Tixagevimab-Cilgavimab Effectively Prevents COVID-19 Infection in Patients with End-Stage Kidney Disease
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
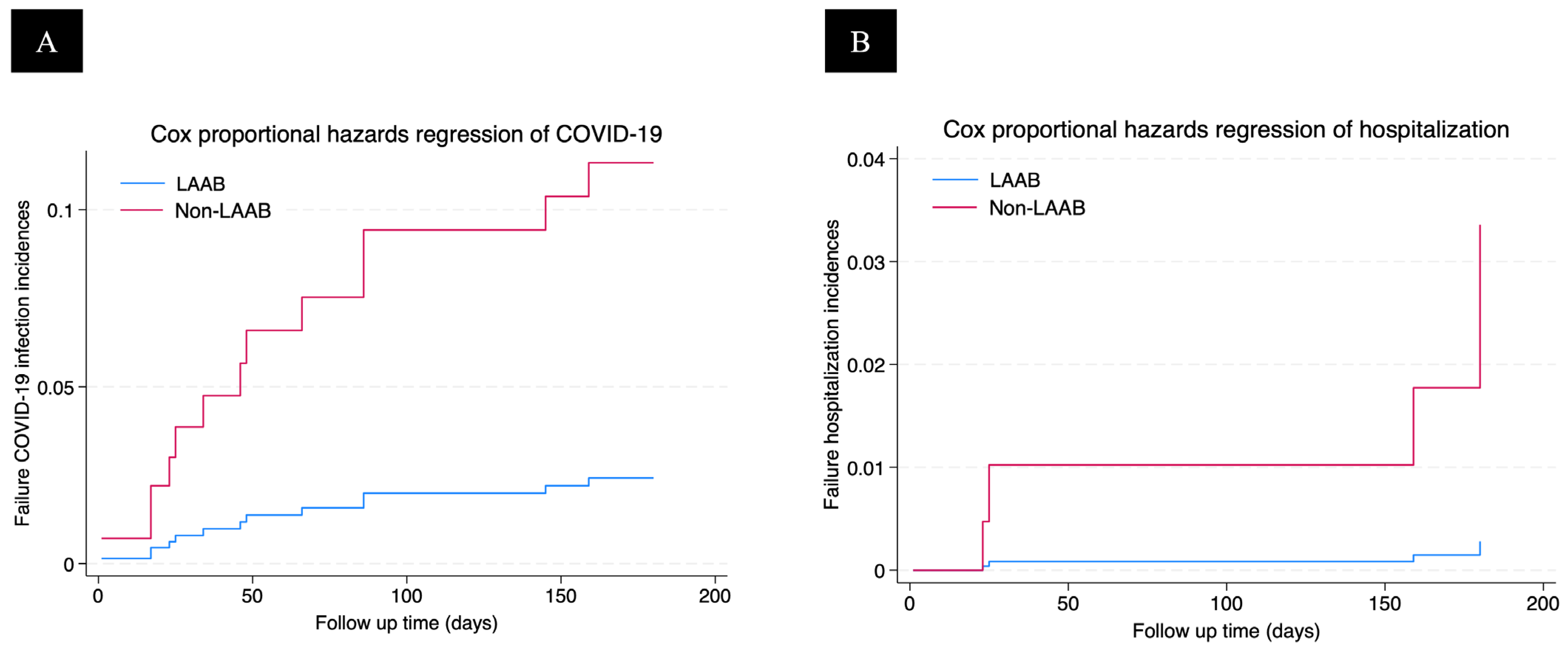
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yen, J.-S.; Wang, I.-K.; Yen, T.-H. COVID-19 Vaccination and Dialysis Patients: Why the Variable Response. QJM Int. J. Med. 2021, 114, 440–444. [Google Scholar] [CrossRef]
- Ozturk, S.; Turgutalp, K.; Arici, M.; Odabas, A.R.; Altiparmak, M.R.; Aydin, Z.; Cebeci, E.; Basturk, T.; Soypacaci, Z.; Sahin, G.; et al. Mortality Analysis of COVID-19 Infection in Chronic Kidney Disease, Haemodialysis and Renal Transplant Patients Compared with Patients without Kidney Disease: A Nationwide Analysis from Turkey. Nephrol. Dial. Transplant. 2020, 35, 2083–2095. [Google Scholar] [CrossRef]
- Li, P.; Guan, Y.; Zhou, S.; Wang, E.; Sun, P.; Fei, G.; Zeng, D.; Wang, R. Mortality and Risk Factors for COVID-19 in Hemodialysis Patients: A Systematic Review and Meta-Analysis. Sci. Prog. 2022, 105, 00368504221110858. [Google Scholar] [CrossRef]
- Martins, M.P.; de Oliveira, R.B. COVID-19 and Chronic Kidney Disease: A Narrative Review. COVID 2023, 3, 1092–1105. [Google Scholar] [CrossRef]
- Zitt, E.; Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Mutschlechner, B.; Ulmer, H.; Benda, M.A.; Sprenger-Mähr, H.; Winder, T.; Lhotta, K. The Safety and Immunogenicity of the mRNA-BNT162b2 SARS-CoV-2 Vaccine in Hemodialysis Patients. Front. Immunol. 2021, 12, 704773. [Google Scholar] [CrossRef]
- Yau, K.; Abe, K.T.; Naimark, D.; Oliver, M.J.; Perl, J.; Leis, J.A.; Bolotin, S.; Tran, V.; Mullin, S.I.; Shadowitz, E.; et al. Evaluation of the SARS-CoV-2 Antibody Response to the BNT162b2 Vaccine in Patients Undergoing Hemodialysis. JAMA Netw. Open 2021, 4, e2123622. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of COVID-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef] [PubMed]
- Implementation of AZD7442 (Tixagevimab/Cilgavimab) COVID-19 Pre-Exposure Prophylaxis (PrEP) in the Largest Health Maintenance Organization in Israel: Real-World Uptake and Sociodemographic and Clinical Characteristics Across Immunocompromised Patient Groups | Infectious Diseases and Therapy. Available online: https://link.springer.com/article/10.1007/s40121-024-00981-8 (accessed on 16 August 2025).
