Afrina barna-like Virus, a Novel Virus Associated with Afrina sporoboliae, the Drop Seed Gall-Forming Nematode
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Origin, RNA Extraction, High-Throughput Sequencing, and Sequence Analysis
2.2. RT-PCR Validation, Sanger Sequencing of the Genome, and Phylogenetic Analysis
3. Results
3.1. High-Throughput Sequencing Analysis of the Nematode Samples
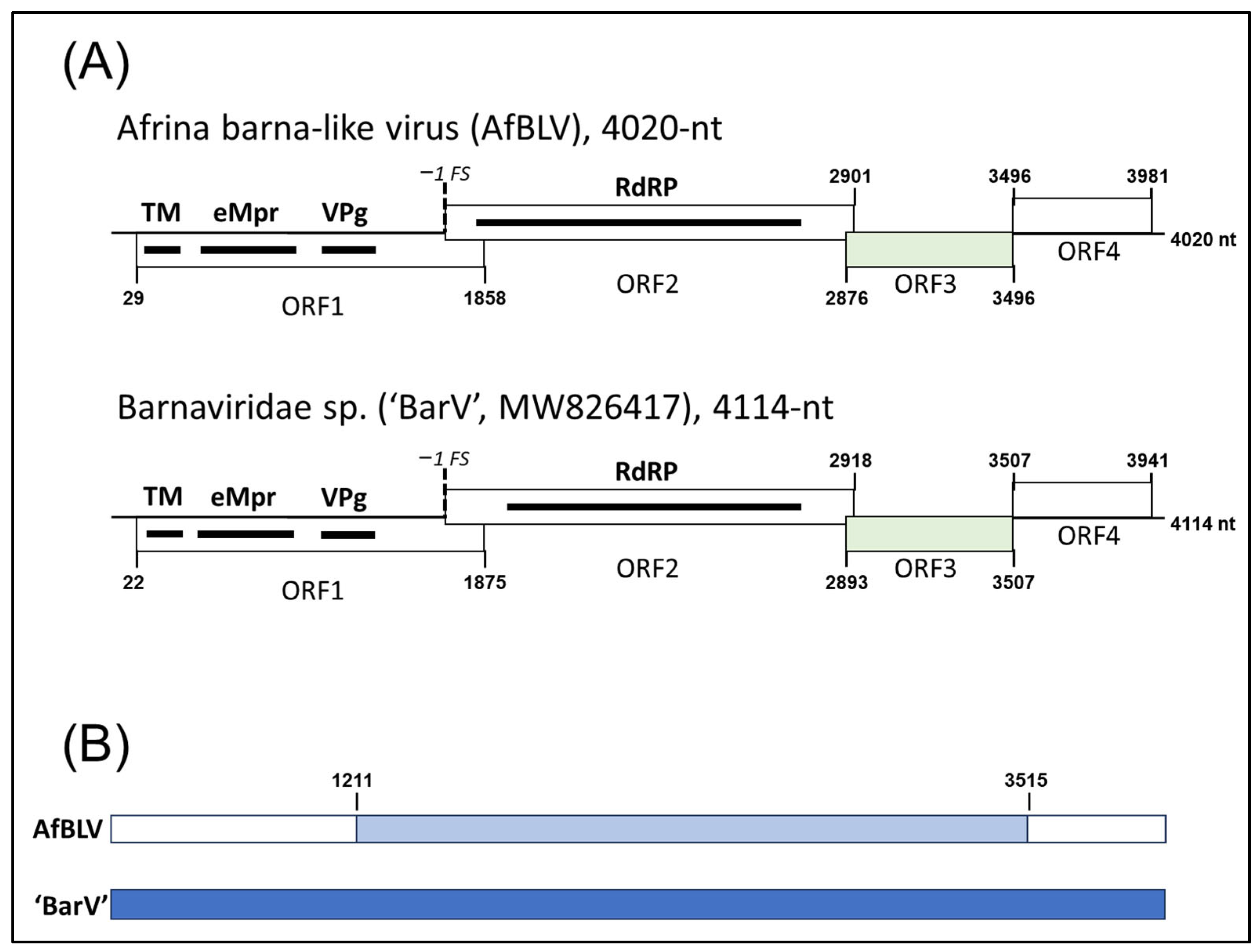
3.2. Sanger Sequencing of the Afrina barna-like Virus Genome
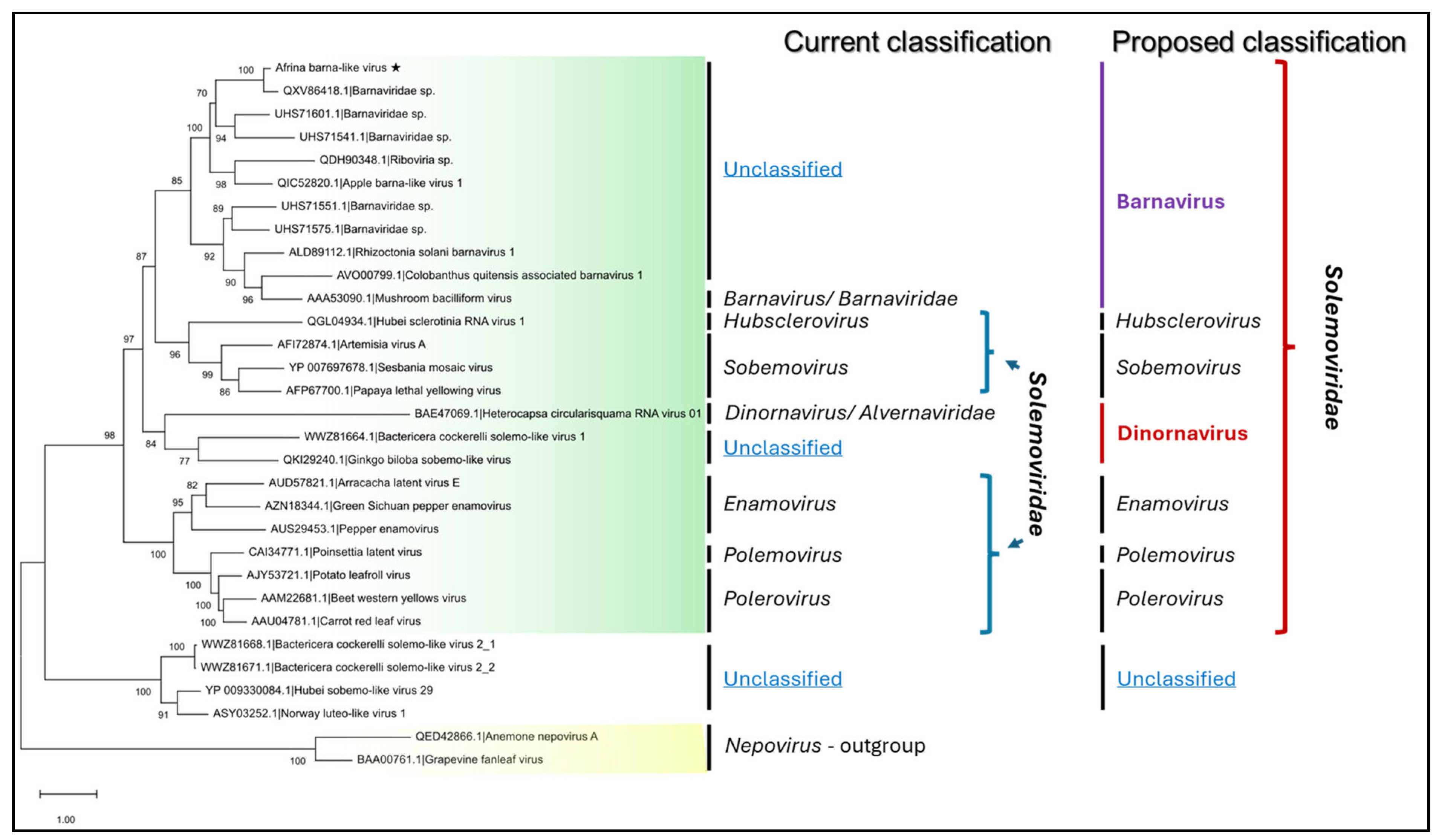
3.3. Afrina barna-like Virus Is a New Species in the Genus Barnavirus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revill, P.A. Family Barnaviridae. In Virus Taxonomy. Classification and Nomenclature of Viruses. Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2012; pp. 961–964. [Google Scholar]
- Revill, P.A.; Davidson, A.D.; Wright, P.J. The Nucleotide Sequence and Genome Organization of Mushroom Bacilliform Virus: A Single-Stranded RNA Virus of Agaricus Bisporus (Lange) Imbach. Virology 1994, 202, 904–911. [Google Scholar] [CrossRef]
- Nibert, M.L.; Manny, A.R.; Debat, H.J.; Firth, A.E.; Bertini, L.; Caruso, C. A Barnavirus Sequence Mined from a Transcriptome of the Antarctic Pearlwort Colobanthus Quitensis. Arch. Virol. 2018, 163, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.A.; Cross, A.R.; Harper, S.J. A Bushel of Viruses: Identification of Seventeen Novel Putative Viruses by RNA-Seq in Six Apple Trees. PLoS ONE 2020, 15, e0227669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yang, J.; Lu, S.; Jin, D.; Pu, J.; Wu, S.; Luo, X.L.; Liu, L.; Li, Z.; Xu, J. RNA Virus Diversity in Birds and Small Mammals From Qinghai–Tibet Plateau of China. Front. Microbiol. 2022, 13, 780651. [Google Scholar] [CrossRef] [PubMed]
