Seroprevalence of Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), and Hepatitis C Virus (HCV) Among Blood Donors in Borgou, Benin in 2023: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Study Population
2.2. Data Collection
2.3. Laboratory Screening Test
- -
- HIV: Genscreen ULTRA HIV Ag-Ab—Bio-Rad;
- -
- HBV: Monolisa HBsAg ULTRA—Bio-Rad;
- -
- HCV: Monolisa HCV Ag-Ab ULTRA V2—Bio-Rad.
2.4. Data Analysis
2.5. Ethical Consideration
3. Results
3.1. Socio-Demographic Characteristics of the Study Population
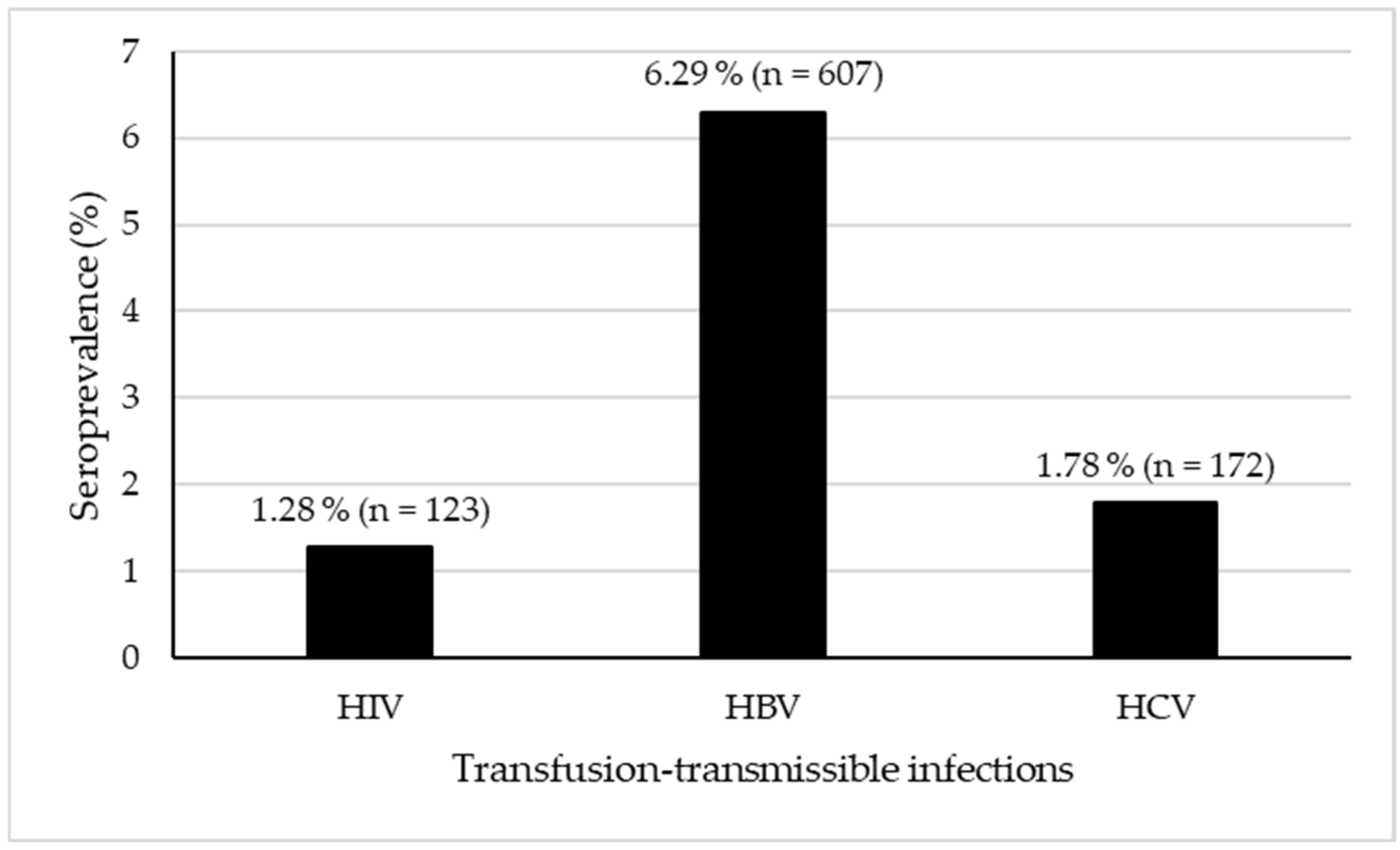
3.2. Seroprevalence of Transfusion-Transmittable Infections
3.3. Seroprevalence of HIV, HBV, and HCV by Donor Characteristics: Distribution and Association Analysis
3.4. Transfusion-Transmissible Infections and Associated Risk Factors Among Blood Donors in Borgou
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LBPs | labile blood products |
| TTVIs | transfusion-transmitted viral infections |
| HIV | human immunodeficiency virus |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| SPSS | Statistical Package for the Social Sciences |
| HBsAg | hepatitis B surface antigen |
| HCV Ag-Ab | hepatitis C virus antigen-antibody combination |
| ELISA | enzyme-linked immunosorbent assay |
References and Note
- Lefrère, J.-J.; Rouger, P. Pratique Nouvelle de la Transfusion Sanguine, 1st ed.; Masson Elsevier: Paris, France, 2009; 159p, Available online: https://linkinghub.elsevier.com/retrieve/pii/B9782294707346X00013 (accessed on 5 July 2025).
- World Health Organization. Blood Transfusion. Available online: https://www.who.int/news-room/facts-in-pictures/detail/blood-transfusion (accessed on 6 July 2025).
- Organisation Mondiale de la Santé (OMS). Hépatite B: Aide-Mémoire N°204; Organisation Mondiale de la Santé: Genève, Switzerland, 2016. [Google Scholar]
- Alassad, A.; Al Rahwanji, M.J.; Yousfan, A.; Al Moualem, S.; Farhat, A.; Youssef, L.A. Seroprevalence and trends of hepatitis B virus, hepatitis C virus and human immunodeficiency virus in Syrian blood donors at Damascus University Blood Center between 2004 and 2021. Front. Public Health 2023, 11, 1174638. [Google Scholar] [CrossRef]
