Kyasanur Forest Disease Virus: Epidemiological Insights, Pathogenesis, Therapeutic Strategies, and Advances in Vaccines and Diagnostics
Abstract
1. Introduction
2. KFDV Epidemiology
2.1. Transmission of KFDV
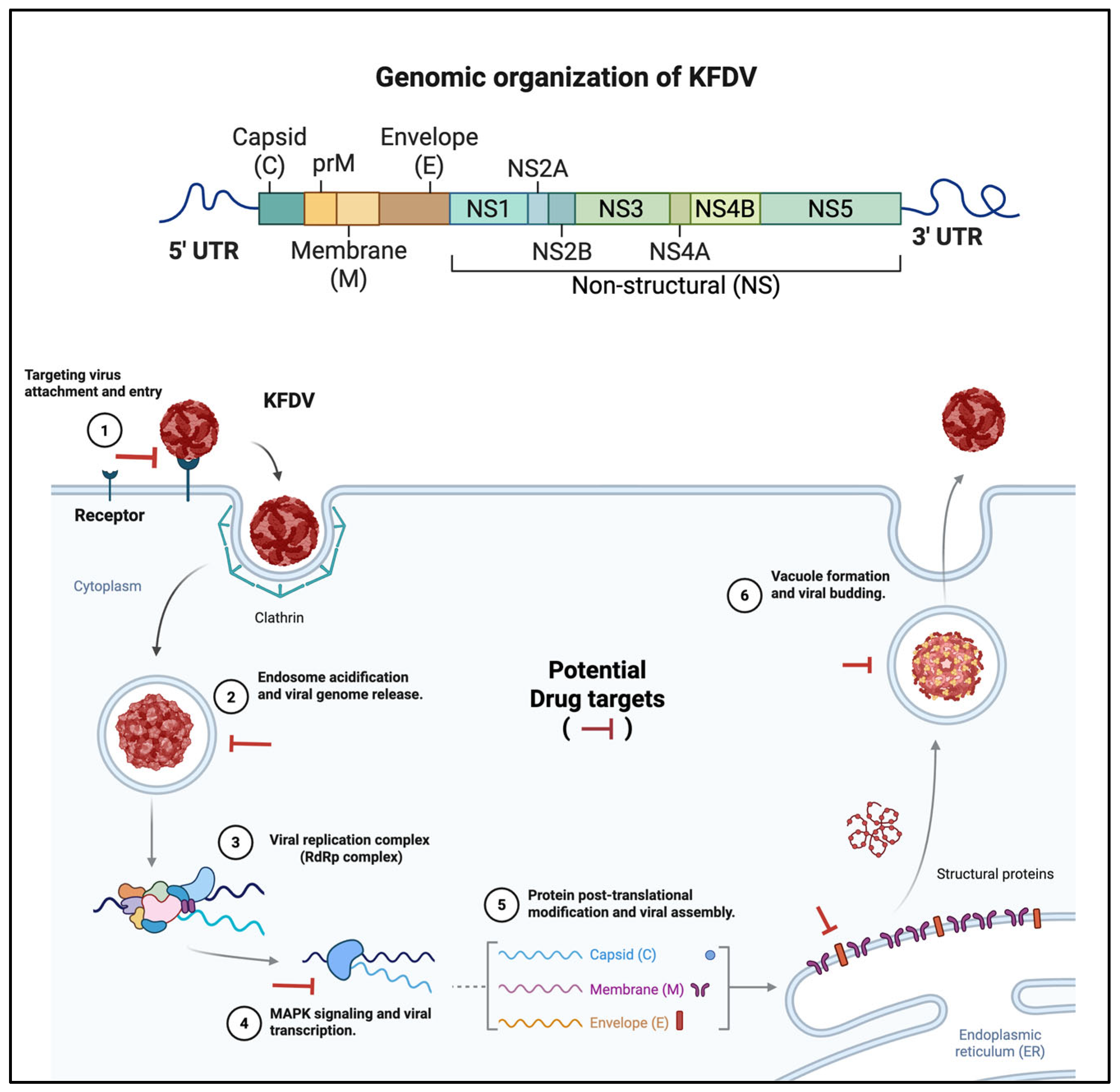
2.2. KFD Virus
2.3. KFDV Pathogenesis
2.4. Therapeutic Options for KFDV
2.5. Current Status
2.6. Repurposed Drugs
| Therapeutic Agent | Mechanism of Action | Evidence of Activity | Current Status | Notes for KFDV Potential |
|---|---|---|---|---|
| NITD008 | Nucleoside analog; inhibits RNA synthesis | In vitro activity against KFDV, DENV, Zika [21,44,55] | Preclinical; discontinued due to toxicity | Demonstrated KFDV inhibition; safer analogs needed; AG129 model testing pending. |
| Favipiravir | RNA-dependent RNA polymerase inhibitor | In vitro/vivo activity against Ebola, DENV [56,57] | Clinically approved (e.g., influenza); not tested for KFDV | Broad-spectrum; potential for KFDV NS5 targeting; clinical trials required. |
| Sofosbuvir | NS5B polymerase inhibitor | In vitro activity against HCV, DENV [46,58] | Clinically approved (HCV); not tested for KFDV | Flavivirus cross-reactivity possible; in vivo KFDV studies needed. |
| Niclosamide | Induces autophagy; inhibits NS2B-NS3 protease | In vitro and in vivo activity [52,53] against DENV and SARS-CoV-2 [59] | FDA-approved (anthelmintic); not tested for KFDV | Promising for KFDV due to flavivirus similarity; AG129 model adaptation suggested. |
| Monoclonal Antibodies | Neutralizes E protein; blocks viral entry | Effective in DENV passive immunization [60] | Preclinical/early clinical for DENV; none for KFDV | KFDV-specific antibodies needed; risk of cross-reactivity with other flaviviruses. |
2.7. Challenges in Developing Therapeutics for KFDV
2.8. Vaccines
2.9. Available Vaccine for KFDV
2.10. Novel Vaccine Candidates
| Vaccine Name | Platform/ Technology | Evidence of Efficacy | Current Status | Notes for KFDV Potential |
|---|---|---|---|---|
| Formalin-Inactivated Vaccine | Inactivated whole virus (mouse brain-derived) | 62.4% efficacy (2 doses), 82.9% (with boosters) [14,43] | In use since the 1960s; suspended in 2022 | Partial protection; waning immunity; production challenges; booster dependency. |
| VSV-Based Vaccine | Recombinant vesicular stomatitis virus (VSV) expressing KFDV E protein | 100% protection in BALB/c mice [16]; reduced viral load in macaques [15]; cross-protects against AHFV | Preclinical (mice, macaques) | Promising efficacy and safety; Phase I/II trials needed; scalable production is potential. |
| Multi-Epitope Subunit Vaccine | Recombinant subunit (in silico designed E protein epitopes) | Strong B/T-cell responses predicted in silico; binds TLR-2 [18] | Preclinical (in silico) | Cost-effective; in vivo validation pending; potential AHFV cross-protection. |
| mRNA Vaccine | mRNA encoding KFDV antigens | Effective for Zika, SARS-CoV-2 [65,66,67] | None of the study done for KFDV | Rapid development potential; adaptable to strains; requires KFDV-specific design. |
| Live-Attenuated Vaccine | Attenuated KFDV strain | Successful for yellow fever [68] 2017, DENV [69] | Not developed for KFDV | Could induce robust immunity; safety concerns need addressing; preclinical testing needed. |
2.11. Challenges in KFDV Vaccination and Development
3. Diagnostics
