Assessment of Mammalian Scavenger and Wild White-Tailed Deer Activity at White-Tailed Deer Farms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area: CWD
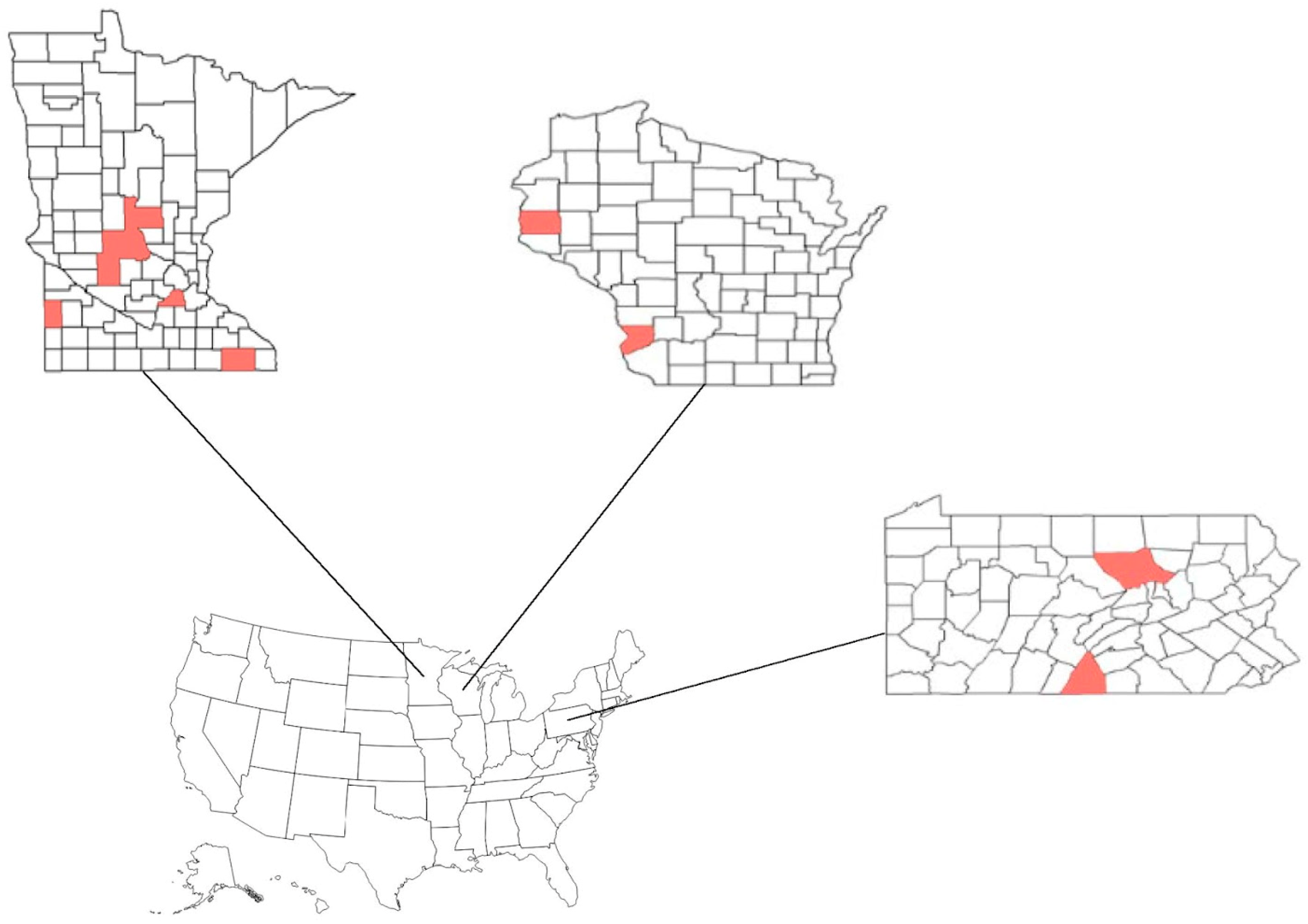
2.2. Description of Farms
2.3. Field Methods
2.4. Camera Placement
2.5. Data Processing
2.5.1. Landscape Metrics
2.5.2. Independent Observations
2.6. Statistical Analysis
3. Results
3.1. Wildlife at Farm Fences
3.2. Wildlife at Farmed Deer Feeders
3.3. Wild and Farmed Deer Interaction
3.4. Assessment of Association with Landscape Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edmunds, D.R.; Albeke, S.E.; Grogan, R.G.; Lindzey, F.G.; Legg, D.E.; Cook, W.E.; Schumaker, B.A.; Kreeger, T.J.; Cornish, T.E. Chronic Wasting Disease Influences Activity and Behavior in White-Tailed Deer. J. Wildl. Manag. 2018, 82, 138–154. [Google Scholar] [CrossRef]
- Mori, J.; Rivera, N.; Novakofski, J.; Mateus-Pinilla, N. A review of chronic wasting disease (CWD) spread, surveillance, and control in the United States captive cervid industry. Prion 2024, 18, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, D.R.; Kauffman, M.J.; Schumaker, B.A.; Lindzey, F.G.; Cook, W.E.; Kreeger, T.J.; Grogan, R.G.; Cornish, T.E. Chronic Wasting Disease Drives Population Decline of White-Tailed Deer. PLoS ONE 2016, 11, e0161127. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.M.; Haus, J.M.; Eyler, T.B.; Duda, M.D.; Bowman, J.L. Revisiting Hunter Perceptions toward Chronic Wasting Disease: Changes in Behavior over Time. Animals 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Heffelfinger, J.R. Taxonomy, Evolutionary History and Distribution. In Biology and Management of White-Tailed Deer; Hewitt, D.G., Ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Associates, S. Hunting in America: An Economic Force. 2018. Available online: https://www.fishwildlife.org/application/files/3815/3719/7536/Southwick_Assoc_-_NSSF_Hunting_Econ.pdf (accessed on 3 March 2023).