- Poonvivatchailarn, U. Pre-Exposure Prophylaxis with Tixagevimab/Cilgavimab for COVID-19 Among Patients with End Stage Renal Disease Among Patients with End Stage Renal Diseasein Nakhonpathom Hospitalin Nakhonpathom Hospital. Reg. 4-5 Med. J. 2023, 42, 311–318. [Google Scholar]
- Choi, H.; Kim, A.-Y.; Park, I.; Lee, H.; Lee, M.-J. COVID-19 Infection in Patients with End-Stage Kidney Disease Undergoing Renal Replacement Therapies in Korea. Kidney Res. Clin. Pract. 2025, 44, 522–534. [Google Scholar] [CrossRef]
- Boongird, S.; Srithongkul, T.; Sethakarun, S.; Bruminhent, J.; Kiertiburanakul, S.; Nongnuch, A.; Kitiyakara, C.; Sritippayawan, S. Tixagevimab–Cilgavimab for Preventing Breakthrough COVID-19 in Dialysis Patients: A Prospective Study. Clin. Kidney J. 2024, 17, sfae309. [Google Scholar] [CrossRef]
- Wheeler, K. FDA Approves Treatment for Uremic Pruritis Korsuva Is Approved for Treating Moderate to Severe Pruritis Associated with Chronic Kidney Disease. Drug Top. 2022, 166, 35. [Google Scholar]
- Sanayei, A.M.; Montalvan, A.; Faria, I.; Ochalla, J.; Pavlakis, M.; Blair, B.M.; Alonso, C.D.; Curry, M.; Saberi, B. Tixagevimab-Cilgavimab Decreases the Rate of SARS-CoV-2 Infection Among Solid Organ Transplant Recipients. Transplant. Proc. 2023, 55, 1784–1792. [Google Scholar] [CrossRef]
- Nassar, M.K.; Sabry, A.; Elgamal, M.; Zeid, Z.; Abdellateif Abdelghany, D.; Tharwat, S. Tixagevimab and Cilgavimab (Evusheld) Boosts Antibody Levels to SARS-CoV-2 in End-Stage Renal Disease Patients on Chronic Hemodialysis: A Single-Center Study. Medicina 2023, 59, 2109. [Google Scholar] [CrossRef]
- Khorramnia, S.; Navidi, Z.; Orandi, A.; Iravani, M.M.; Orandi, A.; Malekabad, E.S.; Moghadam, S.H.P. Tixagevimab/Cilgavimab Prophylaxis against COVID-19 in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis. Clin. Transpl. Res. 2024, 38, 136–144. [Google Scholar] [CrossRef]
- Abdeldaim, D.T.; Schindowski, K. Fc-Engineered Therapeutic Antibodies: Recent Advances and Future Directions. Pharmaceutics 2023, 15, 2402. [Google Scholar] [CrossRef] [PubMed]