- Barrantes-Infante, B.L.; Schroeder, B.K.; Subbotin, S.A.; Murray, T.D. Afrina sporoboliae sp. n. (Nematoda: Anguinidae) Associated with Sporobolus cryptandrus from Idaho, United States: Phylogenetic Relationships and Population Structure. Phytopathology 2018, 108, 768–779. [Google Scholar] [CrossRef]
- Murray, T.D.; Duarte, A.; Luster, D.G.; McKirdy, S.J.; Rogers, E.E.; Schroeder, B.K.; Subbotin, S.A. Seed Gall Nematodes and Their Association with Toxigenic Bacteria. Annu. Rev. Phytopathol. 2025, 63. [Google Scholar] [CrossRef]
- Murray, T.D.; Schroeder, B.K.; Schneider, W.L.; Luster, D.G.; Sechler, A.; Rogers, E.E.; Subbotin, S.A. Rathayibacter toxicus, Other Rathayibacter Species Inducing Bacterial Head Blight of Grasses, and the Potential for Livestock Poisonings. Phytopathology 2017, 107, 804–815. [Google Scholar] [CrossRef]
- Davis, E.W.; Tabima, J.F.; Weisberg, A.J.; Lopes, L.D.; Wiseman, M.S.; Wiseman, M.S.; Pupko, T.; Belcher, M.S.; Sechler, A.J.; Tancos, M.A.; et al. Evolution of the U.S. Biological Select Agent Rathayibacter toxicus. mBio 2018, 9, e01280-18. [Google Scholar] [CrossRef]
- Bekal, S.; Domier, L.L.; Niblack, T.L.; Lambert, K.N. Discovery and Initial Analysis of Novel Viral Genomes in the Soybean Cyst Nematode. J. Gen. Virol. 2011, 92, 1870–1879. [Google Scholar] [CrossRef]
- Ruark, C.L.; Koenning, S.R.; Davis, E.L.; Opperman, C.H.; Lommel, S.A.; Mitchum, M.G.; Sit, T.L. Soybean Cyst Nematode Culture Collections and Field Populations from North Carolina and Missouri Reveal High Incidences of Infection by Viruses. PLoS ONE 2017, 12, e0171514. [Google Scholar] [CrossRef]
