A Strand-Specific Quantitative RT-PCR Method for Detecting vRNA, cRNA, and mRNA of H7N9 Avian Influenza Virus in a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Facilities
2.2. Mouse Study
2.3. RNA Extraction
2.4. In Vitro Generation of RNA
2.5. Hot-Start Reverse Transcription with a Tagged Primer
2.6. TaqMan qRT-PCR
2.7. Data Acquisition and Analysis
3. Results
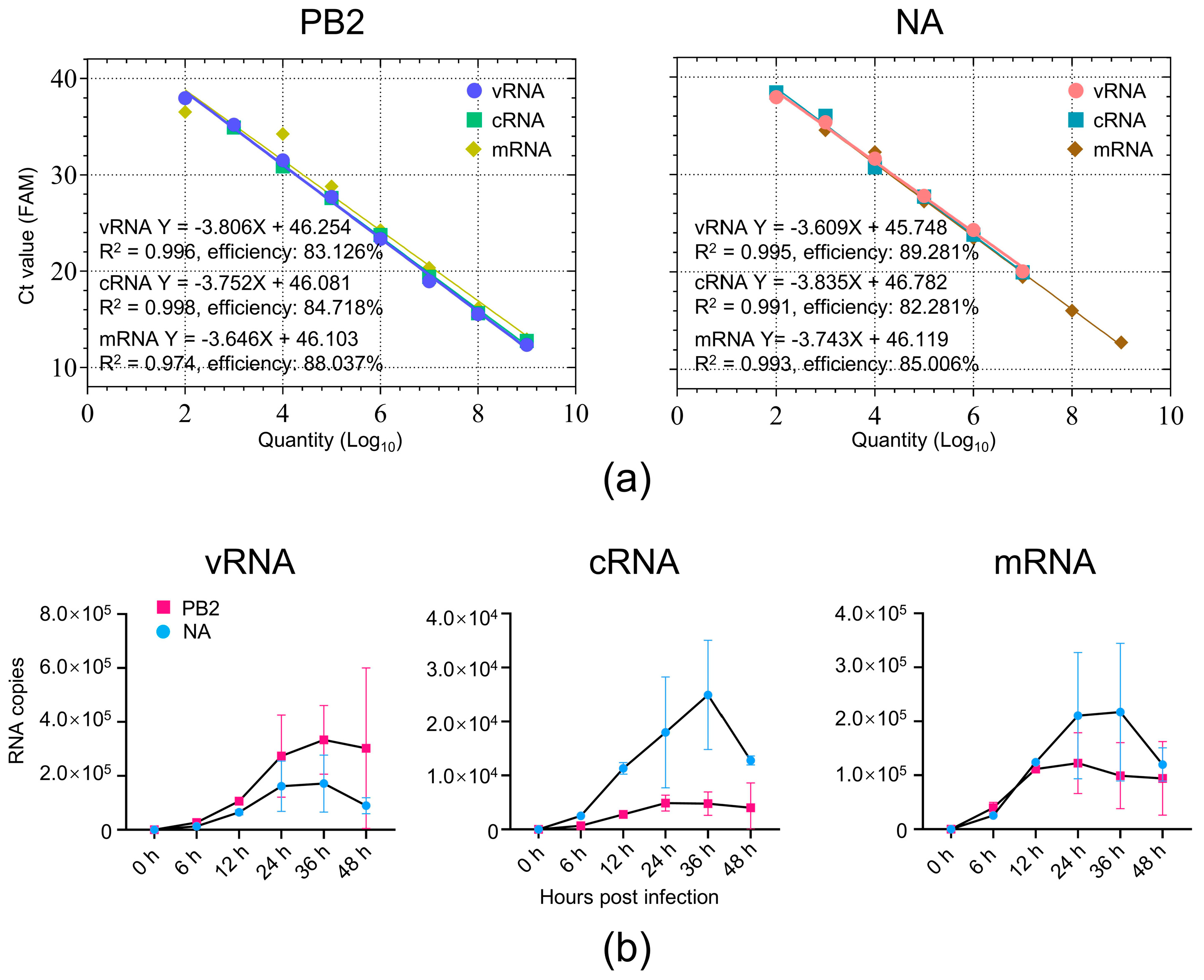
3.1. Detection of PB2 and NA vRNA, cRNA, and mRNA of H7N9 AIV in Mice
3.2. Absolute Quantification of Viral RNAs of H7N9 AIV in Mouse Lung Tissue
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, X.; Li, J.; Wang, Y.; Yu, X.; He, X.; Shi, J.; Deng, G.; Zeng, X.; Tian, G.; Li, Y.; et al. H7N9 virus infection triggers lethal cytokine storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells. Natl. Sci. Rev. 2022, 9, nwab137. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microbes Infect. 2023, 12, 2155072. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Deng, G.; Kong, H.; Gu, C.; Ma, S.; Yin, X.; Zeng, X.; Cui, P.; Chen, Y.; Yang, H.; et al. H7N9 virulent mutants detected in chickens in China pose an increased threat to humans. Cell Res. 2017, 27, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- de Jong, R.M.; Stockhofe-Zurwieden, N.; Verheij, E.S.; de Boer-Luijtze, E.A.; Ruiter, S.J.; de Leeuw, O.S.; Cornelissen, L.A. Rapid emergence of a virulent PB2 E627K variant during adaptation of highly pathogenic avian influenza H7N7 virus to mice. Virol. J. 2013, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Fukuyama, S.; Yamada, S.; Zhao, D.; Murakami, S.; Uraki, R.; Watanabe, T.; Tomita, Y.; Neumann, G.; Kawaoka, Y. Amino acids substitutions in the PB2 protein of H7N9 influenza A viruses are important for virulence in mammalian hosts. Sci. Rep. 2015, 5, 8039. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; An, S.H.; Kim, I.; Go, D.M.; Kim, D.Y.; Choi, J.G.; Lee, Y.J.; Kim, J.H.; Kwon, H.J. Prerequisites for the acquisition of mammalian pathogenicity by influenza A virus with a prototypic avian PB2 gene. Sci. Rep. 2017, 7, 10205. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Peng, O.; Shen, X.; Gong, L.; Xue, C.; Cao, Y. Multiple amino acid substitutions involved in the adaption of three avian-origin H7N9 influenza viruses in mice. Virol. J. 