- Reindl-Schwaighofer, R.; Heinzel, A.; Raab, L.; Strassl, R.; Herz, C.T.; Regele, F.; Doberer, K.; Helk, O.; Spechtl, P.; Aschauer, C.; et al. Cilgavimab and Tixagevimab as Pre-Exposure Prophylaxis in Vaccine Non-Responder Kidney Transplant Recipients during a Period of Prevalent SARS-CoV-2 BA.2 and BA.4/5 Variants—A Prospective Cohort Study (RESCUE-TX). eBioMedicine 2024, 109, 105417. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Hirsch, J.S.; Wanchoo, R.; Sachdeva, M.; Sakhiya, V.; Hong, S.; Jhaveri, K.D.; Fishbane, S.; Abate, M.; Andrade, H.P.; et al. Outcomes of Patients with End-Stage Kidney Disease Hospitalized with COVID-19. Kidney Int. 2020, 98, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.G.; Tam, T.A.; Elliott, M.J.; Ahmed, S.B.; Riehl-Tonn, V.; Swamy, A.K.R.; Benham, J.L.; Peterson, J.; MacRae, J.M. Sex Differences in COVID-19 Symptoms and Outcomes in People with Kidney Failure Treated with Dialysis: A Prospective Cohort Study. J. Nephrol. 2022, 36, 851–860. [Google Scholar] [CrossRef]
- Tylicki, L.; Biedunkiewicz, B.; Puchalska-Reglińska, E.; Gellert, R.; Burnier, M.; Wolf, J.; Dȩbska-Ślizień, A. COVID-19 Vaccination Reduces Mortality in Patients on Maintenance Hemodialysis. Front. Med. 2022, 9, 937167. [Google Scholar] [CrossRef]
- Yildiz, A.; Tufan, F. Lower Creatinine as a Marker of Malnutrition and Lower Muscle Mass in Hemodialysis Patients. Clin. Interv. Aging 2015, 10, 1593–1594. [Google Scholar] [CrossRef]
- He, Z.; Peng, Z.; Gao, N.; Zhong, S.; Yu, F.; Tang, Z.; Liao, Z.; Zhao, S.; Umwiza, G.; Chen, M.; et al. Risk Factors for the Mortality of Hemodialysis Patients with COVID-19 in Northern Hunan Province, China. BMC Nephrol. 2025, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Rilat, M.L.A.; Fenoglio, R.; Foddai, S.G.; Radin, M.; Cecchi, I.; Cinnirella, G.; Crosasso, P.; Guidetti, M.G.; Barinotti, A.; et al. Safety and Efficacy of Pre-Exposure Prophylaxis with Tixagevimab/Cilgavimab (Evusheld) in Patients with Glomerular Diseases Who Received Rituximab. Clin. Kidney J. 2023, 16, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Dluzynski, D.; Ssentongo, P.; Hale, C.M.; Shaheen, S.K.; Maglakelidze, N.; Sivik, J.M.; Henao, M.P.; Chinchilli, V.M.; Paules, C.I. Dose-Dependent Impact of Tixagevimab–Cilgavimab as Primary Prevention against SARS-CoV-2 in Immunocompromised Individuals. Sci. Rep. 2025, 15, 17578. [Google Scholar] [CrossRef]
- Soeroto, A.Y.; Yanto, T.A.; Kurniawan, A.; Hariyanto, T.I. Efficacy and Safety of Tixagevimab-Cilgavimab as Pre-Exposure Prophylaxis for COVID-19: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2023, 33, e2420. [Google Scholar] [CrossRef]