3.1. Current Diagnostic Tools
3.2. Challenges in KFDV Diagnosis
4. Discussion
4.1. Research Gaps
4.2. Future Directions
4.3. Global Implications and Integrated Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Work, T.H.; Trapido, H.; Murthy, D.P.N.; Rao, R.L.; Bhatt, P.N.; Kulkarni, K.G. Kyasanur forest disease III. A preliminary report on the nature of the infection and clinical manifestations in human beings. Indian J. Med. Sci. 1957, 11, 619–645. [Google Scholar] [PubMed]
- Work, T.H. Russian spring-summer virus in India: Kyasanur Forest disease. Prog. Med. Virol. 1958, 1, 248–279. [Google Scholar] [PubMed]
- Trapido, H.; Rajagopalan, P.K.; Work, T.H.; Varma, M.G. Kyasanur Forest disease. VIII. Isolation of Kyasanur Forest disease virus from naturally infected ticks of the genus Haemaphysalis. Indian J. Med. Res. 1959, 47, 133–138. [Google Scholar] [PubMed]
- Holbrook, M.R. Ocular manifestations of Kyasanur forest disease (a clinical study). Indian J. Ophthalmol. 1983, 31, 700–702. [Google Scholar]
- Wadia, R.S. Neurological Involvement in Kyasanur Forest Disease. Neurol. India 1975, 23, 115–120. [Google Scholar] [PubMed]
- Muraleedharan, M. Kyasanur Forest Disease (KFD): Rare Disease of Zoonotic Origin. J. Nepal Health Res. Counc. 2016, 14, 214–218. [Google Scholar] [PubMed]
- Chakraborty, S.; Andrade, F.C.D.; Ghosh, S.; Uelmen, J.; Ruiz, M.O. Historical Expansion of Kyasanur Forest Disease in India from 1957 to 2017: A Retrospective Analysis. Geohealth 2019, 3, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Srilekha, N.; Kandi, V.; Jayashankar, C.A.; Harshitha, A.; Akshay, A.S.; Kapil, C.; Palacholla, P.S. Kyasanur Forest Disease: A Comprehensive Review. Cureus 2024, 16, e65228. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Anand, A.; Singh, A.; Khare, A.; Neyazi, A.; Rustagi, S.; Kukreti, N.; Gaidhane, A.M.; Zahiruddin, Q.S.; Satapathy, P. Kyasanur Forest Disease: Clinical manifestations and molecular dynamics in a zoonotic landscape. Clin. Infect. Pract. 2024, 21, 100352, ISSN 2590-1702. [Google Scholar] [CrossRef]
- Ajesh, K.; Nagaraja, B.K.; Sreejith, K. Kyasanur forest disease virus breaking the endemic barrier: An investigation into ecological effects on disease emergence and future outlook. Zoonoses Public Health 2017, 64, e73–e80. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Lendrum, D.; Manga, L.; Bagayoko, M.; Sommerfeld, J. Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20130552. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Agrawal, R.; Murmu, J.; Kanungo, S.; Pati, S. Does the rise in cases of Kyasanur forest disease call for the implementation of One Health in India? IJID Reg. 2023, 7, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, C.N.; Desai, G.B.; Achar, T.R.; Banerjee, K. Field evaluation of formalin inactivated Kyasanur forest disease virus tissue culture vaccine in three districts of Karnataka state. Indian J. Med. Res. 1994, 99, 152–158. [Google Scholar] [PubMed]
