shape disease outcomes. Collectively, these findings highlight the dual roles of anti-NS1 antibodies in dengue protection and pathogenesis and emphasize the importance of fine epitope targeting and Fc effector programming (Table 1).

Table 1.
Protective and pathogenic roles of anti-NS1 antibodies in Flavivirus infection.
3. IgG Subclasses and Functions
Antibody effector functions play a central role in bridging adaptive and innate immunity and are critical for viral clearance. In humans, IgG is the most abundant antibody isotype in serum, comprising four subclasses—IgG1, IgG2, IgG3, and IgG4—which differ in their constant (Fc) regions between the hinge and CH2 domains of the heavy chain [73]. Of these, IgG1 is the most prevalent (~60%), followed by IgG2 (32%), IgG3 (4%), and IgG4 (4%) [73]. Increasing evidence suggests that IgG subclass bias significantly influences protection versus pathology in DENV infection. Studies have shown elevated levels of anti-DENV IgG1 and IgG4 in individuals with dengue hemorrhagic fever (DHF) during the acute phase, yet the specific viral antigens targeted were not identified [74]. Higher levels of anti-E and anti-NS1 total IgG, IgG4, and greater Fc effector functions have been reported in children protected from symptomatic dengue [75]. In these cases, Fc-silent IgG4 antibodies may compete with IgG1 for antigen binding, thereby modulating immune activation and limiting inflammation [75]. Notably, anti-NS1 IgG1 levels are consistently higher in patients with acute or past DHF, whereas anti-NS1 IgG3 responses are more prominent in those with milder dengue fever (DF) [11,68], suggesting distinct roles for these subclasses in shaping disease outcome.
Different IgG subclasses engage distinct Fcγ receptors (FcγRs) with varying affinities, thereby influencing the outcome of immune responses [73,76,77]. Fcγ receptor engagement is a key mechanism linking antibody specificity to antiviral immunity across flaviviruses. In West Nile virus (WNV) and Zika virus (ZIKV) infections, antibody binding to surface NS1 in infected cells triggers activating FcγR-mediated functions, leading to protection in mice [48,51]. In dengue, antibody−FcγR interactions are more complex due to the potential for ADE. Nonetheless, protective effects have been demonstrated in mouse models using NS1-specific monoclonal antibodies whose efficacy depends on FcγR engagement [49,53]. These findings underscore the importance of FcγR affinity, subclass distribution, and immune complex geometry in shaping the outcome of flavivirus infections. However, it is important to note that FcγR expression patterns and functional profiles differ between mice and humans [78], limiting the direct extrapolation of murine findings to clinical contexts. While most evidence comes from mouse models, human studies confirming NS1-specific FcγR engagement are scarce. Future functional studies—such as ADCC or ADCP with receptor blocking in human PBMCs using anti-NS1 antibodies—could directly link subclass to receptor signaling. Analyzing FcR gene polymorphisms in clinical isolate may shed light on in vivo significance.
Beyond FcγRs, IgG subclasses also vary in their ability to activate the classical complement pathway via C1q binding [79,80]. The efficiency of complement activation is influenced by antibody concentration, epitope density, and subclass [81]. Human IgG3 is the most potent activator of complement, especially under low antigen density, while IgG1 becomes more effective under high antigen concentrations [81]. In WNV infection, complement-mediated neutralization relies on C1q and specific IgG subclasses, with IgG1 and IgG3 demonstrating the greatest activity and IgG4 showing little to no effect [80]. In dengue, the bias toward anti-NS1 IgG1 over IgG3 in DHF cases may contribute to enhanced inflammatory and complement-activating responses, potentially exacerbating disease [11,68]. Engagement of FcγRIIB by IgG3 may help suppress excessive immune activation and thus be protective. Supporting this, studies on anti-E antibodies have shown that FcγRIIB engagement by immune complexes can suppress phagocytosis and reduce ADE in monocytes [82]. At present, the direct association between specific anti-NS1 IgG subclass titers and in vivo complement activation—as measured by circulating complement products—remains unclear and warrants further investigation. The summary of IgG subclasses and dengue outcome is shown in Table 2.

Table 2.
IgG subclasses, complement activation, and dengue outcome.
4. Fc Glycosylation and Disease Severity
Although neutralizing antibody titers have traditionally been used as correlates of protection, multiple studies show they do not consistently predict dengue disease severity [83]. Instead, the glycosylation status of the IgG Fc region—particularly the presence or absence of core fucose at position N297—has emerged as a more reliable predictor of clinical outcomes [83,84,85].
Under normal conditions, most circulating IgG is fucosylated, which restricts its interaction with FcγRIIIA, limiting pro-inflammatory signaling [86]. In contrast, afucosylated IgG1 binds FcγRIIIA with a significantly higher affinity, thereby enhancing Fc-dependent effector functions such as ADCC and amplifying inflammatory responses, especially in severe viral infections [84,86,87,88]. This functional relevance was confirmed in dengue mouse models (FcγR-humanized mice), where disease severity was mitigated by blocking the afucosylated IgG1–FcγRIIIA interaction using a nanobody [89].
In human dengue, particularly during secondary infections, elevated levels of afucosylated IgG1 targeting both E and NS1 proteins have been strongly linked to severe outcomes [83]. Mechanistically, these antibodies facilitate immune complex formation and overactivation of effector cells through FcγRIIIA, contributing to immunopathology [84]. Moreover, their enrichment has been correlated with reduced platelet counts, suggesting a role in thrombocytopenia [84].
