APOBEC3B Promotes SARS-CoV-2 Through Activation of PKR/eIF2⍺ and AMPD2 Dysregulation
Abstract
1. Introduction
2. Results
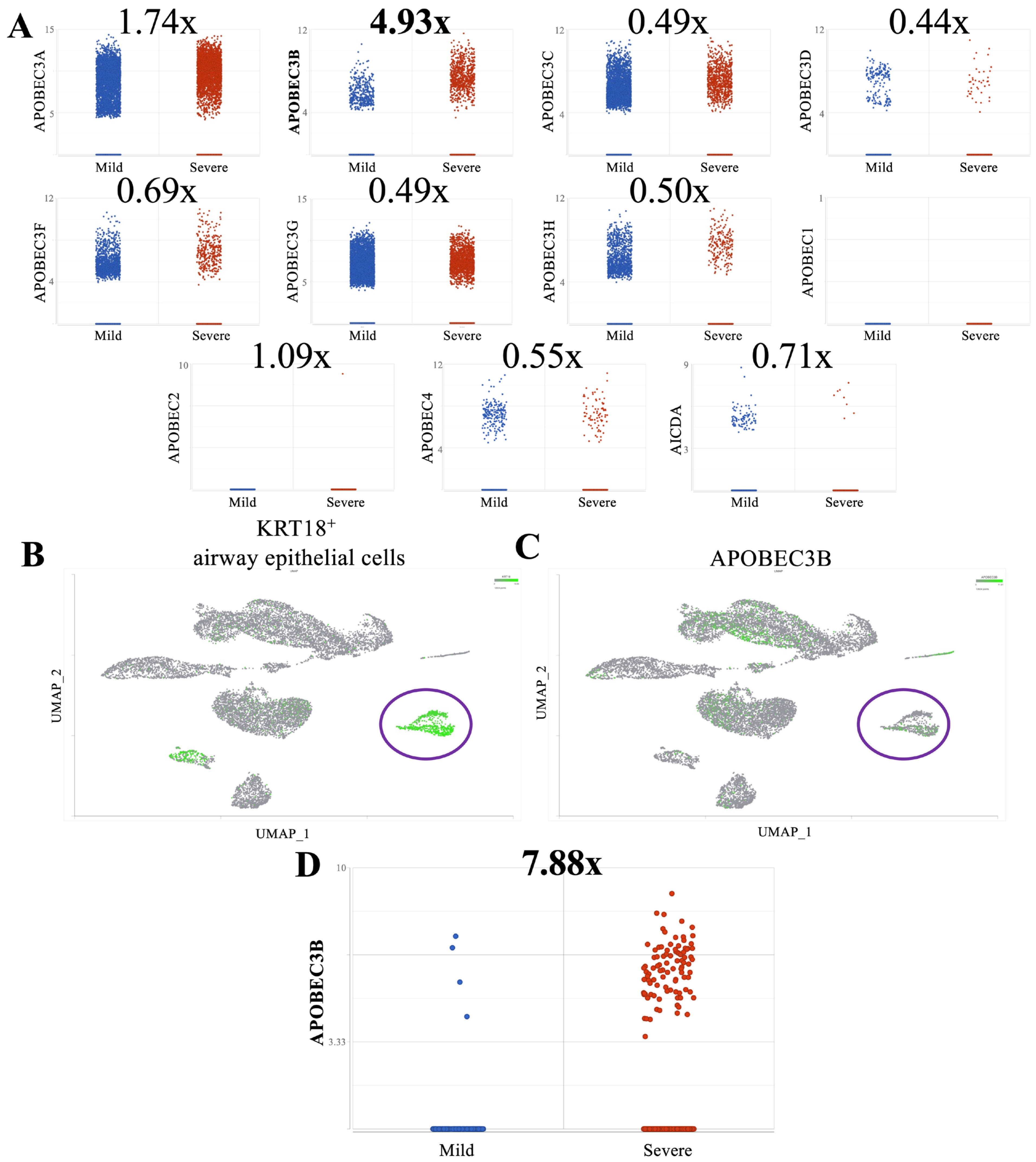
2.1. A3B Is Overexpressed in Broncho-Alveolar Lavage Fluid from Patients with Severe SARS-CoV-2 Infection
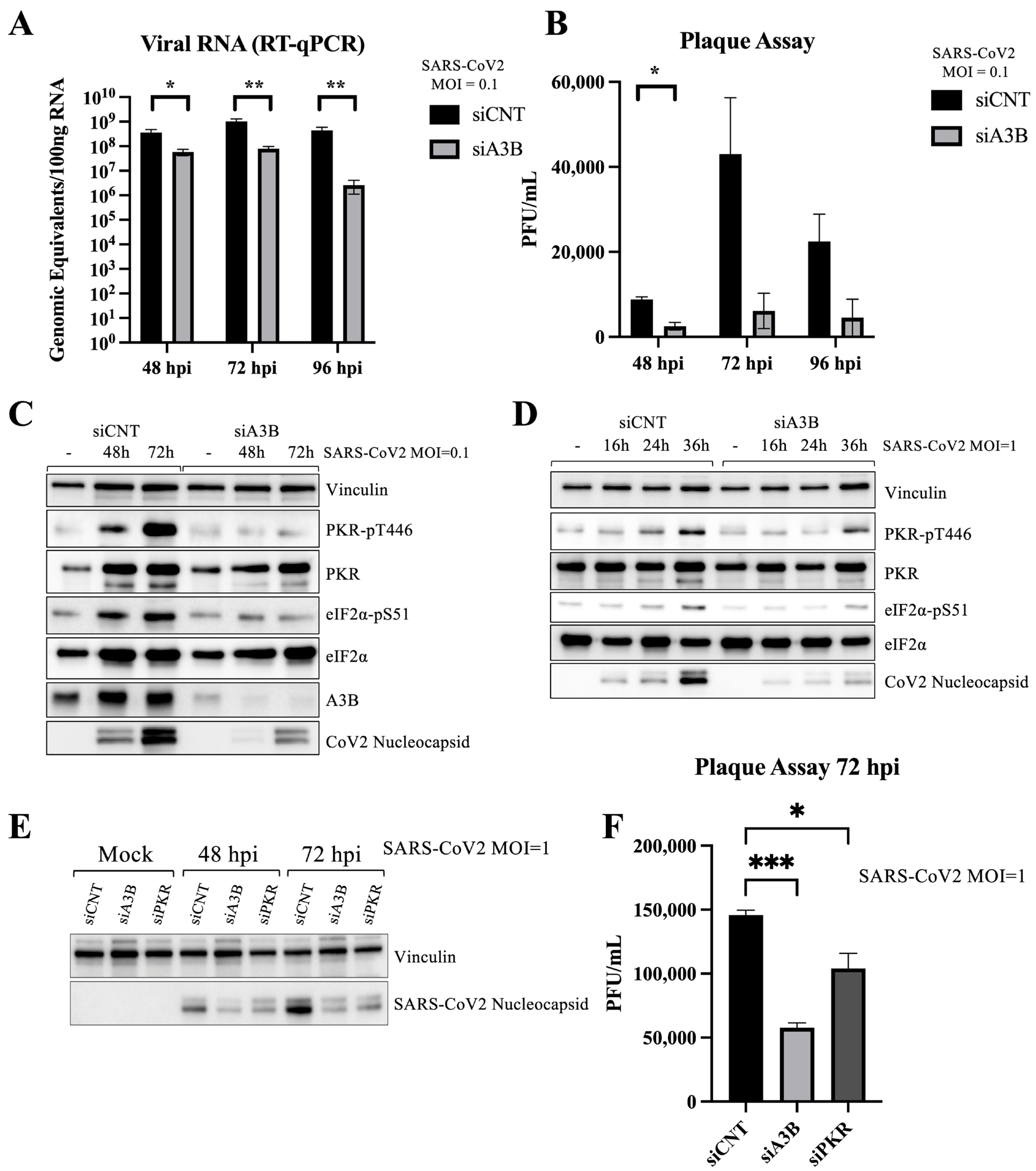
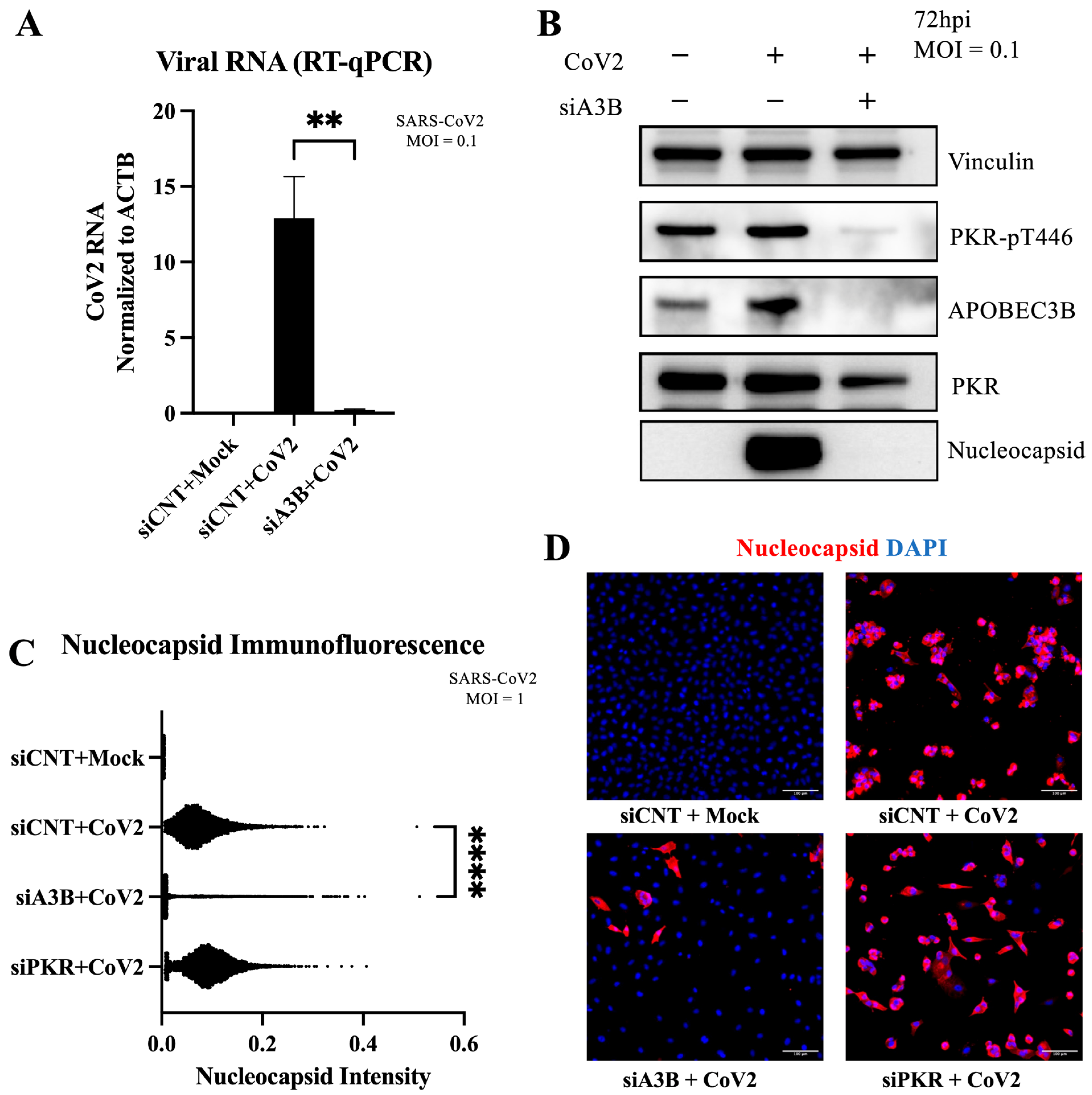
2.2. A3B Knockdown Significantly Reduces SARS-CoV-2 Infectivity in Caco-2 Cells
2.3. A3B Knockdown Reduces SARS-CoV-2 Infectivity in Caco-2 via Attenuation of PKR/eIF2⍺ Pathway
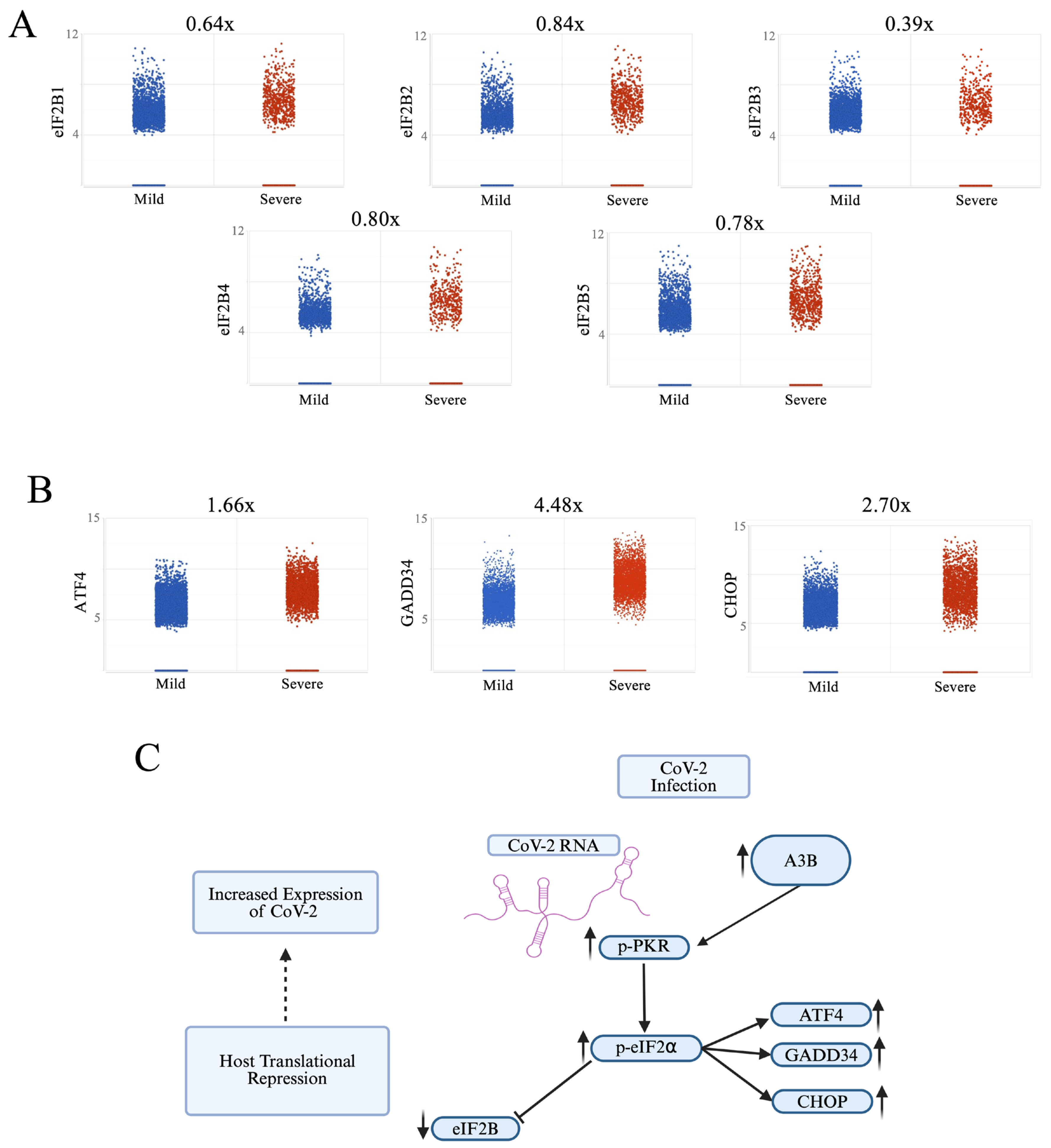
2.4. Severe COVID-19 Patient BALF Cells Show Signs of p-PKR/p-eIF2⍺ Pathway Activation
2.5. A3B Knockdown Reduces SARS-CoV-2 Infectivity in A549-ACE2 Independently of PKR Activation
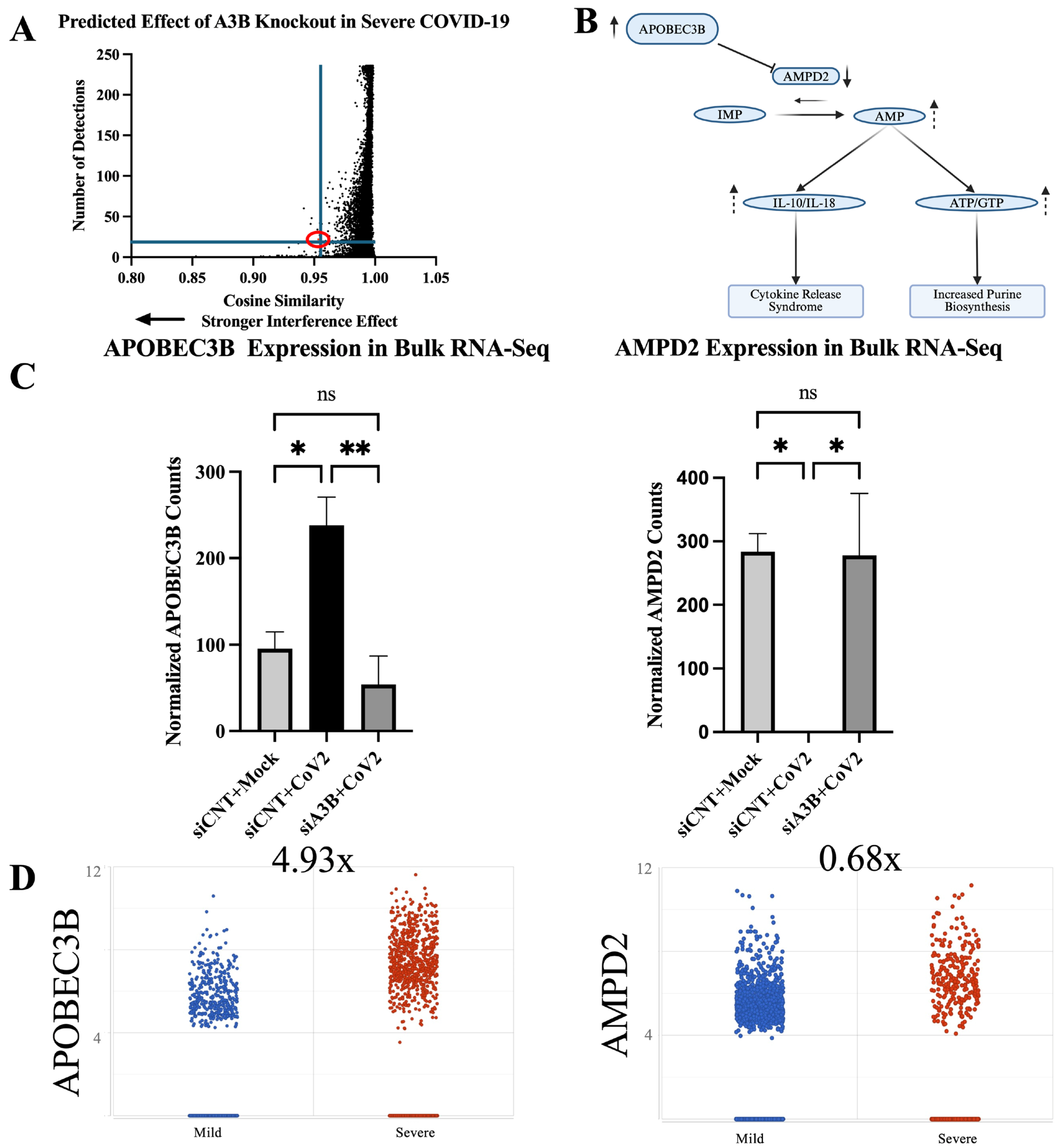
2.6. Geneformer Predicts A3B Knockout Dysregulates AMPD2 in the Context of Severe SARS-CoV-2 Infection
2.7. AMPD2 Is Downregulated in Severe COVID-19 Infection
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. RNA Interference
4.3. SARS-CoV-2 Virus Infections
4.4. Reverse Transcription-Quantitative Polymerase Chain Reaction
4.5. Western Blots
4.6. Immunofluorescence
4.7. Bulk RNA Sequencing
4.8. Bulk RNA Sequencing Analysis
4.9. Single-Cell RNA Sequencing Analysis and Cell Type Identification
4.10. Geneformer
4.11. Antibodies
4.12. Statistical Analysis and Plotting
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salter, J.D.; Bennett, R.P.; Smith, H.C. The APOBEC protein family: United by structure, divergent in function. Trends Biochem. Sci. 2016, 41, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Jarmuz, A.; Chester, A.; Bayliss, J.; Gisbourne, J.; Dunham, I.; Scott, J.; Navaratnam, N. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics 2002, 79, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.R. Role and mechanism of action of the APOBEC3 family of antiretroviral resistance factors. J. Virol. 2006, 80, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.C.; Bennett, R.P.; Kizilyer, A.; McDougall, W.M.; Prohaska, K.M. Functions and Regulation of the APOBEC Family of Proteins; Elsevier: Amsterdam, The Netherlands, 2012; pp. 258–268. [Google Scholar]
