Viral Inactivation by Light-Emitting Diodes: Action Spectra Reveal Genomic Damage as the Primary Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Host Cell Strains
2.2. Irradiation of Viral Suspensions by LEDs
2.3. Measurements of Virus Infectivity
2.4. RNA Extraction and Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.5. Dot-Blot Analysis
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
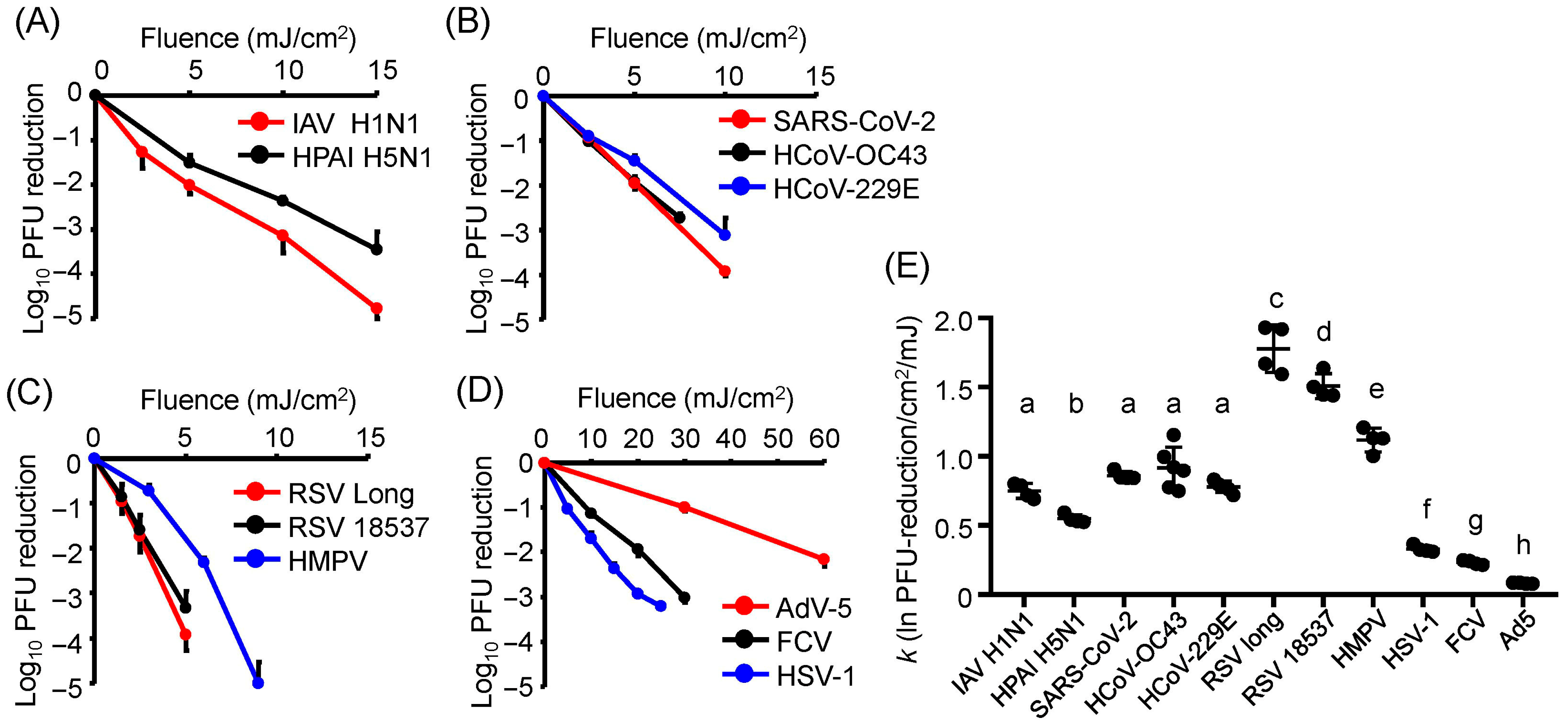
3.1. Differences in U280-LED Responses Were Attributed to Viral Strains and Components
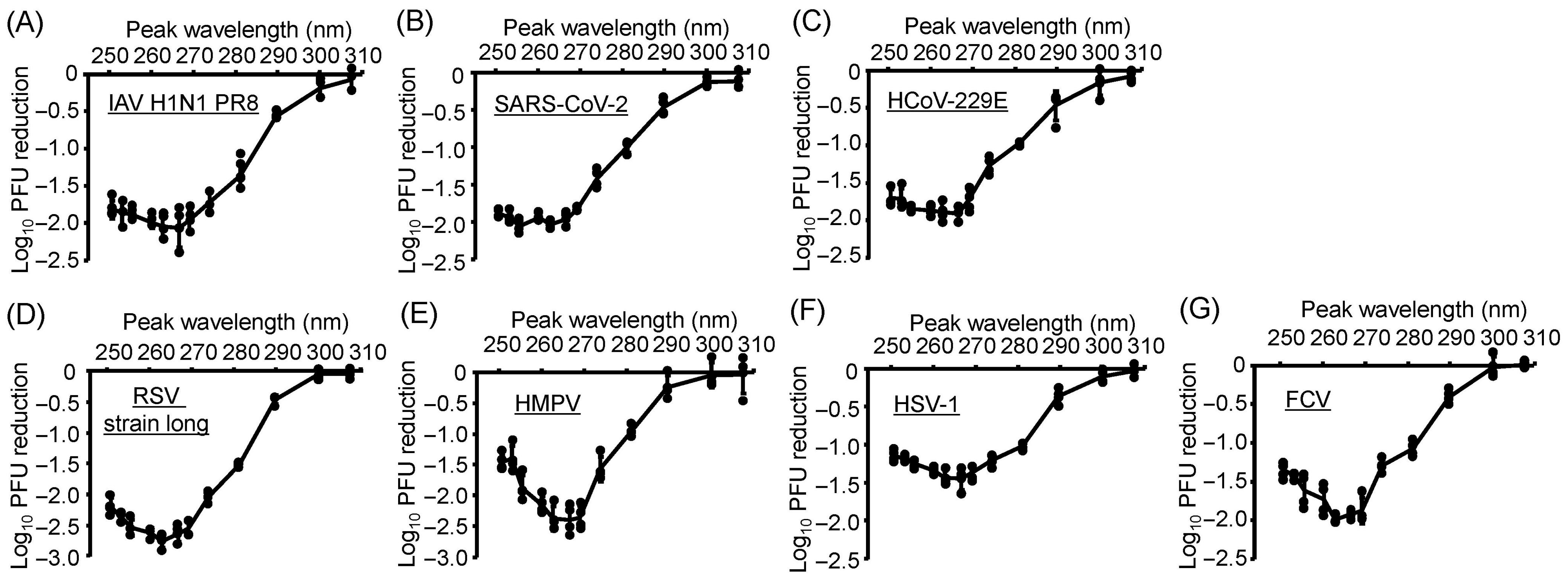
3.2. All Virus Strains Exhibited Similar Action Spectra, and U267- and U270-LEDs Displayed the Greatest Virucidal Efficiency
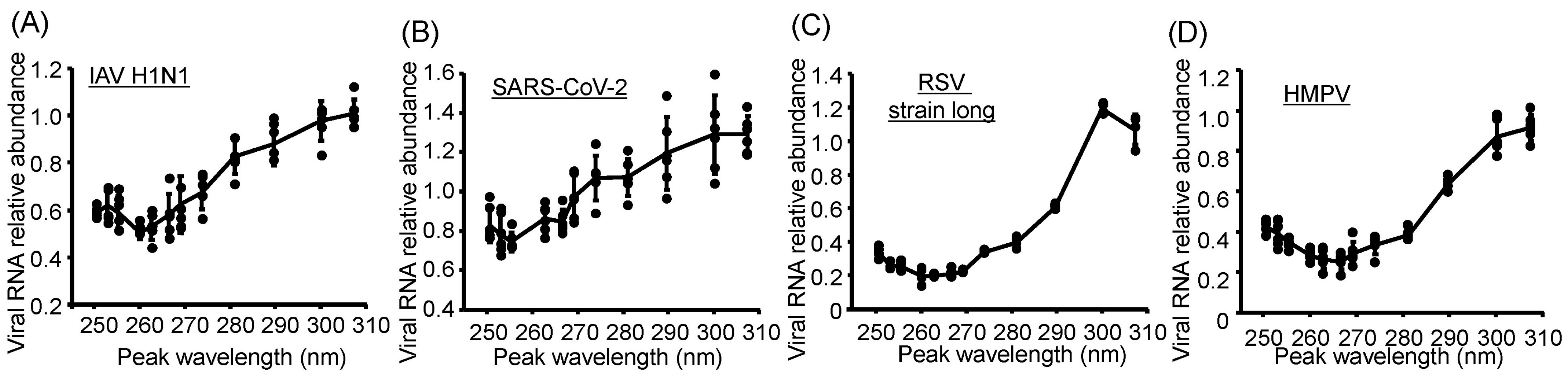
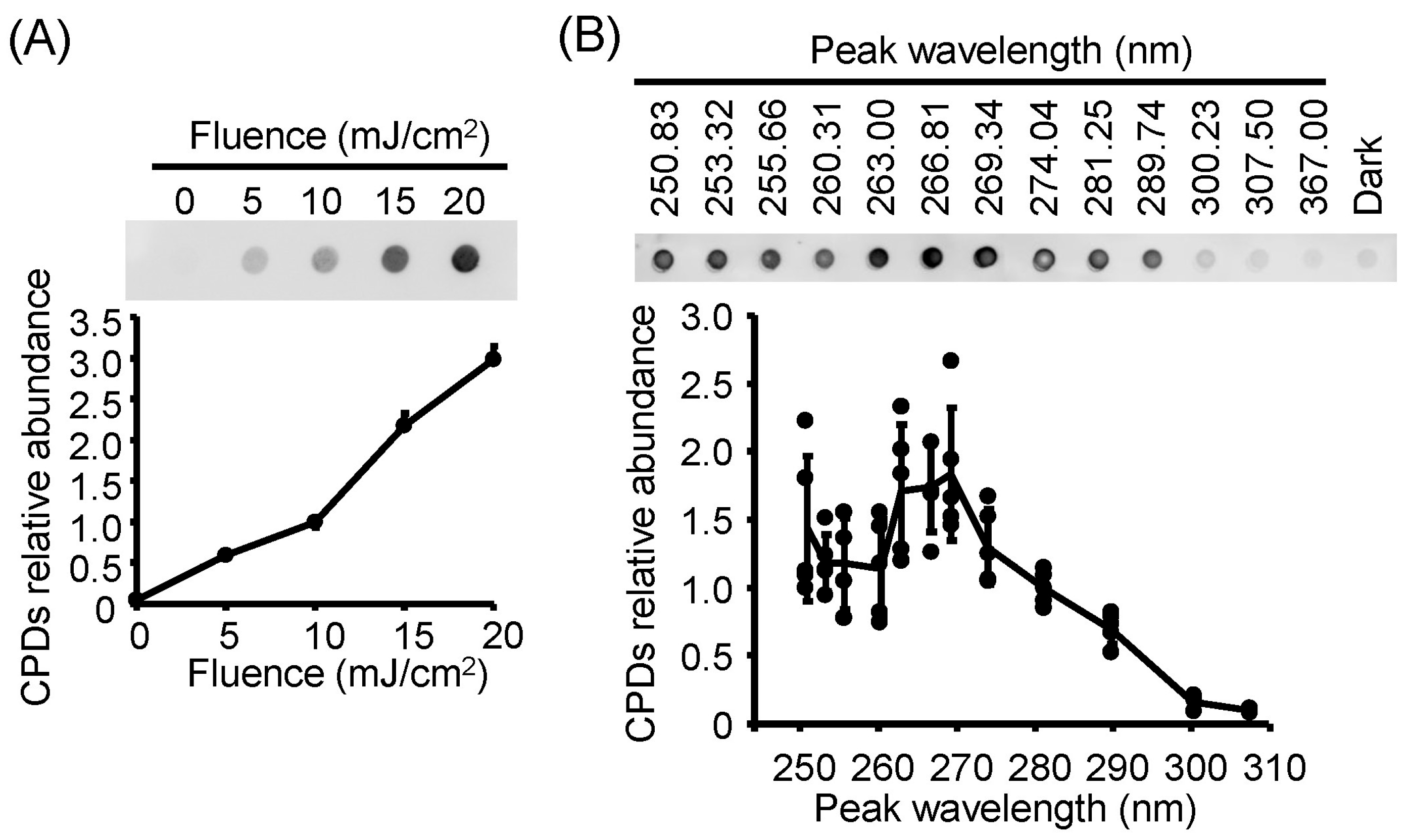
3.3. Damage to Viral Genomic RNA Was Induced by U280-LED Irradiation
3.4. Action Spectra of Infectivity Reduction by UV-LED Irradiation Were Related to Viral RNA Damage
3.5. Action Spectra of Infectivity Reduction by UV-LED Irradiation Were Related to Viral DNA Damage
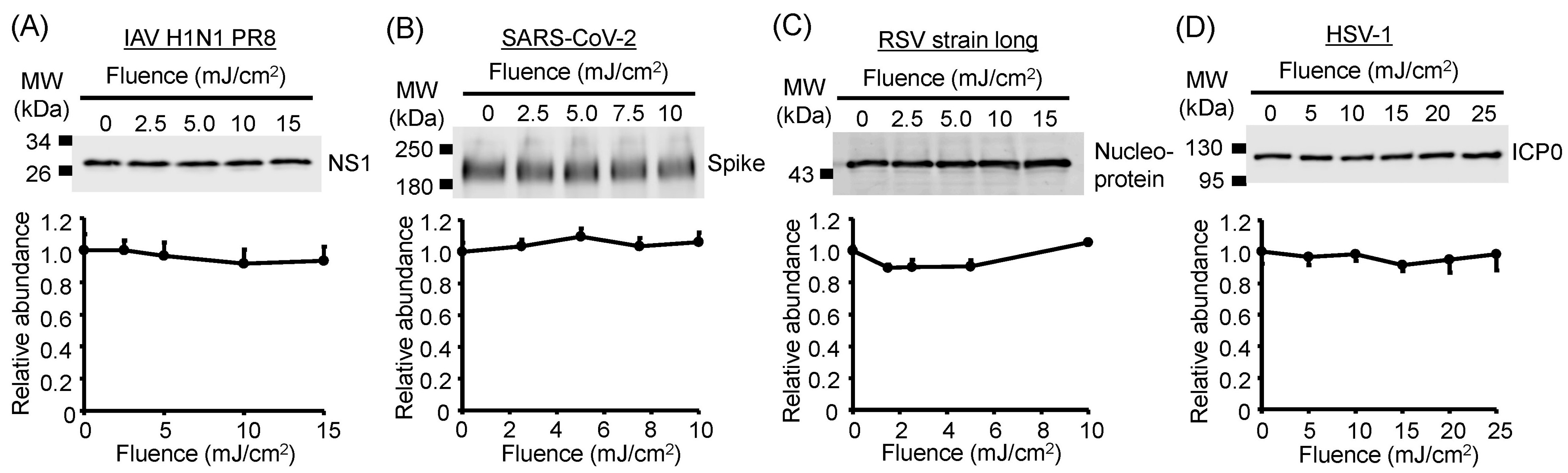
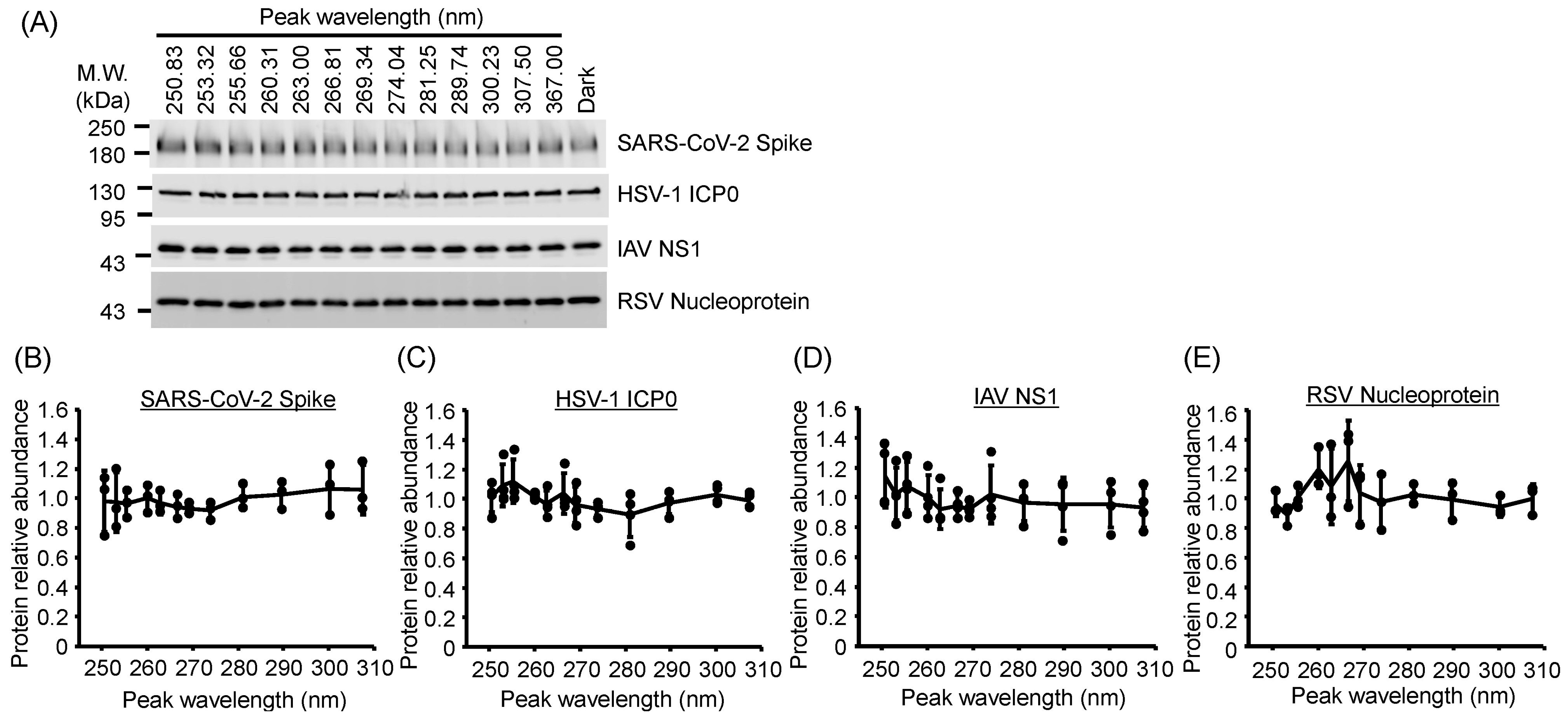
3.6. Viral Protein Degradation Was Not Induced by UV-LED Irradiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV | ultraviolet |
| LEDs | light-emitting diodes |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| HCoV | human coronavirus |
| IAV | influenza A virus |
| RSVs | respiratory syncytial viruses |
| HMPV | human metapneumovirus |
| HSV | herpes simplex virus |
| FCV | feline calicivirus |
| AdV | adenovirus |
| PFUs | plaque-forming units |
References
- Cyranoski, D. Profile of a killer: The complex biology powering the coronavirus pandemic. Nature 2020, 581, 22–26. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9, e57309. [Google Scholar] [CrossRef]
- Li, F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020, 39, e105114. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, M.U.; Colaneri, M.; Seminari, E.M.; Baldanti, F.; Bruno, R. Low risk of SARS-CoV-2 transmission by fomites in real-life conditions. Lancet Infect. Dis. 2021, 21, e112. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. Efficacy of ethanol against viruses in hand disinfection. J. Hosp. Infect. 2018, 98, 331–338. