Abstract
Peste des petits ruminants virus (PPRV) has been classified into four lineages based on the nucleocapsid and fusion genes, with lineage IV strains being the most widely distributed. In Africa, recent epidemiological data revealed that PPRV lineage IV is increasingly displacing other lineages in prevalence, suggesting a competitive advantage in viral transmission and adaptability. Moreover, a lineage IV strain was the only confirmed strain in Europe and Asia. In this study, a one-step Taqman quantitative real-time reverse transcription polymerase chain reaction (RT-qPCR) assay for lineage IV PPRV was established by targeting the hemagglutinin (H) gene. The results indicated that this method could detect approximately six copies of PPRV RNA, indicating high sensitivity. No cross-reactions with related viruses or other lineages of PPRV were observed. The results of a repeatability test indicated that the coefficient of variation values were low in both the inter-assay and intra-assay experimental groups. Detection of field samples indicated that all positive samples could be detected successfully using the developed method. This RT-qPCR assay provides a valuable tool to facilitate targeted surveillance and rapid differential diagnosis in regions with active circulation of PPRV lineage IV, enabling timely epidemiological investigations and strain-specific identification.
1. Introduction
Peste des petits ruminants (PPR) is a highly contagious disease caused by the peste des petits ruminants virus (PPRV), which primarily affects goats, sheep, and small wild ruminants. The incubation period of PPR is typically 4 to 6 days but may range from 3 to 10 days. Fever; increased ocular, oral, and nasal discharges; gastritis; diarrhea; and pneumonia are usually observed in infected animals [1]. PPRV belongs to the species Small ruminant morbillivirus, genus Morbilliviruses, subfamily Orthoparamyxovirinae, and family Paramyxoviridae and has a linear negative-stranded RNA genome [2,3]. The PPRV genome encodes six structural proteins, including the nucleocapsid (N), matrix (M), phosphoprotein (P), fusion (F), hemagglutinin (H), polymerase (L) proteins, and two nonstructural proteins (C and V), in the order of 3′N-P/C/V-M-F-H-L-5′ [4]. Although only one serotype of PPRV was confirmed, according to the N or F gene, PPRV can be classified into four lineages [2]. Lineages I and II are mainly distributed in West Africa, lineage III is mainly distributed in East Africa, and lineage IV is widely distributed in the Arabian Peninsula, the Middle East, and Southern and Eastern Asia [5]. Huge economic losses have been associated with PPRV, with direct or indirect losses reaching USD 2.1 billion each year. Once newly introduced, PPRV can infect up to 90% of a herd and cause 70% death of infected animals. Under the Global Framework for the Progressive Control of Transboundary Animal Disease (GF-TADs), the World Organization for Animal Health (WOAH) has identified PPR for global control and eradication by 2030.
PPR was first discovered in Cote d’Ivoire in 1942 and mainly circulated in Africa and Asia. However, it continues to spread to new areas despite various control methods having been implemented. In July 2024, PPR re-emerged in Europe (Bulgaria previously reported one case of PPR in 2018), including cases in Greece, Romania, and Hungary. Consequently, PPR epidemic-free certification of these countries was suspended, and according to the World Animal Health Information System (WAHIS), PPR is still circulating in these countries, indicating that the area influenced by PPRV has further expanded. The results of viral phylogenetic analysis indicated that the virus isolated from Greece and Romania belonged to lineage IV and shared high genetic similarity with the strains circulating in Georgia [6]. In 2007, China first reported PPR in Tibet, and in 2013, PPR re-emerged in the Xinjiang Uygur Autonomous Region and rapidly spread to almost all provinces [7,8]. Subsequently, various control methods were implemented, including culling, restriction on transportation of susceptible animals, compulsory vaccination, and active surveillance. Benefitting from these measures, the PPR situation in China has stabilized in recent years, with only a few PPR cases being reported.
