Reactivated CMV Proctitis/Anitis Presenting as a Localized Proximal Anal Swelling and Anal Pain in a Diabetic Patient: Case Report and Literature Review
Abstract
1. Introduction
2. Materials and Methods
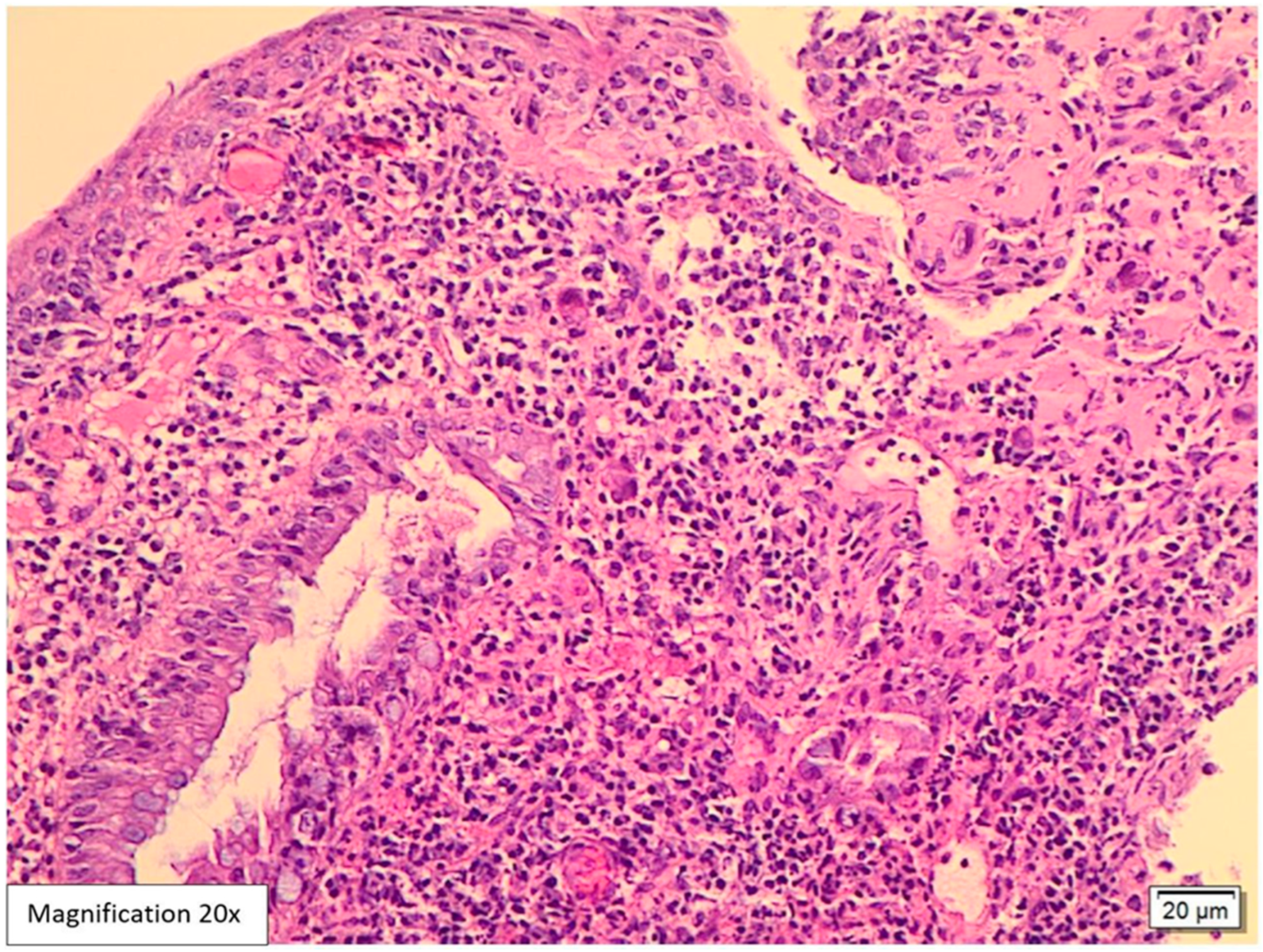
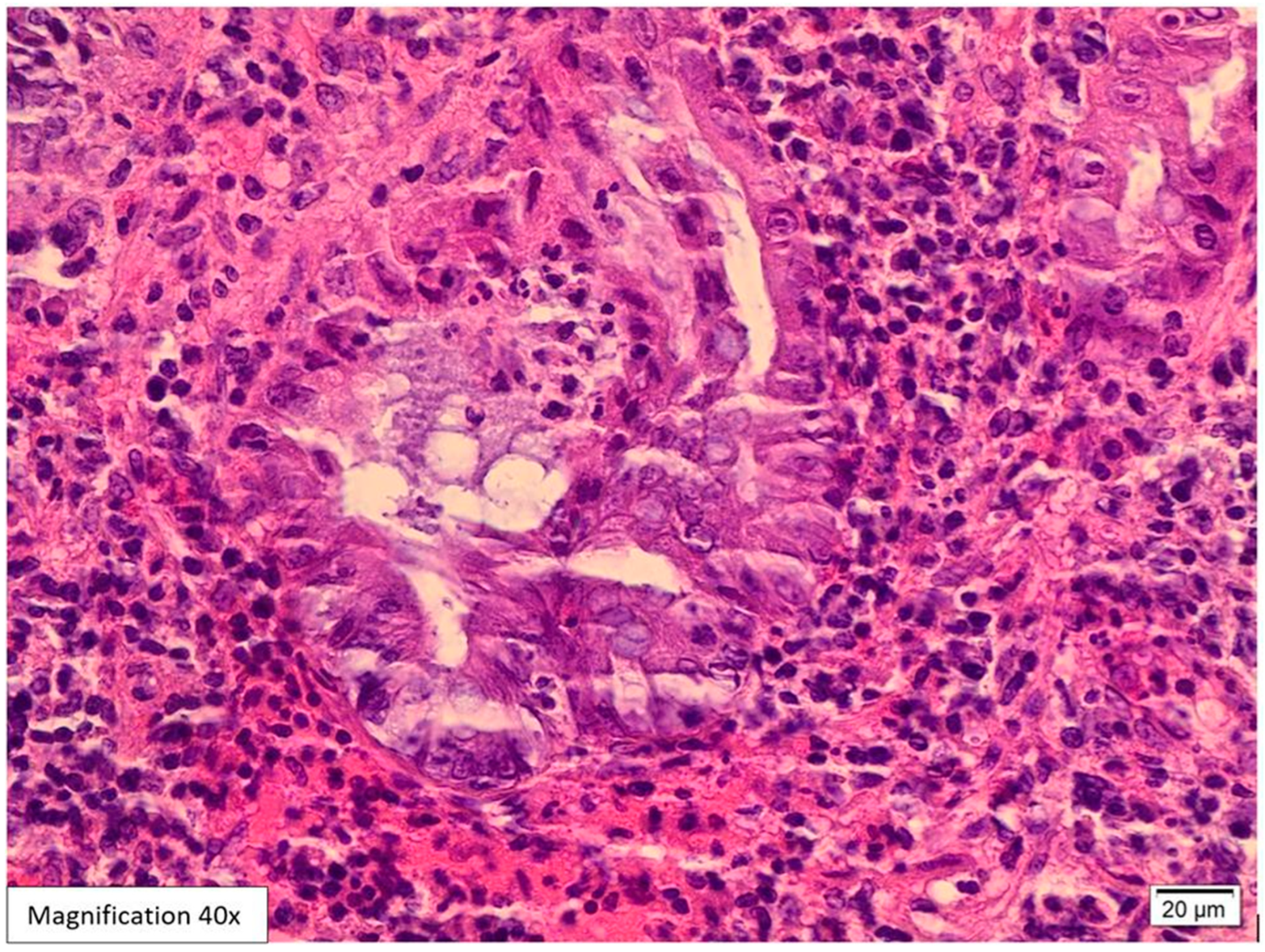
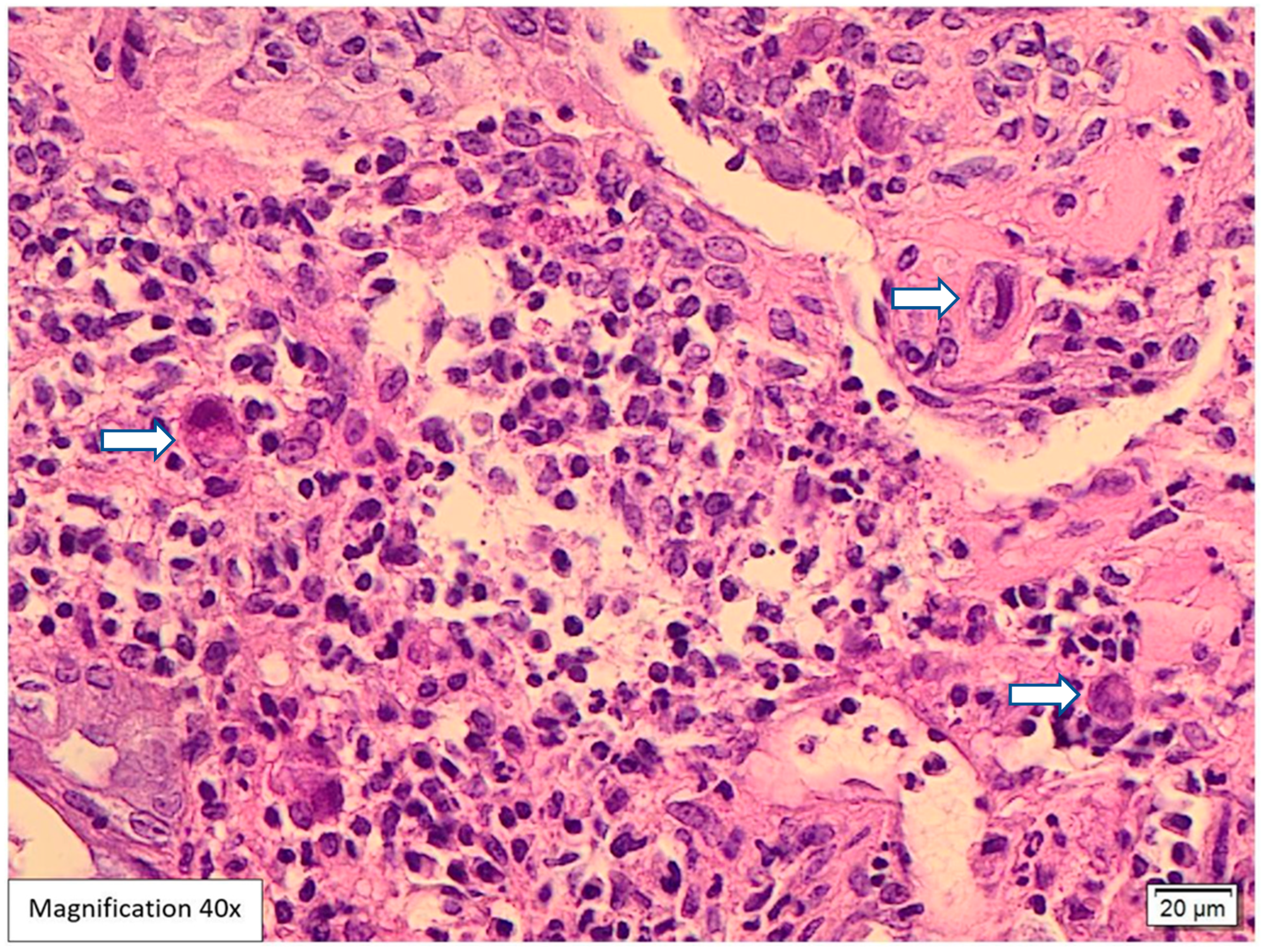
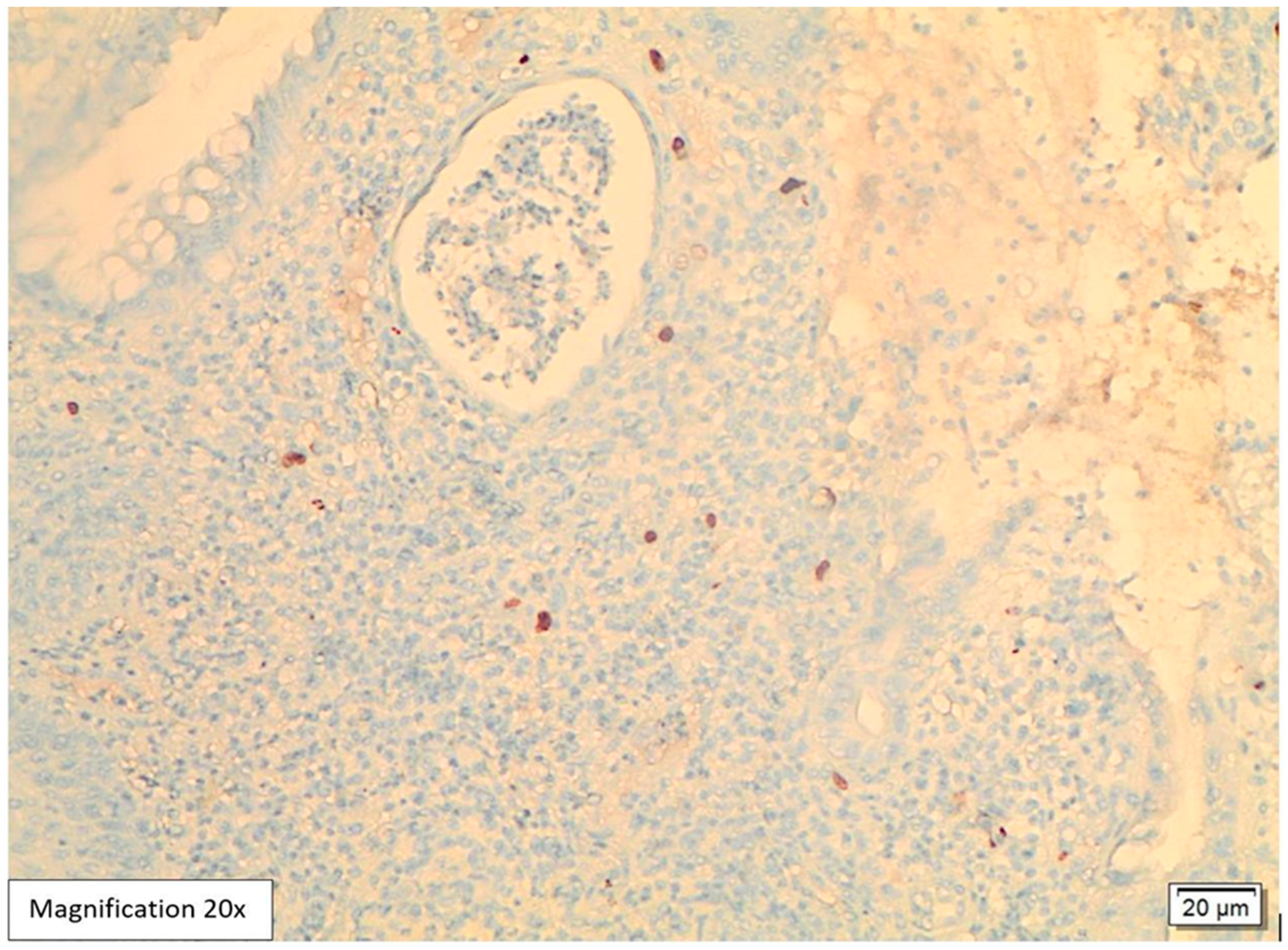
Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMV | Cytomegalovirus |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemistry |
| CT | Computed tomography |
| IBD | Inflammatory bowel disease |
| COVID-19 | Coronavirus disease 2019 |
| DM | Diabetes mellitus |
| PCR | Polymerase chain reaction |
| ICU | Intensive care unit |
References
- Yerushalmy-Feler, A.; Padlipsky, J.; Cohen, S. Diagnosis and Management of CMV Colitis. Curr. Infect. Dis. Rep. 2019, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Li, X. Human cytomegalovirus: Pathogenesis, prevention, and treatment. Mol. Biomed. 2024, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pang, J.; Dong, L.; Yu, X. Structural basis for genome packaging, retention, and ejection in human cytomegalovirus. Nat. Commun. 2021, 12, 4538. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, P.I.; Mourtzoukou, E.G.; Varbobitis, I.C.; Falagas, M.E. Severe cytomegalovirus infection in apparently immunocompetent patients: A systematic review. Virol. J. 2008, 5, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azer, S.A.; Limaiem, F. Cytomegalovirus Colitis [Updated 1 May 2022]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542231 (accessed on 7 July 2025).