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aaerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Sharafeldin, T.A.; Goyal, S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbound. Emerg. Dis. 2021, 68, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Best practices for disinfection of noncritical environmental surfaces and equipment in health care facilities: A bundle approach. Am. J. Infect. Control. 2019, 47, A96–A105. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, N.; Stucki, M.; Späth, P.J.; Kempf, C. Virus safety of intravenous immunoglobulin: Future challenges. Clin. Rev. Allergy Immunol. 2005, 29, 333–344. [Google Scholar] [CrossRef]
- Gröner, A.; Broumis, C.; Fang, R.; Nowak, T.; Popp, B.; Schäfer, W.; Roth, N.J. Effective inactivation of a wide range of viruses by pasteurization. Transfusion 2018, 58, 41–51. [Google Scholar] [CrossRef]
- Beck, S.E.; Rodriguez, R.A.; Hawkins, M.A.; Hargy, T.M.; Larason, T.C.; Linden, K.G.; Dozois, C.M. Comparison of UV-induced inactivation and RNA damage in MS2 phage across the germicidal UV spectrum. Appl. Environ. Microbiol. 2015, 82, 1468–1474. [Google Scholar] [CrossRef]
- Ma, B.; Linden, Y.S.; Gundy, P.M.; Gerba, C.P.; Sobsey, M.D.; Linden, K.G. Inactivation of coronaviruses and phage Phi6 from irradiation across UVC wavelengths. Environ. Sci. Technol. Lett. 2021, 8, 425–430. [Google Scholar] [CrossRef]
- Brooke, K.; Mayer, B.K.; Yang, Y.; Gerrity, D.W.; Abbaszadegan, M. The Impact of Capsid Proteins on Virus Removal and Inactivation During Water Treatment Processes. Microbiol. Insights 2015, 8, 15–28. [Google Scholar] [CrossRef]
- Kingsley, D.H. High pressure processing and its application to the challenge of virus-contaminated foods. Food Environ. Virol. 2013, 5, 1–12. [Google Scholar] [CrossRef]
- Ma, B.; Gundy, P.M.; Gerba, C.P.; Sobsey, M.D.; Linden, K.G. UV Inactivation of SARS-CoV-2 across the UVC spectrum: KrCl* excimer, mercury-vapor, and light-emitting-diode (LED) sources. Appl. Environ. Microbiol. 2021, 87, e153221. [Google Scholar] [CrossRef]
- Nishisaka-Nonaka, R.; Mawatari, K.; Yamamoto, T.; Kojima, M.; Shimohata, T.; Uebanso, T.; Nakahashi, M.; Emoto, T.; Akutagawa, M.; Kinouchi, Y.; et al. Irradiation by ultraviolet light-emitting diodes inactivates influenza a viruses by inhibiting replication and transcription of viral RNA in host cells. J. Photochem. Photobiol. B Biol. 2018, 189, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Araud, E.; Fuzawa, M.; Shisler, J.L.; Li, J.; Nguyen, T.H.; Schaffner, D.W. UV inactivation of rotavirus and tulane virus targets different components of the virions. Appl. Environ. Microbiol. 2020, 86, e02436-19. [Google Scholar] [CrossRef]