It is very difficult to differentiate PPR from rinderpest because of their similar clinical sign presentations; therefore, all PPR cases should be confirmed via laboratory diagnostic methods. Timely diagnosis is critical to curbing the transmission of PPRV. Viral isolation is regarded as the gold standard among all diagnostic methods; however, it is time-consuming and is technically difficult, which has limited its wide application [9]. Enzyme-linked immunosorbent assays (ELISAs) are mainly used for PPRV antibody detection. However, using current technology, it is impossible to distinguish between antibodies produced by natural wild virus infection and those generated through vaccination. Consequently, ELISA is usually applied to monitor antibody levels and for immune efficacy evaluation. Several new detection methods for PPR were also established recently, such as loop-mediated isothermal amplification (LAMP) [10] and clustered regularly interspaced short palindromic repeats (CRISPR) [11], which are easy to operate and observe; however, their cost remains relatively high compared with conventional methods. Quantitative real-time reverse transcription polymerase chain reaction (RT-qPCR) is still the most commonly used method for PPRV detection and is recommended by WOAH because of its accuracy, sensitivity, and economy. Compared to conventional PCR, it exhibits higher sensitivity, simpler operation, and requires less time. The PPRV N gene is preferentially targeted in RT-qPCR assays because of its proximity to the viral promoter region, which is responsible for the N protein’s status as the most abundantly expressed viral protein during replication [3,12]. The F gene has also been selected as a target because of its conserved sequence. Several primers and probes with high sensitivity and specificity are available to detect PPRV. A rapid RT-qPCR assay based on the N and F genes, established by Batten and Flannery, could detect 10 viral genomic copies of PPRV, and all four lineages could be successfully detected [13]. Kwiatek et al. established an RT-qPCR method based on the N gene, with a detection limit of approximately 32 viral genome copies for all lineages of PPRV [14]. Based on the N gene, Polci designed primers and a probe that could detect approximately 20 copies of the virus with a 95% probability, and no amplification signals were recorded when the method was applied to viruses closely related or clinically similar to PPRV- or PPR-negative blood samples [15].
In Africa, lineages I to III viruses were widespread before 2008. However, lineage IV was found to replace the other lineages and spread widely in relevant African countries since then [16,17,18,19]. In the Arabian Peninsula, the only PPRV that circulated before was lineage III. However, no cases of lineage III were reported in recent years; conversely, the PPRV cases reported in the vast majority of Asian countries belong to lineage IV [20]. In Europe, the circulating PPRV in a newly affected region was also confirmed as lineage IV [6]. Lineage IV of PPRV has emerged as the predominant strain with the broadest global distribution. Several years ago, we designed a pair of primers and a probe for PPRV detection based on the N gene [21]. However, according to in silico evaluation of PPRV-RT-qPCR assays performed by Flannery [13], the sensitivity of this primer pair was relatively low when compared with other assays. Consequently, in this study, we aimed to develop a novel H gene-based RT-qPCR assay for PPRV lineage IV and systematically evaluated its diagnostic performance through sensitivity analysis, specificity assessment, and repeatability testing. The established method demonstrated significant potential as an effective diagnostic alternative for PPR surveillance and control programs.
2. Materials and Methods
2.1. Primer and Probe Design
PPRV full genomic sequences of lineages I to IV were collected from GenBank and aligned using Snapgene software (GSL Biotech LLC, Boston, MA, USA). The primers and probe were designed based on the conserved regions of the PPRV H gene from lineage IV PPRV. The forward primer matched position 7974–7996 (5′-AACGTGTCCTCAGTGTTTACCRT-3′); the Taqman probe bound to position 8014–8043 (FAM-CGGAAGAACATATACYGTCTGGAGATCCG-BHQ1), whereas the reverse primer matched position 8076–8101 (5′-ATCTCGAAGACTCTTAAAAATGGCC-3′) (Reference strain: China/XJYL/2013, GenBank: KM091959.1).