- Navarro, F.; Bollman, B.; Chen, H.; König, R.; Yu, Q.; Chiles, K.; Landau, N.R. Complementary function of the two catalytic domains of APOBEC3G. Virology 2005, 333, 374–386. [Google Scholar] [CrossRef]
- Yang, H.; Ito, F.; Wolfe, A.D.; Li, S.; Mohammadzadeh, N.; Love, R.P.; Yan, M.; Zirkle, B.; Gaba, A.; Chelico, L. Understanding the structural basis of HIV-1 restriction by the full length double-domain APOBEC3G. Nat. Commun. 2020, 11, 632. [Google Scholar] [CrossRef]
- Harris, R.S.; Liddament, M.T. Retroviral restriction by APOBEC proteins. Nat. Rev. Immunol. 2004, 4, 868–877. [Google Scholar] [CrossRef]
- Sheehy, A.M.; Gaddis, N.C.; Choi, J.D.; Malim, M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 2002, 418, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Belanger, K.; Savoie, M.; Rosales Gerpe, M.C.; Couture, J.-F.; Langlois, M.-A. Binding of RNA by APOBEC3G controls deamination-independent restriction of retroviruses. Nucleic Acids Res. 2013, 41, 7438–7452. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.S.; Dudley, J.P. APOBECs and virus restriction. Virology 2015, 479, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Ooms, M.; Krikoni, A.; Kress, A.K.; Simon, V.; Münk, C. APOBEC3A, APOBEC3B, and APOBEC3H haplotype 2 restrict human T-lymphotropic virus type 1. J. Virol. 2012, 86, 6097–6108. [Google Scholar] [CrossRef] [PubMed]
- Beggel, B.; Münk, C.; Däumer, M.; Hauck, K.; Häussinger, D.; Lengauer, T.; Erhardt, A. Full genome ultra-deep pyrosequencing associates G-to-A hypermutation of the hepatitis B virus genome with the natural progression of hepatitis B. J. Viral Hepat. 2013, 20, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Lucifora, J.; Xia, Y.; Reisinger, F.; Zhang, K.; Stadler, D.; Cheng, X.; Sprinzl, M.F.; Koppensteiner, H.; Makowska, Z.; Volz, T. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 2014, 343, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Narvaiza, I.; Linfesty, D.C.; Greener, B.N.; Hakata, Y.; Pintel, D.J.; Logue, E.; Landau, N.R.; Weitzman, M.D. Deaminase-independent inhibition of parvoviruses by the APOBEC3A cytidine deaminase. PLoS Pathog. 2009, 5, e1000439. [Google Scholar] [CrossRef]