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Li, J.; Zou, R.; Pan, J.; Jin, T.; Li, L.; Liu, P.; Zhao, Y.; Yu, X.; Wang, H.; et al. Dynamic PB2-E627K substitution of influenza H7N9 virus indicates the in vivo genetic tuning and rapid host adaptation. Proc. Natl. Acad. Sci. USA 2020, 117, 23807–23814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, L.; Yan, Z.; Gan, T.; Li, L.; Chen, R.; Chen, R.; Zheng, Z.; Hong, W.; Wang, J.; et al. Dual E627K and D701N mutations in the PB2 protein of A(H7N9) influenza virus increased its virulence in mammalian models. Sci. Rep. 2015, 5, 14170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, A.; Wan, Y.; Liu, X.; Qiu, C.; Xi, X.; Ren, Y.; Wang, J.; Dong, Y.; Bao, M.; et al. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc. Natl. Acad. Sci. USA 2014, 111, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Guo, J.; Li, L.; Chang, C.; Li, Y.; Bian, C.; Xu, K.; Chen, H.; Sun, B. The PB2 E627K mutation contributes to the high polymerase activity and enhanced replication of H7N9 influenza virus. J. Gen. Virol. 2014, 95, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Deng, G.; Ma, S.; Zeng, X.; Yin, X.; Li, M.; Zhang, B.; Cui, P.; Chen, Y.; Yang, H.; et al. Rapid Evolution of H7N9 Highly Pathogenic Viruses that Emerged in China in 2017. Cell Host Microbe 2018, 24, 558–568.e7. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Revol, R.; Ostbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.R.; Wilson, G.M.; Coon, J.J.; Mehle, A. Post-Translation Regulation of Influenza Virus Replication. Annu. Rev. Virol. 2020, 7, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Carter, T.; Iqbal, M. The Influenza A Virus Replication Cycle: A Comprehensive Review. Viruses 2024, 16, 316. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, E.; Watanabe, T.; Fujii, K.; Goto, H.; Watanabe, S.; Noda, T.; Kawaoka, Y. Strand-specific real-time RT-PCR for distinguishing influenza vRNA, cRNA, and mRNA. J. Virol. Methods. 2011, 173, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.; Fay, E.J.; Lee, Z.; Aron, S.; Hu, W.S.; Langlois, R.A. Segment-specific kinetics of mRNA, cRNA and vRNA accumulation during influenza infection. J. Virol. 2021, 95, e02102-20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, J.; Deng, G.; Guo, J.; Zeng, X.; He, X.; Kong, H.; Gu, C.; Li, X.; Liu, J.; et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 2013, 341, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Deng, G.; Cui, P.; Zeng, X.; Li, B.; Wang, D.; He, X.; Yan, C.; Zhang, Y.; Li, J.; et al. Evolution of H7N9 highly pathogenic avian influenza virus in the context of vaccination. Emerg. Microbes Infect. 2024, 13, 2343912. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, Z.; Shi, J.; Deng, G.; Kong, H.; Tao, S.; Li, C.; Liu, L.; Guan, Y.; Chen, H. Glycine at Position 622 in PB1 Contributes to the Virulence of H5N1 Avian Influenza Virus in Mice. J. Virol. 