2.2. Virus and Plasmids
Goat pox virus (GPV), Orf virus (ORFV) and Foot-and-Mouth disease virus (FMDV) were collected from clinical samples. PPRV China/XJYL/2013 (lineage IV) and China/Tibet/2007 (lineage IV) were stored and prepared at the National Reference Laboratory for Peste des Petits Ruminants (at the China Animal Health and Epidemiology Center (CAHEC), Qingdao, China). Partial H genes from PPRV/Cote_dIvoire/1989 (lineage I), Ghana/NK1/2010 (lineage II), SnDK11/13 (lineage II), KN5/2011 (lineage III), UAE 1986 (lineage III), PPRV/Oman 1983 (lineage III), and the Mprocco 2008 strain (lineage IV) were commercially synthesized and cloned into vector pUC57. All plasmids were constructed and verified by Shanghai Sangon Biotechnology Company (Shanghai, China). The PPRV/XJYL/2013 virus standard used for sensitivity and repeatability assays was provided from the National Reference Laboratory for Peste des Petits Ruminants.
2.3. RNA Extraction and Real-Time Quantitative RT-PCR
All clinical samples were processed in a biosafety level III (BSL-3) laboratory. Viral RNA was extracted using a magnetic bead-based viral DNA/RNA extraction kit (Xi’an Tianlong Science and Technology Co., Ltd., Xi’an, China) according to the manufacturer’s instructions. qPCR amplification and detection were performed using a Bio-Rad PCR machine using a one-step RT-qPCR kit (RR600A, Takara, Shiga, Japan). Each reaction system comprised 25 μL: 12.5 μL of one-step PrimeScript III RT-qPCR Mix (1×), 1 μL of forward primer (0.4 μM), 1 μL of reverse primer (0.4 μM), 0.5 μL of probe (0.2 μM), 0.5 μL of ROX reference dye (50 nM), 3 μL of the RNA sample, and 6.5 μL of RNA-free water. All samples were detected in duplicate. The cycling conditions were set as follows: reverse transcription at 50 °C for 10 min, followed by reverse transcriptase inactivation and DNA polymerase activation at 95 °C for 5 min, and 40 cycles of amplification (denaturation at 95 °C for 15 s and annealing at 60 °C for 30 s). Fluorescence signals were collected at 60 °C in each cycle, and cycle thresholds (Cts) were assigned to each sample in the exponential phase of the amplification plot of each cycle.
2.4. Sensitivity Test
Viral standard nucleic acids were serially diluted 10-fold to concentrations ranging from 6 × 100 to 106 copies/μL, respectively. The diluted viral nucleic acids were used as templates for detection using the designed primers and probe.
2.5. Repeatability Test
Viral standard nucleic acids were serially diluted 10-fold to concentrations ranging from 6 × 100 to 106 copies/μL, respectively. Different concentrations of viral nucleic acid were detected using primer and probe, and the inter-assay repeatability and intra-assay repeatability were all evaluated, respectively.
2.6. Specificity Test
The specificity of the assay was assessed by detecting various viruses, including GPV, ORFV, FMDV, PPRV China/XJYL/2013 and China/Tibet/2007 strains, PPRV/Cote_dIvoire/1989, Ghana/NK1/2010, SnDK11/13, KN5/2011, UAE 1986, PPRV/Oman 1983, and Mprocco 2008 plasmids.
2.7. Field Samples
One hundred field samples comprising swabs and tissues were collected by our laboratory. PPRV was inactivated using 1% (w/v) NaOH in a BSL-3 laboratory, and viral RNA was extracted, followed by reverse transcription in a BSL-2 laboratory. All clinical samples were detected and confirmed as PPRV-positive or PPRV-negative using conventional RT-PCR [22] before the primers and probe designed in this study were used for the detection of these samples.
3. Results
3.1. Multiple Sequence Alignments of Primers and Probe Target Sites
The sequence alignment analysis presented in Figure 1 demonstrates that both the primers and the probe developed in this study exhibit high specificity for conserved genomic regions within PPRV lineage IV.