- Shieh, A.C.; Guler, E.; Tirumani, S.H.; Dumot, J.; Ramaiya, N.H. Clinical, imaging, endoscopic findings, and management of patients with CMV colitis: A single- institute experience. Emerg. Radiol. 2020, 27, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Germi, R.; Lupo, J.; Laverrière, M.-H.; Masse, V.; Morand, P.; Gavazzi, G. Symptomatic cytomegalovirus gastrointestinal infection with positive quantitative real-time PCR findings in apparently immunocompetent patients: A case series. Clin. Microbiol. Infect. 2015, 21, 1121.e1–1121.e7. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Peck, K.R.; Lee, W.J.; Lee, J.Y.; Cho, S.Y.; Ha, Y.E.; Kang, C.-I.; Chung, D.R.; Kim, Y.-H.; Lee, N.Y.; et al. Clinical presentation and risk factors for Cytomegalovirus colitis in immunocompetent adult patients. Clin. Infect. Dis. 2015, 60, e20–e26. [Google Scholar] [CrossRef] [PubMed]
- Bontà, J.; Zeitz, J.; Frei, P.; Biedermann, L.; Sulz, M.C.; Vavricka, S.R.; Scharl, S.; Fried, M.; Rogler, G.; Scharl, M. Cytomegalovirus disease in inflammatory bowel disease: Epidemiology and disease characteristics in a large single-centre experience. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, A.M.; Santos, F.M.; Ortiz-Agostinho, C.L.; Nishitokukado, I.; Frota, C.S.; Gomes, F.U.; Leite, A.Z.d.A.; Pannuti, C.S.; Boas, L.S.V.; Teixeira, M.G.; et al. Cytomegalovirus infection in inflammatory bowel disease is not associated with worsening of intestinal inflammatory activity. PLoS ONE 2014, 9, e111574. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, S.; O’Sullivan, A.; Adesina, T.; Gwozdz, A.M.; Rees, J.; Satta, G. Cytomegalovirus proctitis mimicking rectal cancer in an immunocompetent elderly patient: A case report. BMC Res. Notes 2014, 7, 799. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Kumar, N.L.; McNabb-Baltar, J.Y. Cytomegalovirus colitis presenting as a rectal mass. J. Dig. Endosc. 2017, 8, 137–139. [Google Scholar] [CrossRef]
- Nadella, D.; Tandon, A.; Dara, P. A Case of Cytomegalovirus Infection Mimicking Rectal Malignancy in an Immunocompetent Patient. J. Hosp. Med. 2020. Available online: https://shmabstracts.org/abstract/a-case-of-cytomegalovirus-infection-mimicking-rectal-malignancy-in-an-immunocompetent-patient/ (accessed on 15 August 2022).
- Hirayama, Y.; Ando, T.; Hirooka, Y.; Watanabe, O.; Miyahara, R.; Nakamura, M.; Yamamura, T.; Goto, H. Characteristic endoscopic findings and riskfactors for cytomegalovirus-associated colitis in patients with active ulcerative colitis. World J. Gastrointest Endosc. 2016, 8, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.M.; Guo, F.P.; Copland, A.P.; Pai, R.K.; Pinsky, B.A. A comparison of CMV detection in gastrointestinal mucosal biopsies using immunohistochemistryand PCR performed on formalin-fixed, paraffinembedded tissue. Am. J. Surg. Pathol. 2013, 37, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, W.; Lv, H.; Wu, D.; Feng, Y.; Shu, H.; Jin, M.; Hu, L.; Wang, Q.; Wu, D.; et al. The association between CMV viremia or endoscopic features and histopathological characteristics of CMV colitis in patients with underlying ulcerative colitis. Inflamm. Bowel Dis. 2017, 23, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Aomatsu, K.; Nakamura, M.; Aomatsu, N.; Aomatsu, K. Cytomegalovirus colitis followed by ischemic colitis in a nonimmunocompromised adult: A case report. World J. Gastroenterol. 2015, 21, 3750–3754. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Zayyani, N.R. Cytomegalovirus colitis masquerading asrectal malignancy in an immunocompetent patient. Indian J. Pathol. Microbiol. 2015, 58, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Bahng, S.; Kang, K.J.; Ku, B.-H.; Jo, Y.C.; Kim, J.Y.; Chang, D.K.; Son, H.J.; Rhee, P.-L.; Kim, J.J.; et al. Cytomegalovirus colitis in patients without inflammatory bowel disease: A single center study. Scand. J. Gastroenterol. 2010, 45, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Klauber, E.; Briski, L.E.; Khatib, R. Cytomegalovirus colitis in the immuncompetent host: An overview. Scand. J. Infect. Dis. 1998, 30, 559–564. [Google Scholar] [PubMed]