- Qiao, Z.; Ye, Y.; Chang, P.H.; Thirunarayanan, D.; Wigginton, K.R. Nucleic acid photolysis by UV. Environ. Sci. Technol. 2018, 52, 10408–10415. [Google Scholar] [CrossRef]
- Song, K.; Taghipour, F.; Mohseni, M. Microorganisms inactivation by wavelength combinations of ultraviolet light-emitting diodes (UV-LEDs). Sci. Total Environ. 2019, 665, 1103–1110. [Google Scholar] [CrossRef]
- Kojima, M.; Mawatari, K.; Emoto, T.; Nishisaka-Nonaka, R.; Bui, T.K.N.; Shimohata, T.; Uebanso, T.; Akutagawa, M.; Kinouchi, Y.; Wada, T.; et al. Irradiation by a combination of different peak-wavelength ultraviolet-light emitting diodes enhances the inactivation of influenza A viruses. Microorganisms 2020, 8, 1014. [Google Scholar] [CrossRef]
- Bui, T.K.N.; Mawatari, K.; Emoto, T.; Fukushima, S.; Shimohata, T.; Uebanso, T.; Akutagawa, M.; Kinouchi, Y.; Takahashi, A. UV-LED irradiation reduces the infectivity of herpes simplex virus type 1 by targeting different viral components depending on the peak wavelength. J. Photochem. Photobiol. B Biol. 2022, 228, 112410. [Google Scholar] [CrossRef]
- Hamamoto, A.; Mori, M.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Akutagawa, M.; Ikehara, T.; Nakaya, Y.; Kinouchi, Y. New water disinfection system using UVA light-emitting diodes. J. Appl. Microbiol. 2007, 103, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, A.; Bandou, C.; Nakano, M.; Mawatari, K.; Lian, X.; Yamato, M.; Harada, N.; Akutagawa, M.; Kinouchi, Y.; Nakaya, Y.; et al. Differences in stress response after UVC or UVA irradiation in Vibrio parahaemolyticus. Environ. Microbiol. Rep. 2010, 2, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, M.; Mawatari, K.; Hirata, A.; Maetani, M.; Shimohata, T.; Uebanso, T.; Hamada, Y.; Akutagawa, M.; Kinouchi, Y.; Takahashi, A. Simultaneous irradiation with different wavelengths of ultraviolet light has synergistic bactericidal effect on Vibrio parahaemolyticus. Photochem. Photobiol. 2014, 90, 1397–1403. [Google Scholar] [CrossRef]
- Bhattarai, T.; Ebong, A.; Raja, M. A review of light-emitting diodes and ultraviolet light-emitting diodes and their applications. Photonics 2024, 11, 491. [Google Scholar] [CrossRef]