- Chen, H.; Lilley, C.E.; Yu, Q.; Lee, D.V.; Chou, J.; Narvaiza, I.; Landau, N.R.; Weitzman, M.D. APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr. Biol. 2006, 16, 480–485. [Google Scholar] [CrossRef]
- Zhu, B.; Xiao, Y.; Yeager, M.; Clifford, G.; Wentzensen, N.; Cullen, M.; Boland, J.F.; Bass, S.; Steinberg, M.K.; Raine-Bennett, T. Mutations in the HPV16 genome induced by APOBEC3 are associated with viral clearance. Nat. Commun. 2020, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.J.; Xu, T.; Guo, K.; Griffin, L.M.; Westrich, J.A.; Lee, D.; Lambert, P.F.; Santiago, M.L.; Pyeon, D. APOBEC3A functions as a restriction factor of human papillomavirus. J. Virol. 2015, 89, 688–702. [Google Scholar] [CrossRef]
- Cheng, A.Z.; Yockteng-Melgar, J.; Jarvis, M.C.; Malik-Soni, N.; Borozan, I.; Carpenter, M.A.; McCann, J.L.; Ebrahimi, D.; Shaban, N.M.; Marcon, E. Epstein–Barr virus BORF2 inhibits cellular APOBEC3B to preserve viral genome integrity. Nat. Microbiol. 2019, 4, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Bekerman, E.; Jeon, D.; Ardolino, M.; Coscoy, L. A role for host activation-induced cytidine deaminase in innate immune defense against KSHV. PLoS Pathog. 2013, 9, e1003748. [Google Scholar] [CrossRef]
- Weisblum, Y.; Oiknine-Djian, E.; Zakay-Rones, Z.; Vorontsov, O.; Haimov-Kochman, R.; Nevo, Y.; Stockheim, D.; Yagel, S.; Panet, A.; Wolf, D.G. APOBEC3A is upregulated by human cytomegalovirus (HCMV) in the maternal-fetal interface, acting as an innate anti-HCMV effector. J. Virol. 2017, 91, e01296-17. [Google Scholar] [CrossRef]
- Pautasso, S.; Galitska, G.; Dell’Oste, V.; Biolatti, M.; Cagliani, R.; Forni, D.; De Andrea, M.; Gariglio, M.; Sironi, M.; Landolfo, S. Strategy of human cytomegalovirus to escape interferon beta-induced APOBEC3G editing activity. J. Virol. 2018, 92, e01224-18. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.A.; Holland, T.C.; Bhagwat, A.S. Human herpes simplex virus-1 depletes APOBEC3A from nuclei. Virology 2019, 537, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Moraes, S.N.; Becker, J.T.; Moghadasi, S.A.; Shaban, N.M.; Auerbach, A.A.; Cheng, A.Z.; Harris, R.S. Evidence linking APOBEC3B genesis and evolution of innate immune antagonism by gamma-herpesvirus ribonucleotide reductases. eLife 2022, 11, e83893. [Google Scholar] [CrossRef]