- Mathiason, C.K.; Powers, J.G.; Dahmes, S.J.; Osborn, D.A.; Miller, K.V.; Warren, R.J.; Mason, G.L.; Hays, S.A.; Hayes-Klug, J.; Seelig, D.M.; et al. Infectious Prions in the Saliva and Blood of Deer with Chronic Wasting Disease. Science 2006, 314, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Conner, M.M. Epidemiology of chronic wasting disease in free-ranging mule deer: Spatial, temporal, and demographic influences on observed prevalence patterns. J. Wildl. Dis. 2005, 41, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.E.; Bartz, J.C.; Vercauteren, K.C.; Bartelt-Hunt, S.L. Enzymatic Digestion of Chronic Wasting Disease Prions Bound to Soil. Environ. Sci. Technol. 2010, 44, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Zabel, M.; Ortega, A. The Ecology of Prions. Microbiol. Mol. Biol. Rev. 2017, 81, e00001-17. [Google Scholar] [CrossRef] [PubMed]
- Schultze, M.L.; Horn-Delzer, A.; Glaser, L.; Hamberg, A.; Zellner, D.; Wolf, T.M.; Wells, S.J. Herd-level risk factors associated with chronic wasting disease-positive herd status in Minnesota, Pennsylvania, and Wisconsin cervid herds. Prev. Vet. Med. 2023, 218, 106000. [Google Scholar] [CrossRef] [PubMed]
- VerCauteren, K.C.; Pilon, J.L.; Nash, P.B.; Phillips, G.E.; Fischer, J.W. Prion Remains Infectious after Passage through Digestive System of American Crows (Corvus brachyrhynchos). PLoS ONE 2012, 7, e45774. [Google Scholar] [CrossRef] [PubMed]
- Baune, C.; Wolfe, L.L.; Schott, K.C.; Griffin, K.A.; Hughson, A.G.; Miller, M.W.; Race, B. Reduction of Chronic Wasting Disease Prion Seeding Activity following Digestion by Mountain Lions. mSphere 2021, 6, e00812-21. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.J.; Carlson, C.M.; Schneider, J.R.; Johnson, C.J.; Greenlee, J.J. Increased Attack Rates and Decreased Incubation Periods in Raccoons with Chronic Wasting Disease Passaged through Meadow Voles. Emerg. Infect. Dis. 2022, 28, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Cassmann, E.D.; Frese, A.J.; Moore, S.J.; Greenlee, J.J. Transmission of Raccoon-Passaged Chronic Wasting Disease Agent to White-Tailed Deer. Viruses 2022, 14, 1578. [Google Scholar] [CrossRef] [PubMed]
- Inzalaco, H.N.; Brandell, E.E.; Wilson, S.P.; Hunsaker, M.; Stahler, D.R.; Woelfel, K.; Walsh, D.P.; Nordeen, T.; Storm, D.J.; Lichtenberg, S.S.; et al. Detection of prions from spiked and free-ranging carnivore feces. Sci. Rep. 2024, 14, 3804. [Google Scholar] [CrossRef] [PubMed]
- Pennsylvania Game Commission. History of CWD in PA. 2023. Available online: https://www.pa.gov/content/dam/copapwp-pagov/en/pgc/documents/wildlife/wildlifehealth/documents/cwd%20history%20of%20cwd%20in%20pa%20bwm%20202304.pdf (accessed on 10 May 2024).