Emerging evidence suggests Fc glycosylation patterns may also influence vertical transmission. Infants born to mothers with high levels of afucosylated anti-dengue IgG1 are more susceptible to symptomatic dengue, indicating that maternal Fc glycan profiles can shape disease susceptibility in early life [90].
The diverse effector mechanisms mediated by anti-NS1 antibodies—shaped by glycosylation status and Fc receptor interactions—are summarized in Figure 1.
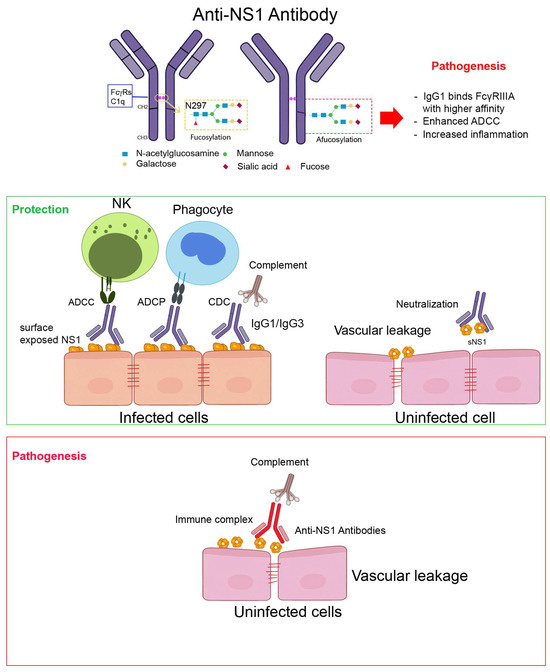
Figure 1.
Fc-mediated effector functions of anti-NS1 antibodies in dengue infection. This schematic illustrates how anti-NS1 antibodies engage both protective and pathogenic effector functions, depending on their epitope specificity, IgG subclass, and Fc glycosylation profile. Protective functions are typically seen with high-affinity, surface-accessible anti-NS1 antibodies of the IgG1/IgG3 subclass, while pathogenic effects may arise when immune complexes accumulate or Fc effector activity is dysregulated. NS1 is present on the surface of infected cells and in soluble hexameric form in circulation. Antibodies targeting accessible NS1 epitopes can activate various Fc-mediated pathways, including antibody-dependent cellular cytotoxicity (ADCC) by NK cells, antibody-dependent cellular phagocytosis (ADCP) by monocytes/macrophages, and complement-dependent cytotoxicity (CDC). These functions are typically associated with protective outcomes (green). In contrast, immune complex formation and deposition on endothelial cells may lead to inflammation and vascular pathology (red). The Fc domain of IgG antibodies contains an N-linked glycosylation site at N297, where the presence or absence of core fucose (afucosylation) critically modulates Fcγ receptor binding and downstream immune activation.
5. Clinical Relevance and Translational Potential
To induce protective Fc-dependent antibodies, dengue vaccines should include antigens that are accessible to effector mechanisms such as ADCC and phagocytosis (ADCP). The dengue NS1 is a promising candidate in this regard due to its surface expression on infected cells and absence from the virion, reducing the risk of ADE [91,92]. Epitope mapping should focus on surface-accessible regions of NS1 that are expressed on infected cells but lack homology to host proteins, thereby minimizing cross-reactive autoimmunity [12,50,62,64,93].
Comparative studies across flaviviruses indicate that anti-NS1 antibodies can confer protection in models of DENV, WNV, and ZIKV through Fc-mediated mechanisms [47,52,53,72]. However, NS1 also induces tissue-specific endothelial dysfunction, with differential patterns of vascular leakage depending on the target cells [94]. These virus-specific differences highlight the need for careful evaluation of NS1 as a vaccine or therapeutic target in dengue, as its pathogenic and protective roles may differ from other flaviviruses.
To optimize the quality of the humoral response, vaccines should aim to elicit a favorable IgG subclass profile—preferably IgG1 and IgG3—by incorporating Th1-skewing adjuvants such as CpG, poly(I:C), or TLR7/9 agonists, which enhance cellular immunity [95,96]. Balanced Fc glycosylation is also critical; vaccines should avoid inducing excessive afucosylation, as this has been linked to enhanced FcγRIIIA binding and increased immunopathology [84]. Thus, functional protection—defined by Fc-mediated effector activities rather than neutralizing titers alone—should be a key criterion in vaccine design.
Beyond prophylaxis, passive immunotherapy represents a complementary strategy for dengue treatment. Engineered monoclonal antibodies with modified Fc domains—such as N297A or LALA mutations—can retain or enhance ADCC activity, making them promising therapeutic candidates [89,97,98]. Furthermore, emerging evidence indicates that differences in Fc glycan profiles, such as increased afucosylation, correlate with disease severity. These glycan signatures may serve as valuable biomarkers for predicting clinical outcomes and guiding patient management [83,87,99].
6. Conclusion and Future Perspectives
The dengue virus NS1 is highly immunogenic and absent from viral particles, making it an appealing target for vaccine development. Advances in molecular and structural biology have enabled epitope-based approaches that elicit protective antibodies. However, beyond epitope specificity, Fc-mediated effector functions—such as ADCC, ADCP, and CDC—are increasingly recognized as essential components of protective immunity and should be considered in vaccine and antibody design.