- Peretti, A.; Geoghegan, E.M.; Pastrana, D.V.; Smola, S.; Feld, P.; Sauter, M.; Lohse, S.; Ramesh, M.; Lim, E.S.; Wang, D. Characterization of BK polyomaviruses from kidney transplant recipients suggests a role for APOBEC3 in driving in-host virus evolution. Cell Host Microbe 2018, 23, 628–635.e7. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Rosenthal, R.; Kanu, N.; McGranahan, N.; Bartek, J.; Quezada, S.; Hare, J.; Harris, R.; Swanton, C. Perspective: APOBEC mutagenesis in drug resistance and immune escape in HIV and cancer evolution. Ann. Oncol. 2018, 29, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Calabrese, P.; Wang, S.; Qin, C.; Rao, Y.; Feng, P.; Chen, X.S. The roles of APOBEC-mediated RNA editing in SARS-CoV-2 mutations, replication and fitness. Sci. Rep. 2022, 12, 14972. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, L.; Oh, S.; Ortega, P.; Bouin, A.; Bournique, E.; Sanchez, A.; Martensen, P.M.; Auerbach, A.A.; Becker, J.T.; Seldin, M. APOBEC3B drives PKR-mediated translation shutdown and protects stress granules in response to viral infection. Nat. Commun. 2023, 14, 820. [Google Scholar] [CrossRef]
- Pavitt, G.D. Regulation of translation initiation factor eIF2B at the hub of the integrated stress response. Wiley Interdiscip. Rev. RNA 2018, 9, e1491. [Google Scholar] [CrossRef]
- McCormick, C.; Khaperskyy, D.A. Translation inhibition and stress granules in the antiviral immune response. Nat. Rev. Immunol. 2017, 17, 647–660. [Google Scholar] [CrossRef]
- Wei, L.-H.; Sun, Y.; Guo, J.U. Genome-wide CRISPR screens identify noncanonical translation factor eIF2A as an enhancer of SARS-CoV-2 programmed—1 ribosomal frameshifting. Cell Rep. 2023, 42, 112987. [Google Scholar] [CrossRef]
