Abstract
The rise in antibiotic-resistant bacteria has made the management of bacterial diseases increasingly challenging. As a result, bacteriophages have gained attention as a promising alternative to antibiotics for combating bacterial pathogens. However, the usage of phages as biocontrol agents faces many challenges, including environmental stability, delivery efficiency, host specificity, and potential bacterial resistance. Advancements in genetic engineering and nanotechnology have been explored to enhance the stability, efficacy, and adaptability of phage-based treatments. In this review, we discuss the key barriers to the effective implementation of phage therapy and highlight innovative strategies to overcome these challenges. By addressing these limitations, this review aims to provide insights into optimizing phage-based approaches for widespread therapeutic and biocontrol applications.
1. Introduction
Bacteriophages were first independently characterized by Felix d’Herrelle and Frederick Twort (Figure 1) over a century ago [1,2]. The name, coined by d’Herelle, originates from bacteriophage, meaning ‘bacteria-eater,’ derived from the Greek phago, which means ‘to eat’ or ‘to devour’ [1]. Since then, their potential applications have remained a subject of ongoing scientific investigation and discourse (Figure 1). Due to their potent antibacterial properties, phages were rapidly adopted to prevent and treat both plant and human infections (a clinical approach commonly called ‘bacteriophage therapy’ or ‘phage therapy’) shortly after their discovery. Phage therapy emerged as a promising approach for combating bacterial diseases in the early 20th century, particularly in hot and humid climates, where such diseases often caused devastating crop losses [3,4,5].
The first bacteriophage patent for control of bacterial plant diseases was granted to L. R. E. Jackson [6] and identified broad-range viral h-mutants as effective phages against plant pathogenic bacteria, aiding in the prevention and control of plant diseases and ice nucleation (Figure 1). Several phage-based products have since received approval from the U.S. Environmental Protection Agency (EPA). The first registered product for the use of bacteriophages for control of bacterial plant diseases, AgriphageTM, targets Xanthomonas campestris pv. vesicatoria and Pseudomonas syringae pv. tomato on peppers and tomatoes [7]. Another notable example is a phage cocktail developed to combat Pierce’s disease in grapevines caused by Xylella fastidiosa subsp. fastidiosa [8]. However, despite these advancements, field studies often report inconsistent results due to environmental challenges such as weather variability, as well as the need to optimize delivery methods and timing for effective biocontrol. To address these challenges, standardized protocols and strategies have been recommended to improve the reliability and efficacy of phage-based applications [9,10]. An interesting project, Xylencer, from Wageningen University leverages synthetic biology and bacteriophages to target Xylella fastidiosa and has shown promising results [11]. Additionally, growing regulatory restrictions on agricultural antibiotic use have heightened interest in utilizing bacteriophages to manage antimicrobial resistance in agriculture.

Figure 1.
Chronological events in the use of bacteriophages for control of plant diseases (1917–2024). a Twort first independently characterized bacteriophage in 1915. In 1917, Felix d’Herelle independently discovered bacteriophage and described the lytic principle. b Mallmann and Hemstreet showed that a filtrate from rotting cabbage (containing phages) could inhibit the black rot pathogen Xanthomonas campestris pv. campestris. c Kotila and Coons [12] demonstrated that phages could prevent soft rot in potato and carrot slices caused by Pectobacterium species (then Bacillus carotovorus), indicating phage potential to curb post-harvest rot. d Thomas [13] conducted the first phage field trials by treating corn seeds with phage to control Stewart’s wilt (caused by Pantoea stewartii). The phage treatment cut disease incidence from 18% in controls to ~1.5%, a remarkable early success. e L.R.E. Jackson discovered “viral h-mutants”–phage mutants with broadened host range, that could kill plant-pathogenic Pseudomonas syringae (cause of bean halo blight, etc.), including strains resistant to parent phages. This work led to a patent in 1989 describing mixtures of such host-range mutant phages to prevent bacterial plant diseases and frost injury. f Flaherty et al. [14] published a study evaluating host-range mutant (h-mutant) bacteriophage cocktails to control bacterial spot (Xanthomonas campestris pv. vesicatoria) on tomato. In both greenhouse and field conditions (1997–1998), phage treatments significantly reduced disease incidence and improved plant vigor. The phage-treated plants showed 17–25% reduction in disease severity and up to 24% increase in extra-large fruit yield compared to untreated controls, outperforming traditional copper-based bactericides. This work was one of the earliest and most compelling demonstrations of phage cocktail efficacy in real-world crop production. g Balogh et al. [15] used phage protective formulations found to reduce bacterial spot disease in greenhouse and field, and application of bacteriophages in the evening to improve disease control. h AgriPhage received full U.S. Environmental Protection Agency approval, making it the first bacteriophage-based pesticide for plant disease. Developed by Omnilytics Inc., AgriPhage initially targeted bacterial spot and speck in tomatoes and peppers (Xanthomonas campestris pv. vesicatoria and Pseudomonas syringae pv. tomato). This milestone marked the transition of phage therapy from lab/field trials to a regulated commercial agricultural product. i Researchers began demonstrating that mixtures of phages (cocktails) improve efficacy and overcome the narrow host range of single phages. For example, in Florida, Balogh et al. [16] applied a four-phage cocktail (formulated in a protective skim-milk carrier) to citrus seedlings and achieved ~50–60% reduction in disease severity for citrus canker and bacterial spot. This was among the first modern studies showing that phage cocktails can effectively suppress plant diseases in greenhouse conditions. j Chan et al. [17] and others formally proposed phage cocktails as a necessity for robust biocontrol, especially in heterogeneous field environments where a single phage often can’t infect all pathogenic strains. k A breakthrough in managing Pierce’s disease of grapevines (caused by Xylella fastidiosa) was reported by Das et al. [8]. A cocktail of four lytic phages was injected into diseased grapevines, significantly reducing Xylella levels and halting disease progression. This study demonstrated phage cocktails as a viable strategy against a lethal, systemic plant disease, garnering wide attention. l The first bacteriophage products for agriculture emerged in Europe. In Hungary, an “Erwiphage™ Plus” cocktail targeting fire blight (Erwinia amylovora) was released in 2018 [18]. Around the same time, a UK company (APS Biocontrol) introduced Biolyse®, a phage-based solution to prevent soft rot in stored potatoes [19]. These mark the entry of phage biocontrol into the European market, despite regulatory hurdles. m Multiple field trials in the late 2010s validated that phage cocktails can control plant pathogens under real-world conditions. For example, in 2019, Wang et al. [20] showed an 80% reduction of tomato wilt caused by Ralstonia solanacearum in field plots using a four-phage cocktail. Similarly, Carstens et al. [21] showed that a six-phage mix against potato soft rot/blackleg (Pectobacterium atrosepticum) cut disease incidence by ~60%. These studies confirmed that carefully formulated cocktails can significantly reduce plant disease in both greenhouse and field settings. n In 2024 the “Xylencer” project (Wageningen University) engineered phages to combat Xylella in olive trees, showing promising results against this quarantine pathogen.
1.1. What Are Bacteriophages?
Bacteriophages, commonly referred to as phages, are viruses that specifically target bacterial hosts and exhibit typical viral characteristics. They cannot replicate independently and must rely on a bacterial host for reproduction. Phages are believed to be among the oldest known biological entities, dating back approximately 3 billion years [22]. They are also the most abundant organisms in the world, with ocean water containing approximately 107 phage particles per milliliter and the biosphere harboring an estimated ∼1031 virus-like particles, outnumbering bacteria by a factor of ten [23,24].
Phages typically measure between 50 and 200 nm and carry genetic instructions that facilitate rapid and efficient replication. The ability of a bacteriophage strain to infect different bacterial strains defines its host range and varies widely [25]. Some phages exhibit a narrow host range, infecting a few bacterial strains, while others have a broad host range, capable of infecting multiple strains within a bacterial species or different bacterial species within the same genus or even across different genera [25,26,27,28]. Cross-genera infection is rare and is usually limited to closely related bacteria. Host range plays a pivotal role in determining the efficacy of phage-based applications [25].
Despite their microscopic size, phages are visible under electron microscopy, showcasing a diversity of shapes and structures. The majority of environmental phages belong to the order Caudovirales, which are characterized by double-stranded DNA (dsDNA) and tails. Morphologically, these phages are classified into three main types based on their tail structures: Siphoviridae (long, non-contractile tails), Myoviridae (contractile tails consisting of a sheath and a central tube), and Podoviridae (short, non-contractile tails) (Table 1) [29,30].

Table 1.
Summary of phages and their usage against plant bacterial diseases.
1.2. Mechanism of Lytic Phages
Most bacteriophages used in phage therapy predominantly follow a lytic lifecycle (Figure 2), characterized by the rapid infection and destruction of host bacterial cells [66]. The process begins with the phage attaching to specific receptors on the host cell surface in two distinct stages: an initial reversible interaction followed by irreversible binding. Once firmly attached, the phage employs lytic enzymes to degrade the host cell wall, facilitating the injection of its genetic material into the host cytoplasm [67]. Inside the host, the phage genome takes control of cellular processes, redirecting them from their normal functions to support viral replication and protein synthesis. This includes degradation of the host genome, replication of phage DNA, and production of viral proteins, including capsids. These components are then assembled into mature phage particles. Finally, lysis of the host cell occurs, driven by key phage proteins. Holins disrupt the host cell membrane, creating pores, while endolysins degrade the peptidoglycan layer of the cell wall [68]. This compromise of membrane integrity and loss of selective permeability causes osmotic imbalance, leading to cell lysis. The newly formed phage particles are subsequently released to infect other host cells, perpetuating the lytic cycle [69].

Figure 2.
Lytic lifecycle of bacteriophage. It begins with specific recognition of a susceptible bacterial host, followed by adsorption to the bacterial surface. Phages are strain-specific—although multiple phages may be present in the environment, only those capable of recognizing a given bacterial strain can bind to it. (Note that the red-colored phage particle attaches to the receptor on the bacterial cell, while the blue-colored phage particle does not attach). Upon successful adsorption, the phage injects its nucleic acid into the host cell, initiating replication of the phage genome and synthesis of viral components. These components are assembled into mature virions, which are then released through lysis of the bacterial cell. The released phages can subsequently infect new bacterial hosts.
While lytic phages are typically favored in phage therapy for their immediate bactericidal effect, some lytic phages may be temperate and capable of entering a lysogenic lifecycle under certain conditions. This is a concern, as temperate phages integrate into the host genome and replicate alongside the bacterium, often without destroying it. Temperate phages pose several risks: they can carry toxin genes or pathogenicity islands that enhance bacterial virulence, participate in generalized or specialized transduction that facilitates horizontal gene transfer, and confer superinfection immunity to the lysogenized host, making it resistant to further phage infection [70]. These features can undermine the therapeutic efficacy of phage therapy and potentially worsen clinical outcomes. Thus, obligately lytic phage, those that do not undergo lysogeny, are preferred for clinical applications. The presence of lysogeny in therapeutic phages must therefore be carefully screened and avoided to mitigate these risks [71].