- Studemeister, A. Cytomegalovirus proctitis: A rare and disregarded sexually transmitted disease. Sex. Transm. Dis. 2011, 38, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Bromley, T.; Lewis, K.; Fitzpatrick, C.; Richardson, D. Factors Associated with Cytomegalovirus (CMV) Procto-Colitis in Immunocompetent Adults: A Systematic Review. Eur. J. Ther. 2024, 30, 409–418. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chen, Y.-H.; Lu, P.-L. Reactivated cytomegalovirus proctitis in an immunocompetent patient presenting as nosocomial diarrhea: A case report and literature review. BMC Infect. Dis. 2017, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.-H.; Chau, T.-N.; Cheung, T.-C.; Kng, C.; Wong, S.-Y.; Ng, W.-F.; Lee, K.-C.; Chan, E.; Lai, S.-T.; Yuen, W.-C.; et al. Cytomegalovirus colitis in individuals without apparent cause of immunodeficiency. Dig. Dis. Sci. 1999, 44, 945–952. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.H.; Post, K.; Sen, J.D.; Chang, H.Y.; Zhao, Z.; Fan, R.; Chen, S.; Leland, D.; Cheng, L.; Lin, J. qPCR increases sensitivity to detect cytomegalovirus in formalinfixed, paraffin-embedded tissue of gastrointestinal biopsies. Hum. Pathol. 2014, 45, 48–53. [Google Scholar] [CrossRef] [PubMed]
- De la Hoz, R.E.; Stephens, G.; Sherlock, C. Diagnosis and Treatment Approaches to CMV Infections in Adult Patients. J. Clin. Virol. 2002, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kishore, J.; Ghoshal, U.; Ghoshal, U.C.; Krishnani, N.; Kumar, S.; Singh, M.; Ayyagari, A. Infection with Cytomegalovirus in Patients with Inflammatory Bowel Disease: Prevalence, Clinical Significance and Outcome. J. Med. Microbiol. 2004, 53, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Maresca, R.; Varca, S.; Di Vincenzo, F.; Ainora, M.E.; Mignini, I.; Papa, A.; Scaldaferri, F.; Gasbarrini, A.; Giustiniani, M.C.; Zocco, M.A.; et al. Cytomegalovirus Infection: An Underrated Target in Inflammatory Bowel Disease Treatment. J. Clin. Med. 2024, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Garrido, E.; Carrera, E.; Manzano, R.; Lopez-Sanroman, A. Clinical significance of cytomegalovirus infection in patients with inflammatory bowel disease. World J. Gastroenterol. 2013, 19, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Shimbo, T.; Sekine, K.; Tanaka, S.; Niikura, R.; Mezaki, K.; Morino, E.; Yazaki, H.; Igari, T.; Ohmagari, N.; et al. Combined endoscopy, aspiration, andbiopsy analysis for identifying infectious colitis in patients with ileocecal ulcers. Clin. Gastroenterol. Hepatol. 2013, 11, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.N.; Prasad, V.; Klein, M.D. Cytomegalovirus enteritis mimicking Crohn’s disease in a lupus nephritis patient: A case report. World J. Gastroenterol. 2009, 15, 4327–4330. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Tange, T. Prevalence of cytomegalovirus infection ininflammatory bowel disease patients. Dis. Colon. Rectum 2004, 47, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Rezania, D.; Ouban, A.; Marcet, J.; Kelley, S.; Coppola, D. CMV colitis mimicking recurrent inflammatory bowel disease: Report of three cases. Am. Surg. 2007, 73, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Drew, W.L. Cytomegalovirus infection in patients with AIDS. J. Infect. Dis. 1988, 158, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Galiatsatos, P.; Shrier, I.; Lamoureux, E.; Szilagyi, A. Meta-analysis of outcome of cytomegalovirus colitis in immunocompetent hosts. Dig. Dis. Sci. 2005, 50, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Harano, Y.; Kotajima, L.; Arioka, H. Case of cytomegalovirus colitis in an immunocompetent patient: A rare cause of abdominal pain and diarrhea in the elderly. Int. J. Gen. Med. 2015, 3, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. 