- Slobodin, B.; Sehrawat, U.; Lev, A.; Hayat, D.; Zuckerman, B.; Fraticelli, D.; Ogran, A.; Ben-Shmuel, A.; Bar-David, E.; Levy, H. Cap-independent translation and a precisely located RNA sequence enable SARS-CoV-2 to control host translation and escape anti-viral response. Nucleic Acids Res. 2022, 50, 8080–8092. [Google Scholar] [CrossRef] [PubMed]
- Renner, D.M.; Parenti, N.A.; Bracci, N.; Weiss, S.R. Betacoronaviruses Differentially Activate the Integrated Stress Response to Optimize Viral Replication in Lung-Derived Cell Lines. Viruses 2025, 17, 120. [Google Scholar] [CrossRef]
- Ricciardi-Jorge, T.; da Rocha, E.L.; Gonzalez-Kozlova, E.; Rodrigues-Luiz, G.F.; Ferguson, B.J.; Sweeney, T.; Irigoyen, N.; Mansur, D.S. PKR-mediated stress response enhances dengue and Zika virus replication. Mbio 2023, 14, e0093423. [Google Scholar] [CrossRef]
- Qin, C.; Rao, Y.; Yuan, H.; Wang, T.-Y.; Zhao, J.; Espinosa, B.; Liu, Y.; Zhang, S.; Savas, A.C.; Liu, Q. SARS-CoV-2 couples evasion of inflammatory response to activated nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2122897119. [Google Scholar] [CrossRef]
- Xiao, N.; Nie, M.; Pang, H.; Wang, B.; Hu, J.; Meng, X.; Li, K.; Ran, X.; Long, Q.; Deng, H. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021, 12, 1618. [Google Scholar] [CrossRef]
- Theodoris, C.V.; Xiao, L.; Chopra, A.; Chaffin, M.D.; Al Sayed, Z.R.; Hill, M.C.; Mantineo, H.; Brydon, E.M.; Zeng, Z.; Liu, X.S. Transfer learning enables predictions in network biology. Nature 2023, 618, 616–624. [Google Scholar] [CrossRef]
- Chen, H.; Venkatesh, M.S.; Ortega, J.G.; Mahesh, S.V.; Nandi, T.N.; Madduri, R.K.; Pelka, K.; Theodoris, C.V. Quantized multi-task learning for context-specific representations of gene network dynamics. bioRxiv 2024. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Zupin, L.; Fontana, F.; Clemente, L.; Ruscio, M.; Ricci, G.; Crovella, S. Effect of short time of SARS-CoV-2 infection in Caco-2 cells. Viruses 2022, 14, 704. [Google Scholar] [CrossRef] [PubMed]
- Pires De Souza, G.A.; Le Bideau, M.; Boschi, C.; Wurtz, N.; Colson, P.; Aherfi, S.; Devaux, C.; La Scola, B. Choosing a cellular model to study SARS-CoV-2. Front. Cell. Infect. Microbiol. 2022, 12, 1003608. [Google Scholar] [CrossRef]