Controlled human infection models (CHIMs) offer valuable platforms for studying dengue immunity in a human-specific context [100,101]. These models enable real-time assessment of antibody kinetics, Fc effector function, IgG subclass distribution, and glycan profiles during natural infection or vaccine-primed responses [75]. When integrated with systems serology, CHIMs can help define correlates of protection and inform rational vaccine and therapeutic strategies.
Emerging high-throughput platforms for Fc engineering and functional profiling also hold promise for both diagnostics and therapeutics. Distinct Fc glycan patterns and subclass distributions may serve as biomarkers of disease severity. For example, quantifying afucosylated IgG1 specific to NS1 or E protein using glycan-sensitive ELISAs could enable early risk stratification or serve as companion diagnostics in vaccine trials.
Combining precise epitope targeting with optimized Fc effector profiles will be key to developing safe, effective, and broadly protective dengue vaccines and antibody-based interventions. However, several knowledge gaps remain. It is still unclear how Fc glycosylation evolves throughout the course of natural dengue infection, or whether glycoform profiles differ between primary and secondary cases. In addition, the role of host Fcγ receptor polymorphisms in modulating effector function and disease severity in endemic populations is poorly understood. Standardized, field-deployable assays to assess Fc-mediated function in human samples are urgently needed to translate these mechanistic insights into diagnostic and clinical practice.
Funding
This paper was supported by the Alliance of International Science Organizations (ANSO-CR-PP-2021-08).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The author acknowledges the use of ChatGPT (OpenAI) version 4o to assist in improving the language clarity, grammar, and structural refinement of the manuscript. The author reviewed and verified all content for accuracy and originality.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, G. WHO recommends additional tests for Sanofi’s dengue vaccine after safety concerns. BMJ 2018, 361, k1765. [Google Scholar] [CrossRef]
- Daniels, B.C.; Ferguson, N.M.; Dorigatti, I. Efficacy, public health impact and optimal use of the Takeda dengue vaccine. Nat. Med. 2025, 31, 2663–2672. [Google Scholar] [CrossRef]
- Tricou, V.; Yu, D.; Reynales, H.; Biswal, S.; Saez-Llorens, X.; Sirivichayakul, C.; Lopez, P.; Borja-Tabora, C.; Bravo, L.; Kosalaraksa, P.; et al. Long-term efficacy and safety of a tetravalent dengue vaccine (TAK-003): 4·5-year results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2024, 12, e257–e270. [Google Scholar] [CrossRef]
- Nogueira, M.L.; Cintra, M.A.T.A.; Moreira, J.; Patiño, E.G.; Braga, P.E.; Tenório, J.C.V.; Alves, L.B.d.O.; Infante, V.; Silveira, D.H.R.; de Lacerda, M.V.G.; et al. Efficacy and safety of Butantan-DV in participants aged 2–59 years through an extended follow-up: Results from a double-blind, randomised, placebo-controlled, phase 3, multicentre trial in Brazil. Lancet Infect. Dis. 2024, 24, 1234–1244. [Google Scholar] [CrossRef]
- Nanaware, N.; Banerjee, A.; Bagchi, S.M.; Bagchi, P.; Mukherjee, A. Dengue Virus Infection: A Tale of Viral Exploitations and Host Responses. Viruses 2021, 13, 1967. [Google Scholar] [CrossRef]
- Urcuqui-Inchima, S.; Patiño, C.; Torres, S.; Haenni, A.-L.; Díaz, F.J. Recent developments in understanding dengue virus replication. Adv. Virus Res. 2010, 77, 1–39. [Google Scholar] [CrossRef]
- Praneechit, H.; Thiemmeca, S.; Prayongkul, D.; Kongmanas, K.; Mairiang, D.; Punyadee, N.; Songjaeng, A.; Tangthawornchaikul, N.; Angkasekwinai, N.; Sriruksa, K.; et al. Whole-blood model reveals granulocytes as key sites of dengue virus propagation, expanding understanding of disease pathogenesis. mBio 2024, 15, e0150524. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-Reacting Antibodies Enhance Dengue Virus Infection in Humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Jayathilaka, D.; Gomes, L.; Jeewandara, C.; Jayarathna, G.S.B.; Herath, D.; Perera, P.A.; Fernando, S.; Wijewickrama, A.; Hardman, C.S.; Ogg, G.S.; et al. Role of NS1 antibodies in the pathogenesis of acute secondary dengue infection. Nat. Commun. 2018, 9, 5242. [Google Scholar] [CrossRef] [PubMed]