- Organisation Mondiale de la Santé (OMS). Dépistage des Infections Transmissibles par le Sang. Recommandations; Organisation Mondiale de la Santé: Genève, Switzerland, 2010; 75p, Available online: https://iris.who.int/handle/10665/112663 (accessed on 6 July 2025).
- Belkacemi, M.; Merbouh, M.A. Seroprevalence of Human Immunodeficiency Virus, Hepatitis C Virus, and Hepatitis B Virus Among Blood Donors in Sidi Bel Abbes, West Algeria. Cureus 2023, 15, e47066. [Google Scholar] [CrossRef] [PubMed]
- Negash, M.; Ayalew, M.; Geremew, D.; Workineh, M. Seroprevalence and associated risk factors for HIV, Hepatitis B and C among blood Donors in South Gondar District blood Bank, Northwest Ethiopia. BMC Infect. Dis. 2019, 19, 430. [Google Scholar] [CrossRef]
- Allain, J.-P.; Owusu-Ofori, S.; Bates, I. Blood Transfusion in Sub-Saharan Africa. Transfus. Altern. Transfus. Med. 2004, 6, 16–23. [Google Scholar] [CrossRef]
- Kra, O.; N’dri, N.; Ehui, E.; Ouattara, B.; Bissagnene, E. Prévalence de l’antigène HBs chez les donneurs de sang au centre régional de transfusion sanguine de Bouaké (Côte d’Ivoire) en 2001. Bull. Soc. Pathol. Exot. 2007, 100, 127–129. [Google Scholar] [PubMed]
- Allain, J.-P.; Candotti, D.; Soldan, K.; Sarkodie, F.; Phelps, B.; Giachetti, C.; Shyamala, V.; Yeboah, F.; Anokwa, M.; Owusu-Ofori, S.; et al. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood 2003, 101, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Okoroiwu, H.U.; Okafor, I.M.; Asemota, E.A.; Okpokam, D.C. Seroprevalence of transfusion-transmissible infections (HBV, HCV, syphilis and HIV) among prospective blood donors in a tertiary health care facility in Calabar, Nigeria; an eleven years evaluation. BMC Public Health 2018, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Nagalo, M.B.; Sanou, M.; Bisseye, C.; Kaboré, M.I.; Nebie, Y.K.; Kienou, K.; Kiba, A.; Dahourou, H.; Ouattara, S.; Zongo, J.D.; et al. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis among blood donors in Koudougou (Burkina Faso) in 2009. Blood Transfus. 2011, 9, 419–424. [Google Scholar]