- Kneissl, M.; Seong, T.-Y.; Han, J.; Amano, H. The emergence and prospects of deep-ultraviolet light-emitting diode technologies. Nat. Photonics 2019, 13, 233–244. [Google Scholar] [CrossRef]
- Beck, S.E.; Ryu, H.; Boczek, L.A.; Cashdollar, J.L.; Jeanis, K.M.; Rosenblum, J.S.; Lawal, O.R.; Linden, K.G. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Res. 2017, 109, 207–216. [Google Scholar] [CrossRef]
- Tanaka, T.; Nogariya, O.; Shionoiri, N.; Maeda, Y.; Arakaki, A. Integrated molecular analysis of the inactivation of a non-enveloped virus, feline calicivirus, by UV-C radiation. J. Biosci. Bioeng. 2018, 126, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.E.; Hull, N.M.; Poepping, C.; Linden, K.G. Wavelength-dependent damage to adenoviral proteins across the germicidal UV spectrum. Environ. Sci. Technol. 2018, 52, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, T.; Koma, T.; Suzuki, A.; Mizuno, T.; Nagamatsu, K.; Arimochi, H.; Tsuchiya, K.; Matsuoka, K.; Yasui, T.; Yasutomo, K.; et al. Quantitative evaluation of SARS-CoV-2 inactivation using a deep ultraviolet light-emitting diode. Sci. Rep. 2021, 11, 5070. [Google Scholar] [CrossRef]
- Oguma, K.; Rattanakul, S.; Masaike, M. Inactivation of health-related microorganisms in water using UV light-emitting diodes. Water Supply 2019, 19, 1507–1514. [Google Scholar] [CrossRef]
- Ishida, K.; Onoda, Y.; Kadomura-Ishikawa, Y.; Nagahashi, M.; Yamashita, M.; Fukushima, S.; Aizawa, T.; Yamauchi, S.; Fujikawa, Y.; Tanaka, T.; et al. Development of a standard evaluation method for microbial UV sensitivity using light-emitting diodes. Heliyon 2024, 10, e27456. [Google Scholar] [CrossRef]
- Onoda, Y.; Nagahashi, M.; Yamashita, M.; Fukushima, S.; Aizawa, T.; Yamauchi, S.; Fujikawa, Y.; Tanaka, T.; Kadomura-Ishikawa, Y.; Ishida, K.; et al. Accumulated melanin in molds provides wavelength-dependent UV tolerance. Photochem. Photobiol. Sci. 2024, 23, 1791–1806. [Google Scholar] [CrossRef]
- Ishida, K.; Matsubara, M.; Nagahashi, M.; Onoda, Y.; Aizawa, T.; Yamauchi, S.; Fujikawa, Y.; Tanaka, T.; Kadomura-Ishikawa, Y.; Uebanso, T.; et al. Efficacy of ultraviolet-light emitting diodes in bacterial inactivation and DNA damage via sensitivity evaluation using multiple wavelengths and bacterial strains. Arch. Microbiol. 2025, 207, 130. [Google Scholar] [CrossRef]
- Lu, Y.H.; Shi, X.R.; Li, W.S.; Lai, A.C.K. Wavelength-specific inactivation mechanisms and efficacies of germicidal UVC for airborne human coronavirus. J. Hazard. Mater. 2025, 484, 136666. [Google Scholar] [CrossRef]