- Hertz, T.; Beatty, P.R.; MacMillen, Z.; Killingbeck, S.S.; Wang, C.; Harris, E. Antibody Epitopes Identified in Critical Regions of Dengue Virus Nonstructural 1 Protein in Mouse Vaccination and Natural Human Infections. J. Immunol. 2017, 198, 4025–4035. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell. Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef]
- Westaway, E.G.; Goodman, M.R. Variation in distribution of the three flavivirus-specified glycoproteins detected by immunofluorescence in infected Vero cells. Arch. Virol. 1987, 94, 215–228. [Google Scholar] [CrossRef]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. FLAVIVIRUS GENOME ORGANIZATION, EXPRESSION, AND REPLICATION. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef]
- Winkler, G.; Randolph, V.B.; Cleaves, G.R.; Ryan, T.E.; Stollar, V. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology 1988, 162, 187–196. [Google Scholar] [CrossRef]
- Flamand, M.; Megret, F.; Mathieu, M.; Lepault, J.; Rey, F.A.; Deubel, V. Dengue Virus Type 1 Nonstructural Glycoprotein NS1 Is Secreted from Mammalian Cells as a Soluble Hexamer in a Glycosylation-Dependent Fashion. J. Virol. 1999, 73, 6104–6110. [Google Scholar] [CrossRef]
- Shu, B.; Ooi, J.S.G.; Tan, A.W.K.; Ng, T.-S.; Dejnirattisai, W.; Mongkolsapaya, J.; Fibriansah, G.; Shi, J.; Kostyuchenko, V.A.; Screaton, G.R.; et al. CryoEM structures of the multimeric secreted NS1, a major factor for dengue hemorrhagic fever. Nat. Commun. 2022, 13, 6756. [Google Scholar] [CrossRef]
- Gutsche, I.; Coulibaly, F.; Voss, J.E.; Salmon, J.; D’ALayer, J.; Ermonval, M.; Larquet, E.; Charneau, P.; Krey, T.; Mégret, F.; et al. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc. Natl. Acad. Sci. USA 2011, 108, 8003–8008. [Google Scholar] [CrossRef]
- Yap, S.S.L.; Nguyen-Khuong, T.; Rudd, P.M.; Alonso, S. Dengue Virus Glycosylation: What Do We Know? Front. Microbiol. 2017, 8, 1415. [Google Scholar] [CrossRef] [PubMed]
- Winkler, G.; Maxwell, S.E.; Ruemmler, C.; Stollar, V. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology 1989, 171, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.M.; Jones, M.K.; Young, P.R. Immunolocalization of the Dengue Virus Nonstructural Glycoprotein NS1 Suggests a Role in Viral RNA Replication. Virology 1996, 220, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J. Virol. 1997, 71, 9608–9617. [Google Scholar] [CrossRef]
- Pryor, M.J.; Wright, P.J. Glycosylation Mutants of Dengue Virus NS1 Protein. J. Gen. Virol. 1994, 75, 1183–1187. [Google Scholar] [CrossRef]
- Avirutnan, P.; Zhang, L.; Punyadee, N.; Manuyakorn, A.; Puttikhunt, C.; Kasinrerk, W.; Malasit, P.; Atkinson, J.P.; Diamond, M.S.; Buchmeier, M.J. Secreted NS1 of Dengue Virus Attaches to the Surface of Cells via Interactions with Heparan Sulfate and Chondroitin Sulfate, E. PLoS Pathog. 2007, 3, e183. [Google Scholar] [CrossRef]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-Linked Immunosorbent Assay Specific to Dengue Virus Type 1 Nonstructural Protein NS1 Reveals Circulation of the Antigen in the Blood during the Acute Phase of Disease in Patients Experiencing Primary or Secondary Infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef]
- Youn, S.; Li, T.; McCune, B.T.; Edeling, M.A.; Fremont, D.H.; Cristea, I.M.; Diamond, M.S. Evidence for a Genetic and Physical Interaction between Nonstructural Proteins NS1 and NS4B That Modulates Replication of West Nile Virus. J. Virol. 2012, 86, 7360–7371. [Google Scholar] [CrossRef]
- Akey, D.L.; Brown, W.; Jose, J.; Kuhn, R.J.; Smith, J.L. Structure-guided insights on the role of NS1 in flavivirus infection. BioEssays 2015, 37, 489–494. [Google Scholar] [CrossRef]
- Akey, D.L.; Brown, W.C.; Dutta, S.; Konwerski, J.; Jose, J.; Jurkiw, T.J.; DelProposto, J.; Ogata, C.M.; Skiniotis, G.; Kuhn, R.J.; et al. Flavivirus NS1 Structures Reveal Surfaces for Associations with Membranes and the Immune System. Science 2014, 343, 881–885. [Google Scholar] [CrossRef]