- Kasabi, G.S.; Murhekar, M.V.; Sandhya, V.K.; Raghunandan, R.; Kiran, S.K.; Channabasappa, G.H.; Mehendale, S.M.; Bausch, D.G. Coverage and effectiveness of Kyasanur forest disease (KFD) vaccine in Karnataka, South India, 2005–2010. PLoS Negl. Trop. Dis. 2013, 7, e2025. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.; Tang-Huau, T.-L.; Feldmann, F.; Hanley, P.W.; Rosenke, R.; Shaia, C.; Marzi, A.; Feldmann, H. Single-dose VSV-based vaccine protects against Kyasanur Forest disease in nonhuman primates. Sci. Adv. 2023, 9, eadj1428. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.; Meade-White, K.; Haddock, E.; Feldmann, F.; Marzi, A.; Feldmann, H. A live-attenuated viral vector vaccine protects mice against lethal challenge with Kyasanur Forest disease virus. NPJ Vaccines 2021, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Kumar, V.; Madhukalya, R.; Gupta, R.; Agarwal, V.; Choudhary, S.; Bhutkar, M.; Tomar, S.; Kumar, D.; Kumar, R. Purification and characterization of kyasanur forest disease virus EDIII domain of major envelope glycoprotein. J. Virol. Methods. 2025, 333, 115089. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Varamballi, P. In-silico design of envelope based multi-epitope vaccine candidate against Kyasanur forest disease virus. Sci. Rep. 2021, 11, 17118. [Google Scholar] [CrossRef] [PubMed]
- Kasibhatla, S.M.; Rajan, L.; Shete, A.; Jani, V.; Yadav, S.; Joshi, Y.; Sahay, R.; Patil, D.Y.; Mohandas, S.; Majumdar, T.; et al. Construction of an immunoinformatics-based multi-epitope vaccine candidate targeting Kyasanur forest disease virus. PeerJ. 2025, 13, e18982. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.B.; Jeyachandran, A.; Pariyapurath, N.K.; Jagadibabu, S.; Rajaiah, P.; Channappa, S.K.; Pachamuthu, R.G.; Premkumar, A.A.; Jagannathan, S. Advancements in Vaccine Development: A Comprehensive Design of a Multi-Epitopic Immunodominant Peptide Vaccine Targeting Kyasanur Forest Disease via Reverse Vaccinology; Cold Spring Harbor Laboratory: New York, NY, USA, 2025. [Google Scholar] [CrossRef]
- Lo, M.K.; Shi, P.Y.; Chen, Y.L.; Flint, M.; Spiropoulou, C.F. In vitro antiviral activity of adenosine analog NITD008 against tick-borne flaviviruses. Antivir. Res. 2016, 130, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Sharma, S.; Dash, P.K.; Dhankher, S.; Sandhya, V.K.; Kiran, S.K. Dry-down probe free qPCR for detection of KFD in resource limited settings. PLoS ONE 2023, 18, e0284559. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; De Silva, I.; Millar, D.S. Latest Advances in Arbovirus Diagnostics. Microorganisms 2023, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L. The Impacts of Climate Change on Ticks and Tick-Borne Disease Risk. Annu. Rev. Entomol. 2021, 66, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, P. Kyasanur forest disease: An epidemiological view in India. Rev. Med. Virol. 2006, 16, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Munivenkatappa, A.; Sahay, R.R.; Yadav, P.D.; Viswanathan, R.; Mourya, D.T. Clinical & epidemiological significance of Kyasanur forest disease. Indian J. Med. Res. 2018, 148, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Rajaiah, P. Kyasanur Forest Disease in India: Innovative options for intervention. Hum. Vaccin. Immunother. 2019, 15, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Yadav, P.D.; Sahina, S.; Nadh, V.A. The species distribution of ticks & the prevalence of Kyasanur forest disease virus in questing nymphal ticks from Western Ghats of Kerala, South India. Indian J. Med. Res. 2021, 154, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Vedachalam, S.K.; Rajput, B.L.; Choudhary, S.; Narayanaswamy, D.; Chandra, S.; Pallavi, M.D.; Rajagopal, P.M.; Dikid, T. Kyasanur Forest Disease: An Epidemiological Investigation and Case-Control Study in Shivamogga, Karnataka, India-2022. Int. J. Public Health 2024, 69, 1606715. [Google Scholar] [CrossRef] [PubMed]