- WDNR. CWD-Affected Counties. Available online: https://p.widencdn.net/uuer0c/cwdaffectedcountiesdifferences (accessed on 8 May 2024).
- Hansen, C. A Deadly Disease in Deer; How Hunters Are Helping to Track It. Natural Resources Institute. Available online: https://naturalresources.extension.wisc.edu/a-deadly-disease-in-deer-how-hunters-are-helping-to-track-it/ (accessed on 11 May 2024).
- University of Minnesota. Chronic Wasting Disease—Where It Is. 2020. Available online: https://www.house.mn.gov/comm/docs/7fea8c5d-8ec7-4efd-be58-b89bdb8f94ac.pdf (accessed on 10 May 2024).
- MNDNR. CWD Test Results: July 1, 2024 to Present. Available online: https://www.dnr.state.mn.us/cwdcheck/index.html (accessed on 14 May 2024).
- Dewitz, J. National Land Cover Database; U.S. Geological Survey: Reston, VA, USA, 2021. [CrossRef]
- U.S. Census Bureau; Department of Commerce. TIGER/Line Shapefile, 2019, State, Pennsylvania, Primary and Secondary Roads State-Based Shapefile. 2019. Available online: https://catalog.data.gov/dataset/tiger-line-shapefile-2019-state-pennsylvania-primary-and-secondary-roads-state-based-shapefile (accessed on 24 May 2024).
- Minnesota Department of Transportation. Metadata: Roads, Minnesota. 2012. Available online: https://resources.gisdata.mn.gov/pub/gdrs/data/pub/us_mn_state_dot/trans_roads_mndot_tis/metadata/metadata.html (accessed on 24 May 2024).
- Wisconsin Department of Natural Resources. Roads Wisconsin (US Census). Available online: https://geodata.wisc.edu/catalog/B347457B-71C2-478A-AF31-AAD2E58B02FB (accessed on 24 May 2024).
- Beery, S.; Morris, D.; Yang, S.; Simon, M.; Norouzzadeh, A.; Joshi, N. Efficient Pipeline for Automating Species ID in new Camera Trap Projects. Biodivers. Inf. Sci. Stand. 2019, 3, e37222. [Google Scholar] [CrossRef]
- Hesselbarth, M.; Sciani, M.; Wiegand, K.; Nowosad, J. landscapemetrics: An open-source R tool to calculate landscape metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef]
- Pebesma, E. Simple Features for R: Standardized Support for Spatial Vector Data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef]
- Hijmans, R. terra: Spatial Data Analysis. 2024. Available online: https://CRAN.R-project.org/package=terra (accessed on 3 March 2023).