- Biering, S.B.; Akey, D.L.; Wong, M.P.; Brown, W.C.; Lo, N.T.N.; Puerta-Guardo, H.; de Sousa, F.T.G.; Wang, C.; Konwerski, J.R.; Espinosa, D.A.; et al. Structural basis for antibody inhibition of flavivirus NS1–triggered endothelial dysfunction. Science 2021, 371, 194–200. [Google Scholar] [CrossRef]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anupriya, M.G.; Modak, A.; Sreekumar, E. Dengue virus or NS1 protein induces trans-endothelial cell permeability associated with VE-Cadherin and RhoA phosphorylation in HMEC-1 cells preventable by Angiopoietin-1. J. Gen. Virol. 2018, 99, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Song, H.; Liu, L.; Bletchly, C.; Brillault, L.; Amarilla, A.A.; Xu, X.; Qi, J.; Chai, Y.; Cheung, S.T.M.; et al. A broadly protective antibody that targets the flavivirus NS1 protein. Science 2021, 371, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Biering, S.B.; de Sousa, F.T.G.; Shu, J.; Glasner, D.R.; Li, J.; Blanc, S.F.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Disassembly of Intercellular Junctions Leading to Barrier Dysfunction and Vascular Leak in a GSK-3β-Dependent Manner. Pathogens 2022, 11, 615. [Google Scholar] [CrossRef]
- Wang, C.; Puerta-Guardo, H.; Biering, S.B.; Glasner, D.R.; Tran, E.B.; Patana, M.; Gomberg, T.A.; Malvar, C.; Lo, N.T.N.; Espinosa, D.A.; et al. Endocytosis of flavivirus NS1 is required for NS1-mediated endothelial hyperpermeability and is abolished by a single N-glycosylation site mutation. PLoS Pathog. 2019, 15, e1007938. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E.; Kuhn, R.J. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef]
- Glasner, D.R.; Ratnasiri, K.; Puerta-Guardo, H.; Espinosa, D.A.; Beatty, P.R.; Harris, E.; Kuhn, R.J. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. 2017, 13, e1006673. [Google Scholar] [CrossRef]
- Pan, P.; Li, G.; Shen, M.; Yu, Z.; Ge, W.; Lao, Z.; Fan, Y.; Chen, K.; Ding, Z.; Wang, W.; et al. DENV NS1 and MMP-9 cooperate to induce vascular leakage by altering endothelial cell adhesion and tight junction. PLoS Pathog. 2021, 17, e1008603. [Google Scholar] [CrossRef]
- Chen, H.-R.; Chuang, Y.-C.; Lin, Y.-S.; Liu, H.-S.; Liu, C.-C.; Perng, G.-C.; Yeh, T.-M.; Harris, E. Dengue Virus Nonstructural Protein 1 Induces Vascular Leakage through Macrophage Migration Inhibitory Factor and Autophagy. PLoS Neglected Trop. Dis. 2016, 10, e0004828. [Google Scholar] [CrossRef]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef]
- Modhiran, N.; Watterson, D.; Blumenthal, A.; Baxter, A.G.; Young, P.R.; Stacey, K.J. Dengue virus NS1 protein activates immune cells via TLR4 but not TLR2 or TLR6. Immunol. Cell Biol. 2017, 95, 491–495. [Google Scholar] [CrossRef]
- Avirutnan, P.; Fuchs, A.; Hauhart, R.E.; Somnuke, P.; Youn, S.; Diamond, M.S.; Atkinson, J.P. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J. Exp. Med. 2010, 207, 793–806. [Google Scholar] [CrossRef]
- Avirutnan, P.E.; Hauhart, R.; Somnuke, P.; Blom, A.M.; Diamond, M.S.; Atkinson, J.P. Binding of Flavivirus Nonstructural Protein NS1 to C4b Binding Protein Modulates Complement Activation. J. Immunol. 2011, 187, 424–433. [Google Scholar] [CrossRef]
- Thiemmeca, S.; Tamdet, C.; Punyadee, N.; Prommool, T.; Songjaeng, A.; Noisakran, S.; Puttikhunt, C.; Atkinson, J.P.; Diamond, M.S.; Ponlawat, A.; et al. Secreted NS1 Protects Dengue Virus from Mannose-Binding Lectin–Mediated Neutralization. J. Immunol. 2016, 197, 4053–4065. [Google Scholar] [CrossRef]
- Conde, J.N.; da Silva, E.M.; Allonso, D.; Coelho, D.R.; Andrade, I.d.S.; de Medeiros, L.N.; Menezes, J.L.; Barbosa, A.S.; Mohana-Borges, R.; Diamond, M.S. Inhibition of the Membrane Attack Complex by Dengue Virus NS1 through Interaction with Vitronectin and Terminal Complement Proteins. J. Virol. 2016, 90, 9570–9581. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.J.; Duehr, J.; Dulin, H.; Broecker, F.; Brown, J.A.; Arumemi, F.O.; González, M.C.B.; Leyva-Grado, V.H.; Evans, M.J.; Simon, V.; et al. Human antibodies targeting Zika virus NS1 provide protection against disease in a mouse model. Nat. Commun. 2018, 9, 4560. [Google Scholar] [CrossRef] [PubMed]
- Wessel, A.W.; Kose, N.; Bombardi, R.G.; Roy, V.; Chantima, W.; Mongkolsapaya, J.; Edeling, M.A.; Nelson, C.A.; Bosch, I.; Alter, G.; et al. Antibodies targeting epitopes on the cell-surface form of NS1 protect against Zika virus infection during pregnancy. Nat. Commun. 2020, 11, 5278. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.-W.; Chen, P.-W.; Chen, C.-Y.; Lai, Y.-C.; Chu, Y.-T.; Hung, C.-Y.; Lee, H.; Wu, H.F.; Chuang, Y.-C.; Lin, J.; et al. Therapeutic Effects of Monoclonal Antibody against Dengue Virus NS1 in a STAT1 Knockout Mouse Model of Dengue Infection. J. Immunol. 2017, 199, 2834–2844. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Chuang, Y.-C.; Liu, C.-C.; Ho, T.-S.; Lin, Y.-S.; Anderson, R.; Yeh, T.-M. Antibodies Against Modified NS1 Wing Domain Peptide Protect Against Dengue Virus Infection. Sci. Rep. 2017, 7, 6975. [Google Scholar] [CrossRef]