- Graobe, B.B.; Gake, B.; Balkissou, A.D.; Minsia, C.; Taoufick, Y.; Gnowe, G.; Toumpim, M.; Mballa, A.G.E.; Nukenine, E.N. Seroprevalence of HIV, Hepatitis B and C Viruses, and Treponema Pallidum among Blood Donors Attending the Garoua Regional Hospital Blood Bank, North Cameroon, a Cross-Sectional Study. Int. J. Med. Sci. Clin. Res. Stud. 2023, 3, 1486–1494. [Google Scholar] [CrossRef]
- Attinsounon, C.A.; Saké, K.; Tchouya Djofang, K.P.; Koudoukpo, C. Séroprévalence des hépatites virales B et C, du VIH et de la syphilis chez les nouveaux donneurs de sang à Parakou en 2017. 2018; Livret des abstracts: 49p.
- Nébié, K.Y.; Olinger, C.M.; Kafando, E.; Dahourou, H.; Diallo, S.; Kientega, Y.; Domo, Y.; Kienou, K.; Ouattara, S.; Sawadogo, I.; et al. Lack of knowledge among blood donors in Burkina Faso (West Africa); potential obstacle to transfusion security. Transfus. Clin. Biol. 2007, 14, 446–452. [Google Scholar] [CrossRef]
- Bah, A.; Traoré, M.K.; Kassogué, A.; Coulibaly, D.; Sogodogo, I.; Diallo, H.; Diallo S, S.; Keita, M.; Koné, S.I.; Kanté, M.; et al. Séroprévalence des donneurs de sang à l’hôpital Nianankoro Fomba de Ségou. Rev. Malienne Infect. Microbiol. 2019, 13, 41–46. [Google Scholar] [CrossRef]
- Tagny, C.T.; Murphy, E.L.; Lefrère, J.-J. Le groupe de recherches transfusionnelles d’Afrique francophone: Bilan des cinq premières années. Transfus. Clin. Biol. 2014, 21, 37–42. [Google Scholar] [CrossRef]
- Noubiap, J.J.N.; Joko, W.Y.A.; Nansseu, J.R.N.; Tene, U.G.; Siaka, C. Sero-epidemiology of human immunodeficiency virus, hepatitis B and C viruses, and syphilis infections among first-time blood donors in Edéa, Cameroon. Int. J. Infect. Dis. 2013, 17, e832–e837. [Google Scholar] [CrossRef] [PubMed]
- Kakisingi, C.N.; Mukuku, O.; Matanda, S.K.; Manika, M.M.; Kyabu, V.K.; Kasamba, E.I.; Mawaw, P.M.; Mwamba, C.M.; Kapend, L. Profil épidémiologique et séroprévalence des donneurs de sang aux cliniques universitaires de Lubumbashi, République Démocratique du Congo. Pan Afr. Med. J. 2016, 23, 175. [Google Scholar] [CrossRef]