- Vedachalam, S.K.; Rajput, B.L.; Choudhary, S.; Narayanaswamy, D.; Chandra, S.; Pallavi, M.D.; Rajagopal, P.M.; Dikid, T. Descriptive epidemiology of Kyasanur forest disease in Thirthahalli taluk, Shivamogga, Karnataka, 2018–2022. Discov. Public Health 2025, 22, 130. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sander, W.; Allan, B.F.; Andrade, F.C.D. Sociodemographic factors associated with Kyasanur forest disease in India-a retrospective study. IJID Reg. 2024, 10, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bhat, H.R.; Naik, S.V. Transmission of Kyasanur forest disease virus by Haemaphysalis wellingtoni Nuttall and Warburton, 1907 (Acarina: Ixodidae). Indian J. Med. Res. 1978, 67, 697–703. [Google Scholar] [PubMed]
- Zhang, C.; Li, Y.; Samad, A.; He, H.; Ma, H.; Chen, Y.; Jin, T. Kyasanur Forest disease virus NS3 helicase: Insights into structure, activity, and inhibitors. Int. J. Biol. Macromol. 2024, 254, 127856. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.W.; Cutts, T.A.; Court, D.A.; Theriault, S. The generation of a reverse genetics system for Kyasanur Forest Disease Virus and the ability to antagonize the induction of the antiviral state in vitro. Virus Res. 2012, 163, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.W.M.; Ranadheera, C.; Nikiforuk, A.M.; Cutts, T.A.; Kobasa, D.; Court, D.A.; Theriault, S.S.; Singh, S.K. Limited Effects of Type I Interferons on Kyasanur Forest Disease Virus in Cell Culture. PLoS Negl. Trop. Dis. 2016, 10, e0004871. [Google Scholar] [CrossRef] [PubMed]
- Sirmarova, J.; Salat, J.; Palus, M.; Hönig, V.; Langhansova, H.; Holbrook, M.R.; Ruzek, D. Kyasanur Forest disease virus infection activates human vascular endothelial cells and monocyte-derived dendritic cells. Emerg. Microbes. Infect. 2018, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Sawatsky, B.; McAuley, A.J.; Holbrook, M.R.; Bente, D.A. Comparative pathogenesis of Alkhumra hemorrhagic fever and Kyasanur forest disease viruses in a mouse model. PLoS Negl. Trop. Dis. 2014, 8, e2934. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.R.; Yadav, P.D.; Shete, A.; Chaubal, G.; Mohandas, S.; Sahay, R.R.; Jain, R.; Mote, C.; Kumar, S.; Kaushal, H.; et al. Study of Kyasanur forest disease viremia, antibody kinetics, and virus infection in target organs of Macaca radiata. Sci. Rep. 2020, 10, 12561. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.J.; Mitzel, D.N.; Taylor, R.T.; Best, S.M.; Bloom, M.E. Tick-borne flaviviruses: Dissecting host immune responses and virus countermeasures. Immunol. Res. 2009, 43, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Avirutnan, P.; Hauhart, R.E.; Somnuke, P.; Blom, A.M.; Diamond, M.S.; Atkinson, J.P. Binding of flavivirus nonstructural protein NS1 to C4b binding protein modulates complement activation. J. Immunol. 