2. A Renewed Interest in Phage Therapy
The resurgence of interest in phage therapy is largely driven by concerns over rising antibiotic resistance in an era where traditional antibiotics are becoming less effective [72,73]. While a few phage-based treatments have been developed, their widespread clinical use remains limited. The growing resistance to antibiotics primarily stems from their excessive and improper use, creating selective pressures that drive bacterial adaptation [74].
Despite their potential for treating bacterial infections, phage therapy has yet to see widespread adoption. What are the key obstacles preventing its success, and can they be overcome? In this review, we explore the challenges hindering effective therapeutic use of phages and evaluate their prospects for future development.
2.1. Phage Survival and Persistence
Phage viability can be affected by exposure to a variety of environmental parameters, with the most detrimental parameter being ultraviolet (UV) radiation. UV radiation, particularly UV-B (280–315 nm), has been shown to cause significant damage to the DNA of bacteriophages, thereby impairing their ability to replicate [75,76]. Ozone depletion has led to an increase in UV-B intensity, with each 1% reduction in ozone concentration resulting in a 1.2% increase in UV-B levels. According to a 1994 report from the Palmer Station in Antarctica, seasonal stratospheric ozone depletion increased the UV-B radiation reaching terrestrial Antarctic habitats by up to 50%. This was also accompanied by shorter bursts of UV-B penetrating throughout the rest of the atmosphere [77]. An infective phage must contend with this increased UV-B exposure when it is applied to a potential host. UV-C radiation can have similar effects as UV-B, but it is thought to also affect phage adsorption to the host cells [78]. Overall, though, UV exposure of any kind generally reduces bacteriophage longevity. For instance, Erwinia amylovora phage Y2 exhibited a 99.79% reduction in PFUs after 5 min of UV exposure [79], while Dickeya solani phages became undetectable after 10 min. Similarly, the population of Xanthomonas axonopodis pv. citrumelo phage declined from 106 to 0 PFUs within 4 h when sprayed on tomato leaves [80].
To mitigate the adverse effects of UV radiation, phages can be applied during evening hours [10]. Studies have demonstrated that phage application after sunset significantly extends their persistence within the phyllosphere, providing an extended window for bacterial infection and control [81]. This strategy underscores the importance of optimizing phage application timing to maximize their efficacy in agricultural systems. Mixing bacteriophage suspensions with various compounds (i.e., formulations) has been used to protect phage longevity upon UV exposure. Balogh et al. [15] demonstrated increased phage longevity and efficacy when using formulations containing (i) pregelatinized corn flour (PCF) and sucrose; (ii) casecrete, sucrose, and PCF; and (iii) skim milk and sucrose (M + S). Another study investigated using readily available and natural sources as protective formulations for infective phages [82]. These formulations included extracts from carrot, red pepper, beetroot, casein, and soy peptone, as well as astaxanthin, aromatic amino acids, and Tween 80. All these compounds were found to significantly increase infective phage particle half-lives when irradiated with UV. In general, compounds with the ability to absorb detrimental UV can make good phage protectants.
In addition to UV radiation, phages are subject to hostile environmental conditions in the phyllosphere (leaf surface of a plant) and rhizosphere (region surrounding the roots). In the phyllosphere, phages are subjected to adverse conditions such as fluctuating temperature that may increase desiccation, chemicals such as copper pesticide residues that may be present on plant surfaces [80,83], and rain-induced leaching that reduces the number of phages on the leaf surface [84]. These issues prevent phages from coming into contact with potential hosts. Strategies to limit UV damage to phages are also generally effective at reducing damage from these environmental challenges, such as using protective formulations [81,85] or applying phages in the evening or at dawn. Another strategy is to maintain phage populations using propagating bacterial strains [10]. Bacteriophages mixed with a non-pathogenic bacterial species were used to enhance phage persistence on the leaf surface and were shown to improve biocontrol of black rot disease of broccoli plants [58].
In the rhizosphere, there is high heterogeneity in the soil due to variation in soil particle size and composition, soil biota, and microbiome diversity [86], which influences the movement or diffusion of phages through the soil. For instance, sandy soils with larger particles have more chances of phage movement owing to greater pore space and better water flow than poorly structured soils, like clay soils. Poor distribution and dispersal of phages were shown to create a barrier that prevented phages from contacting their host bacteria and, hence, were ineffective for disease control [87]. The stability of phages in the soil is also driven by temperature [88], soil moisture [89], and plant root exudates or litter composition [83]. Stability of phages can also be affected by anthropogenic factors such as continuous cropping, which could affect the abundance of the viral community in the soil [90]. Continuous cropping practices allowed for viral priming and affected soil viral abundance, where viruses remaining in the soil previous season adapted to infect the juvenile rhizosphere of the hosts in the next cropping season [89]. In contrast, crop rotation reduced viral priming activity in the rhizosphere [83]. In the context of soil phage therapy, this could influence a co-evolution and persistence of phage in the absence of their host to closely related host strains found in the rhizosphere [85]. Applying phages as seed coating can also improve phage persistence in the rhizosphere, especially for seed-borne diseases. Coating Xcc-contaminated brassica seeds with phages reduced the bacterial titer between 1 and 2 log units [59]. Likewise, maize seeds infected with Clavibacter michiganensis subsp. nebraskensis (Cmn) that causes Goss’ wilt were coated with phages and polymer stabilizers such as polyvinyl alcohol (PVOH), and the phage treatment was shown to reduce the bacterial titres of the pathogen by 76% on the seed surface and 51% in internally infected seed and 78% in the seedling tissue [91]. Coating of melon seeds with phage ACPWH resulted in 95% germination and 95% survival of the seedlings after soil inoculation with the bacterial fruit blotch pathogen, Acidovorax citrulli, as compared to 13% germination and 0% survival in non-coated seeds (Table 1) [31]. These studies show bacteriophages can persist on seeds even after germination without any negative effects on plants [58]. However, this could be limited to seedborne diseases and be effective against early stages of bacterial infection. The combination of seed coating and soil drenching of the phage could provide a long-lasting protection against the bacterial pathogen.
2.2. Phage Storage Challenges Under Laboratory and Commercial Conditions
In addition to UV radiation, many other environmental parameters can affect phage survival during storage and affect biocontrol efficacy. Temperature is one of the most important factors of successful phage storage, as elevated temperatures can adversely affect phage viability and infectivity [92]. Phages are therefore typically stored at low temperatures at 4 °C, −20 °C, or −80 °C [93], with increased longevity at the lowest temperatures. Other important considerations are the techniques used to make a phage suspension, storage media composition, conditions of the storage area, and methods used to apply the phages to the host cells [10,92]. In general, keeping phages cold and away from light is sufficient to maintain phage survival; however, the composition of the storage medium can also affect phage viability. Under experimental conditions, phages are typically stored in SM buffer. This buffer includes Tris-HCI, a compound that is not approved by the FDA for use as a food additive [94]. While this allows phages to be stored stably for long periods for experimental purposes, it cannot be used commercially for food applications, limiting its use for controlling plant pathogens. Many other potential storage media cannot be added to food due to their effect on the food’s aesthetic or practical characteristics, including flavor and odor [95]. Other potential food-safe media options do not seem to significantly affect phage survival and viability, and thus still require cold temperatures for continued viability [95]. These limitations can make it difficult to store phages. One option to get around some common storage issues is to store phages as a dry powder through lyophilization, or by freeze drying, which eliminates the need for temperature control during storage and shipping [96]. However, this method is costly and requires special equipment [95]. Another method to increase phage longevity during storage is to force the phages to undergo adaptive evolution in response to thermal stress, resulting in phage strains adapted to thermal stress and therefore making them easier to store [92].
2.3. Phage Host Range and Selection of Phage Candidates for Therapy
The high degree of specificity of phages in phage therapy is one of the advantages of managing plant bacterial diseases, by sparing beneficial microbes and maintaining the microbial ecosystem (Table 1). Some phages are so specific that they can only infect one bacterial species or even a few strains within a species [17,27]. However, this limits the use of these phages in targeting different phytopathogens in the field. A phage host range profile can be subjectively determined by the spectrum of strains that can be successfully infected and killed by the phage. The lysis of these strains is determined by the specificity of a phage’s host binding proteins that allow for attachment of the phage to the bacterial cell, and molecular interactions between the phage and bacterial cell, which lead to the injection of the phage genetic material and hijacking of the bacterial metabolism and production of new offspring [25,97]. Developing a phage biocide that eliminates every strain of a particular bacterial species can be an adsorption challenge. In recent times, to mitigate this challenge, multiple phages are often mixed, creating phage cocktails to target different pathogens of either different species causing similar disease or different strains in one species [14]. Several studies have shown the effectiveness of phage cocktails against plant bacterial pathogens. For example, phage combinations consisting of four phage types isolated from tomato fields decreased the incidence of bacterial wilt disease caused by Ralstonia solanacearum by up to 80% in field experiments [20]. In a study by Carstens et al. [21] a six-phage cocktail reduced both disease incidence and disease severity of black leg disease of potato stems by 61% and 64%, which is caused by Pectobacterium atrosepticum (Table 1). In addition, phage cocktail formulations containing several phages have protected plants against plant pathogenic bacterial species such as Xylella fastidiosa [8], Xanthomonas campestris pv. vesicatoria [60,98], Xanthomonas axonopodis pv. citrumelo [59], Xanthomonas axonopodis pv. citri [59], Xanthomonas campestris pv. pelargonii [57], Pseudomonas syringae pv. porri [42], Dickeya solani [99], and Xanthomonas axonopodis pv. allii [56]. In addition, the commercially available phage mixture product, AgriPhageTM, is effective against several bacterial diseases caused by Xanthomonas campestris pv. vesicatoria, Pseudomonas syringae pv. tomato, Erwinia amylovora, Clavibacter michiganensis subsp. michiganensis, Xanthomonas arboricola pv. pruni, and Xanthomonas arboricola pv. juglandis [100].
For an effective phage cocktail application, a cocktail of lytic phages isolated from various sources, with diverse receptor binding proteins and strong adsorption, should be used to avoid or minimize resistance development [41,47]. Prior to phage cocktail preparation, each required phage host range should be determined, including its genomic features, application requirements, and efficiency against pathogens [17]. This is to avoid inefficiency and to minimize targeting potentially beneficial bacteria. Phages in a cocktail may also have a synergistic effect, where one phage may increase and augment the virulence of the other phages against the growth of the target bacteria [56,101]. Hence, careful attention should be given to each of the phages used in cocktails. In addition, due to the complexity of plant pathogen interactions, it should be emphasized that a single multidimensional cocktail for all bacterial phytopathogens may not be plausible to develop. Constant surveillance of emerging phage-resistant bacteria and modification of phage cocktail formulations should be performed as needed to ensure the effective killing of target pathogens [102]. Even in AgriPhageTM, they constantly monitor the phage population and update their product [100].