- Goita, D.; Traore, M.; Kassogue, O.; Sogoba, D.; Guindo, S.; Keita, B.; Dembele, K.; Dissa, M.; Berthe, A.; Dao, S. Séroprévalence du VIH, des Virus des Hépatites B et C et de la Syphilis chez les Donneurs de Sang à l’Hôpital de Sikasso, Mali. Health Sci. Dis. 2019, 20, 43–48. [Google Scholar]
- Namululi, B.A.; Guerrieri, C.; Dramaix, M. Impact du mode de recrutement des donneurs de sang sur la prévalence du VIH et du VHB à Bukavu, République démocratique du Congo. Med. Sante Trop. 2012, 22, 69–74. [Google Scholar]
- Tounkara, A.; Sarro, Y.S.; Kristensen, S.; Dao, S.; Diallo, H.; Diarra, B.; Noumsi, T.; Guindo, O. Seroprevalence of HIV/HBV coinfection in Malian blood donors. J. Int. Assoc. Physicians AIDS Care 2009, 8, 47–51. [Google Scholar] [CrossRef]
- Rajab, J.A.; Muchina, W.P.; Orinda, D.A.O.; Scott, C.S. Blood donor haematology parameters in two regions of Kenya. East Afr. Med. J. 2005, 82, 124–128. [Google Scholar] [CrossRef]
- Kpossou, A.R.; Paraiso, M.N.; Sokpon, C.N.; Alassan, K.S.; Vignon, R.K.; Keke, R.K.; Bigot, C.; Domonhédo, C.; Gbédo, S.E.; Séhonou, J.; et al. Hépatite virale B lors d’une campagne de dépistage en population générale au Bénin: Séroprévalence et facteurs associés. Pan Afr. Med. J. 2020, 37, 247. [Google Scholar] [CrossRef] [PubMed]
- Dicko, M.Y.; Sidibé, S.D.; Katile, D.; Samaké, D.K.; Coulibaly, S.H.; Tounkara, M.S. Impact de l’instabilité sociale sur les Infections par le Virus de l’Immunodéficience Humaine et les Virus des hépatites B et C. Cas de Gao. J. Anaesth. Crit. Care Case Rep. 2021, 3, 24–28. [Google Scholar]
- N’dri, N.; Thot’o, A.S.; Okon, A.J.-B.; Assi, C.; Allah-Kouadio, E.; Soro, D.; Diakité, M.; Koné, A.; Koné, S.; Lohouès-Kouacou, M.J.; et al. Prevalence of HBs Ag among blood donors in Transfusion Center, Abidjan (Ivory Coast). Open J. Gastroenterol. 2013, 3, 165–169. [Google Scholar] [CrossRef]
- Bartonjo, G. Prevalence and Factors Associated with Transfusion Transmissible Infections Among Blood Donors at Regional Blood Transfusion Center Nakuru and Tenwek Mission Hospital, Kenya. Master’s Thesis, Jomo Kenyatta University of Agriculture and Technology, Juja, Kenya, 2013. Available online: http://repository.kemri.go.ke:8080/xmlui/handle/123456789/288 (accessed on 6 July 2025).