- Gerchman, Y.; Mamane, H.; Friedman, N.; Mandelboim, M. UV-LED disinfection of Coronavirus: Wavelength effect. J. Photochem. Photobiol. B Biol. 2020, 212, 112044. [Google Scholar] [CrossRef]
- Kim, J.K.; Patel, D.; Choi, B.S. Contrasting structural impacts induced by cis-syn cyclobutane dimer and (6-4) adduct in DNA duplex decamers: Implication in mutagenesis and repair activity. Photochem. Photobiol. 1995, 62, 44–50. [Google Scholar] [CrossRef]
- Takagi, T.; Nishikawa, J.; Yanagihara, M.; Fukuda, S.; Kubota, N.; Kobayashi, Y.; Otsuyama, K.; Nojima, J.; Tsuneoka, H.; Sakai, K.; et al. Microbicidal effect of deep ultraviolet light-emitting diode irradiation. Lasers. Med. Sci. 2021, 36, 927–931. [Google Scholar] [CrossRef]
- Beck, S.E.; Rodriguez, R.A.; Linden, K.G.; Hargy, T.M.; Larason, T.C.; Wright, H.B. Wavelength dependent UV inactivation and DNA damage of adenovirus as measured by cell culture infectivity and long range quantitative PCR. Environ. Sci. Technol. 2014, 48, 591–598. [Google Scholar] [CrossRef]
- Schuit, M.A.; Larason, T.C.; Krause, M.L.; Green, B.M.; Holland, B.P.; Wood, S.P.; Grantham, S.; Zong, Y.; Zarobila, C.J.; Freeburger, D.L.; et al. SARS-CoV-2 inactivation by ultraviolet radiation and visible light is dependent on wavelength and sample matrix. J. Photochem. Photobiol. B Biol. 2022, 233, 112503. [Google Scholar] [CrossRef] [PubMed]
- Poblete, S.; Božič, A.; Kanduč, M.; Podgornik, R.; Guzman, H.V. RNA secondary structures regulate adsorption of fragments onto flat substrates. ACS Omega 2021, 6, 32823–32831. [Google Scholar] [CrossRef] [PubMed]
- Köndgen, S.; Oh, D.-Y.; Thürmer, A.; Sedaghatjoo, S.; Patrono, L.V.; Calvignac-Spencer, S.; Biere, B.; Wolff, T.; Dürrwald, R.; Fuchs, S.; et al. A robust, scalable, and cost-efficient approach to whole genome sequencing of RSV directly from clinical samples. J. Clin. Microbiol. 2024, 62, e01111–e01123. [Google Scholar] [CrossRef]
- Sibert, B.S.; Kim, J.Y.; Yang, J.E.; Ke, Z.; Stobart, C.C.; Moore, M.L.; Wright, E.R. Assembly of respiratory syncytial virus matrix protein lattice and its coordination with fusion glycoprotein trimers. Nat. Commun. 2024, 15, 5923. [Google Scholar] [CrossRef]
- Ouyang, Y.; Liao, H.; Hu, Y.; Luo, K.; Hu, S.; Zhu, H. Innate immune evasion by human respiratory syncytial virus. Front. Microbiol. 2022, 13, 865592. [Google Scholar] [CrossRef] [PubMed]