- Chung, K.M.; Thompson, B.S.; Fremont, D.H.; Diamond, M.S. Antibody Recognition of Cell Surface-Associated NS1 Triggers Fc-γ Receptor-Mediated Phagocytosis and Clearance of West Nile Virus-Infected Cells. J. Virol. 2007, 81, 9551–9555. [Google Scholar] [CrossRef]
- Chung, K.M.; Nybakken, G.E.; Thompson, B.S.; Engle, M.J.; Marri, A.; Fremont, D.H.; Diamond, M.S. Antibodies against West Nile Virus Nonstructural Protein NS1 Prevent Lethal Infection through Fc γ Receptor-Dependent and -Independent Mechanisms. J. Virol. 2006, 80, 1340–1351. [Google Scholar] [CrossRef]
- Tien, S.-M.; Chang, P.-C.; Lai, Y.-C.; Chuang, Y.-C.; Tseng, C.-K.; Kao, Y.-S.; Huang, H.-J.; Hsiao, Y.-P.; Liu, Y.-L.; Lin, H.-H.; et al. Therapeutic efficacy of humanized monoclonal antibodies targeting dengue virus nonstructural protein 1 in the mouse model. PLoS Pathog. 2022, 18, e1010469. [Google Scholar] [CrossRef]
- Costa, S.M.; Paes, M.V.; Barreto, D.F.; Pinhão, A.T.; Barth, O.M.; Queiroz, J.L.; Armôa, G.R.; Freire, M.S.; Alves, A.M. Protection against dengue type 2 virus induced in mice immunized with a DNA plasmid encoding the non-structural 1 (NS1) gene fused to the tissue plasminogen activator signal sequence. Vaccine 2006, 24, 195–205. [Google Scholar] [CrossRef]
- Wan, S.-W.; Lu, Y.-T.; Huang, C.-H.; Lin, C.-F.; Anderson, R.; Liu, H.-S.; Yeh, T.-M.; Yen, Y.-T.; Wu-Hsieh, B.A.; Lin, Y.-S.; et al. Protection against Dengue Virus Infection in Mice by Administration of Antibodies against Modified Nonstructural Protein 1. PLoS ONE 2014, 9, e92495. [Google Scholar] [CrossRef]
- Falgout, B.; Bray, M.; Schlesinger, J.J.; Lai, C.J. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J. Virol. 1990, 64, 4356–4363. [Google Scholar] [CrossRef]
- Dias, A.G.; Duarte, E.M.; Zambrana, J.V.; Cardona-Ospina, J.A.; Bos, S.; Roy, V.; Huffaker, J.; Kuan, G.; Balmaseda, A.; Alter, G.; et al. Anti-dengue virus antibodies that elicit complement-mediated lysis of Zika virion correlate with protection from severe dengue disease. Cell Rep. 2025, 44, 115613. [Google Scholar] [CrossRef]
- Sanchez-Vargas, L.A.; Mathew, A.; Salje, H.; Sousa, D.A.; Casale, N.; Farmer, A.; Buddhari, D.; Anderson, K.; Iamsirithaworn, S.; Kaewhiran, S.; et al. Protective Role of NS1-Specific Antibodies in the Immune Response to Dengue Virus Through Antibody-Dependent Cellular Cytotoxicity. J. Infect. Dis. 2024, 230, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Glasner, D.R.; Watkins, H.; Puerta-Guardo, H.; Kassa, Y.A.; Egan, M.; Dean, H.; Harris, E. Magnitude and Functionality of the NS1-Specific Antibody Response Elicited by a Live-Attenuated Tetravalent Dengue Vaccine Candidate. J. Infect. Dis. 2020, 221, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Avirutnan, P.; Punyadee, N.; Noisakran, S.; Komoltri, C.; Thiemmeca, S.; Auethavornanan, K.; Jairungsri, A.; Kanlaya, R.; Tangthawornchaikul, N.; Puttikhunt, C.; et al. Vascular Leakage in Severe Dengue Virus Infections: A Potential Role for the Nonstructural Viral Protein NS1 and Complement. J. Infect. Dis. 2006, 193, 1078–1088. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lin, J.; Lin, Y.-S.; Wang, S.; Yeh, T.-M. Dengue Virus Nonstructural Protein 1–Induced Antibodies Cross-React with Human Plasminogen and Enhance Its Activation. J. Immunol. 2016, 196, 1218–1226. [Google Scholar] [CrossRef]
- Liu, I.-J.; Chiu, C.-Y.; Chen, Y.-C.; Wu, H.-C. Molecular Mimicry of Human Endothelial Cell Antigen by Autoantibodies to Nonstructural Protein 1 of Dengue Virus. J. Biol. Chem. 2011, 286, 9726–9736. [Google Scholar] [CrossRef]
- Chu, Y.-T.; Lin, C.-F.; Chang, C.-P.; Yeh, T.-M.; Anderson, R.; Wan, S.-W.; Yang, Y.-W.; Lin, Y.-S. Anti-dengue virus nonstructural protein 1 antibodies contribute to platelet phagocytosis by macrophages. Thromb. Haemost. 2016, 115, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Falconar, A.K.I. The dengue virus nonstructural-1 protein (NS1) generatesantibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to humanendothelial cells: Potential implications in haemorrhagic fever pathogenesis. Arch. Virol. 1997, 142, 897–916. [Google Scholar] [CrossRef]
- Cheng, H.-J.; Lin, C.-F.; Lei, H.-Y.; Liu, H.-S.; Yeh, T.-M.; Luo, Y.-H.; Lin, Y.-S. Proteomic Analysis of Endothelial Cell Autoantigens Recognized by Anti-Dengue Virus Nonstructural Protein 1 Antibodies. Exp. Biol. Med. 2009, 234, 63–73. [Google Scholar] [CrossRef]