2016, 90, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.L.; Bui, C.T.; Mok, C.K.; Ng, M.M.; Nicholls, J.M.; Peiris, J.S.; Chan, M.C.; Chan, R.W. Evaluation of the human adaptation of influenza A/H7N9 virus in PB2 protein using human and swine respiratory tract explant cultures. Sci. Rep. 2016, 6, 35401. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; An, S.H.; Choi, J.G.; Lee, Y.J.; Kim, J.H.; Kwon, H.J. Rank orders of mammalian pathogenicity-related PB2 mutations of avian influenza A viruses. Sci. Rep. 2020, 10, 5359. [Google Scholar] [CrossRef] [PubMed]
- Frensing, T.; Kupke, S.Y.; Bachmann, M.; Fritzsche, S.; Gallo-Ramirez, L.E.; Reichl, U. Influenza virus intracellular replication dynamics, release kinetics, and particle morphology during propagation in MDCK cells. Appl. Microbiol. Biotechnol. 2016, 100, 7181–7192. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.A.; Haddock, L.A., 3rd; Braun, K.M.; Meliopoulos, V.; Livingston, B.; Honce, R.; Schaack, G.A.; Boehm, E.; Higgins, C.A.; Barry, G.L.; et al. Influenza A virus undergoes compartmentalized replication in vivo dominated by stochastic bottlenecks. Nat. Commun. 2022, 13, 3416. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Shi, J.; Wang, C.; Zhang, Y.; Xing, X.; Kong, H.; Yan, C.; Zeng, X.; Liu, L.; Tian, G.; et al. Global dissemination of H5N1 influenza viruses bearing the clade 2.3.4.4b HA gene and biologic analysis of the ones detected in China. Emerg. Microbes Infect. 2022, 11, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Wang, G.; Wang, Y.-h.; Liu, X.; Li, M.; Kong, H.; Chen, H.; Jiang, L.; Li, C. A Strand-Specific Quantitative RT-PCR Method for Detecting vRNA, cRNA, and mRNA of H7N9 Avian Influenza Virus in a Mouse Model. Viruses 2025, 17, 1007. https://doi.org/10.3390/v17071007
Wang B, Wang G, Wang Y-h, Liu X, Li M, Kong H, Chen H, Jiang L, Li C. A Strand-Specific Quantitative RT-PCR Method for Detecting vRNA, cRNA, and mRNA of H7N9 Avian Influenza Virus in a Mouse Model. Viruses. 2025; 17(7):1007. https://doi.org/10.3390/v17071007
Chicago/Turabian StyleWang, Bo, Guangwen Wang, Yi-han Wang, Xuwei Liu, Manman Li, Huihui Kong, Hualan Chen, Li Jiang, and Chengjun Li. 2025. "A Strand-Specific Quantitative RT-PCR Method for Detecting vRNA, cRNA, and mRNA of H7N9 Avian Influenza Virus in a Mouse Model" Viruses 17, no. 7: 1007. https://doi.org/10.3390/v17071007
APA StyleWang, B., Wang, G., Wang, Y.-h., Liu, X., Li, M., Kong, H., Chen, H., Jiang, L., & Li, C. (2025). A Strand-Specific Quantitative RT-PCR Method for Detecting vRNA, cRNA, and mRNA of H7N9 Avian Influenza Virus in a Mouse Model. Viruses, 17(7), 1007. https://doi.org/10.3390/v17071007