- Millhouse, S.; Wang, X.; Fraser, N.W.; Faber, L.; Block, T.M. Direct evidence that HSV DNA damaged by ultraviolet (UV) irradiation can be repaired in a cell type-dependent manner. J. Neurovirology 2012, 18, 231–243. [Google Scholar] [CrossRef][Green Version]
- Canh, V.D.; Yasui, M.; Torii, S.; Oguma, K.; Katayama, H. Susceptibility of enveloped and non-enveloped viruses to ultraviolet light-emitting diode (UV-LED) irradiation and implications for virus inactivation mechanisms. Environ. Sci. Water Res. Technol. 2023, 9, 2283–2292. [Google Scholar] [CrossRef]
- McDevitt, J.J.; Lai, K.M.; Rudnick, S.N.; Houseman, E.A.; First, M.W.; Milton, D.K. Characterization of UVC light sensitivity of vaccinia virus. Appl. Environ. Microbiol. 2007, 73, 5760–5766. [Google Scholar] [CrossRef]
- Beck, S.E.; Wright, H.B.; Hargy, T.M.; Larason, T.C.; Linden, K.G. Action spectra for validation of pathogen disinfection in medium-pressure ultraviolet (UV) systems. Water Res. 2015, 70, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.I.; Davies, M.J. Actions of ultraviolet light on cellular structures. In Cancer: Cell Structures, Carcinogens and Genomic Instability; Springer: Berlin/Heidelberg, Germany, 2006; pp. 131–157. [Google Scholar] [CrossRef]
- Lo, C.-W.; Matsuura, R.; Iimura, K.; Wada, S.; Shinjo, A.; Benno, Y.; Nakagawa, M.; Takei, M.; Aida, Y. UVC disinfects SARS-CoV-2 by induction of viral genome damage without apparent effects on viral morphology and proteins. Sci. Rep. 2021, 11, 13804. [Google Scholar] [CrossRef] [PubMed]
- Hessling, M.; Haag, R.; Sieber, N.; Vatter, P. The impact of far-UVC radiation (200–230 nm) on pathogens, cells, skin, and eyes—a collection and analysis of a hundred years of data. GMS Hyg. Infect. Control 2021, 16, Doc07. [Google Scholar] [CrossRef] [PubMed]
- Simonet, J.; Gantzer, C. Inactivation of Poliovirus 1 and F-specific RNA Phages and Degradation of Their Genomes by UV Irradiation at 254 Nanometers. Appl. Environ. Microbiol. 2006, 72, 7671–7677. [Google Scholar] [CrossRef]
- Szeto, W.; Yam, W.C.; Huang, H.; Leung, D.Y.C. The efficacy of vacuum-ultraviolet light disinfection of some common environmental pathogens. BMC Infect. Dis. 2020, 20, 127. [Google Scholar] [CrossRef]