- Garcia, M.; Meurs, E.; Esteban, M. The dsRNA protein kinase PKR: Virus and cell control. Biochimie 2007, 89, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Bass, A.; Liu, Y.; Dakshanamurthy, S. Single-cell and bulk RNASeq profiling of COVID-19 patients reveal immune and inflammatory mechanisms of infection-induced organ damage. Viruses 2021, 13, 2418. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xu, X.; Fan, J.; Chen, H.; Zhao, Y.; Huang, W.; Liu, W.; Zhang, Z.; Cui, Q.; Li, Q. APOBEC3-related mutations in the spike protein-encoding region facilitate SARS-CoV-2 evolution. Heliyon 2024, 10, e32139. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; He, B.; Carver, K.; Vanheyningen, D.; Parkin, B.; Garmire, L.X.; Olszewski, M.A.; Deng, J.C. Heterogeneity of neutrophils and inflammatory responses in patients with COVID-19 and healthy controls. Front. Immunol. 2022, 13, 970287. [Google Scholar] [CrossRef]
- Ehlers, L.; Kuppe, A.; Damerau, A.; Wilantri, S.; Kirchner, M.; Mertins, P.; Strehl, C.; Buttgereit, F.; Gaber, T. Surface AMP deaminase 2 as a novel regulator modifying extracellular adenine nucleotide metabolism. FASEB J. 2021, 35, e21684. [Google Scholar] [CrossRef]
- Leonard, B.; McCann, J.L.; Starrett, G.J.; Kosyakovsky, L.; Luengas, E.M.; Molan, A.M.; Burns, M.B.; McDougle, R.M.; Parker, P.J.; Brown, W.L. The PKC/NF-κB signaling pathway induces APOBEC3B expression in multiple human cancers. Cancer Res. 2015, 75, 4538–4547. [Google Scholar] [CrossRef] [PubMed]
- Faure-Dupuy, S.; Riedl, T.; Rolland, M.; Hizir, Z.; Reisinger, F.; Neuhaus, K.; Schuehle, S.; Remouchamps, C.; Gillet, N.; Schönung, M. Control of APOBEC3B induction and cccDNA decay by NF-κB and miR-138-5p. JHEP Rep. 2021, 3, 100354. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-W.; Zhou, S.-F. Inflammation, Cytokines, the IL-17/IL-6/STAT3/NF-κB Axis, and Tumorigenesis; Taylor & Francis: Abingdon, UK, 2015; pp. 2941–2946. [Google Scholar]
- Liu, W.; Wu, J.; Yang, F.; Ma, L.; Ni, C.; Hou, X.; Wang, L.; Xu, A.; Song, J.; Deng, Y. Genetic polymorphisms predisposing the interleukin 6–induced APOBEC3B-UNG imbalance increase HCC risk via promoting the generation of APOBEC-signature HBV mutations. Clin. Cancer Res. 2019, 25, 5525–5536. [Google Scholar] [CrossRef]