2011, 187, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Maguire, T.; Hileman, R.E.; Fromm, J.R.; Esko, J.D.; Linhardt, R.J.; Marks, R.M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 1997, 3, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Kroschewski, H.; Allison, S.L.; Heinz, F.X.; Mandl, C.W. Role of heparan sulfate for attachment and entry of tick-borne encephalitis virus. Virology 2003, 308, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Devadiga, S.; McElroy, A.K.; Prabhu, S.G.; Arunkumar, G. Dynamics of human B and T cell adaptive immune responses to Kyasanur Forest disease virus infection. Sci. Rep. 2020, 10, 15306. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, Y.-L.; Schul, W.; Wang, Q.-Y.; Gu, F.; Duraiswamy, J.; Kondreddi, R.R.; Niyomrattanakit, P.; Lakshminarayana, S.B.; Goh, A. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. USA 2009, 106, 20435–20439. [Google Scholar] [CrossRef] [PubMed]
- Bixler, S.L.; Bocan, T.M.; Wells, J.; Wetzel, K.S.; Van Tongeren, S.A.; Dong, L.; Garza, N.L.; Donnelly, G.; Cazares, L.H.; Nuss, J.; et al. Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus. Antivir. Res. 2018, 151, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Sofosbuvir: A review of its use in patients with chronic hepatitis C. Drugs 2014, 74, 1127–1146. [Google Scholar] [CrossRef] [PubMed]
- Marlin, R.; Desjardins, D.; Contreras, V.; Lingas, G.; Solas, C.; Roques, P.; Naninck, T.; Pascal, Q.; Behillil, S.; Maisonnasse, P.; et al. Antiviral efficacy of favipiravir against Zika and SARS-CoV-2 viruses in non-human primates. Nat. Commun. 2022, 13, 5108. [Google Scholar] [CrossRef] [PubMed]
- Morrey, J.; Taro, B.; Siddharthan, V.; Wang, H.; Smee, D.; Christensen, A.; Furuta, Y. Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents. Antivir. Res. 2008, 80, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Shafer, K.; Smee, D.F.; Morrey, J.D.; Furuta, Y. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob. Agents Chemother. 2009, 53, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Reis, P.A.; de Freitas, C.S.; Sacramento, C.Q.; Hoelz, L.V.B.; Bastos, M.M.; Mattos, M.; Rocha, N.; Quintanilha, I.G.D.A.; Pedrosa, C.D.S.G.; et al. Beyond Members of the Flaviviridae Family, Sofosbuvir also Inhibits Chikungunya Virus Replication. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, C.Q.; de Melo, G.R.; de Freitas, C.S.; Rocha, N.; Hoelz, L.V.B.; Miranda, M.; Fintelman-Rodrigues, N.; Marttorelli, A.; Ferreira, A.C.; Barbosa-Lima, G.; et al. The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication. Sci. Rep. 2017, 7, 40920. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.-C.; HuangFu, W.-C.; Tsai, T.-T.; Ho, M.-R.; Jhan, M.-K.; Shen, T.-J.; Tseng, P.-C.; Wang, Y.-T.; Lin, C.-F.; Beasley, D.W. The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR. PLoS Negl. Trop. Dis. 2018, 12, e0006715. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Papies, J.; Bajaj, T.; Emanuel, J.; Dethloff, F.; Chua, R.L.; Trimpert, J.; Heinemann, N.; Niemeyer, C.; Weege, F.; et al. SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals. Nat. Commun. 2021, 12, 3818. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Brecher, M.; Deng, Y.-Q.; Zhang, J.; Sakamuru, S.; Liu, B.; Huang, R.; Koetzner, C.A.; Allen, C.; Jones, S.A.; et al. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell. Res. 2017, 27, 1046–1064. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.-Q.; Zhang, N.-N.; Li, C.-F.; Tian, M.; Hao, J.-N.; Xie, X.-P.; Shi, P.-Y.; Qin, C.-F. Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus. Open Forum Infect. Dis. 2016, 3, ofw175. [Google Scholar] [CrossRef] [PubMed]