- Khan, B.A.; Pagsinohin, M.; Lu, L.M.; Tan, P.; Teo, R.; Khan, B.A.; Pagsinohin, M.; Lu, L.M.; Tan, P.; Teo, R. Tixagevimab and Cilgavimab Administration for Hemodialysis Patients at Community-Based Dialysis Centers in Singapore as Pre-Exposure Prophylaxis for SARS-CoV-2 Infection. Cureus 2023, 15, e41297. [Google Scholar] [CrossRef]
- Moon, R.; Tien, A.; Chung, J.; Pinnelas, R.; Lee, R.; Hwang, J.; Brasfield, F.; Sahota, A. Safety and Efficacy of Intramuscular Tixagevimab-Cilgavimab in Prevention of COVID-19 in Patients Who Are Immunocompromised. Perm J. 2023, 27, 44–54. [Google Scholar] [CrossRef]
- Lu, W.; Li, X.; You, W.; Gong, R. Rhabdomyolysis in a Patient with End-Stage Renal Disease and SARS-CoV-2 Infection: A Case Report. Medicine 2023, 102, e36360. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | LAAB | No LAAB | p-Value |
|---|---|---|---|
| (n = 58) | (n = 59) | ||
| Gender | |||
| Male | 33 (56.9) | 32 (54.2) | 0.853 |
| Female | 25 (43.1) | 27 (45.8) | |
| Age (year), Mean ± SD | 59.7 ± 14.5 | 59.1 ± 15.2 | 0.831 |
| Comorbidity | |||
| Diabetes mellitus | 27 (46.6) | 34 (57.6) | 0.269 |
| Hypertension | 58 (100) | 55 (93.2) | 0.119 |
| Dyslipidemia | 48 (82.8) | 40 (67.8) | 0.086 |
| Cancer | 5 (8.6) | 2 (3.4) | 0.272 |
| History of COVID-19 infection | 9 (15.5) | 20 (33.9) | 0.031 |
| Systolic blood pressure (mmHg), Mean ± SD | 145.7 ± 26.9 | 134.8 ± 18.8 | 0.013 |
| Diastolic blood pressure (mmHg), Mean ± SD | 77.6 ± 17.2 | 70.5 ± 15.4 | 0.021 |
| Number of vaccine (doses), Mean ± SD | 2.4 ± 0.9 | 2.2 ± 1.1 | 0.256 |
| Primary kidney disease | 0.277 | ||
| Diabetes mellitus | 21 (36.2) | 30 (50.9) | |
| Hypertension | 26 (44.8) | 18 (30.5) | |
| Lupus nephritis | 9 (15.5) | 8 (13.6) | |
| Renal stone | 1 (1.7) | 3 (5.1) | |
| ADPKD | 1 (1.7) | 0 | |
| Renal replacement therapy | 0.366 | ||
| Peritoneal dialysis | 23 (39.7) | 16 (27.1) | |
| Hemodialysis | 31 (53.5) | 38 (64.4) | |
| Kidney transplantation | 4 (6.9) | 5 (8.5) | |
| Hospitalization | 4 (6.9) | 8 (13.6) | 0.362 |
| Hemoglobin (g/dL), Mean ± SD | 10.5 ± 1.6 | 10.2 ± 1.7 | 0.352 |
| White blood cell (109/L), Mean ± SD | 73.9 ± 3.6 | 73.3 ± 1.8 | 0.910 |
| Platelet (109/L), Mean ± SD | 219.4 ± 70.5 | 250.6 ± 88.2 | 0.037 |
| BUN (mmol/L), Mean ± SD | 41.0 ± 15.5 | 44.2 ± 22.2 | 0.374 |
| Serum creatinine (mg/dL), Mean ± SD | 8.4 ± 3.6 | 7.6 ± 3.7 | 0.273 |