2.4. The Difficulty of Applying Phages Uniformly over a Large Area
Application over large areas requires a higher volume of phage. Phage titers of 1 × 106 to 1 × 1010 PFU/mL have been used for plant disease control (Table 1). Maintaining a high phage titer is essential for effective disease control. While achieving this in small-scale applications may be relatively straightforward, large-scale deployment in fruit orchards, ranging from 29 to 49,535 acres, would require substantial phage volumes [103,104]. For example, one quart of AgriPhage—Tomato Spot/Speck- is recommended for one acre and costs approximately 50 USD [105]. Keeping in mind, phages should be applied repeatedly due to their instability in harsh environmental conditions. The application to large farms over a long time may not be viable. Although there has been modeling and research on lowering the cost of production of phages, commercial applications are still expensive when dealing with large acreages of farms.
According to Vu and Oh [106], bacteriophages are mostly applied in the rhizosphere through soil drenching, in the phyllosphere via spraying, and in the stem by infiltrating. These modes of application can be effective in herbaceous plants and shrubs due to the small canopy and root area.
Phage viability is compromised in the phyllosphere. In a previous study by Balogh [107], phage concentrations at 104 PFU/g of leaf tissue were found to be ineffective (Supplemental Table S1). In a field study, bacteriophage PFUs plummeted below 104 PFU/g in the tomato phyllosphere within 4 h post-application applied prior to periods of high UVA + B in May and June in Florida, USA [80], indicating a loss of effective residual activity for disease control. Furthermore, phage residual activity was not detected the following day for those treatments. In contrast, an earlier study has shown that copper treatments exhibited residual efficacy on the tomato phyllosphere for at least 7 days after application [108]. Hence, the application of phage for disease control can be challenging compared to copper-based compounds in large areas, given that phage needs to be applied more frequently to maintain effective residual activity.
Additionally, spraying large trees or vines uniformly can be physically challenging [109]. One way to combat this is the application of phage through the vascular system [110]. For example, XylPhi-PDâ is a commercially available grapevine vascular injection that can be used to control Pierce’s disease of grapes caused by the vascular bacterial pathogen, Xylella fastidiosa subsp. Fastidiosa [111].
2.5. Possible Development of Phage Resistance in Bacterial Host
In nature, bacteria constantly face attacks from phages and have evolved a diverse array of defense mechanisms to counteract these infections. In response, phages have developed counterstrategies to evade bacterial defenses, leading to a dynamic evolutionary arms race between phages and their hosts [112]. This ongoing coevolution presents a major challenge for phage therapy, as bacterial resistance mechanisms can limit the long-term efficacy of phage-based treatments. Phage resistance in bacteria is a multifaceted process, driven by genetic adaptations, phase variation, and molecular modifications, which collectively enable bacteria to evade or neutralize phage infections [110,111].
One of the most common resistance mechanisms is blocking phage adsorption by altering the bacterial surface receptors. In many Gram-negative phytopathogens, this often involves mutations in cell envelope components such as lipopolysaccharides (LPS) or other outer-membrane structures that serve as phage receptors [113]. For example, the potato pathogen Dickeya solani can become resistant to lytic phage ΦD5 through mutations in genes required for LPS core or O-antigen synthesis. In one study, random Tn5 insertions in D. solani yielded multiple phage-resistant mutants, all showing small but effective modifications in their LPS structure that completely blocked ΦD5 adsorption. Notably, gel analysis revealed no major LPS band differences, suggesting that even minor structural modifications were sufficient to prevent phage binding. Similar LPS biosynthesis mutations conferring phage resistance have been reported in soft-rot Pectobacterium species (P. atrosepticum, P. carotovorum, and P. brasiliense) [113]. Likewise, in Xanthomonas oryzae (rice blight bacterium), spontaneous mutations in a glycosyltransferase gene involved in LPS assembly were shown to reduce phage adsorption by altering the O-antigen structure [114].
Another illustrative case is Pseudomonas syringae, a foliar plant pathogen. Laboratory experiments showed that phage-resistant P. syringae mutants carried mutations in the rfbA and rfbD genes, which are essential for LPS biosynthesis. Loss or alteration of these components eliminated the phage receptor, rendering the bacteria resistant to infection. However, this resistance comes at a cost: while mutants exhibited normal growth in rich media, they showed reduced virulence on tomato plants, likely due to the impaired LPS affecting bacterial fitness. Such fitness trade-offs are common in bacteria that evade phages through receptor loss. For example, transposon knockouts in P. carotovorum resulted in phage-resistant mutants that lost some ability to cause potato tuber rot [113].
Beyond surface modifications, phytopathogenic bacteria can acquire novel genetic elements that actively counteract phage infections. One of the most well-studied genetic resistance mechanisms is the CRISPR-Cas system, an adaptive immune mechanism found in many bacteria, including phytopathogens. The CRISPR-Cas system allows bacteria to capture short DNA fragments (spacers) from invading phages and store them in their genome. These spacers are later used as a molecular “memory” to recognize and degrade future phage infections [113,115,116,117]. The CRISPR-Cas system was first identified in yogurt bacteria (Streptococcus thermophilus) where it provided immunity against lytic phages, and subsequent studies have shown it can impose a fitness cost in some contexts (e.g., slowed growth due to the metabolic burden of maintaining the system) [118,119]. In phytopathogens, CRISPR-Cas loci are likewise present—for instance, P. atrosepticum carries multiple CRISPR spacer arrays—and they likely contribute to phage resistance by similar spacer acquisition and phage DNA targeting [117] (though detailed examples in planta are still emerging). In addition to CRISPR, bacteria often harbor or acquire other phage defense systems encoded by specific genes. Restriction-modification (R-M) systems involve restriction enzymes that cleave foreign (phage) DNA at specific sequences, paired with methylases that protect the host’s own DNA. R-M systems can be horizontally transferred on plasmids or mobile genetic elements, providing immediate resistance to many phages by degrading their genomes upon entry [120,121,122]. Another strategy is through abortive infection (Abi) systems, where a bacterium sacrifices itself upon phage invasion by triggering a self-destruct pathway, thereby aborting the phage replication cycle and protecting clonal bacterial neighbors. Genes for various Abi systems (such as toxin-antitoxin modules or phage trigger sensors) can be found on plasmids, transposons, or prophages in many bacteria [120]. In the context of plant pathogens, these horizontally acquired defense genes have not been studied as extensively as receptor mutations, but they likely operate under the surface. For example, some Xanthomonas and Pseudomonas strains possess plasmid-borne endonucleases and other defense proteins that could target phage DNA (paralleling what has been observed in human-pathogenic bacteria. By understanding the specific resistance mechanisms (and their costs) in phytopathogens, we can better design phage applications that either circumvent resistance or exploit the trade-offs associated with it [121].
2.6. Regulatory Issues
Regulatory authorities classify bacteriophages as biological substances, placing them under the purview of pharmaceutical legislation. Due to their classification as biological substances, they are subject to regulations mandating extensive data collection on safety, efficacy, and any potential environmental impact. In the European Union, the regulatory framework mandates that medicinal products produced through industrial processes require marketing authorization. This entails demonstrating safety, efficacy, and quality, with manufacturing conducted under Good Manufacturing Practices (GMP) [122]. However, achieving GMP compliance involves significant financial investment, posing a major barrier for new entrepreneurs in new phage product commercialization [123]. Legislation also demands rigorous qualitative and quantitative assessments of all components within a medicinal product. For phages, these criteria include the absence of prophages and antibiotic resistance in host bacteria, the exclusive lytic activity of phages against target pathogens, and stringent controls for impurities such as endotoxins and residual reagents. Given the limitations of the current regulatory framework, individual Member States within the European Union are adopting national-level strategies to address the regulation of phage therapy [124].
For phages targeting plant pathogenic bacteria, they must be approved for use as a biopesticide by the regulatory bodies overseeing a particular region [125]. In the United States, the Environmental Protection Agency evaluates potential new biopesticides under the guidance of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) to determine any potential risks to both human health and the health of the environment. The potential biopesticide or phage treatment must also meet the guidelines surrounding allowable levels of pesticide residues on the crop after harvest as outlined by the Federal Food, Drug, and Cosmetics Act (FFDCA) and the Food Quality Protection Act (FQPA). The Endangered Species Act also sets guidelines on biopesticide use to avoid off-target effects of pesticide use on any endangered species. Once a new phage treatment or biopesticide has gone through all the necessary evaluations, it can be approved for use on crops in the fields. As of now, only a few phage treatments have been approved as biopesticides for agricultural use by the EPA as of now.
3. Innovation over the Century to Tackle Challenges
3.1. Controlled Delivery Strategies
The application of bacteriophage-based biocontrol in agriculture faces significant challenges due to harsh environmental conditions, including UV radiation, desiccation, temperature fluctuations, and enzymatic degradation [81]. Unlike controlled environments in medical and veterinary settings, where pH fluctuations and enzymatic degradation within mammalian systems are the primary concerns, agricultural phage applications must contend with variable and often extreme abiotic stressors that can reduce phage viability and efficacy.
To overcome these challenges, advanced controlled delivery systems have been developed to enhance phage stability and effectiveness. These systems offer several advantages, including increased stability, localized and sustained availability, protection from UV degradation, improved adherence to target sites, and precision delivery to sites where the target organism resides. One of the most promising approaches for phage stabilization is encapsulation, where phages are embedded within protective matrices such as alginate, liposomes, chitosan, or biodegradable polymers. This method shields phages from environmental stressors, including UV exposure, desiccation, pH extremes, and enzymatic degradation, thereby improving storage stability, eliminating dependence on cold-chain logistics, and enabling phages to withstand manufacturing and field application stressors [126,127]. Recent studies have demonstrated the effectiveness of nano formulations in improving phage persistence in agricultural environments. For example, Nano N-Acetylcysteine-Zinc Sulfide has been used to enhance the phyllosphere persistence of phages, leading to a 16.4% reduction in bacterial spot disease severity in tomatoes [128].
The prospect of using bacteriophages for plant disease control is promising. The efficacy of bacteriophage can be improved by increasing stability in adverse environment (UV exposure, high pH, and temperature), improving the delivery and release methods, and increasing bactericidal activity by encapsulating the phages with different components. The following are substances used for coating bacteriophages to increase their effectiveness:
3.1.1. Nanomaterials
A material is classified as a nanomaterial if its size or one of its dimensions is in the range of 1 to 100 nm [129]. Various nanomaterials have been tested for their ability to enhance the stability of bacteriophages. In a study by Choudhary et al. [128], nano–N–Acetylcysteine–Zinc Sulfide (nano-NAC-ZnS) formulated phage ΦXp06-02-1 was assessed for its persistence in UV light in vitro and phyllosphere. In the study, phage persistence in the phyllosphere was 15-fold higher when formulated with nano-NAC-ZnS compared to non-formulated after 8 h of sunlight exposure. Additionally, nano-NAC-ZnS had some bactericidal effect after 24 h of incubation against two strains of Xanthomonas euvesicatoria pv. perforans, known to cause bacterial spot of tomato, help the phage for disease control.
