Non-Influenza and Non-SARS-CoV-2 Viruses Among Patients with Severe Acute Respiratory Infections in Tanzania: A Post-COVID-19 Pandemic Snapshot
Abstract
1. Introduction
2. Materials and Methods
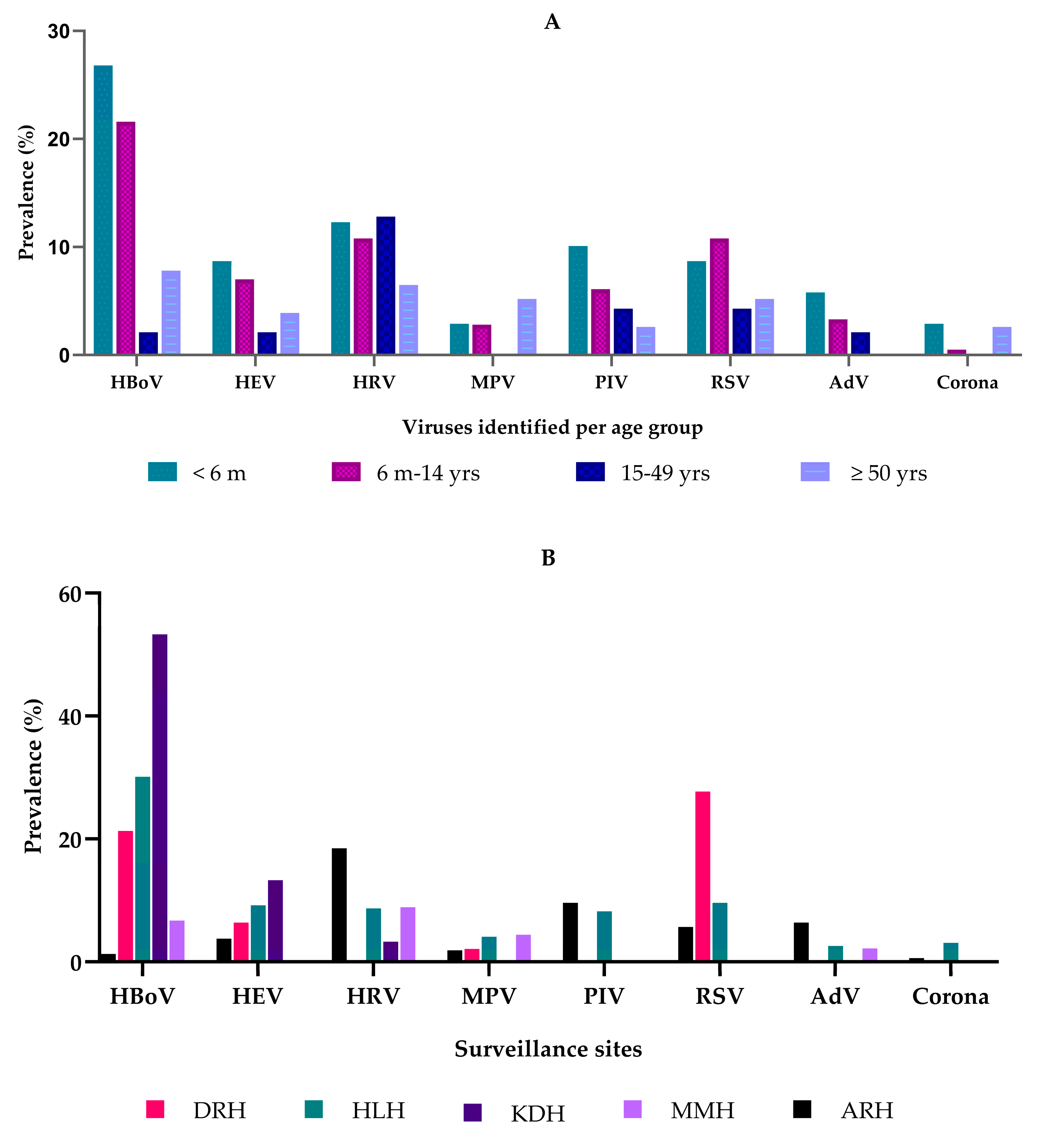
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okesanya, O.J.; Manirambona, E.; Buban, J.M.A.; Olaleke, N.O.; Lucero-Prisno, D.E. Coronavirus Disease 2019 emergency is over but the pandemic is not: Implications for a new global order. Int. J. Surg. Glob. Health 2023, 6, e0207. [Google Scholar] [CrossRef]
- Sarker, R.; Roknuzzaman, A.S.M.; Hossain, M.J.; Islam, M.R. Benefits and probable ill effects of WHO’s declaration of end of COVID-19 pandemic: A way back to pandemic-free normal life. Ann. Med. Surg. 2023, 85, 3199–3201. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.B.; Mahé, C.; Chaves, S.S. Uncertain effects of the pandemic on respiratory viruses. Science 2021, 372, 1043–1044. [Google Scholar] [CrossRef]
- Groves, H.E.; Piché-Renaud, P.P.; Peci, A.; Farrar, D.S.; Buckrell, S.; Bancej, C.; Sevenhuysen, C.; Campigotto, A.; Gubbay, J.B.; Morris, S.K. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Reg. Health Am. 2021, 1, 100015. [Google Scholar] [CrossRef]
- Han, P.; Shen, K. Covid lockdown and repaying the immunity debt in children. Glob. Pediatr. 2024, 9, 100195. [Google Scholar] [CrossRef]
- Munro, A.P.S.; House, T. Cycles of Susceptibility: Immunity Debt Explains Altered Infectious Disease Dynamics Post-Pandemic. Clin. Infect. Dis. 2024, ciae493. [Google Scholar] [CrossRef]
- McCabe, R.; Whittaker, C.; Sheppard, R.J.; Abdelmagid, N.; Ahmed, A.; Alabdeen, I.Z.; Brazeau, N.F.; Ahmed Abd Elhameed, A.E.; Bin-Ghouth, A.S.; Hamlet, A.; et al. Alternative epidemic indicators for COVID-19 in three settings with incomplete death registration systems. Sci. Adv. 2023, 9, eadg7676. [Google Scholar] [CrossRef] [PubMed]
- Tessema, G.A.; Kinfu, Y.; Dachew, B.A.; Tesema, A.G.; Assefa, Y.; Alene, K.A.; Aregay, A.F.; Ayalew, M.B.; Bezabhe, W.M.; Bali, A.G.; et al. The COVID-19 pandemic and healthcare systems in Africa: A scoping review of preparedness, impact and response. BMJ Glob. Health 2021, 6, e007179. [Google Scholar] [CrossRef]
- Kelly, M.E.; Gharpure, R.; Shivji, S.; Matonya, M.; Moshi, S.; Mwafulango, A.; Mwalongo, V.; Mghamba, J.; Simba, A.; Balajee, S.A.; et al. Etiologies of influenza-like illness and severe acute respiratory infections in Tanzania, 2017–2019. PLoS Glob. Public. Health 2023, 3, e0000906. [Google Scholar] [CrossRef]