| Serum calcium (mg/dL), Mean ± SD | 9.2 ± 1.1 | 8.9 ± 0.9 | 0.164 |
| Serum phosphorus (mg/dL), Mean ± SD | 4.2 ± 1.5 | 4.1 ± 1.5 | 0.669 |
| Serum albumin (g/dL), Mean ± SD | 3.6 ± 0.5 | 3.7 ± 0.7 | 0.744 |
| iPTH (pg/mL), median (IQR) | 215.1 (139.7–528.3) | 184.8 (77–356.2) | 0.128 |
| Potential Factor | COVID-19 | Hospitalization | ||
|---|---|---|---|---|
| Adjusted HR | p-Value | Adjusted HR | p-Value | |
| Long-acting antibody | 0.20 (0.04–0.95) | 0.043 | 0.08 (0.01–1.56) | 0.096 |
| Male gender | 1.49 (0.40–5.55) | 0.550 | 0.95 (0.12–7.83) | 0.963 |
| Age ≥ 60 years | 0.56 (0.10–3.01) | 0.498 | 0.78 (0.01–13.34) | 0.861 |
| Diabetes mellitus | 2.19 (0.42–11.34) | 0.351 | 0.13 (0.01–4.62) | 0.264 |
| Dyslipidemia | 3.07 (0.61–15.46) | 0.175 | 1.23 (0.14–11.14) | 0.853 |
| History of COVID-19 infection | 0.12 (0.02–0.91) | 0.040 | 2.72 (0.28–26.62) | 0.390 |
| Number of vaccine (doses) | 1.39 (0.73–2.63) | 0.313 | 1.06 (0.44–2.51) | 0.902 |
| Systolic blood pressure | 0.99 (0.96–1.02) | 0.620 | 1.04 (0.97–1.11) | 0.287 |
| Diastolic blood pressure | 1.08 (1.02–1.13) | 0.003 | 1.06 (0.97–1.16) | 0.199 |
| Serum Creatinine | 1.18 (0.96–1.45) | 0.111 | 0.92 (0.64–1.32) | 0.665 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongtal, N.; Pichitsiri, W.; Sirilak, S.; Wannakittirat, A.; Sontham, B.; Inkate, S.; Thammathiwat, T. Tixagevimab-Cilgavimab Effectively Prevents COVID-19 Infection in Patients with End-Stage Kidney Disease. Viruses 2025, 17, 1216. https://doi.org/10.3390/v17091216
Kongtal N, Pichitsiri W, Sirilak S, Wannakittirat A, Sontham B, Inkate S, Thammathiwat T. Tixagevimab-Cilgavimab Effectively Prevents COVID-19 Infection in Patients with End-Stage Kidney Disease. Viruses. 2025; 17(9):1216. https://doi.org/10.3390/v17091216
Chicago/Turabian StyleKongtal, Noppakao, Watchara Pichitsiri, Supinda Sirilak, Anyarin Wannakittirat, Busakorn Sontham, Sagoontee Inkate, and Theerachai Thammathiwat. 2025. "Tixagevimab-Cilgavimab Effectively Prevents COVID-19 Infection in Patients with End-Stage Kidney Disease" Viruses 17, no. 9: 1216. https://doi.org/10.3390/v17091216
APA StyleKongtal, N., Pichitsiri, W., Sirilak, S., Wannakittirat, A., Sontham, B., Inkate, S., & Thammathiwat, T. (2025). Tixagevimab-Cilgavimab Effectively Prevents COVID-19 Infection in Patients with End-Stage Kidney Disease. Viruses, 17(9), 1216. https://doi.org/10.3390/v17091216