Encapsulation of phage with nanomaterials has also shown promise in protecting them from extreme temperatures and pH conditions. Phage HK6, effective against Enterobacter cloacae, exhibited enhanced bactericidal activity and improved thermal and pH stability when encapsulated with chitosan nanoparticles (CS-NPs) [130]. Both encapsulated and free phages displayed similar stability at 25 °C, 50 °C, and 60 °C, as well as within a pH range of 5–12. However, encapsulation significantly improved phage stability at higher temperatures (70 °C and 80 °C) and extreme pH levels (3, 11, and 12).
Similarly, T7 phage engineered with a silver nanoparticle-binding peptide in capsid, when armed with silver nanoparticles, significantly reduced E. coli biofilm compared to phage alone [126]. This experiment also confirmed the non-toxicity of the recombinant phage bound with silver nanomaterials to eukaryotic cells, confirming its safety. Additionally, phage, PEL1, immobilized onto Fe3O4-based magnetic colloidal nanoparticle clusters coated with chitosan (PELI-CS-Fe3O4), demonstrated a significant reduction in mixed biofilms of P. aeruginosa and E. coli compared to PELI1 or CS-Fe3O4 alone [127], highlighting the synergistic effect of nano formulations with phage to manage bacteria.
3.1.2. Hydrogels
Hydrogels are highly absorbent, 3D crosslinked polymer networks that retain large amounts of water while providing softness, flexibility, durability, and biocompatibility [131]. They can serve as a controlled delivery system for phages (Figure 3A) in targeted tissues by adjusting the porosity of the gel [132]. Co-delivery of phages has been successfully tested with various hydrogels, including carboxymethyl cellulose (CMC) [133,134] and alginate hydrogel [135]. Additionally, bacteriophage encapsulated with PEG-4MAL (poly (ethylene glycol)-4-maleimide) hydrogel was used to treat murine radial segmental defects infected with P. aeruginosa in mice without compromising the metabolic activity of human mesenchymal stromal cells or the bactericidal activity of the phage [136]. Notably, a 4.7-fold reduction in P. aeruginosa count was observed at the infection site by using phage treated with hydrogels compared to hydrogels alone, highlighting their potential for targeted antibacterial therapy.
3.1.3. Liposomes for Encapsulation of Phages
Liposomes are microscopic, laboratory-made vesicular structures composed of single or multilayered phospholipid bilayers enclosing an aqueous compartment [137]. Their phospholipid layer can merge with cell membranes, facilitating the controlled release of their contents [138]. Liposomes have been used in human [139] and animal systems for drug delivery [138]. Encapsulation of the bacteriophage UAB_Ph87 in liposomes (Figure 3B) significantly enhanced its stability, increasing its resistance by 3.5-fold in simulated gastric fluid (pH 2.8) after 60 min of incubation compared to the non-encapsulated phage [140]. Furthermore, in a burn wound infection with Klebsiella pneumonia B5055 in mice, treatment with a phage cocktail loaded in liposomes resulted in a lower bacterial load in the skin, blood, and liver compared to liposomes alone or untreated control. This suggests that liposome encapsulation enhances the bactericidal activity of phages [141].
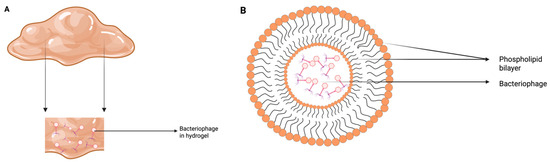
Figure 3.
Bacteriophage encapsulation methods designed to protect phages from UV radiation and other environmental stresses in order to enhance phage stability and efficacy: (A) Hydrogel, (B) Liposome. These figures were inspired by Durr and Leipzig [142] and recreated by the authors.
3.2. Genetic Engineering for Phages
Engineered bacteriophages offer a promising alternative to traditional antibiotics by overcoming key challenges such as host specificity, bacterial resistance, and immune system evasion. These phages can be tailored to degrade bacterial biofilms, evade bacterial defense mechanisms, and deliver antimicrobial payloads, making them a valuable tool in combating antibiotic-resistant infections [143,144]. A range of genome editing technologies has been employed to engineer bacteriophages for diverse applications. These technologies include homologous recombination [145], CRISPR (Clustered regularly interspaced short palindromic repeats) system [143], BRED (bacteriophage recombineering of electroporated DNA) [146], and in vivo recombineering [144]. These techniques have expanded phages’ potential applications in targeted bacterial eradication and microbiome modulation.
One notable example is phage Y2, a lytic phage targeting Erwinia amylovora, the fire blight pathogen. Since E. amylovora produces an exopolysaccharide (EPS) shield that hinders phage infection, Born et al. [147] enhanced Y2’s antimicrobial activity by introducing the dpoL1 depolymerase gene via homologous recombination, creating the engineered phage (Y2::dpoL1-C) to degrade the EPS barrier. This modification enabled Y2 to degrade the EPS barrier, significantly enhanced bacterial killing, reducing E. amylovora counts in lab and field conditions, and preventing colonization of apple blossoms.
Another key advancement in phage engineering involves modifying host specificity through altering receptor-binding proteins. Mahichi et al. [148] successfully swapped the tail fiber gene of coliphage T2 with that from phage IP008, enabling T2 to infect a new host. Similarly, Marzari et al. [149] fused a receptor-binding domain from phage, broadening its host range.
To counteract bacterial resistance mechanisms, Qin et al. [150] engineered Pseudomonas phages to carry anti-CRISPR genes (AcrIF1, AcrIF2, AcrIF3), effectively disabling the bacterial CRISPR-Cas system and increasing phage infection success. Additionally, Yehl et al. [151] developed phage libraries with diverse tail fiber mutants, preventing the emergence of resistant bacterial clones and ensuring long-term efficacy in biocontrol strategies. These genetic modifications enhance phage efficacy, providing a powerful tool for sustainable biocontrol in agriculture. These approaches can be applied in agriculture to target specific phytopathogens or expand phage coverage across multiple bacterial species, improving biocontrol efficacy while minimizing bacterial resistance.
3.3. Combining Phages with Other Disease-Control Strategies for Managing Bacterial Diseases
Bacteriophages have been reported to be an effective biocontrol against several plant bacterial diseases (Table 1) and are a good alternative to antibiotics, as phages are abundant, highly specific, and have fewer environmental concerns. However, to improve the efficiency of phages in phage therapy and minimize phage resistance by bacterial pathogens, research studies have been geared toward supporting phage therapy with other chemical substances, thus combining phages with other disease control strategies (Table 2). Two studies [152,153] showed that a combination of bacteriophage and Systemic Acquired Resistance (SAR) inducers, such as acibenzolar-S-methyl (ASM), significantly reduced bacterial spot disease and gave more efficient disease control than phage alone or SAR alone. In another study, Abrahamian et al. [154] reported that bacteriophage applications against X. perforans were only effective in the field when in combination with other treatments such as copper octanoate (bactericides) and ASM (SAR inducers). Furthermore, treatments containing bacteriophage mixtures with plant activators or copper were compared for a more sustainable and effective control of Xanthomonas axonopodis pv. allii causing Xanthomonas leaf blight of onion [55]. They showed that the integration of bacteriophages with inducers such as acibenzolar-S-methyl (ASM) was equally effective or superior to the integration of phages with copper in reducing the disease severity. However, the use of bacteriophages with plant activators may produce a more viable option than using copper products, since plant defense stimulators are more sustainable, and they readily degrade in the ecosystem [155,156]. Not many studies have explored the combination of phages with other strategies; however, the combination of phages with other treatments should be aimed at reducing pathogen populations or improving the environment for phages, while ensuring the other treatments do not negatively affect the phage virions or interfere with phage replication [157]. In vitro assays, adding chemical or biological agents to phages to improve the lysis of target bacteria, further show potential IPM that can be used in field studies. Carvacrol combined with cocktail phages provided a higher efficiency of lysing Pseudomonas syringae pv. actinidae, the pathogen causing bacterial canker of kiwifruit, than using cocktail and individual phages alone [158]. Pantoea agglomerans served as a carrier strain for Erwinia phages and improved the concentration and in vitro lysis efficiency of Erwinia amylovora [159,160]. Furthermore, Kim et al. [161] showed a synergistic effect of cocktail phages with the antibiotic, kasugamycin, which resulted in a significant reduction in Erwinia amylovora in vitro and in immature wound-infected apples. These are potential IPM strategies that could be explored when using phages as biocontrol agents.

Table 2.
Use of phages with other chemical agents for integrated disease management.
4. What Are the Practical Considerations Before and After Use?
The poor persistence of phages in the phyllosphere and rhizosphere leads to another important practical consideration when using phages for biocontrol. Previous studies have found a sharp decline in phage titer over only a few short hours, due to the deleterious conditions present in the phyllosphere, such as UV radiation, high temperatures, and exposure to chemical pesticides. Iriarte et al. [80] found that a phage targeting Xanthomonas perforans titers of 106 or 108 PFU/mL were able to reduce bacterial spot on tomatoes inoculated with 108 CFU/mL of Xanthomonas perforans. However, phage titers of 104 PFU/mL or lower were unable to reduce bacterial infection [107]. This decrease in titer also occurs naturally over a few hours, meaning that phage suspensions will quickly become ineffective in a short time after they are originally applied. Optimal timing of bacteriophage treatments, therefore, varies depending on the bacterial disease being treated and the environment of the bacteriophage application. It is difficult to describe overall best practices for the timing of phage applications; in general, however, it appears to be most effective to apply the phage treatment directly before bacterial inoculation.
Practically speaking, this means that phage suspension must be applied to the crop shortly before the bacterial species enters the plant. Given that it is impossible to determine exactly when a particular pathogenic bacterium will enter plants in the field, this means that the best chance of control comes from applying the phage suspension frequently. While a daily application may not be necessary for a particular pathosystem, phage concentrations tend to fall dramatically after 24 h, and so the phages should be applied frequently. Applying the phage suspensions in the evening hours aids longevity and persistence of phage activity on the plant, since that limits damage from UV radiation and higher temperatures during the day [15].