- Durbeej, B.; Eriksson, L.A. On the Formation of Cyclobutane Pyrimidine Dimers in UV-irradiated DNA: Why Are Thymines More Reactive? Photochem. Photobiol. 2003, 78, 159–167. [Google Scholar] [CrossRef]
- Wurtmann, E.J.; Wolin, S.L. RNA Under Attack: Cellular Handling of RNA Damage. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 34–49. [Google Scholar] [CrossRef]
- Bosshard, F.; Riedel, K.; Schneider, T.C.; Geiser, C.; Bucheli, M.; Egli, T. Protein oxidation and aggregation in UVA-irradiated Escherichia coli cells as signs of accelerated cellular senescence. Environ. Microbiol. 2010, 12, 2931–2945. [Google Scholar] [CrossRef]
- Santos, A.L.; Oliveira, V.; Baptista, I.; Henriques, I.; Gomes, N.C.M.; Almeida, A.; Correia, A.; Cunha, A. Wavelength dependence of biological damage induced by UV radiation on bacteria. Arch. Microbiol. 2013, 195, 63–74. [Google Scholar] [CrossRef]
- Lytle, C.D.; Jose-Luis Sagripanti, J.L. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J. Virol. 2005, 79, 14244–14252. [Google Scholar] [CrossRef] [PubMed]
- Longest, A.K.; Rockey, N.C.; Lakdawala, S.S.; Marr, L.C. Review of factors affecting virus inactivation in aerosols and droplets. J. R. Soc. Interface. 2024, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Raeiszadeh, M.; Adeli, B. A critical review on ultraviolet disinfection systems against COVID-19 outbreak: Applicability, validation, and safety considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef] [PubMed]


| Virus | Host Cells | ||||
|---|---|---|---|---|---|
| Envelope | Genome | Virus Strain | Provider | Cell Strain | Provider |
| Enveloped | ss-RNA | IAV strain A/Puerto Rico /8/1934 (H1N1) | Dr. Adachi 1 | MDCK | ATCC |
| HPAI strain A/crow /Kyoto/53/2004 (H5N1) | Dr. Nakaya 2 | MDCK | ATCC | ||
| SARS-CoV-2/Hu/DP /Kng/19-020 | KPIPH | Vero E6 /TMPRSS2 | JCRB | ||
| HCoV-OC43 | ATCC | Vero E6 /TMPRSS2 | JCRB | ||
| HCoV-229E | ATCC | HeLa /ACE2TMPRSS2 | JCRB | ||
| RSV strain long | ATCC | Hep-2 | JCRB | ||
| RSV strain CH18537 | ATCC | Hep-2 | JCRB | ||
| HMPV strain TN/83-1211 | BEI Resources | Hep-2 | JCRB | ||
| ds-DNA | HSV-1 strain KOS | ATCC | Vero | ATCC | |
| Non- enveloped | ss-RNA | FCV strain F-9 | ATCC | CRFK | ATCC |
| ds-DNA | AdV-5 strain Adenoid 75 | ATCC | Vero | ATCC | |
| Virus Strain | Peak Wavelength of LEDs (nm) | Fluence (mJ/cm2) | Reference | ||
|---|---|---|---|---|---|
| Log10 Infectivity Reduction | |||||
| −1 | −2 | −3 | |||
| Human coronavirus OC43 | 279 | 3.5 | 5.5 | 7.0 | Gerchman [40] |