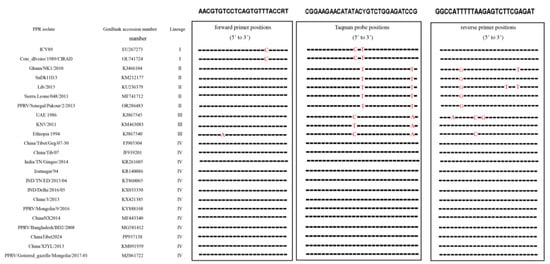
Figure 1.
Alignment of the primer and probe targeted to the gene among multiple PPRV strains. The sequences of the forward primers, probe and reverse primer are shown in boxes; red represented different nucleotides from the primer and probe.
3.2. Sensitivity and Standard Curve of the Real-Time RT-qPCR
The 10-fold gradient dilutions of the China/XJYL/2013 strain-derived RNA were detected. The minimum template concentration detected by this method was 6 copies of RNA, with a corresponding Ct value of 38.11. The dynamic range of the assay over a 10-log-unit span of viral RNA concentrations ranged from 6 to 6 × 106 RNA copies/μL. The standard curve for the H-gene RT-qPCR assay was also performed. (Figure 2). The cut-off value was determined to be 38.69 through testing of negative samples.
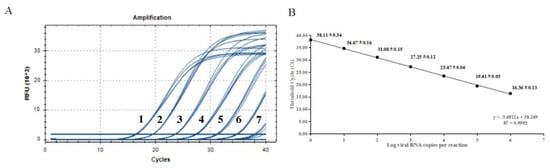
Figure 2.
Sensitivity tests and standard curve for RT-qPCR based on the H gene. (A) Amplification curves of templates with different concentrations, (B) Standard curve of developed method. The data for each concentration is presented as CT value ± SD. The lowest copy number that could be determined was up to 6 copies/μL; the optical standard formula is y = −3.6921x + 38.269, and the correlation coefficient is 0.9992.
3.3. Repeatability of the Real-Time RT-qPCR
Different copies of PPRV China/XJYL/2013 RNA were assayed for the repeatability test. The results showed that for the intra-assay repeatability, the CV values were all less than 1.50%. The CV values of the inter-assay repeatability were all less than 1.67% (Table 1).

Table 1.
The inter-assay (upper) and intra-assay (lower) repeatability of developed method.
3.4. Specificity of the Real-Time RT-qPCR
Results indicated that only PPRV China/XJYL/2013, China/Tibet/2007, and Morocco 2008 were detected by a developed method, and no cross-reaction with other viruses or plasmids was observed (Table 2).

Table 2.
The specificity of developed method.
3.5. Fields Samples
One hundred field samples collected from a national epidemiological investigation project on PPR or reported PPR cases in China were detected using conventional RT-PCR and the novel RT-qPCR method, respectively. The results indicated that all the positive samples confirmed by conventional RT-PCR were detected successfully using the novel method, demonstrating 100% correlation (Table 3).

Table 3.
Detective results of fields samples.
4. Discussion
PPR is classified as a list A disease in China because of its biological risk and threat to domestic and wild small ruminants. Since the outbreak of PPR in 2007, a national epidemiological investigation project on PPR has been conducted in all provinces, autonomous regions, and municipalities. Compulsory vaccination has also been implemented in almost all areas in China since then. The PPR attenuated vaccine developed using the Nigeria 75/1 strain has been widely applied in China and has been confirmed to confer strong protective immunity with a long duration in sheep and goats [23]. The PPR Monitoring and Assessment Tool (PMAT) is based on four different stages identified in the Global Strategy for the progressive control and eradication of PPR, which correspond to a combination of decreasing levels of epidemiological risk and increasing levels of prevention and control. Vaccination is one of the key tools to control PPR and was identified as the main option in stage 2 and stage 3 of PMAT. However, in stage 4 (post-eradication), vaccinations need to be suspended and the capacity of laboratory diagnosis needs to be strengthened. In addition, various wild ruminants demonstrated susceptibility to PPR, including gemsbok, goitered gazelle, bharal, alpine ibex, and argali [9,24,25,26]. In contrast to domestic animals, wild populations present unique challenges for PPR vaccination because of their limited human contact and free-ranging behaviors. This barrier creates a critical gap in current epidemic prevention and control strategies, particularly with regard to effective immunization coverage. To address this vulnerability, implementation of active surveillance via advanced diagnostic technologies with enhanced sensitivity and specificity becomes imperative, enabling accurate detection and surveillance of PPR virus circulation in wild reservoirs.