5. Conclusions
Bacteriophage therapy has undergone a remarkable evolution since its inception over a century ago, emerging as a viable alternative to traditional antimicrobial strategies, particularly in the face of rising antibiotic resistance. While its high specificity offers precision in targeting bacterial hosts, it simultaneously poses challenges related to narrow host ranges, resistance development, and practical implementation. Nevertheless, innovative advancements such as phage cocktails, genetic engineering, and novel delivery mechanisms have significantly expanded the scope and applicability of phage therapy across medicine, agriculture, and biotechnology.
To maximize the potential of phage therapy, a deeper understanding of phage–host dynamics is crucial, particularly in diverse and complex environments such as the rhizosphere, phyllosphere, and human microbiota. Research should also focus on the synergistic integration of phages with other biocontrol agents and therapeutic approaches to ensure both effectiveness and sustainability. Advances in genetic engineering can enable the customization of phages for broader host ranges, improved stability, and enhanced efficacy against resistant strains, potentially revolutionizing their use in both plant and human health.
In agricultural applications, overcoming challenges related to phage stability under harsh environmental conditions, large-scale application logistics, and regulatory compliance remains critical. Addressing these issues will require collaboration among researchers, industry stakeholders, and policymakers to establish standardized protocols, streamline approval processes, and develop cost-effective production and storage methods. Furthermore, the role of bacteriophages in shaping microbial ecosystems and their evolutionary interplay with bacterial hosts underscores their ecological importance beyond therapeutic applications. As research expands into uncovering novel phage types and mechanisms, the potential for groundbreaking discoveries in phage biology remains vast.
Phage therapy represents a promising paradigm shift toward precision-targeted microbial management. Its success, however, will depend on sustained investments in interdisciplinary research, innovation in delivery systems, and global regulatory frameworks that facilitate its safe and effective use. By addressing these multifaceted challenges, bacteriophage therapy can become a cornerstone in the fight against bacterial diseases, offering sustainable and adaptable solutions for global health and food security.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v17081033/s1, Table S1: “Effect of phage concentration and copper-mancozeb treatment on bacterial spot disease development on tomato. Table from M.S. thesis by Balogh [102]”.
Author Contributions
Conceptualization, M.C., I.A.B., S.T.M., A.P., M.P., B.B., M.L.P. and J.B.J.; resources, M.L.P. and J.B.J.; data curation M.C., I.A.B., S.T.M., A.P., M.P., B.B., M.L.P. and J.B.J.; writing—original draft preparation M.C., I.A.B., S.T.M., A.P., M.P., B.B., M.L.P. and J.B.J.; writing—review and editing, M.C., I.A.B., S.T.M., A.P., M.P., B.B., M.L.P. and J.B.J.; visualization, M.L.P. and J.B.J., supervision, M.L.P. and J.B.J.; project administration, M.L.P. and J.B.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- d’Hérelle, F. The Bacteriophage, Its Rôle in Immunity; Williams & Wilkins Company: Baltimore, MD, USA, 1922. [Google Scholar]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- Katznelson, H. Bacteriophage in relation to plant diseases. Bot. Rev. 1937, 3, 499–521. [Google Scholar] [CrossRef]
- Mallmann, W.L.; Hemstreet, C. Isolation of an inhibitory substance from plants. J. Agric. Res. 1924, 28, 599–602. [Google Scholar]
- Moore, E.S. D’Herelle’s bacteriophage in relation to plant parasites. S. Afr. J. Sci. 1926, 23, 306. [Google Scholar]
- Jackson, L.R.E. Bacteriophage Prevention and Control of Harmful Plant Bacteria. WO1990013631A1, 3 May 1989. [Google Scholar]
- United States Environmental Protection Agency AGRIPHAGE: Bactericide for Use on Tomatoes and Peppers. 2006. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/067986-00001-20060622.pdf (accessed on 6 February 2025).
- Das, M.; Bhowmick, T.S.; Ahern, S.J.; Young, R.; Gonzalez, C.F. Control of Pierce’s disease by phage. PLoS ONE 2015, 10, e0128902. [Google Scholar] [CrossRef]
- Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.A.; Momol, M.T.; Jones, J.B.; Vallad, G.E. Soil-based systemic delivery and phyllosphere in vivo propagation of bacteriophages: Two possible strategies for improving bacteriophage persistence for plant disease control. Bacteriophage 2012, 2, e23530. [Google Scholar] [CrossRef]
- Jones, J.B.; Vallad, G.E.; Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.C.; Momol, M.T. Considerations for using bacteriophages for plant disease control. Bacteriophage 2012, 2, 208–214. [Google Scholar] [CrossRef]
- Toussaint, B.; Munoz, A.P.; Pirnay, J.-P. Overview and Outlook of Phage Therapy and Phage Biocontrol; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- Kotila, J.E.; Coons, G.H. Investigations on the Blackleg Disease of Potato; Michigan State University, Agricultural Experiment Station: East Lansing, MI, USA, 1925. [Google Scholar]
- Thomas, R.C. A bacteriophage in relation to Stewards disease of corn. Phytopathology 1935, 25, 371–372. [Google Scholar]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Balogh, B.; Jones, J.B.; Momol, M.T.; Olson, S.M.; Obradovic, A.; King, P.; Jackson, L.E. Improved Efficacy of Newly Formulated Bacteriophages for Management of Bacterial Spot on Tomato. Plant Disease 2003, 87, 949–954. [Google Scholar] [CrossRef]
- Balogh, B.; Canteros, B.I.; Stall, R.E.; Jones, J.B. Control of Citrus Canker and Citrus Bacterial Spot with Bacteriophages. Plant Disease 2008, 92, 1048–1052. [Google Scholar] [CrossRef]
- Kering, K.K.; Kibii, B.J.; Wei, H. Biocontrol of phytobacteria with bacteriophage cocktails. Pest Manag. Sci. 2019, 75, 1775–1781. [Google Scholar] [CrossRef]
- Enviroinvest Zrt. Available online: https://www.enviroinvest.hu/ (accessed on 17 April 2025).
- APS Biocontrol. Available online: https://www.apsbiocontrol.com (accessed on 17 April 2025).
- Wang, X.; Wei, Z.; Yang, K.; Wang, J.; Jousset, A.; Xu, Y.; Shen, Q.; Friman, V.-P. Phage combination therapies for bacterial wilt disease in tomato. Nat. Biotechnol. 2019, 37, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Carstens, A.B.; Djurhuus, A.M.; Kot, W.; Hansen, L.H. A novel six-phage cocktail reduces Pectobacterium atrosepticum soft rot infection in potato tubers under simulated storage conditions. FEMS Microbiol. Lett. 2019, 366, fnz101. [Google Scholar] [CrossRef] [PubMed]
- Keen, E.C. A century of phage research: Bacteriophages and the shaping of modern biology. Bioessays 2015, 37, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Chibani-Chennoufi, S.; Bruttin, A.; Dillmann, M.-L.; Brüssow, H. Phage-host Interaction: An ecological perspective. J. Bacteriol. 2004, 186, 3677–3686. [Google Scholar] [CrossRef]
- Mushegian, A.R. Are there 1031 virus particles on Earth, or more, or fewer? J. Bacteriol. 2020, 202, e00052-20. [Google Scholar] [CrossRef]
- Hyman, P.; Abedon, S.T. Chapter 7—Bacteriophage host range and bacterial resistance. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2010; Volume 70, pp. 217–248. [Google Scholar]
- Anand, T.; Vaid, R.K.; Bera, B.C.; Barua, S.; Riyesh, T.; Virmani, N.; Yadav, N.; Malik, P. Isolation and characterization of a bacteriophage with broad host Range, displaying potential in preventing Bovine diarrhoea. Virus Genes 2015, 51, 315–321. [Google Scholar] [CrossRef]
- Ross, A.; Ward, S.; Hyman, P. More Is Better: Selecting for Broad Host Range Bacteriophages. Front. Microbiol. 2016, 7, 1352. [Google Scholar] [CrossRef]
- Weinbauer, M.G. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef]
- Ackermann, H.W. Ackermann, H.W. Ackermann bacteriophage classification. In Bacteriophages: Biology and Applications; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-203-49175-1. [Google Scholar]
- Xu, J.; Chen, M.; He, L.; Zhang, S.; Ding, T.; Yao, H.; Lu, C.; Zhang, W. Isolation and characterization of a T4-like Phage with a relatively wide host range within Escherichia coli. J. Basic Microbiol. 2016, 56, 405–421. [Google Scholar] [CrossRef]
- Rahimi-Midani, A.; Choi, T.-J. Transport of phage in Melon plants and inhibition of progression of bacterial fruit blotch. Viruses 2020, 12, 477. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Vaerenbergh, J.V.; Vandenheuvel, D.; Dunon, V.; Ceyssens, P.-J.; Proft, M.D.; Kropinski, A.M.; Noben, J.-P.; Maes, M.; Lavigne, R. T4-related bacteriophage LIMEstone isolates for the control of soft rot on potato caused by ‘Dickeya solani’. PLoS ONE 2012, 7, e33227. [Google Scholar] [CrossRef] [PubMed]
- Kmoch, M.; Vacek, J.; Loubová, V.; Petrzik, K.; Brázdová, S.; Ševčík, R. Potential of Limestonevirus bacteriophages for ecological control of Dickeya solani causing bacterial potato blackleg. Agriculture 2024, 14, 497. [Google Scholar] [CrossRef]
- Boulé, J.; Sholberg, P.L.; Lehman, S.M.; O’gorman, D.T.; Svircev, A. MIsolation and characterization of eight bacteriophages infecting Erwinia amylovora and their potential as biological control agents in British Columbia, Canada. Can. J. Plant Pathol. 2011, 33, 308–317. [Google Scholar] [CrossRef]
- Sabri, M.; El Handi, K.; Valentini, F.; De Stradis, A.; Achbani, E.H.; Benkirane, R.; Resch, G.; Elbeaino, T. Identification and characterization of Erwinia Phage IT22: A new bacteriophage-based biocontrol against Erwinia amylovora. Viruses 2022, 14, 2455. [Google Scholar] [CrossRef]