1), S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.K.; Samsom, M.; Jones, K.L.; Horowitz, M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001, 24, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Schimke, K.; Chubb, S.A.; Davis, W.A.; Philipst, P.; Davis, T.M. Antiplatelet therapy, Helicobacter pylori infection and complicated peptic ulcer disease in diabetes: The fremantle diabetes study. Diabet. Med. 2009, 26, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.X.; Papaioannou, G.; Karga, H.; Roussos, A.; Mantzaris, G.J. Helicobacter pylori infection and endocrine disorders: Is there a link? World J. Gastroenterol. 2009, 15, 2701–2707. [Google Scholar] [CrossRef] [PubMed]
- Gunji, T.; Matsuhashi, N.; Sato, H.; Fujibayashi, K.; Okumura, M.; Sasabe, N.; Urabe, A. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter 2009, 14, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Elhawary, E.I.; Mahmoud, G.F.; El-Daly, M.A.; Mekky, F.A.; Esmat, G.G.; Abdel-Hamid, M. Association of UVV with diabetes mellitus: An Egyptian case-control study. Virol. J. 2011, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.M.; Bour, J.B.; Galland-Jos, C.; Minello, A.; Verges, B.; Guiguet, M. Risk factors for diabetes mellitus and insulin resistance in chronic hepatitis C. J. Hepatol. 2001, 35, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Serin, E.; Göktürk, S.; Ozturk, N.A.; Kulaksizoglu, S.; Ylmaz, U. The prevalence of occult hepatitis B virus infection in type 2 diabetes mellitus patients. Eu. J. Gastroenterol. Hepatol. 2008, 20, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.D.; Perz, J.F. Eliminating the blood: Ongoing outbreaks of hepatitis B virus infection and the need for innovative glucose monitoring technologies. J. Diabetes Sci. Technol. 2009, 3, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.-J.; Wu, R.-C.; Chen, C.-L.; Chiu, C.-T.; Lai, M.-W.; Chen, C.-C.; Chiu, C.-H.; Pan, Y.-B.; Lin, W.-R.; Le, P.-H. Cytomegalovirus Diseases of the Gastrointestinal Tract in Immunocompetent Patients: A Narrative Review. Viruses 2024, 16, 346. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Affolter, K.; Boynton, K.; Chen, X.; Valentine, J.; Peterson, K. CMV Disease in IBD: Comparison of Diagnostic Tests and Correlation with Disease Outcome. Inflamm. Bowel Dis. 2018, 24, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, G.; Moss, A.C. Cytomegalovirus in inflammatory bowel disease: Pathogen or innocent bystander? Inflamm. Bowel Dis. 2010, 16, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Platt, M.; Platt, S.; Royston, D.D. Lymphocyte proliferation: Dichotomy of effect of related anaesthetic agents. Br. J. Anaesth. 1986, 58, 132. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuquteish, D.; Al Oqaily, A.; Bataineh, L.; Khater, B. Reactivated CMV Proctitis/Anitis Presenting as a Localized Proximal Anal Swelling and Anal Pain in a Diabetic Patient: Case Report and Literature Review. Viruses 2025, 17, 1023. https://doi.org/10.3390/v17081023
Abuquteish D, Al Oqaily A, Bataineh L, Khater B. Reactivated CMV Proctitis/Anitis Presenting as a Localized Proximal Anal Swelling and Anal Pain in a Diabetic Patient: Case Report and Literature Review. Viruses. 2025; 17(8):1023. https://doi.org/10.3390/v17081023
Chicago/Turabian StyleAbuquteish, Dua, Ayat Al Oqaily, Lama Bataineh, and Bashar Khater. 2025. "Reactivated CMV Proctitis/Anitis Presenting as a Localized Proximal Anal Swelling and Anal Pain in a Diabetic Patient: Case Report and Literature Review" Viruses 17, no. 8: 1023. https://doi.org/10.3390/v17081023
APA StyleAbuquteish, D., Al Oqaily, A., Bataineh, L., & Khater, B. (2025). Reactivated CMV Proctitis/Anitis Presenting as a Localized Proximal Anal Swelling and Anal Pain in a Diabetic Patient: Case Report and Literature Review. Viruses, 17(8), 1023. https://doi.org/10.3390/v17081023