- Lonsinger, R.C.; Dart, M.M.; Larsen, R.T.; Knight, R.N. Efficacy of machine learning image classification for automated occupancy-based monitoring. Remote. Sens. Ecol. Conserv. 2023, 10, 56–61. [Google Scholar] [CrossRef]
- Vélez, J.; McShea, W.; Shamon, H.; Castiblanco, P.; Tabak, M.; Chalmers, C.; Fergus, P.; Fieberg, J. An evaluation of platforms for processing camera-trap data using artificial intelligence. Methods Ecol. Evol. 2022, 14, 459–477. [Google Scholar] [CrossRef]
- O’Brien, T.; Kinnaird, M.; Wibisono, H. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. 2003, 6, 131–139. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2016, 67, 1–48. [Google Scholar] [CrossRef]
- Rhodes, J.R.; McaAlpine, C.A.; Zuur, A.F.; Smith, G.M.; Ieno, E.N. GLMM Applied on the Spatial Distribution of Koalas in a Fragmented Landscape. In Mixed Effects Models and Extensions in Ecology with R; Springer: Berlin/Heidelberg, Germany, 2009; pp. 469–472. [Google Scholar]
- Akaike, K. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike, Proceedings of the 2nd International Symposium on Information Theory, Tsahkadsor, Armenia, 2–8 September 1971; Springer: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Jennelle, C.S.; Samuel, M.D.; Nolden, C.A.; Berkley, E.A. Deer Carcass Decomposition and Potential Scavenger Exposure to Chronic Wasting Disease. J. Wildl. Manag. 2009, 73, 655–662. [Google Scholar] [CrossRef]
- Rovero, F.; Marshall, A.R. Camera trapping photographic rate as an index of density in forest ungulates. J. Appl. Ecol. 2009, 46, 1011–1017. [Google Scholar] [CrossRef]
- Sollmann, R. A gentle introduction to camera-trap data analysis. Afr. J. Ecol. 2018, 56, 740–749. [Google Scholar] [CrossRef]
- Peral, C.; Landman, M.; Kerley, G.I.H. The inappropriate use of time-to-independence biases estimates of activity patterns of free-ranging mammals derived from camera traps. Ecol. Evol. 2022, 12, e9408. [Google Scholar] [CrossRef] [PubMed]
- Kays, R.; Arbogast, B.S.; Baker-Whatton, M.; Beirne, C.; Boone, H.M.; Bowler, M.; Burneo, S.F.; Cove, M.V.; Ding, P.; Espinosa, S.; et al. An empirical evaluation of camera trap study design: How many, how long and when? Methods Ecol. Evol. 2020, 11, 700–713. [Google Scholar] [CrossRef]
- Huang, M.H.J.; Demarais, S.; Banda, A.; Strickland, B.K.; Welch, A.G.; Hearst, S.; Lichtenberg, S.; Houston, A.; Pepin, K.M.; VerCauteren, K.C. Expanding CWD disease surveillance options using environmental contamination at deer signposts. Ecol. Solut. Evid. 2024, 5, e12298. [Google Scholar] [CrossRef]



| Site | State | Size (Sq. km) | Type | Season |
|---|---|---|---|---|
| 1 | MN | 0.078 | B | S, W |
| 2 | MN | 0.028 | B | S, F, W |
| 3 | MN | 0.018 | B | S, W |
| 4 | WI | 0.478 | HR | S, F, W |
| 5 | MN | 0.004 | B | S |
| 6 | MN | 0.021 | B | S |
| 7 | MN | 0.041 | B | S |
| 8 | MN | 0.005 | B | S |
| 9 | MN | 0.069 | B | S |
| 10 | PA | 8.328 | HR | S |
| 11 | WI | 0.058 | B | S |
| 12 | PA | 0.072 | B | S |
| 13 | MN | 0.051 | B | S |