As previously mentioned, the N and F genes have been commonly selected as potential targets to develop PPRV detection methods. Mahapatra developed a nested PCR method based on the N gene, which demonstrated high sensitivity in detecting all PPRV lineages [27]. Zhang established a kind of real-time reverse transcription recombinase-aided amplification (RT-RAA) assay based on the N gene, which could detect 103 copies of PPRV, with 99.4% concordance with conventional RT-PCR [28]. Additionally, Ke designed a lyophilized real-time fluorescent PCR assay targeting the F gene, which simultaneously detected 11 pathogens affecting sheep and goats [29]. During PPRV infection, most of the neutralizing antibodies are directed against the surface hemagglutinin (H) glycoprotein H [30]. The H glycoprotein serves as a principal target for neutralizing antibodies, making it a preferred candidate to develop serological detection methods, such as blocking and competitive ELISA assays [31,32]. However, despite the protein’s immunodominance in humoral responses, the H gene has been underutilized in nucleic acid-based diagnostic platforms compared with the more frequently targeted N and F genes because of its low sequence conservation. An earlier RT-qPCR assay targeting the H gene was evaluated; however, computational analysis using PCRv software revealed its lower sensitivity compared with that of N or F gene-based detection methods [13]. To provide a new choice for PPRV diagnosis, herein, we developed a novel H gene-specific RT-qPCR assay, which demonstrated significantly improved sensitivity and specificity. This advance establishes a reliable molecular detection tool that complements existing PPR diagnostic approaches while expanding the genetic targets available for comprehensive outbreak monitoring.
Lineage IV of PPRV has become the main epidemic strain around the world in recent years and thus should be paid more attention. According to related studies, no lineage I strains have been identified in the last 10 years, whereas lineage II and III strains were occasionally detected in some African countries [33,34,35,36]. As a transboundary animal disease, PPRV may be introduced into previously unaffected regions through the movement and commercial trade of live animals. Consequently, a rapid diagnosis method applicable for PPRV lineage IV is essential for the control of PPR. RT-qPCR has been widely used for PPR diagnosis and will continue to contribute to the global eradication program. RT-qPCR methods are usually evaluated according to their specificity and sensitivity. According to our results, the new method could detect as few as six copies of PPRV, showing higher sensitivity compared with other PPRV detection assays. PPR is sometimes misdiagnosed as other diseases because of their similar symptoms. Importantly, the developed method enables accurate differentiation of PPR from other prevalent pathologies affecting small ruminants. The low intra-assay and inter-assay CV values observed in the repeatability tests demonstrate the stability of our method, which is essential for a robust and practical detection method. However, some limitations of our research should be addressed here. In our research, plasmids served as a practical alternative for accessing genetic material from economically significant viruses. While this approach allowed us to bypass the reverse transcription (RT) step—effectively converting the RT-qPCR into a standard qPCR assay—one potential solution would be to generate RNA templates via in vitro transcription of the plasmids. However, this method could complicate the evaluation of certain RT-qPCR performance characteristics. Additionally, as noted in the manuscript, another limitation is that we did not benchmark our assay against the virus isolation, which is regarded as the gold standard for PPRV detection.