- Biosca, E.G.; Delgado Santander, R.; Morán, F.; Figàs-Segura, À.; Vázquez, R.; Català-Senent, J.F.; Álvarez, B. First European Erwinia amylovora lytic bacteriophage cocktails effective in the host: Characterization and prospects for fire blight biocontrol. Biology 2024, 13, 176. [Google Scholar] [CrossRef]
- Gdanetz, K.; Dobbins, M.R.; Villani, S.M.; Outwater, C.A.; Slack, S.M.; Nesbitt, D.; Svircev, A.M.; Lauwers, E.M.; Zeng, Q.; Cox, K.D.; et al. Multisite field evaluation of bacteriophages for fire blight management: Incorporation of ultraviolet radiation protectants and impact on the apple flower microbiome. Phytopathology® 2024, 114, 1028–1038. [Google Scholar] [CrossRef]
- Vique, G.; Mendoza-Barberá, E.; Ramos-Barbero, M.D.; Blanco-Picazo, P.; Sala-Comorera, L.; Quirós, P.; Atares, S.; Salaet, I.; Muniesa, M.; Rodríguez-Rubio, L. Efficacy of Erwinia amylovora and Xanthomonas campestris pv. campestris phages to control fire blight and black rot in vivo. Microbiol. Spectr. 2025, 13, e00280-25. [Google Scholar] [CrossRef]
- Czajkowski, R.; Ozymko, Z.; de Jager, V.; Siwinska, J.; Smolarska, A.; Ossowicki, A.; Narajczyk, M.; Lojkowska, E. Genomic, Proteomic and morphological characterization of two novel broad host lytic bacteriophages ΦPD10.3 and ΦPD23.1 infecting pectinolytic Pectobacterium spp. and Dickeya spp. PLoS ONE 2015, 10, e0119812. [Google Scholar] [CrossRef]
- Zaczek-Moczydłowska, M.A.; Young, G.K.; Trudgett, J.; Fleming, C.C.; Campbell, K.; O’Hanlon, R. Genomic characterization, formulation and efficacy in planta of a Siphoviridae and Podoviridae protection cocktail against the bacterial plant pathogens Pectobacterium spp. Viruses 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.T.; Kim, H.; Lee, S.; Hwang, I.S.; Kwon, C.-T.; Oh, C.-S. Bacteriophage cocktail for biocontrol of soft rot disease caused by Pectobacterium species in Chinese cabbage. Appl. Microbiol. Biotechnol. 2023, 108, 11. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, S.; Volckaert, A.; Venneman, S.; Declercq, B.; Vandenheuvel, D.; Allonsius, C.N.; Van Malderghem, C.; Jang, H.B.; Briers, Y.; Noben, J.P.; et al. Characterization of novel bacteriophages for biocontrol of bacterial blight in Leek caused by Pseudomonas syringae pv. porri. Front. Microbiol. 2016, 7, 279. [Google Scholar] [CrossRef] [PubMed]
- Flores, O.; Retamales, J.; Núñez, M.; León, M.; Salinas, P.; Besoain, X.; Yañez, C.; Bastías, R. Characterization of bacteriophages against Pseudomonas syringae pv. actinidiae with potential use as natural antimicrobials in kiwifruit plants. Microorganisms 2020, 8, 974. [Google Scholar] [CrossRef]
- Rabiey, M.; Roy, S.R.; Holtappels, D.; Franceschetti, L.; Quilty, B.J.; Creeth, R.; Sundin, G.W.; Wagemans, J.; Lavigne, R.; Jackson, R.W. Phage biocontrol to combat Pseudomonas syringae pathogens causing disease in cherry. Microb. Biotechnol. 2020, 13, 1428–1445. [Google Scholar] [CrossRef]
- Nguyen, H.T.D.; Yoon, S.; Kim, M.-H.; Kim, Y.-K.; Yoon, M.-Y.; Cho, Y.-H.; Lim, Y.; Shin, S.H.; Kim, D.-E. Characterization of bacteriophage ϕPto-Bp6g, a novel phage that lyses Pseudomonas tolaasii causing brown blotch disease in mushrooms. J. Microbiol. Methods 2012, 91, 514–519. [Google Scholar] [CrossRef]
- Fujiwara, A.; Fujisawa, M.; Hamasaki, R.; Kawasaki, T.; Fujie, M.; Yamada, T. Biocontrol of Ralstonia solanacearum by treatment with lytic bacteriophages. Appl. Environ. Microbiol. 2011, 77, 4155–4162. [Google Scholar] [CrossRef]
- Wei, C.; Liu, J.; Maina, A.N.; Mwaura, F.B.; Yu, J.; Yan, C.; Zhang, R.; Wei, H. Developing a bacteriophage cocktail for biocontrol of potato bacterial wilt. Virol. Sin. 2017, 32, 476–484. [Google Scholar] [CrossRef]
- Elhalag, K.; Nasr-Eldin, M.; Hussien, A.; Ahmad, A. Potential use of soilborne lytic Podoviridae phage as a biocontrol agent against Ralstonia solanacearum. J. Basic Microbiol. 2018, 58, 658–669. [Google Scholar] [CrossRef]
- Álvarez, B.; López, M.M.; Biosca, E.G. Biocontrol of the major plant pathogen Ralstonia solanacearum in irrigation water and host plants by novel waterborne lytic bacteriophages. Front. Microbiol. 2019, 10, 2813. [Google Scholar] [CrossRef]
- Ramírez, M.; Neuman, B.W.; Ramírez, C.A. Bacteriophages as promising agents for the biological control of Moko disease (Ralstonia solanacearum) of banana. Biol. Control 2020, 149, 104238. [Google Scholar] [CrossRef]
- Umrao, P.D.; Kumar, V.; Kaistha, S.D. Biocontrol potential of bacteriophage ɸsp1 against bacterial wilt-causing Ralstonia solanacearum in Solanaceae crops. Egypt. J. Biol. Pest Control 2021, 31, 61. [Google Scholar] [CrossRef]
- Thapa Magar, R.; Lee, S.Y.; Kim, H.J.; Lee, S.-W. Biocontrol of bacterial wilt in tomato with a cocktail of lytic bacteriophages. Appl. Microbiol. Biotechnol. 2022, 106, 3837–3848. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Huang, M.; He, Y.; Guo, S.; Yang, K.; Wang, N.; Sun, T.; Yang, H.; Yang, T.; et al. Phages enhance both phytopathogen density control and rhizosphere microbiome suppressiveness. mBio 2024, 15, e03016-23. [Google Scholar] [CrossRef] [PubMed]
- McKenna, F.; El-Tarabily, K.A.; Hardy, G.E.S.T.J.; Dell, B. Novel in vivo use of a polyvalent Streptomyces phage to disinfest Streptomyces scabies-infected seed potatoes. Plant Pathol. 2001, 50, 666–675. [Google Scholar] [CrossRef]
- Lang, J.M.; Gent, D.H.; Schwartz, H.F. Management of Xanthomonas leaf Blight of onion with bacteriophages and a plant activator. Plant Dis. 2007, 91, 871–878. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Tran, T.N.; Holtappels, D.; Kim Ngan, N.L.; Hao, N.P.; Vallino, M.; Tien, D.T.K.; Khanh-Pham, N.H.; Lavigne, R.; Kamei, K.; et al. Phage biocontrol of bacterial leaf blight disease on welsh onion caused by Xanthomonas axonopodis pv. allii. Antibiotics 2021, 10, 517. [Google Scholar] [CrossRef]
- Flaherty, J.E.; Harbaugh, B.K.; Jones, J.B.; Somodi, G.C.; Jackson, L.E. H-mutant bacteriophages as a potential biocontrol of bacterial blight of geranium. HortScience 2001, 36, 98–100. [Google Scholar] [CrossRef]
- Nagai, H.; Miyake, N.; Kato, S.; Maekawa, D.; Inoue, Y.; Takikawa, Y. Improved control of black rot of broccoli caused by Xanthomonas campestris pv. campestris using a bacteriophage and a nonpathogenic Xanthomonas sp. strain. J. Gen. Plant Pathol. 2017, 83, 373–381. [Google Scholar] [CrossRef]
- Holtappels, D.; Fortuna, K.J.; Moons, L.; Broeckaert, N.; Bäcker, L.E.; Venneman, S.; Rombouts, S.; Lippens, L.; Baeyen, S.; Pollet, S.; et al. The Potential of bacteriophages to control Xanthomonas campestris pv. campestris at different stages of disease development. Microb. Biotechnol. 2022, 15, 1762–1782. [Google Scholar] [CrossRef]
- Ibrahim, Y.E.; Saleh, A.A.; Al-Saleh, M.A. Management of Asiatic citrus canker under field conditions in Saudi Arabia using bacteriophages and acibenzolar-S-Methyl. Plant Dis. 2017, 101, 761–765. [Google Scholar] [CrossRef]
- Gašić, K.; Kuzmanović, N.; Ivanović, M.; Prokić, A.; Šević, M.; Obradović, A. Complete genome of the Xanthomonas euvesicatoria specific bacteriophage KΦ1, its survival and potential in control of pepper bacterial spot. Front. Microbiol. 2018, 9, 2021. [Google Scholar] [CrossRef]
- Chae, J.-C.; Nguyen, B.H.; Yu, S.-M.; Lee, H.K.; Lee, Y.H. Diversity of bacteriophages infecting Xanthomonas oryzae pv. oryzae in paddy fields and its potential to control bacterial leaf blight of rice. J. Microbiol. Biotechnol. 2014, 24, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Chen, J.; Zhang, M.; Wang, L.; Masum, M.M.I.; Yan, C.; An, Q.; Li, B.; Chen, J. Identification and characterization of five new OP2-pelated Myoviridae bacteriophages infecting different strains of Xanthomonas oryzae pv. oryzae. J. Plant Pathol. 2019, 101, 263–273. [Google Scholar] [CrossRef]
- Liu, M.; Hu, R.; Xia, M.; He, X.; Jin, Y. Novel broad-spectrum bacteriophages against Xanthomonas oryzae and their biocontrol potential in rice bacterial diseases. Environ. Microbiol. 2023, 25, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Shkoporov, A.N.; Lood, C.; Millard, A.D.; Dutilh, B.E.; Alfenas-Zerbini, P.; van Zyl, L.J.; Aziz, R.K.; Oksanen, H.M.; Poranen, M.M.; et al. Abolishment of morphology-based taxa and change to binomial species Names: 2022 taxonomy update of the ICTV bacterial viruses subcommittee. Arch. Virol. 2023, 168, 74. [Google Scholar] [CrossRef] [PubMed]
- Young, R. Phage Lysis: Three steps, Three Choices, One outcome. J. Microbiol. 2014, 52, 243–258. [Google Scholar] [CrossRef]
- Catalão, M.J.; Gil, F.; Moniz-Pereira, J.; São-José, C.; Pimentel, M. Diversity in bacterial lysis systems: Bacteriophages show the way. FEMS Microbiol. Rev. 2013, 37, 554–571. [Google Scholar] [CrossRef]
- Wang, I.-N.; Smith, D.L.; Young, R. Holins: The protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 2000, 54, 799–825. [Google Scholar] [CrossRef]
- Young, R. Bacteriophage lysis: Mechanism and regulation. Microbiol. Rev. 1992, 56, 430–481. [Google Scholar] [CrossRef]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage therapy: Going temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef]
- Hyman, P. Phages for phage therapy: Isolation, characterization, and host range breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial use and resistance in plant agriculture: A one health perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- IARC working group on the evaluation of carcinogenic risks to humans. “Solar and ultraviolet radiation”. In Radiation; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Pfeifer, G.P. Formation and processing of UV photoproducts: Effects of DNA sequence and chromatin environment. Photochem. Photobiol. 1997, 65, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Frederick, J.E.; Lubin, D. Solar ultraviolet irradiance at palmer Station, Antarctica. In Ultraviolet Radiation in Antarctica: Measurements and Biological Effects; American Geophysical Union (AGU): Washington, DC, USA, 1994; pp. 43–52. ISBN 978-1-118-66794-1. [Google Scholar]