- Fitzner, J.; Qasmieh, S.; Mounts, A.W.; Alexander, B.; Besselaar, T.; Briand, S.; Brown, C.; Clark, S.; Dueger, E.; Gross, D.; et al. Revision of clinical case definitions: Influenza-like illness and severe acute respiratory infection. Bull. World Health Organ. 2018, 96, 122. [Google Scholar] [CrossRef]
- Han, T.; Wang, Y.; Zhang, D.; Li, Y.; Zhang, L.; Yan, J.; Li, C.; Yang, S.; Guo, L.; Yan, H. Changes in infant respiratory pathogens pre-during, and post-COVID-19 non-pharmacological interventions in Beijing. Ital. J. Pediatr. 2025, 51, 8. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Z.; Jiang, M.; Lu, Q.; Tu, Y. The circulating characteristics of common respiratory pathogens in Ningbo, China, both before and following the cessation of COVID-19 containment measures. Dent. Sci. Rep. 2024, 14, 25876. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.P.; Zhao, Y.N.; Wang, Z.K.; Wang, M.Z.; Li, R.; Zhang, J.; Li, N.; Zhang, Z.F.; Rong, R.J.; Sun, Y.C.; et al. Prevalence of respiratory pathogens among hospitalised patients with acute respiratory infection during and after the COVID-19 pandemic in Shijiazhuang, China. Front. Cell Infect. Microbiol. 2024, 14, 1486953. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, Y.; Wang, J.; Li, Y.; Wang, Y.; Gao, Y.; Zhao, M.; Zhao, M.; Tan, H.; Tie, Y.; et al. Epidemiology of respiratory pathogens in patients with acute respiratory infections during the COVID-19 pandemic and after easing of COVID-19 restrictions. Microbiol. Spectr. 2024, 12, e0116124. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-I.; Hsueh, P.-R.; Chuang, J.-H.; Liu, M.T. Changing epidemic patterns of infectious diseases during and after COVID-19 pandemic in Taiwan. J. Microbiol. Immunol. Infect. 2024, 57, 685–690. [Google Scholar] [CrossRef]
- Park, M.; Choi, W.S.; Cowling, B. Shifts in patterns in influenza virus and RSV infections in Korea after the COVID-19 pandemic resulting from immunity debt (Preprint). JMIR Public Health Surveill. 2025, 68058. [Google Scholar] [CrossRef]
- Trapani, S.; Caporizzi, A.; Ricci, S.; Indolfi, G. Human Bocavirus in Childhood: A True Respiratory Pathogen or a “Passenger” Virus? A Comprehensive Review. Microorganisms 2023, 11, 1243. [Google Scholar] [CrossRef]
- Oldhoff, E.; Bennet, R.; Eriksson, M.; Allander, T. Human bocavirus 1 epidemiology in children in relation to virus load and codetection. Acta Paediatr. 2023, 112, 1049–1055. [Google Scholar] [CrossRef]
- Christensen, A.; Nordbø, S.A.; Krokstad, S.; Rognlien, A.G.; Døllner, H. Human bocavirus in children: Mono-detection, high viral load and viraemia are associated with respiratory tract infection. J. Clin. Virol. 2010, 49, 158. [Google Scholar] [CrossRef]