- Kud, J.; Dahan, J.; Orellana, G.E.; Dandurand, L.-M.; Karasev, A.V. A Novel Rhabdovirus Associated with the Idaho Population of Potato Cyst Nematode Globodera pallida. Viruses 2022, 14, 2718. [Google Scholar] [CrossRef] [PubMed]
- Ruark, C.L.; Gardner, M.; Mitchum, M.G.; Davis, E.L.; Sit, T.L. Novel RNA Viruses within Plant Parasitic Cyst Nematodes. PLoS ONE 2018, 13, e0193881. [Google Scholar] [CrossRef] [PubMed]
- Kud, J.; Pillai, S.S.; Raber, G.; Caplan, A.; Kuhl, J.C.; Xiao, F.; Dandurand, L.M. Belowground Chemical Interactions: An Insight Into Host-Specific Behavior of Globodera spp. Hatched in Root Exudates From Potato and Its Wild Relative, Solanum sisymbriifolium. Front. Plant Sci. 2022, 12, 802622. [Google Scholar] [CrossRef] [PubMed]
- Gabler, F.; Nam, S.; Till, S.; Mirdita, M.; Steinegger, M.; Söding, J.; Lupas, A.N.; Alva, V. Protein Sequence Analysis Using the MPI Bioinformatics Toolkit. Curr. Protoc. Bioinform. 2020, 72, e108. [Google Scholar] [CrossRef]
- Reyes-Proaño, E.; Knerr, A.J.; Karasev, A.V. Molecular Characterization of Birch Toti-like Virus, a Plant-Associated Member of the New Family Orthototiviridae. Arch. Virol. 2024, 169, 140. [Google Scholar] [CrossRef]
- Green, K.J.; Brown, C.J.; Gray, S.M.; Karasev, A.V. Phylogenetic Study of Recombinant Strains of Potato Virus Y. Virology 2017, 507, 40–52. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Sõmera, M.; Fargette, D.; Hébrard, E.; Sarmiento, C. ICTV Virus Taxonomy Profile: Solemoviridae 2021. J. Gen. Virol. 2021, 102, 001707. [Google Scholar] [CrossRef]