In conclusion, we established a novel RT-qPCR method with high sensitivity and specificity, which could detect PPRV lineage IV. The assay enables virus detection at the early stage of infection or in the presence of low virus levels, thus providing a powerful diagnostic instrument to aid the prevention and control of this animal disease.
Author Contributions
Conceptualization, J.X. and Z.W.; methodology, J.Y.; software, Q.W.; validation, J.X., Y.W. and H.L.; formal analysis, L.L.; investigation, Q.W. and Y.W.; resources, L.L.; data curation, J.Y. and J.B.; writing—original draft preparation, J.X.; writing—review and editing, J.Y. and J.B; visualization, Y.W.; supervision, Z.W; project administration, J.B. and Y.W.; funding acquisition, J.B. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Project for the Prevention and Control of major exotic animal diseases (2022YFD1800500).
Institutional Review Board Statement
All animals were handled in strict accordance with good animal practice according to the Animal Ethics Procedures and Guidelines of the People’s Republic of China. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Animal Welfare Committee of the China Animal Health and Epidemiology Center (protocol code DWFL-2025-2, 15TH February 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The field sample collection involved in this study was conducted by the National PPR Surveillance Project. The diagnosis of the collected samples was carried out by the FAO/WOAH PPR Reference Center/Laboratory at China Animal Health and Epidemiology Center.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Rahman, A.U.; Dhama, K.; Ali, Q.; Hussain, I.; Oneeb, M.; Chaudhary, U.; Wensman, J.J.; Shabbir, M.Z. Peste des petits ruminants in large ruminants, camels and unusual hosts. Vet. Q. 2020, 40, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Parida, S.; Batten, C.; Oura, C.; Kwiaatek, O.; Libeau, G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J. Gen. Virol. 2010, 91, 2885–2897. [Google Scholar] [CrossRef]
- Kumar, N.; Maherchandani, S.; Kashyap, S.K.; Singh, S.V.; Sharma, S.; Chaubey, K.K. Peste des petits ruminants virus infection of small ruminants: A comprehensive review. Viruses 2014, 6, 2287–2327. [Google Scholar] [CrossRef]
- Baron, M.D.; Diallo, A.; Lancelot, R.; Libeau, G. Peste des petits ruminants virus. Adv. Virus Res. 2016, 95, 1–42. [Google Scholar]
- Niu, B.; Liang, R.; Zhang, S.; Sun, X.; Li, F.; Qiu, S.; Zhang, H.; Bao, S.; Zhong, J.; Li, X.; et al. Spatiotemporal characteristics analysis and potential distribution prediction of peste des petits ruminants (PPR) in China from 2007–2018. Transbound. Emerg. Dis. 2022, 69, 2747–2763. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Yusuf, J.; Njeumi, F. Update on peste des petits ruminants in Europe. Vet. Rec. 2024, 195, 211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bao, J.; Wu, X.; Liu, Y.; Li, L.; Liu, C.; Suo, L.; Xie, Z.; Zhao, W.; Zhang, W.; et al. Peste des petits ruminants virus in Tibet, China. Emerg. Infect. Dis. 2009, 15, 299. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Z.; Li, L.; Wu, X.; Sang, P.; Wu, G.; Ding, G.; Suo, L.; Liu, C.; Wang, J.; et al. Detection and genetic characterization of peste des petits ruminants virus in free-living bharals (Pseudois nayaur) in Tibet, China. Res. Vet. Sci. 2011, 90, 238–240. [Google Scholar] [CrossRef]