- Christensen, A.; Nordbø, S.A.; Krokstad, S.; Rognlien, A.G.; Døllner, H. Human bocavirus commonly involved in multiple viral airway infections. J. Clin. Virol. 2008, 41, 34–37. [Google Scholar] [CrossRef]
- Subissi, L.; Bossuyt, N.; Reynders, M.; Gérard, M.; Dauby, N.; Lacor, P.; Daelemans, S.; Lissoir, B.; Holemans, X.; Magerman, K.; et al. Spotlight influenza: Extending influenza surveillance to detect non-influenza respiratory viruses of public health relevance: Analysis of surveillance data; Belgium, 2015 to 2019. Euro Surveill. 2021, 26, 2001104. [Google Scholar] [CrossRef]
- Kara, Y.; Kizil, M.C.; Arslanoglu, M.O.; Kacmaz, E.; Dalokay, N.; Pala, E.; Kiral, E.; Bozan, G.; Us, T.; Kiliç, O.; et al. Unexpected Severe Bocavirus Infections among Hospitalized Children during the COVID-19 Pandemic. J. Pediatr. Infect. Dis. 2022, 18, 199–205. [Google Scholar] [CrossRef]
- Kume, Y.; Hashimoto, K.; Chishiki, M.; Norito, S.; Suwa, R.; Ono, T.; Mochizuki, I.; Mashiyama, F.; Ishibashi, N.; Suzuki, S.; et al. Changes in virus detection in hospitalized children before and after the severe acute respiratory syndrome coronavirus 2 pandemic. Influenza Other Respir. Viruses 2022, 16, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Silva, C.; Vilares, A.T.; Schweitzer, V.; Castanhinha, S.; Martins, A.; Lopes, M.J.; Ascoli-Bartoli, T.; Canelas, G.; Keir, H.R.; Cunha, F.; et al. Non-COVID-19 respiratory viral infection. Breathe 2022, 18, 210151. [Google Scholar] [CrossRef] [PubMed]
- Mijač, M.; Ljubin-Sternak, S.; Ivković-Jureković, I.; Vraneš, J. Comparison of MT-PCR with Quantitative PCR for Human Bocavirus in Respiratory Samples with Multiple Respiratory Viruses Detection. Diagnostics. 2023, 13, 846. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L. Research progress of human bocavirus infection in children. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2023, 35, 548–553. [Google Scholar]
- Srikantiah, P.; Vora, P.; Klugman, K.P. Assessing the Full Burden of Respiratory Syncytial Virus in Young Infants in Low- and Middle-Income Countries: The Importance of Community Mortality Studies. Clin. Infect. Dis. 2021, 73, S177–S179. [Google Scholar] [CrossRef]
| Variables | Total Cases (N = 475) | Negative Cases (256; 53.9%) | Cases with ≥1 Virus Detected (N = 219; 46%) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Age group | ||||||
| <6 months | 138 | 29 | 60 | 23.4 | 78 | 35.6 |
| 6 months–14 years | 213 | 44.8 | 106 | 41.4 | 107 | 48.9 |