- Sun, D.; King, C.; Huang, H.; Shih, Y.; Lee, C.; Tsai, W.; Yu, C.; Chang, H. Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J. Thromb. Haemost. 2007, 5, 2291–2299. [Google Scholar] [CrossRef]
- Lee, P.X.; Ting, D.H.R.; Boey, C.P.H.; Tan, E.T.X.; Chia, J.Z.H.; Idris, F.; Oo, Y.; Ong, L.C.; Chua, Y.L.; Hapuarachchi, C.; et al. Relative contribution of nonstructural protein 1 in dengue pathogenesis. J. Exp. Med. 2020, 217, e20191548. [Google Scholar] [CrossRef] [PubMed]
- Ramu, S.T.; Dissanayake, M.; Jeewandara, C.; Bary, F.; Harvie, M.; Gomes, L.; Wijesinghe, A.; Ariyaratne, D.; Ogg, G.S.; Malavige, G.N. Antibody and memory B cell responses to the dengue virus NS1 antigen in individuals with varying severity of past infection. Immunology 2023, 170, 47–59. [Google Scholar] [CrossRef]
- Muller, D.A.; Choo, J.J.Y.; McElnea, C.; Duyen, H.T.L.; Wills, B.; Young, P.R. Kinetics of NS1 and anti-NS1 IgG following dengue infection reveals likely early formation of immune complexes in secondary infected patients. Sci. Rep. 2025, 15, 6684. [Google Scholar] [CrossRef]
- Papa, M.P.; Mendoza-Torres, E.; Sun, P.; Encinales, L.; Goulet, J.; Defang, G.; Vihasi, J.; Cheng, Y.; Suchowiecki, K.; Rosales, W.; et al. Dengue NS1 Antibodies Are Associated with Clearance of Viral Nonstructural Protein-1. J. Infect. Dis. 2024, 230, e1226–e1234. [Google Scholar] [CrossRef]
- Thiemmeca, S.; Kraivong, R.; Punyadee, N.; Nilchan, N.; Traewachiwiphak, S.; Kongmanus, K.; Luangaram, P.; Prommool, T.; Poraha, R.; Sayboonruan, P.; et al. Epitope-dependent complement activation and ADCC by anti-NS1 antibodies in targeting infected cells: Implications for dengue vaccine design. Immunobiology 2025, 230, 152933. [Google Scholar] [CrossRef]
- Bailey, M.J.; Broecker, F.; Duehr, J.; Arumemi, F.; Krammer, F.; Palese, P.; Tan, G.S. Antibodies Elicited by an NS1-Based Vaccine Protect Mice against Zika Virus. mBio 2019, 10, e02861-18. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Posadas-Mondragón, A.; Aguilar-Faisal, J.L.; Chávez-Negrete, A.; Guillén-Salomón, E.; Alcántara-Farfán, V.; Luna-Rojas, L.; Ávila-Trejo, A.M.; Pacheco-Yépez, J.d.C. Indices of anti-dengue immunoglobulin G subclasses in adult Mexican patients with febrile and hemorrhagic dengue in the acute phase. Microbiol. Immunol. 2017, 61, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.G.; Atyeo, C.; Loos, C.; Montoya, M.; Roy, V.; Bos, S.; Narvekar, P.; Singh, T.; Katzelnick, L.C.; Kuan, G.; et al. Antibody Fc characteristics and effector functions correlate with protection from symptomatic dengue virus type 3 infection. Sci. Transl. Med. 2022, 14, eabm3151. [Google Scholar] [CrossRef]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daëron, M. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef] [PubMed]
- de Taeye, S.W.; Bentlage, A.E.H.; Mebius, M.M.; Meesters, J.I.; Lissenberg-Thunnissen, S.; Falck, D.; Sénard, T.; Salehi, N.; Wuhrer, M.; Schuurman, J.; et al. FcγR Binding and ADCC Activity of Human IgG Allotypes. Front. Immunol. 2020, 11, 740. [Google Scholar] [CrossRef]
- Dekkers, G.; Bentlage, A.E.H.; Stegmann, T.C.; Howie, H.L.; Lissenberg-Thunnissen, S.; Zimring, J.; Rispens, T.; Vidarsson, G. Affinity of human IgG subclasses to mouse Fc gamma receptors. mAbs 2017, 9, 767–773. [Google Scholar] [CrossRef]
- Duncan, A.R.; Winter, G. The binding site for C1q on IgG. Nature 1988, 332, 738–740. [Google Scholar] [CrossRef]
- Mehlhop, E.; Ansarah-Sobrinho, C.; Johnson, S.; Engle, M.; Fremont, D.H.; Pierson, T.C.; Diamond, M.S. Complement Protein C1q Inhibits Antibody-Dependent Enhancement of Flavivirus Infection in an IgG Subclass-Specific Manner. Cell Host Microbe 2007, 2, 417–426. [Google Scholar] [CrossRef]
- Garred, P.; Michaelsen, T.E.; Aase, A. The IgG Subclass Pattern of Complement Activation Depends on Epitope Density and Antibody and Complement Concentration. Scand. J. Immunol. 1989, 30, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.R.; Zhang, S.L.-X.; Tan, H.C.; Chan, Y.K.; Chow, A.; Lim, A.P.C.; Vasudevan, S.G.; Hanson, B.J.; Ooi, E.E. Ligation of Fc gamma receptor IIB inhibits antibody-dependent enhancement of dengue virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 12479–12484. [Google Scholar] [CrossRef]