| 281.3 | 2.7 | 5.2 | 7.7 | This study | |
| Human coronavirus 229E | 282 | 3.7 | 7.4 | 11.1 | Ma [16] |
| 281.3 | 3 | 5.0 | 9.7 | This study | |
| Severe acute respiratory syndrome coronavirus 2 | 282 | 1.9 | 3.8 | 5.7 | Ma [19] |
| 281.3 | 2.0 | 5.0 | 7.5 | This study | |
| Feline calicivirus | 281 | 9.0 | 18.9 | 28.9 | Oguma [35] |
| 281.3 | 8.8 | 20.6 | 30.0 | This study | |
| Virus Strain | Action Spectra for Viral Infectivity (Figure 2) | |||
|---|---|---|---|---|
| Action Spectra for Viral Genomes (Figure 4) | Action Spectra for Viral Proteins (Figure 6) | |||
| r | p | r | p | |
| Influenza A virus H1N1 | 0.966 | <0.001 | −0.319 | 0.312 |
| Severe acute respiratory syndrome coronavirus 2 | 0.959 | <0.001 | 0.750 | 0.008 |
| Respiratory syncytial virus strain long | 0.947 | <0.001 | −0.415 | 0.180 |
| Human metapneumovirus | 0.930 | <0.001 | − | − |
| Herpes simplex virus type 1 | –0.937 | <0.001 | −0.0736 | 0.820 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mawatari, K.; Kadomura-Ishikawa, Y.; Emoto, T.; Onoda, Y.; Ishida, K.; Toda, S.; Uebanso, T.; Aizawa, T.; Yamauchi, S.; Fujikawa, Y.; et al. Viral Inactivation by Light-Emitting Diodes: Action Spectra Reveal Genomic Damage as the Primary Mechanism. Viruses 2025, 17, 1065. https://doi.org/10.3390/v17081065
Mawatari K, Kadomura-Ishikawa Y, Emoto T, Onoda Y, Ishida K, Toda S, Uebanso T, Aizawa T, Yamauchi S, Fujikawa Y, et al. Viral Inactivation by Light-Emitting Diodes: Action Spectra Reveal Genomic Damage as the Primary Mechanism. Viruses. 2025; 17(8):1065. https://doi.org/10.3390/v17081065
Chicago/Turabian StyleMawatari, Kazuaki, Yasuko Kadomura-Ishikawa, Takahiro Emoto, Yushi Onoda, Kai Ishida, Sae Toda, Takashi Uebanso, Toshihiko Aizawa, Shigeharu Yamauchi, Yasuo Fujikawa, and et al. 2025. "Viral Inactivation by Light-Emitting Diodes: Action Spectra Reveal Genomic Damage as the Primary Mechanism" Viruses 17, no. 8: 1065. https://doi.org/10.3390/v17081065
APA StyleMawatari, K., Kadomura-Ishikawa, Y., Emoto, T., Onoda, Y., Ishida, K., Toda, S., Uebanso, T., Aizawa, T., Yamauchi, S., Fujikawa, Y., Tanaka, T., Li, X., Suarez-Lopez, E., Kuhn, R. J., Blatchley III, E. R., III, & Takahashi, A. (2025). Viral Inactivation by Light-Emitting Diodes: Action Spectra Reveal Genomic Damage as the Primary Mechanism. Viruses, 17(8), 1065. https://doi.org/10.3390/v17081065