- Guedj, J.; Piorkowski, G.; Jacquot, F.; Madelain, V.; Nguyen, T.H.T.; Rodallec, A.; Gunther, S.; Carbonnelle, C.; Mentré, F.; Raoul, H.; et al. Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques. PLoS Med. 2018, 15, e1002535. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.J.; Pires de Mello, C.P.; Brown, A.N. Antiviral Evaluation of UV-4B and Interferon-Alpha Combination Regimens Against Dengue Virus. Viruses 2021, 13, 771. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-T.; Colby-Germinario, S.P.; Hassounah, S.A.; Fogarty, C.; Osman, N.; Palanisamy, N.; Han, Y.; Oliveira, M.; Quan, Y.; Wainberg, M.A. Evaluation of Sofosbuvir (β-D-2’-deoxy-2’-α-fluoro-2’-β-C-methyluridine) as an inhibitor of Dengue virus replication. Sci. Rep. 2017, 7, 6345. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Touret, F.; Baronti, C.; Gilles, M.; Hoen, B.; Nougairède, A.; de Lamballerie, X.; Sommer, M.O.A.; Polyak, S.J. Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2). PLoS ONE 2021, 16, e0260958. [Google Scholar] [CrossRef] [PubMed]
- Gunale, B.; Farinola, N.; Kamat, C.D.; Poonawalla, C.S.; Pisal, S.S.; Dhere, R.M.; Miller, C.; Kulkarni, P.S. An observer-blind, randomised, placebo-controlled, phase 1, single ascending dose study of dengue monoclonal antibody in healthy adults in Australia. Lancet Infect. Dis. 2024, 24, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Yeo, K.L.; Chen, Y.-L.; Xu, H.Y.; Dong, H.; Wang, Q.-Y.; Yokokawa, F.; Shi, P.-Y. Synergistic suppression of dengue virus replication using a combination of nucleoside. Antimicrob. Agents Chemother. 2015, 59, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Tien, S.-M.; Chang, P.-C.; Lai, Y.-C.; Chuang, Y.-C.; Tseng, C.-K.; Kao, Y.-S.; Huang, H.-J.; Hsiao, Y.-P.; Liu, Y.-L.; Lin, H.-H.; et al. Therapeutic efficacy of humanized monoclonal antibodies targeting dengue virus nonstructural protein 1 in the mouse model. PLoS Pathog. 2022, 18, e1010469. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Sander, W.E.; Allan, B.F.; Andrade, F.C.D. Retrospective Study of Kyasanur Forest Disease and Deaths among Nonhuman Primates, India, 1957–2020. Emerg. Infect. Dis. 2021, 27, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Suder, E.; Furuyama, W.; Feldmann, H.; Marzi, A.; de Wit, E. The vesicular stomatitis virus-based Ebola virus vaccine: From concept to clinical trials. Hum. Vaccin. Immunother. 2018, 14, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.; Porter, F.; Weissmanm, D. mRNA vaccine—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D.T. Yellow fever live attenuated vaccine: A very successful live attenuated vaccine but still we have problems controlling the disease. Vaccine 2017, 35, 5951–5955. [Google Scholar] [CrossRef] [PubMed]