| 14 | MN | 0.007 | B | S |
| Land-Cover Type | Number of Cameras |
|---|---|
| Agriculture | 38 |
| Developed | 16 |
| Forest | 64 |
| Grassland | 35 |
| Wetland | 10 |
| Species | Number of Observations Inside the Fence (p) | Number of Observations Outside the Fence (p) | Number of Observations on the Fence (p) |
|---|---|---|---|
| black bear | 5 (1.00) | 0 (0.00) | 0 (0.00) |
| bobcat | 4 (0.8) | 1 (0.20) | 0 (0.00) |
| coyote | 1 (0.04) | 25 (0.96) | 0 (0.00) |
| Mustelid spp. | 1 (1.00) | 0 (0.00) | 0 (0.00) |
| red fox | 2 (0.29) | 5 (0.71) | 0 (0.00) |
| opossum | 2 (1.00) | 0 (0.00) | 0 (0.00) |
| raccoon | 50 (0.50) | 46 (0.46) | 4 (0.04) |
| skunk | 2 (0.29) | 5 (0.71) | 0 (0.00) |
| spp. unknown | 11 (0.52) | 9 (0.43) | 1 (0.05) |
| white-tailed deer | 0 (0.00) | 574 (1.00) | 0 (0.00) |
| Site | Season | Number of Observations | Number of Observed Contacts |
|---|---|---|---|
| 1 | F | 0.26 | 0.03 |
| 1 | S | 0.04 | 0.00 |
| 1 | W | 0.14 | 0.00 |
| 2 | F | 0.20 | 0.05 |
| 2 | S | 0.00 | 0.00 |
| 2 | W | 0.08 | 0.00 |
| Predictors | Log Mean | Std. Error | CI | p |
|---|---|---|---|---|
| Intercept | 0.31 | 0.26 | −0.20–0.82 | 0.237 |
| Independent observations of scavengers | 0.16 | 0.10 | −0.04–0.35 | 0.115 |
| Developed land cover | −1.36 | 0.66 | −2.64–−0.07 | 0.039 |
| Number of hours | 0.22 | 0.16 | 0.04–0.65 | 0.025 |
| Random effects | ||||
| Sigma-squared | 1.35 | |||
| Tau | 0.38 | |||
| ICC | 0.22 | |||
| N | 14 | |||
| Observations | 163 | |||
| Marginal R2/Conditional R2 | 0.170/0.352 | |||
| Predictors | Log Mean | Std. Error | CI | p |
|---|---|---|---|---|
| Intercept | −1.16 | 0.36 | −1.87–−0.82 | 0.001 |
| Independent observations of deer | 0.31 | 0.14 | 0.04–0.58 | 0.024 |
| Random effects | ||||
| Sigma-squared | 1.63 | |||
| Tau | 1.12 | |||
| ICC | 0.41 | |||
| N | 14 | |||
| Observations | 163 | |||
| Marginal R2/Conditional R2 | 0.034/0.428 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jack, A.R.; Sansom, W.C.; Wolf, T.M.; Zhang, L.; Schultze, M.L.; Wells, S.J.; Forester, J.D. Assessment of Mammalian Scavenger and Wild White-Tailed Deer Activity at White-Tailed Deer Farms. Viruses 2025, 17, 1024. https://doi.org/10.3390/v17081024
Jack AR, Sansom WC, Wolf TM, Zhang L, Schultze ML, Wells SJ, Forester JD. Assessment of Mammalian Scavenger and Wild White-Tailed Deer Activity at White-Tailed Deer Farms. Viruses. 2025; 17(8):1024. https://doi.org/10.3390/v17081024
Chicago/Turabian StyleJack, Alex R., Whitney C. Sansom, Tiffany M. Wolf, Lin Zhang, Michelle L. Schultze, Scott J. Wells, and James D. Forester. 2025. "Assessment of Mammalian Scavenger and Wild White-Tailed Deer Activity at White-Tailed Deer Farms" Viruses 17, no. 8: 1024. https://doi.org/10.3390/v17081024
APA StyleJack, A. R., Sansom, W. C., Wolf, T. M., Zhang, L., Schultze, M. L., Wells, S. J., & Forester, J. D. (2025). Assessment of Mammalian Scavenger and Wild White-Tailed Deer Activity at White-Tailed Deer Farms. Viruses, 17(8), 1024. https://doi.org/10.3390/v17081024