- Inoue-Nagata, A.K.; Jordan, R.; Kreuze, J.; Li, F.; López-Moya, J.J.; Mäkinen, K.; Ohshima, K.; Wylie, S.J. ICTV Virus Taxonomy Profile: Potyviridae 2022. J. Gen. Virol. 2022, 103, 001738. [Google Scholar] [CrossRef]
- Dahan, J.; Orellana, G.E.; Wald, K.B.; Wenninger, E.J.; Cooper, W.R.; Karasev, A.V. Bactericera Cockerelli Picorna-like Virus and Three New Viruses Found Circulating in Populations of Potato/Tomato Psyllids (Bactericera cockerelli). Viruses 2024, 16, 415. [Google Scholar] [CrossRef]
- Pettersson, J.H.-O.; Shi, M.; Bohlin, J.; Eldholm, V.; Brynildsrud, O.B.; Paulsen, K.M.; Andreassen, Å.; Holmes, E.C. Characterizing the Virome of Ixodes Ricinus Ticks from Northern Europe. Sci. Rep. 2017, 7, 10870. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.-D.; Tian, J.-H.; Chen, L.-J.; Chen, X.; Li, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the Invertebrate RNA Virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Simmonds, P.; Adriaenssens, E.M.; Lefkowitz, E.J.; Oksanen, H.M.; Siddell, S.G.; Zerbini, F.M.; Alfenas-Zerbini, P.; Aylward, F.O.; Dempsey, D.M.; Dutilh, B.E.; et al. Changes to Virus Taxonomy and the ICTV Statutes Ratified by the International Committee on Taxonomy of Viruses (2024). Arch. Virol. 2024, 169, 236. [Google Scholar] [CrossRef]
- Nagasaki, K.; Tomaru, Y. New SsRNA Virus Family Infecting Dinoflagellates: Alvernaviridae. Propos. Int. Comm. Taxon. Viruses 2009, 2009, 016a-iP. Available online: https://ictv.global/ictv/proposals/2009.016a-iP.A.v7.Alvernaviridae.pdf (accessed on 17 July 2025).