- Bournazos, S.; Vo, H.T.M.; Duong, V.; Auerswald, H.; Ly, S.; Sakuntabhai, A.; Dussart, P.; Cantaert, T.; Ravetch, J.V. Antibody fucosylation predicts disease severity in secondary dengue infection. Science 2021, 372, 1102–1105. [Google Scholar] [CrossRef]
- Wang, T.T.; Sewatanon, J.; Memoli, M.J.; Wrammert, J.; Bournazos, S.; Bhaumik, S.K.; Pinsky, B.A.; Chokephaibulkit, K.; Onlamoon, N.; Pattanapanyasat, K.; et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 2017, 355, 395–398. [Google Scholar] [CrossRef]
- Teo, A.; Tan, H.D.; Loy, T.; Chia, P.Y.; Chua, C.L.L.; Evans, M.J. Understanding antibody-dependent enhancement in dengue: Are afucosylated IgG1s a concern? PLoS Pathog. 2023, 19, e1011223. [Google Scholar] [CrossRef]
- Golay, J.; Andrea, A.E.; Cattaneo, I. Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Front. Immunol. 2022, 13, 929895. [Google Scholar] [CrossRef]
- Oosterhoff, J.J.; Larsen, M.D.; van der Schoot, C.E.; Vidarsson, G. Afucosylated IgG responses in humans—Structural clues to the regulation of humoral immunity. Trends Immunol. 2022, 43, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.D.; de Graaf, E.L.; Sonneveld, M.E.; Plomp, H.R.; Nouta, J.; Hoepel, W.; Chen, H.-J.; Linty, F.; Visser, R.; Brinkhaus, M.; et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Sci. 2020, 371, eabc8378. [Google Scholar] [CrossRef]
- Gupta, A.; Kao, K.S.; Yamin, R.; Oren, D.A.; Goldgur, Y.; Du, J.; Lollar, P.; Sundberg, E.J.; Ravetch, J.V. Mechanism of glycoform specificity and in vivo protection by an anti-afucosylated IgG nanobody. Nat. Commun. 2023, 14, 2853. [Google Scholar] [CrossRef]
- Thulin, N.K.; Brewer, R.C.; Sherwood, R.; Bournazos, S.; Edwards, K.G.; Ramadoss, N.S.; Taubenberger, J.K.; Memoli, M.; Gentles, A.J.; Jagannathan, P.; et al. Maternal Anti-Dengue IgG Fucosylation Predicts Susceptibility to Dengue Disease in Infants. Cell Rep. 2020, 31, 107642. [Google Scholar] [CrossRef] [PubMed]
- Kraivong, R.; Traewachiwiphak, S.; Nilchan, N.; Tangthawornchaikul, N.; Pornmun, N.; Poraha, R.; Sriruksa, K.; Limpitikul, W.; Avirutnan, P.; Malasit, P.; et al. Cross-reactive antibodies targeting surface-exposed non-structural protein 1 (NS1) of dengue virus-infected cells recognize epitopes on the spaghetti loop of the β-ladder domain. PLoS ONE 2022, 17, e0266136. [Google Scholar] [CrossRef]
- Carpio, K.L.; Barrett, A.D.T. Flavivirus NS1 and Its Potential in Vaccine Development. Vaccines 2021, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Luangaram, P.; Tamdet, C.; Saengwong, C.; Prommool, T.; Kraivong, R.; Nilchan, N.; Punyadee, N.; Avirutnan, P.; Srisawat, C.; Malasit, P.; et al. Differential critical residues on the overlapped region of the non-structural protein-1 recognized by flavivirus and dengue virus cross-reactive monoclonal antibodies. Sci. Rep. 2022, 12, 21548. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613.e8. [Google Scholar] [CrossRef]
- Visciano, M.L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Effects of adjuvants on IgG subclasses elicited by virus-like Particles. J. Transl. Med. 2012, 10, 4. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. TLR agonists as vaccine adjuvants in the prevention of viral infections: An overview. Front. Microbiol. 2023, 14, 1249718. [Google Scholar] [CrossRef]
- Ramadhany, R.; Hirai, I.; Sasaki, T.; Ono, K.-I.; Ramasoota, P.; Ikuta, K.; Kurosu, T. Antibody with an engineered Fc region as a therapeutic agent against dengue virus infection. Antivir. Res. 2015, 124, 61–68. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Malik, Y.S.; Singh, R.K.; Chaicumpa, W. Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection. Front. Immunol. 2018, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Haslund-Gourley, B.S.; Wigdahl, B.; Comunale, M.A. IgG N-glycan Signatures as Potential Diagnostic and Prognostic Biomarkers. Diagnostics 2023, 13, 1016. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A. Controlled human infection study underpins efficacy of the tetravalent live-attenuated dengue vaccine TV005. J. Clin. Investig. 2024, 134, e177610. [Google Scholar] [CrossRef] [PubMed]
- Pierce, K.K.; Whitehead, S.S.; Diehl, S.A.; Naro, G.; Carmolli, M.C.; He, H.; Tibery, C.M.; Sabundayo, B.P.; Kirkpatrick, B.D.; Durbin, A.P. Evaluation of a new dengue 3 controlled human infection model for use in the evaluation of candidate dengue vaccines. medRxiv 2024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).