- Kallás, E.G.; Cintra, M.A.; Moreira, J.A.; Patiño, E.G.; Braga, P.E.; Tenório, J.C.; Infante, V.; Palacios, R.; de Lacerda, M.V.G.; Pereira, D.B.; et al. Live, Attenuated, Tetravalent Butantan-Dengue Vaccine in Children and Adults. Engl. J. Med. 2024, 390, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Rajak, A.; Kumar, J.S.; Dhankher, S.; Sandhya, V.; Kiran, S.; Golime, R.; Dash, P.K. Development and application of a recombinant Envelope Domain III protein based indirect human IgM ELISA for Kyasanur forest disease virus. Acta Trop. 2022, 235, 106623. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, N.; Hewson, R.; Afrough, B.; Bewley, K.; Arunkumar, G. Development of a quantitative real-time RT-PCR assay that differentiates between Kyasanur Forest disease virus and Alkhurma hemorrhagic fever virus. Ticks Tick Borne Dis. 2020, 11, 101381. [Google Scholar] [CrossRef]
- Sharma, S.; Yadav, P.; Dash, P.K.; Dhankher, S. Molecular epidemiology of Kyasanur forest disease employing ONT-NGS a field forward sequencing. J. Clin. Virol. 2025, 177, 105783. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, T.; Shete, A.; Yadav, P.; Patil, S.; Mali, D.; Waghmare, A.; Gawande, P. Point of care real-time polymerase chain reaction-based diagnostic for Kyasanur forest disease. Int. J. Infect. Dis. 2021, 108, 226–230. [Google Scholar] [CrossRef] [PubMed]


| Diagnostic Methods | Technique | Detection Target | Sensitivity/Specificity | Current Status | Notes on Limitations and Potential Improvements |
|---|---|---|---|---|---|
| RT-PCR (Standard) | Reverse transcription PCR | Viral RNA (envelope gene) | ~95%/~98% [71] | Routine in labs | Limited to the viremic phase (days 3–8); requires infrastructure; dry-down version improves field use. |
| Dry-Down RT-PCR | Lyophilized RT-PCR | Viral RNA (envelope gene) | ~95%/~98% [22] | Emerging (field testing) | Reduces turnaround to 4–6 h; needs validation in remote settings; scalable production needed. |
| ELISA (IgM/IgG) | Enzyme-linked immunosorbent assay | IgM/IgG antibodies | ~92%/~94% (IgM) [70] | Routine in labs | Cross-reactivity with flaviviruses; delayed detection (days 5–14); enhances with recombinant antigens. |
| Next-Generation Sequencing (NGS) | High-throughput sequencing | Whole viral genome | Variable (research-grade [72] | Research tool | Costly and complex; not routine; potential for AI integration to track strains. |
| Point-of-Care (POC) Devices | Lateral flow or RT-PCR-based | KFDV Antigens or RNA | Under validation (~90% est.) [73] | Prototype (development) | Limited validation; needs thermostable, affordable design for rural deployment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohra, B.; Srivastava, K.S.; Raj, A.; Pal, N.; Shukla, R. Kyasanur Forest Disease Virus: Epidemiological Insights, Pathogenesis, Therapeutic Strategies, and Advances in Vaccines and Diagnostics. Viruses 2025, 17, 1022. https://doi.org/10.3390/v17081022
Bohra B, Srivastava KS, Raj A, Pal N, Shukla R. Kyasanur Forest Disease Virus: Epidemiological Insights, Pathogenesis, Therapeutic Strategies, and Advances in Vaccines and Diagnostics. Viruses. 2025; 17(8):1022. https://doi.org/10.3390/v17081022
Chicago/Turabian StyleBohra, Babita, Kumar Saurabh Srivastava, Ayush Raj, Nabanita Pal, and Rahul Shukla. 2025. "Kyasanur Forest Disease Virus: Epidemiological Insights, Pathogenesis, Therapeutic Strategies, and Advances in Vaccines and Diagnostics" Viruses 17, no. 8: 1022. https://doi.org/10.3390/v17081022
APA StyleBohra, B., Srivastava, K. S., Raj, A., Pal, N., & Shukla, R. (2025). Kyasanur Forest Disease Virus: Epidemiological Insights, Pathogenesis, Therapeutic Strategies, and Advances in Vaccines and Diagnostics. Viruses, 17(8), 1022. https://doi.org/10.3390/v17081022