- Nagasaki, K.; Shirai, Y.; Takao, Y.; Mizumoto, H.; Nishida, K.; Tomaru, Y. Comparison of Genome Sequences of Single-Stranded RNA Viruses Infecting the Bivalve-Killing Dinoflagellate Heterocapsa circularisquama. Appl. Environ. Microbiol. 2005, 71, 8888–8894. [Google Scholar] [CrossRef]
- Yang, S.; Shan, T.; Wang, Y.; Yang, J.; Chen, X.; Xiao, Y.; You, Z.; He, Y.; Zhao, M.; Lu, J.; et al. Virome of Riverside Phytocommunity Ecosystem of an Ancient Canal. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Roossinck, M.J. Plant RNA Virus Evolution. Curr. Opin. Microbiol. 2003, 6, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.D. Recombination in Plant Viruses. In Plant Virus Evolution; Roossinck, M.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 133–156. [Google Scholar]
- Gibbs, A.; Ohshima, K. Potyviruses and the Digital Revolution. Annu. Rev. Phytopathol. 2010, 48, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Félix, M.-A.; Ashe, A.; Piffaretti, J.; Wu, G.; Nuez, I.; Bélicard, T.; Jiang, Y.; Zhao, G.; Franz, C.J.; Goldstein, L.D.; et al. Natural and Experimental Infection of Caenorhabditis Nematodes by Novel Viruses Related to Nodaviruses. PLoS Biol. 2011, 9, e1000586. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.J.; Zhao, G.; Félix, M.-A.; Wang, D. Complete Genome Sequence of Le Blanc Virus, a Third Caenorhabditis Nematode-Infecting Virus. J. Virol. 2012, 86, 11940. [Google Scholar] [CrossRef]
- Frézal, L.; Jung, H.; Tahan, S.; Wang, D.; Félix, M.-A. Noda-Like RNA Viruses Infecting Caenorhabditis Nematodes: Sympatry, Diversity, and Reassortment. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef]
- Williams, S.H.; Che, X.; Oleynik, A.; Garcia, J.A.; Muller, D.; Zabka, T.S.; Firth, C.; Corrigan, R.M.; Briese, T.; Jain, K.; et al. Discovery of Two Highly Divergent Negative-Sense RNA Viruses Associated with the Parasitic Nematode, Capillaria hepatica, in Wild Mus Musculus from New York City. J. Gen. Virol. 2019, 100, 1350–1362. [Google Scholar] [CrossRef]
- Bekal, S.; Domier, L.L.; Gonfa, B.; McCoppin, N.K.; Lambert, K.N.; Bhalerao, K. A Novel Flavivirus in the Soybean Cyst Nematode. J. Gen. Virol. 2014, 95, 1272–1280. [Google Scholar] [CrossRef]
- Lin, J.; Ye, R.; Thekke-Veetil, T.; Staton, M.E.; Arelli, P.R.; Bernard, E.C.; Hewezi, T.; Domier, L.L.; Hajimorad, M.R. A Novel Picornavirus-like Genome from Transcriptome Sequencing of Sugar Beet Cyst Nematode Represents a New Putative Genus. J. Gen. Virol. 2018, 99, 1418–1424. [Google Scholar] [CrossRef]
- Vieira, P.; Nemchinov, L.G. A Novel Species of RNA Virus Associated with Root Lesion Nematode Pratylenchus Penetrans. J. Gen. Virol. 2019, 100, 704–708. [Google Scholar] [CrossRef]
- Vieira, P.; Subbotin, S.A.; Alkharouf, N.; Eisenback, J.; Nemchinov, L.G. Expanding the RNA Virome of Nematodes and Other Soil-Inhabiting Organisms. Virus Evol. 2022, 8, veac019. [Google Scholar] [CrossRef]