- Li, S.; Bao, X.; Wang, D.; You, L.; Li, X.; Yang, H.; Bian, J.; Wang, Y.; Yang, Y. APOBEC3B and IL-6 form a positive feedback loop in hepatocellular carcinoma cells. S. China Life Sci. 2017, 60, 617–626. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Tian Ng, A.W.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N. The repertoire of mutational signatures in human cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jia, M.; He, Z.; Liu, X.-S. APOBEC3B and APOBEC mutational signature as potential predictive markers for immunotherapy response in non-small cell lung cancer. Oncogene 2018, 37, 3924–3936. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.B.; Temiz, N.A.; Harris, R.S. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat. Genet. 2013, 45, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Nikkilä, J.; Kumar, R.; Campbell, J.; Brandsma, I.; Pemberton, H.N.; Wallberg, F.; Nagy, K.; Scheer, I.; Vertessy, B.G.; Serebrenik, A.A. Elevated APOBEC3B expression drives a kataegic-like mutation signature and replication stress-related therapeutic vulnerabilities in p53-defective cells. Br. J. Cancer 2017, 117, 113–123. [Google Scholar] [CrossRef]
- Taylor, B.J.; Nik-Zainal, S.; Wu, Y.L.; Stebbings, L.A.; Raine, K.; Campbell, P.J.; Rada, C.; Stratton, M.R.; Neuberger, M.S. DNA deaminases induce break-associated mutation showers with implication of APOBEC3B and 3A in breast cancer kataegis. eLife 2013, 2, e00534. [Google Scholar] [CrossRef] [PubMed]
- Fixman, B.; Díaz-Gay, M.; Qiu, C.; Margaryan, T.; Lee, B.; Chen, X.S. Validation of the APOBEC3A-Mediated RNA Single Base Substitution Signature and Proposal of Novel APOBEC1, APOBEC3B, and APOBEC3G RNA Signatures. J. Mol. Biol. 2024, 436, 168854. [Google Scholar] [CrossRef] [PubMed]
- Fixman, B.B. APOBEC RNA Mutational Signatures and the Role of APOBEC3B in SARS-CoV-2 Infection. Ph.D. Thesis, University of Southern California, Los Angeles, CA, USA, 2025. [Google Scholar]
- Partek. Partek™ Flow™, version 10.0. Computer Software. Illumina: Tokyo, Japan, 2024.