- Xie, D.-D.; Li, J.; Chen, J.-T.; Eyi, U.M.; Matesa, R.A.; Obono, M.M.O.; Ehapo, C.S.; Yang, L.-Y.; Yang, H.; Lin, M.; et al. Seroprevalence of Human Immunodeficiency Virus, Hepatitis B Virus, Hepatitis C Virus, and Treponema pallidum Infections among Blood Donors on Bioko Island, Equatorial Guinea. PLoS ONE 2015, 10, e0139947. [Google Scholar] [CrossRef] [PubMed]
- Eboumbou Moukoko, C.E.; Ngo Sack, F.; Essangui Same, E.G.; Mbangue, M.; Lehman, L.G. HIV, HBV, HCV and T. pallidum infections among blood donors and Transfusion-related complications among recipients at the Laquintinie hospital in Douala, Cameroon. BMC Hematol. 2014, 14, 5. [Google Scholar] [CrossRef]
- Katilé, D.; Konaté, I.; Goïta, D.; Dicko, M.Y.; Konaté, M.K.; Mallé, O.; Sangaré, D. Evaluation de la séroprévalence des hépatites virales B et C chez les donneurs de sang en milieu urbain dans un hôpital régional au Mali: Cas de l’hôpital régional Fousseyni Daou de Kayes. Méd. Afr. Noire 2018, 65, 381–387. [Google Scholar]
- Uwingabiye, J.; Zahid, H.; Unyendje, L.; Hadef, R. Séroprévalence des marqueurs viraux sur les dons du sang au Centre de Transfusion Sanguine, Hôpital Militaire d’Instruction Mohammed V de Rabat. Pan Afr. Med. J. 2016, 25, 185. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Frequency (n) | Percentage (%) | 95% Confidence Interval (CI) |
|---|---|---|---|
| Sex | |||
| Male | 8469 | 87.80 | [87.13–88.44] |
| Female | 1177 | 12.20 | [11.56–12.87] |
| Age group (years) | |||
| 18–24 | 5395 | 55.93 | [54.94–56.92] |
| 25–30 | 1978 | 20.51 | [19.71–21.32] |
| 31–44 | 1718 | 17.81 | [17.06–18.59] |
| 45–64 | 555 | 5.75 | [5.31–6.24] |
| Donor status | |||
| First-time donor | 4572 | 47.40 | [46.40–48.40] |
| Repeat donor | 5074 | 52.60 | [51.60–53.60] |
| Socio-professional category | |||
| Student | 5659 | 58.67 | [57.68–59.65] |
| Salaried worker | 1410 | 14.62 | [13.93–15.34] |
| Artisan | 973 | 10.09 | [9.50–10.70] |
| Medical/paramedical staff | 260 | 2.70 | [2.39–3.04] |
| Military/paramilitary | 186 | 1.93 | [1.67–2.22] |
| Other | 1158 | 12.00 | [11.37–12.67] |
| Donation site | |||
| Mobile unit | 6829 | 70.80 | [69.88–71.70] |
| Fixed site | 2817 | 29.20 | [28.30–30.12] |
| Characteristic | Total Number | HIV n (%) | HBV n (%) | HCV n (%) |
|---|---|---|---|---|
| Sex | ||||
| Male | 8469 | 111 (1.31%) | 543 (6.41%) | 151 (1.78%) |
| Female | 1177 | 12 (1.02%) | 64 (5.44%) | 21 (1.78%) |
| p-value | >0.05 | >0.05 | >0.05 | |
| Age group (years) | ||||
| 18–24 | 5395 | 68 (1.26%) | 349 (6.47%) | 116 (2.15%) |
| 25–30 | 1978 | 20 (1.01%) | 126 (6.37%) | 31 (1.57%) |
| 31–44 | 1718 | 24 (1.4%) | 102 (5.94%) | 20 (1.16%) |
| 45–64 | 555 | 11 (1.98%) | 30 (5.41%) | 5 (0.9%) |
| p-value | >0.05 | >0.05 | <0.05 | |
| Donor status | ||||
| First-time donor | 4572 | 87 (1.9%) | 482 (10.54%) | 119 (2.6%) |
| Repeat donor | 5074 | 36 (0.71%) | 125 (2.46%) | 53 (1.04%) |
| p-value | <0.001 | <0.001 | <0.001 | |
| Socio-professional category | ||||
| Student | 5659 | 71 (1.25%) | 374 (6.61%) | 122 (2.16%) |