- Handelsman, J.; Stabb, E.V. Biocontrol of soilborne plant pathogens. Plant Cell 1996, 8, 1855–1869. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Ozymko, Z.; Zwirowski, S.; Lojkowska, E. Complete genome sequence of a broad-host-range Lytic Dickeya spp. bacteriophage ϕD5. Arch. Virol. 2014, 159, 3153–3155. [Google Scholar] [CrossRef]
- Iriarte, F.B.; Balogh, B.; Momol, M.T.; Smith, L.M.; Wilson, M.; Jones, J.B. Factors affecting survival of bacteriophage on tomato leaf surfaces. Appl. Environ. Microbiol. 2007, 73, 1704–1711. [Google Scholar] [CrossRef]
- Halawa, E.M. Challenges of bacteriophages application in controlling bacterial plant diseases and how to overcome them. J. Genet. Eng. Biotechnol. 2023, 21, 98. [Google Scholar] [CrossRef]
- Born, Y.; Bosshard, L.; Duffy, B.; Loessner, M.J.; Fieseler, L. Protection of Erwinia amylovora bacteriophage Y2 from UV-induced damage by natural compounds. Bacteriophage 2015, 5, e1074330. [Google Scholar] [CrossRef] [PubMed]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Ignoffo, C.M.; Hostetter, D.L.; Sikorowski, P.P.; Sutter, G.; Brooks, W.M. Inactivation of representative species of Entomopathogenic viruses, a bacterium, fungus, and protozoan by an ultraviolet light source. Environ. Entomol. 1977, 6, 411–415. [Google Scholar] [CrossRef]
- Gómez, P.; Buckling, A. Bacteria-phage antagonistic coevolution in soil. Science 2011, 332, 106–109. [Google Scholar] [CrossRef]
- Moldrup, P.; Olesen, T.; Komatsu, T.; Schjønning, P.; Rolston, D.E. Tortuosity, diffusivity, and permeability in the soil liquid and gaseous phases. Soil Sci. Soc. Am. J. 2001, 65, 613–623. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Coclet, C.; Sorensen, P.O.; Karaoz, U.; Wang, S.; Brodie, E.L.; Eloe-Fadrosh, E.A.; Roux, S. Virus diversity and activity is driven by snowmelt and host dynamics in a high-altitude watershed soil ecosystem. Microbiome 2023, 11, 237. [Google Scholar] [CrossRef]
- Santos-Medellín, C.; Blazewicz, S.J.; Pett-Ridge, J.; Firestone, M.K.; Emerson, J.B. Viral but not bacterial community successional patterns reflect extreme turnover shortly after rewetting dry soils. Nat. Ecol. Evol. 2023, 7, 1809–1822. [Google Scholar] [CrossRef]
- Muscatt, G.; Hilton, S.; Raguideau, S.; Teakle, G.; Lidbury, I.D.E.A.; Wellington, E.M.H.; Quince, C.; Millard, A.; Bending, G.D.; Jameson, E. Crop management shapes the diversity and activity of DNA and RNA viruses in the rhizosphere. Microbiome 2022, 10, 181. [Google Scholar] [CrossRef]
- Kimmelshue, C.; Goggi, A.S.; Cademartiri, R. The use of biological seed coatings based on bacteriophages and polymers against Clavibacter michiganensis subsp. nebraskensis in maize seeds. Sci. Rep. 2019, 9, 17950. [Google Scholar] [CrossRef]
- Kering, K.K.; Zhang, X.; Nyaruaba, R.; Yu, J.; Wei, H. Application of adaptive evolution to improve the stability of bacteriophages during storage. Viruses 2020, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- González-Menéndez, E.; Fernández, L.; Gutiérrez, D.; Rodríguez, A.; Martínez, B.; García, P. Comparative analysis of different preservation techniques for the storage of Staphylococcus phages aimed for the industrial development of phage-based antimicrobial products. PLoS ONE 2018, 13, e0205728. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Novel Drug Approvals for 2025 [Internet]; FDA: Silver Spring, MD, USA, 2025. Available online: https://www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2025 (accessed on 1 February 2025).
- Kim, E.-J.; Lim, M.-C.; Woo, M.-A.; Kim, B.S.; Lim, J.-A. Development of stabilizing solution for long-term storage of bacteriophages at room temperature and application to control foodborne pathogens. Viruses 2024, 16, 1155. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, M.; Vervaet, C.; Pirnay, J.-P.; De Vos, D.; Verbeken, G.; Mast, J.; Chanishvili, N.; Vaneechoutte, M. Stability of Staphylococcus aureus phage ISP after freeze-drying (lyophilization). PLoS ONE 2013, 8, e68797. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Muñoz, S.L.; Koskella, B. Chapter Four—Bacteria–phage Interactions in Natural Environments. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 89, pp. 135–183. [Google Scholar]
- Flaherty, J.E.; Jones, J.; Harbaugh, B.K.; Somodi, G.C.; Jackson, L.E. Control of Bacterial Spot on tomato in the greenhouse and field with H-Mutant bacteriophages. HortScience 2000, 35, 882–884. [Google Scholar] [CrossRef]
- Carstens, A.B.; Djurhuus, A.M.; Kot, W.; Jacobs-Sera, D.; Hatfull, G.F.; Hansen, L.H. Unlocking the potential of 46 new bacteriophages for biocontrol of Dickeya solani. Viruses 2018, 10, 621. [Google Scholar] [CrossRef]
- AgriPhage®|Bacterial Control for Crops, Food Safety & Animal Health. Available online: https://agriphage.com/ (accessed on 17 February 2025).
- Schmerer, M.; Molineux, I.J.; Bull, J.J. Synergy as a rationale for phage therapy using phage cocktails. PeerJ 2014, 2, e590. [Google Scholar] [CrossRef]
- Farooq, T.; Hussain, M.D.; Shakeel, M.T.; Tariqjaveed, M.; Aslam, M.N.; Naqvi, S.A.H.; Amjad, R.; Tang, Y.; She, X.; He, Z. Deploying viruses against phytobacteria: Potential use of phage cocktails as a multifaceted approach to combat resistant bacterial plant pathogens. Viruses 2022, 14, 171. [Google Scholar] [CrossRef]
- Balogh, B.; Nga, N.T.T.; Jones, J.B. Relative level of bacteriophage multiplication in vitro or in phyllosphere may not predict in planta efficacy for controlling bacterial leaf spot on tomato Caused by Xanthomonas perforans. Front. Microbiol. 2018, 9, 2176. [Google Scholar] [CrossRef]
- Jo, S.J.; Giri, S.S.; Lee, S.B.; Jung, W.J.; Park, J.H.; Hwang, M.H.; Park, D.S.; Park, E.; Kim, S.W.; Jun, J.W.; et al. Optimization of the large-scale production for Erwinia amylovora bacteriophages. Microb. Cell Factories 2024, 23, 342. [Google Scholar] [CrossRef]
- AgriPhage—Tomato Spot/Speck—1 Quart. Available online: https://www.7springsfarm.com/products/agriphage-tomato-spot-speck-1-quart (accessed on 3 March 2025).
- Vu, N.T.; Oh, C.-S. Bacteriophage Usage for Bacterial Disease Management and Diagnosis in Plants. Plant Pathol. J. 2020, 36, 204–217. [Google Scholar] [CrossRef]
- Balogh, B. Strategies for Improving the Efficacy of Bacteriophages for Controlling Bacterial Spot of Tomato. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2002. [Google Scholar]
- Jones, J.B.; Woltz, S.S.; Kelly, R.O.; Harris, G. The role of ionic copper, total copper, and select bactericides on control of bacterial spot of tomato. Proc. Fla. State Hortic. Soc. 1991, 104, 257–258. [Google Scholar]
- Zhang, M.; Guo, Y.; Powell, C.A.; Doud, M.S.; Yang, C.; Duan, Y. Effective antibiotics against ‘Candidatus Liberibacter asiaticus’ in HLB-affected citrus plants identified via the graft-based evaluation. PLoS ONE 2014, 9, e111032. [Google Scholar] [CrossRef] [PubMed]
- Grace, E.R.; Rabiey, M.; Friman, V.-P.; Jackson, R.W. Seeing the forest for the trees: Use of phages to treat bacterial tree diseases. Plant Pathol. 2021, 70, 1987–2004. [Google Scholar] [CrossRef]
- Kinkhabwala, A. A New Biocontrol Approach for the Reduction of Pierce’s Disease in Vineyards|Progressive Crop Consultant. 2022. Available online: https://progressivecrop.com/2022/01/24/a-new-biocontrol-approach-for-the-reduction-of-pierces-disease-in-vineyards/ (accessed on 12 February 2025).
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, P.; Lewtak, K.; Fiołka, M.; Czaplewska, P.; Narajczyk, M.; Czajkowski, R. Resistance of Dickeya solani strain IPO 2222 to lytic bacteriophage ΦD5 results in fitness tradeoffs for the bacterium during infection. Sci. Rep. 2022, 12, 10725. [Google Scholar] [CrossRef]
- Zhang, M.; Qian, J.; Xu, X.; Ahmed, T.; Yang, Y.; Yan, C.; Elsharkawy, M.M.; Hassan, M.M.; Alorabi, J.A.; Chen, J.; et al. Resistance of Xanthomonas oryzae pv. oryzae to lytic phage X2 by spontaneous mutation of lipopolysaccharide synthesis-related glycosyltransferase. Viruses 2022, 14, 1088. [Google Scholar] [CrossRef]
- Horvath, P.; Romero, D.A.; Coûté-Monvoisin, A.-C.; Richards, M.; Deveau, H.; Moineau, S.; Boyaval, P.; Fremaux, C.; Barrangou, R. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J. Bacteriol. 2008, 190, 1401–1412. [Google Scholar] [CrossRef]
- Deveau, H.; Barrangou, R.; Garneau, J.E.; Labonté, J.; Fremaux, C.; Boyaval, P.; Romero, D.A.; Horvath, P.; Moineau, S. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 2008, 190, 1390–1400. [Google Scholar] [CrossRef]
- Watson, B.N.J.; Staals, R.H.J.; Fineran, P.C. CRISPR-Cas-mediated phage resistance enhances horizontal gene transfer by transduction. mBio 2018, 9, e02406-17. [Google Scholar] [CrossRef]
- Bickle, T.A.; Krüger, D.H. Biology of DNA restriction. Microbiol. Rev. 1993, 57, 434–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pingoud, A.; Jeltsch, A. Structure and function of Type II restriction endonucleases. Nucleic Acids Res. 2001, 29, 3705–3727. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, A.; Tal, N.; Sorek, R. Abortive infection: Bacterial suicide as an antiviral immune Strategy. Annu. Rev. Virol. 2020, 7, 371–384. [Google Scholar] [CrossRef]
- Meaden, S.; Paszkiewicz, K.; Koskella, B. The cost of phage resistance in a plant pathogenic bacterium is context-dependent. Evolution 2015, 69, 1321–1328. [Google Scholar] [CrossRef]
- Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use 2001. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02001L0020-20090807 (accessed on 12 February 2025).