| siRNA | Sequence | Company | Catalog Number |
|---|---|---|---|
| Control | Thermo Fisher Scientific | 4390843 | |
| APOBEC3B | CCUCAGUACCACGCAGAAATT | Thermo Fisher Scientific | s18411 |
| APOBEC3B | GAGAUUCUCAGAUACCUGATT | Thermo Fisher Scientific | s18412 |
| PKR | GGUGAAGGUAGAUCAAAGATT | Thermo Fisher Scientific | s11187 |
| PKR | GACGGAAAGACUUACGUUATT | Thermo Fisher Scientific | s11185 |
| Primer | Sequence | Company |
|---|---|---|
| Actin—Forward | CTGGCACCCAGCACAATG | IDT DNA |
| Actin—Reverse | GCCGATCCACACGGAGTACT | IDT DNA |
| CoV-2 N1—Forward | GGACCCCAAAATCAGCGAAAT | IDT DNA |
| CoV-2 N1—Reverse | TTCTGGTTACTGCCAGTTGAATCTG | IDT DNA |
| Antibody | Isotype | Company | Catalog Number |
|---|---|---|---|
| SARS-CoV-2 Nucleocapsid | Rabbit monoclonal | Cell Signaling Technology (Danvers, MA, USA) | 86326 |
| GAPDH | Rabbit polyclonal | EMD Millipore (Burlington, MA, USA) | ABS16 |
| Vinculin | Mouse monoclonal | Sigma (St. Louis, MO, USA) | V9264 |
| PKR | Mouse monoclonal | BD Biosciences (San Jose, CA, USA) | 610764 |
| PKR-pT446 | Rabbit monoclonal | Abcam (Cambridge, UK) | ab32036 |
| eIF2⍺ | Rabbit monoclonal | Cell Signaling Technology (Danvers, MA, USA) | 5324T |
| eIF2⍺-pS51 | Rabbit monoclonal | Abcam (Cambridge, UK) | 32157 |
| A3B | Rabbit monoclonal | Abcam (Cambridge, UK) | 184990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fixman, B.; Manjunath, L.; Sell, P.; Wang, S.; Margaryan, T.; Qiu, C.; Yang, H.; Buisson, R.; Chen, X.S. APOBEC3B Promotes SARS-CoV-2 Through Activation of PKR/eIF2⍺ and AMPD2 Dysregulation. Viruses 2025, 17, 1176. https://doi.org/10.3390/v17091176
Fixman B, Manjunath L, Sell P, Wang S, Margaryan T, Qiu C, Yang H, Buisson R, Chen XS. APOBEC3B Promotes SARS-CoV-2 Through Activation of PKR/eIF2⍺ and AMPD2 Dysregulation. Viruses. 2025; 17(9):1176. https://doi.org/10.3390/v17091176
Chicago/Turabian StyleFixman, Benjamin, Lavanya Manjunath, Philip Sell, Shanshan Wang, Tamara Margaryan, Connor Qiu, Hanjing Yang, Rémi Buisson, and Xiaojiang S. Chen. 2025. "APOBEC3B Promotes SARS-CoV-2 Through Activation of PKR/eIF2⍺ and AMPD2 Dysregulation" Viruses 17, no. 9: 1176. https://doi.org/10.3390/v17091176
APA StyleFixman, B., Manjunath, L., Sell, P., Wang, S., Margaryan, T., Qiu, C., Yang, H., Buisson, R., & Chen, X. S. (2025). APOBEC3B Promotes SARS-CoV-2 Through Activation of PKR/eIF2⍺ and AMPD2 Dysregulation. Viruses, 17(9), 1176. https://doi.org/10.3390/v17091176