| 15 years–49 years | 47 | 9.9 | 35 | 13.7 | 12 | 5.5 |
| ≥50 years | 77 | 16.2 | 55 | 21.5 | 22 | 10 |
| Sex | ||||||
| Male | 254 | 53.5 | 144 | 56.2 | 110 | 50.2 |
| Female | 221 | 46.5 | 112 | 43.7 | 109 | 49.8 |
| Presence of comorbidity | ||||||
| Any comorbidities | 51 | 10.7 | 37 | 14.4 | 14 | 6.4 |
| Diabetes | 3 | 0.6 | 2 | 0.8 | 1 | 0.5 |
| Heart disease (excluding hypertension) | 27 | 5.7 | 22 | 8.6 | 5 | 2.3 |
| Hypertension | 27 | 5.7 | 21 | 8.2 | 6 | 2.7 |
| HIV | 10 | 2.1 | 7 | 2.7 | 3 | 1.4 |
| Asthma | 3 | 0.6 | 1 | 0.4 | 2 | 0.9 |
| Facility | ||||||
| Arusha Regional Hospital (ARH) | 157 | 33 | 97 | 37.9 | 60 | 27.4 |
| Dodoma Regional Hospital (DRH) | 47 | 9.9 | 24 | 9.4 | 23 | 10.5 |
| Hydom Lutheran Hospital (HLH) | 196 | 41.3 | 87 | 34.0 | 109 | 49.8 |
| Kibondo District Hospital (KDH) | 30 | 6.3 | 11 | 4.3 | 19 | 8.7 |
| Mwananyamala District Hospital (MMH) | 45 | 9.5 | 37 | 14.4 | 8 | 3.7 |
| Month of Hospitalization | ||||||
| March | 180 | 37.9 | 105 | 41.0 | 75 | 34.2 |
| April | 175 | 36.8 | 79 | 30.9 | 96 | 43.8 |
| May | 120 | 25.3 | 72 | 28.1 | 48 | 21.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, M.E.; Msafiri, F.; Averhoff, F.; Danda, J.; Landay, A.; Simba, A.; Mwafulango, A.E.; Mosha, S.; Magesa, A.; Mmbaga, V.; et al. Non-Influenza and Non-SARS-CoV-2 Viruses Among Patients with Severe Acute Respiratory Infections in Tanzania: A Post-COVID-19 Pandemic Snapshot. Viruses 2025, 17, 1042. https://doi.org/10.3390/v17081042
Kelly ME, Msafiri F, Averhoff F, Danda J, Landay A, Simba A, Mwafulango AE, Mosha S, Magesa A, Mmbaga V, et al. Non-Influenza and Non-SARS-CoV-2 Viruses Among Patients with Severe Acute Respiratory Infections in Tanzania: A Post-COVID-19 Pandemic Snapshot. Viruses. 2025; 17(8):1042. https://doi.org/10.3390/v17081042
Chicago/Turabian StyleKelly, Maria Ezekiely, Frank Msafiri, Francisco Averhoff, Jane Danda, Alan Landay, Azma Simba, Ambele Elia Mwafulango, Solomoni Mosha, Alex Magesa, Vida Mmbaga, and et al. 2025. "Non-Influenza and Non-SARS-CoV-2 Viruses Among Patients with Severe Acute Respiratory Infections in Tanzania: A Post-COVID-19 Pandemic Snapshot" Viruses 17, no. 8: 1042. https://doi.org/10.3390/v17081042
APA StyleKelly, M. E., Msafiri, F., Averhoff, F., Danda, J., Landay, A., Simba, A., Mwafulango, A. E., Mosha, S., Magesa, A., Mmbaga, V., & Chaves, S. S. (2025). Non-Influenza and Non-SARS-CoV-2 Viruses Among Patients with Severe Acute Respiratory Infections in Tanzania: A Post-COVID-19 Pandemic Snapshot. Viruses, 17(8), 1042. https://doi.org/10.3390/v17081042