- Reindel, R.; Fiore, C.R. Phage therapy: Considerations and challenges for development. Clin. Infect. Dis. 2017, 64, 1589–1590. [Google Scholar] [CrossRef]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020, 44, 684–700. [Google Scholar] [CrossRef]
- US EPA Biopesticides. Available online: https://www.epa.gov/pesticides/biopesticides (accessed on 3 March 2025).
- Szymczak, M.; Pankowski, J.A.; Kwiatek, A.; Grygorcewicz, B.; Karczewska-Golec, J.; Sadowska, K.; Golec, P. An effective antibiofilm strategy based on bacteriophages armed with silver nanoparticles. Sci. Rep. 2024, 14, 9088. [Google Scholar] [CrossRef]
- Li, L.-L.; Yu, P.; Wang, X.; Yu, S.-S.; Mathieu, J.; Yu, H.-Q.; Alvarez, P.J.J. Enhanced biofilm penetration for microbial control by polyvalent phages conjugated with magnetic colloidal nanoparticle clusters (CNCs). Environ. Sci. Nano 2017, 4, 1817–1826. [Google Scholar] [CrossRef]
- Choudhary, M.; Pereira, J.; Davidson, E.B.; Colee, J.; Santra, S.; Jones, J.B.; Paret, M.L. Improved persistence of bacteriophage formulation with Nano N-Acetylcysteine–Zinc Sulfide and tomato bacterial spot disease Control. Plant Dis. 2023, 107, 3933–3942. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Temsaah, H.R.; Abdelkader, K.; Ahmed, A.E.; Elgiddawy, N.; Eldin, Z.E.; Elshebrawy, H.A.; Kasem, N.G.; El-Gohary, F.A.; Azmy, A.F. Chitosan nano-formulation enhances stability and bactericidal activity of the lytic phage HK6. BMC Biotechnol. 2025, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Dodda, J.M.; Deshmukh, K.; Bezuidenhout, D.; Yeh, Y.-C. Hydrogels: Definition, History, Classifications, Formation, Constitutive Characteristics, and Applications. In Multicomponent Hydrogels; Dodda, J.M., Deshmukh, K., Bezuidenhout, D., Eds.; The Royal Society of Chemistry: London, UK, 2023; pp. 1–25. ISBN 978-1-83916-727-0. [Google Scholar]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Abed, S.; Beig, M.; Barzi, S.M.; Shafiei, M.; Shahraki, A.H.; Sadeghi, S.; Sohrabi, A. Development of phage-containing hydrogel for treating Enterococcus faecalis-infected wounds. PLoS ONE 2024, 19, e0312469. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ponce Benavente, L.; Chittò, M.; Post, V.; Constant, C.; Zeiter, S.; Nylund, P.; D’Este, M.; González Moreno, M.; Trampuz, A.; et al. Combination of bacteriophages and vancomycin in a co-delivery hydrogel for localized treatment of fracture-related infections. npj Biofilms Microbiomes 2024, 10, 77. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Q.; Ma, R.; Huang, W.; Zhang, X.; Liu, Y.; Xu, Z.; Zhang, L.; Li, M.; Zhu, C. Modified alginate-based hydrogel as a carrier of the CB2 agonist JWH133 for bone Engineering. ACS Omega 2021, 6, 6861–6870. [Google Scholar] [CrossRef]
- Wroe, J.A.; Johnson, C.T.; García, A.J. Bacteriophage delivering hydrogels reduce biofilm formation in vitro and infection in vivo. J. Biomed. Mater. Res. Part A 2020, 108, 39–49. [Google Scholar] [CrossRef]
- Abbasi, H.; Kouchak, M.; Mirveis, Z.; Hajipour, F.; Khodarahmi, M.; Rahbar, N.; Handali, S. What we need to know about Liposomes as drug nanocarriers: An Updated Review. Adv. Pharm. Bull. 2023, 13, 7–23. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef]
- Chadha, P.; Katare, O.P.; Chhibber, S. Liposome Loaded Phage Cocktail: Enhanced therapeutic potential in resolving Klebsiella pneumoniae mediated burn wound infections. Burns 2017, 43, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Durr, H.A.; Leipzig, N.D. Advancements in bacteriophage therapies and delivery for bacterial infection. Mater. Adv. 2023, 4, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Kiro, R.; Shitrit, D.; Qimron, U. Efficient engineering of a bacteriophage genome using the Type I-E CRISPR-Cas system. RNA Biol. 2014, 11, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.G.; Goodman, D.B.; Wannier, T.M.; Kaur, D.; Farzadfard, F.; Lu, T.K.; Shipman, S.L.; Church, G.M. High-Throughput functional variant screens via in vivo production of single-stranded DNA. Proc. Natl. Acad. Sci. USA 2021, 118, e2018181118. [Google Scholar] [CrossRef]
- Kilcher, S.; Loessner, M.J. Engineering bacteriophages as versatile biologics. Trends Microbiol. 2019, 27, 355–367. [Google Scholar] [CrossRef]
- Payaslian, F.; Gradaschi, V.; Piuri, M. Genetic manipulation of phages for therapy using BRED. Curr. Opin. Biotechnol. 2021, 68, 8–14. [Google Scholar] [CrossRef]
- Born, Y.; Fieseler, L.; Thöny, V.; Leimer, N.; Duffy, B.; Loessner, M.J. Engineering of bacteriophages Y2::dpoL1-C and Y2::luxAB for efficient control and rapid detection of the fire blight pathogen, Erwinia amylovora. Appl. Environ. Microbiol. 2017, 83, e00341-17. [Google Scholar] [CrossRef]
- Mahichi, F.; Synnott, A.J.; Yamamichi, K.; Osada, T.; Tanji, Y. Site-specific recombination of T2 phage using IP008 long tail fiber genes provides a targeted method for expanding host range while retaining lytic activity. FEMS Microbiol. Lett. 2009, 295, 211–217. [Google Scholar] [CrossRef]
- Marzari, R.; Sblattero, D.; Righi, M.; Bradbury, A. Extending filamentous phage host range by the grafting of a heterologous receptor binding domain. Gene 1997, 185, 27–33. [Google Scholar] [CrossRef]
- Qin, S.; Liu, Y.; Chen, Y.; Hu, J.; Xiao, W.; Tang, X.; Li, G.; Lin, P.; Pu, Q.; Wu, Q.; et al. Engineered bacteriophages containing anti-CRISPR suppress infection of antibiotic-resistant P. aeruginosa. Microbiol. Spectr. 2022, 10, e01602-22. [Google Scholar] [CrossRef]
- Yehl, K.; Lemire, S.; Yang, A.C.; Ando, H.; Mimee, M.; Torres, M.D.T.; de la Fuente-Nunez, C.; Lu, T.K. Engineering phage host-Range and suppressing bacterial resistance through phage tail fiber mutagenesis. Cell 2019, 179, 459–469.e9. [Google Scholar] [CrossRef]
- Obradovic, A.; Jones, J.B.; Momol, M.T.; Balogh, B.; Olson, S.M. Management of tomato bacterial spot in the field by foliar applications of bacteriophages and SAR inducers. Plant Dis. 2004, 88, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, A.; Jones, J.B.; Momol, M.T.; Olson, S.M.; Jackson, L.E.; Balogh, B.; Guven, K.; Iriarte, F.B. Integration of biological control agents and systemic acquired resistance inducers against bacterial spot on tomato. Plant Dis. 2005, 89, 712–716. [Google Scholar] [CrossRef]
- Abrahamian, P.; Jones, J.B.; Vallad, G.E. Efficacy of copper and copper alternatives for management of bacterial spot on tomato under transplant and field production. Crop Prot. 2019, 126, 104919. [Google Scholar] [CrossRef]
- Anith, K.N.; Momol, M.T.; Kloepper, J.W.; Marois, J.J.; Olson, S.M.; Jones, J.B. Efficacy of plant growth-promoting Rhizobacteria, Acibenzolar-S-Methyl, and soil amendment for integrated management of bacterial wilt on tomato. Plant Dis. 2004, 88, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Gent, D.H.; Schwartz, H.F. Management of Xanthomonas leaf blight of onion with a plant activator, biological control Agents, and copper Bactericides. Plant Dis. 2005, 89, 631–639. [Google Scholar] [CrossRef]
- Balogh, B.; Jones, J.B.; Iriarte, F.B.; Momol, M.T. Phage therapy for plant disease control. Curr. Pharm. Biotechnol. 2010, 11, 48–57. [Google Scholar] [CrossRef]
- Ni, P.; Wang, L.; Deng, B.; Jiu, S.; Ma, C.; Zhang, C.; Almeida, A.; Wang, D.; Xu, W.; Wang, S. Combined application of bacteriophages and carvacrol in the control of Pseudomonas syringae pv. actinidiae planktonic and biofilm forms. Microorganisms 2020, 8, 837. [Google Scholar] [CrossRef]
- Gill, J.J.; Svircev, A.M.; Smith, R.; Castle, A.J. Bacteriophages of Erwinia amylovora. Appl. Environ. Microbiol. 2003, 69, 2133–2138. [Google Scholar] [CrossRef]
- Parcey, M.; Gayder, S.; Morley-Senkler, V.; Bakkeren, G.; Úrbez-Torres, J.R.; Ali, S.; Castle, A.J.; Svircev, A.M. Comparative genomic analysis of Erwinia amylovora reveals novel insights in phylogenetic arrangement, plasmid diversity, and streptomycin resistance. Genomics 2020, 112, 3762–3772. [Google Scholar] [CrossRef]
- Kim, S.-G.; Lee, S.-B.; Jo, S.-J.; Cho, K.; Park, J.-K.; Kwon, J.; Giri, S.S.; Kim, S.-W.; Kang, J.-W.; Jung, W.-J.; et al. Phage cocktail in combination with kasugamycin as a potential treatment for fire blight caused by Erwinia amylovora. Antibiotics 2022, 11, 1566. [Google Scholar] [CrossRef]
- Šević, M.; Gašić, K.; Ignjatov, M.; Mijatović, M.; Prokić, A.; Obradović, A. Integration of biological and conventional treatments in control of pepper bacterial spot. Crop Prot. 2019, 119, 46–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).