| Sample | Raw Reads | Post-Trimming Reads | Contigs de novo Assembled | AfBLV 1 | # of Reads Assembled to the AfBLV Genome | # of Reads (and %) Assembled to Fungi Genomes | # of reads (and %) Mapped to A. sporoboliae | # of Reads (and %) Mapped to S. maritimus |
|---|---|---|---|---|---|---|---|---|
| A1 | 53,376,244 | 34,452,026 | 28,061 | + | 157,584 | 2074 (0.0060%) | 30,031,802 (87.17%) | 21,627 (0.0628%) |
| B2 | 62,948,370 | 44,743,366 | 30,048 | + | 191,913 | 2037 (0.0046%) | 40,161,846 (89.76%) | 24,340 (0.0544%) |
| C3 | 58,966,450 | 36,426,824 | 28,292 | + | 140,764 | 1491 (0.0041%) | 30,341,392 (83.29%) | 21,435 (0.0588%) |
| D4 | 50,944,370 | 32,743,486 | 28,154 | + | 187,638 | 1973 (0.0060%) | 28,728,534 (87.74%) | 30,158 (0.0921%) |
| E5 | 56,670,030 | 36,513,632 | 27,370 | + | 152,651 | 1601 (0.0044%) | 30,280,354 (82.93%) | 24,057 (0.0659%) |
| F6 | 50,546,932 | 29,786,438 | 22,846 | + | 79,619 | 891 (0.0030%) | 23,349,988 (78.39%) | 14,400 (0.0483%) |
| G7 | 84,220,492 | 49,407,814 | 23,567 | + | 86,006 | 2126 (0.0043%) | 38,836,542 (78.60%) | 24,243 (0.0491%) |
| H8 | 83,110,782 | 49,872,072 | 24,216 | + | 85,055 | 1866 (0.0037%) | 39,912,720 (80.03%) | 23,123 (0.0464%) |
| I9 | 84,308,846 | 50,441,592 | 25,032 | + | 104,713 | 777 (0.0015%) | 40,338,140 (79.97%) | 19,926 (0.0395%) |
| ‘BarV’ MW826417 | Barnaviridae sp. MZ218180 | Barnaviridae sp. MZ218210 1 | |||||
|---|---|---|---|---|---|---|---|
| nt | aa | nt | aa | nt | aa | ||
| AfBLV | ORF 1 2 | 66.5 | 61.2 | NS | NS | NS | NS |
| ORF 2 | 75.0 | 74.2 | NS | 35.3 | 51.3 | 49.9 | |
| ORF 3 | 64.2 | 69.8 | NS | NS | - | - | |
| ORF 4 | NS 3 | NS | NS | NS | - | - | |
| Genome | 64.7 | - | NS | - | NS | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Proaño, E.; Griffin, A.M.; Duarte, A.; Sheng, H.; Schroeder, B.K.; Murray, T.D.; Karasev, A.V. Afrina barna-like Virus, a Novel Virus Associated with Afrina sporoboliae, the Drop Seed Gall-Forming Nematode. Viruses 2025, 17, 1032. https://doi.org/10.3390/v17081032
Reyes-Proaño E, Griffin AM, Duarte A, Sheng H, Schroeder BK, Murray TD, Karasev AV. Afrina barna-like Virus, a Novel Virus Associated with Afrina sporoboliae, the Drop Seed Gall-Forming Nematode. Viruses. 2025; 17(8):1032. https://doi.org/10.3390/v17081032
Chicago/Turabian StyleReyes-Proaño, Edison, Anna M. Griffin, Aida Duarte, Hongyan Sheng, Brenda K. Schroeder, Timothy D. Murray, and Alexander V. Karasev. 2025. "Afrina barna-like Virus, a Novel Virus Associated with Afrina sporoboliae, the Drop Seed Gall-Forming Nematode" Viruses 17, no. 8: 1032. https://doi.org/10.3390/v17081032
APA StyleReyes-Proaño, E., Griffin, A. M., Duarte, A., Sheng, H., Schroeder, B. K., Murray, T. D., & Karasev, A. V. (2025). Afrina barna-like Virus, a Novel Virus Associated with Afrina sporoboliae, the Drop Seed Gall-Forming Nematode. Viruses, 17(8), 1032. https://doi.org/10.3390/v17081032