| Salaried worker | 1410 | 17 (1.21%) | 54 (3.83%) | 13 (0.92%) |
| Artisan | 973 | 14 (1.44%) | 72 (7.4%) | 14 (1.44%) |
| Medical/paramedical staff | 260 | 1 (0.38%) | 7 (2.69%) | 2 (0.77%) |
| Military/paramilitary | 186 | 2 (1.08%) | 3 (1.61%) | 4 (2.15%) |
| Other | 1158 | 18 (1.55%) | 97 (8.38%) | 17 (1.47%) |
| p-value | 0.775 | <0.001 | 0.013 | |
| Donation site | ||||
| Mobile unit | 6828 | 103 (1.51%) | 496 (7.26%) | 134 (1.96%) |
| Fixed site | 2818 | 20 (0.71%) | 111 (3.94%) | 38 (1.35%) |
| p-value | <0.001 | <0.001 | 0.047 |
| HIV | HBV | HCV | |||||
|---|---|---|---|---|---|---|---|
| Variable | Category | AOR [95% CI] | p-Value | AOR [95% CI] | p-Value | AOR [95% CI] | p-Value |
| Sex | Female | – | – | – | – | – | – |
| Male | 1.65 [0.94–3.19] | 0.104 | 1.67 [1.28–2.22] | p < 0.001 | 1.19 [0.76–1.95] | 0.470 | |
| Age (continuous) | Per year increase | 1.03 [1.01–1.05] | 0.0042 | 1.02 [1.01–1.03] | p < 0.001 | 0.99 [0.96–1.01] | 0.289 |
| Socio-professional category | Medical/paramedical staff | – | – | – | – | – | – |
| Other | 3.22 [0.66–58.3] | 0.257 | 2.48 [1.21–6.00] | p < 0.001 | 1.72 [0.49–10.9] | 0.470 | |
| Salaried/Military | 2.83 [0.58–51.2] | 0.313 | 1.16 [0.56–2.85] | 0.710 | 1.39 [0.39–8.84] | 0.662 | |
| Student/Artisan | 3.30 [0.72–58.6] | 0.239 | 2.12 [1.06–5.04] | 0.0557 | 2.03 [0.63–12.4] | 0.327 | |
| Donor status | First-time donor | – | – | – | – | – | – |
| Repeat donor | 0.38 [0.25–0.57] | p < 0.001 | 0.22 [0.17–0.26] | p < 0.001 | 0.42 [0.30–0.59] | p < 0.001 | |
| Donation site | Fixed site | – | – | – | – | – | – |
| Mobile site | 1.77 [1.08–3.02] | 0.0286 | 1.20 [0.96–1.51] | 0.114 | 0.98 [0.68–1.47] | 0.935 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djaliri, K.-D.; Legba, B.B.; Dougnon, V.; Tidjani, A.; Baba-Moussa, L. Seroprevalence of Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), and Hepatitis C Virus (HCV) Among Blood Donors in Borgou, Benin in 2023: A Cross-Sectional Study. Viruses 2025, 17, 1107. https://doi.org/10.3390/v17081107
Djaliri K-D, Legba BB, Dougnon V, Tidjani A, Baba-Moussa L. Seroprevalence of Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), and Hepatitis C Virus (HCV) Among Blood Donors in Borgou, Benin in 2023: A Cross-Sectional Study. Viruses. 2025; 17(8):1107. https://doi.org/10.3390/v17081107
Chicago/Turabian StyleDjaliri, Kamel-Dine, Brice Boris Legba, Victorien Dougnon, Abdelsalam Tidjani, and Lamine Baba-Moussa. 2025. "Seroprevalence of Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), and Hepatitis C Virus (HCV) Among Blood Donors in Borgou, Benin in 2023: A Cross-Sectional Study" Viruses 17, no. 8: 1107. https://doi.org/10.3390/v17081107
APA StyleDjaliri, K.-D., Legba, B. B., Dougnon, V., Tidjani, A., & Baba-Moussa, L. (2025). Seroprevalence of Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), and Hepatitis C Virus (HCV) Among Blood Donors in Borgou, Benin in 2023: A Cross-Sectional Study. Viruses, 17(8), 1107. https://doi.org/10.3390/v17081107