- Sreenivasa, B.P.; Singh, R.P.; Mondal, B.; Dhar, P.; Bandyopadhyay, S.K. Marmoset B95a cells: A sensitive system for cultivation of peste des petits ruminants (PPR) virus. Vet. Res. Commun. 2006, 30, 103. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.; Howson, E.; Fowler, V.; Batten, C.; Flannery, J.; Selvaraj, M.; Parida, S. Rapid detection of peste des petits ruminants virus (PPRV) nucleic acid using a novel low-cost reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for future use in nascent PPR eradication programme. Viruses 2019, 11, 699. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Zhang, Y.; Wang, S.; Su, N.; Chang, X.; Ren, W.; Zou, Y.; Liu, S.; Li, L.; et al. Establishment of a RAA-CRISPR Cas12a based diagnostic method for peste des petits ruminants virus N gene and M gene. J. Virol. Methods 2024, 329, 114971. [Google Scholar] [CrossRef]
- Batten, C.A.; Banyard, A.C.; King, D.P.; Henstock, M.R.; Edwards, L.; Sanders, A.; Buczkowski, H.; Oura, C.C.L.; Barrett, T. A real time RT-PCR assay for the specific detection of Peste des petits ruminants virus. J. Virol. Methods 2011, 171, 401–404. [Google Scholar] [CrossRef]
- Flannery, J.; Rajko-Nenow, P.; Arnold, H.; Weezep, E.; Rijn, P.A.; Ngeleja, C.; Batten, C. Improved PCR diagnostics using up-to-date in silico validation: An F-gene RT-qPCR assay for the detection of all four lineages of peste des petits ruminants virus. J. Virol. Methods 2019, 274, 113735. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, O.; Keita, D.; Gil, P.; Fernandez-Pinero, J.; Clavero, M.A.J.; Albina, E.; Libeau, G. Quantitative one-step real-time RT-PCR for the fast detection of the four genotypes of PPRV. J. Virol. Methods 2010, 165, 168–177. [Google Scholar] [CrossRef]
- Polci, A.; Cosseddu, G.M.; Ancora, M.; Pinoin, C.; Harrak, M.E.; Sebhatu, T.T.; Ghebremeskel, E.; Sghaier, S.; Lelli, R.; Monaco, F. Development and preliminary evaluation of a new real-time RT-PCR assay for detection of peste des petits ruminants virus genome. Transbound. Emerg. Dis. 2015, 62, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, O.; Ali, Y.H.; Saeed, I.K.; Khalafalla, A.I.; Mohamed, O.I.; Obeida, A.A.; Abdelrahman, M.B.; Osman, H.M.; Taha, K.M.; Abbas, Z.; et al. Asian lineage of peste des petits ruminants virus, Africa. Emerg Infect Dis. 2011, 17, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Cosseddu, G.M.; Pinoni, C.; Polci, A.; Sebhatu, T.; Lelli, R.; Monaco, F. Characterization of Peste des Petits Ruminants Virus, Eritrea, 2002–2011. Emerg. Infect. Dis. 2013, 19, 160–161. [Google Scholar] [CrossRef]
- Maganga, G.D.; Verrier, D.; Zerbinati, R.M.; Drosten, C.; Drexler, J.F.; Leroy, E.M. Molecular typing of PPRV strains detected during an outbreak in sheep and goats in south-eastern Gabon in 2011. Virol. J. 2013, 10, 82. [Google Scholar] [CrossRef]
- Woma, T.Y.; Adombi, C.M.; Yu, D.; Qasim, A.M.M.; Sabi, A.A.; Maurice, N.A.; Olaiya, O.D.; Loitsch, A.; Bailey, D.; Shamaki, D.; et al. Co-circulation of Peste-des-Petits-Ruminants Virus Asian lineage IV with Lineage II in Nigeria. J. Vet. Med. Ser. A 2016, 63, 8. [Google Scholar] [CrossRef]
- Legnardi, M.; Raizman, E.; Beltran-Alcrudo, D.; Cinardi, G.; Robinson, T.; Falzon, L.C.; Djomgang, H.K.; Okori, E.; Parida, S.; Njeumi, F.; et al. Peste des Petits Ruminants in Central and Eastern Asia/West Eurasia: Epidemiological Situation and Status of Control and Eradication Activities after the First Phase of the PPR Global Eradication Programme (2017–2021). Animals 2022, 12, 2030. [Google Scholar] [CrossRef]
- Bao, J.; Li, L.; Wang, Z.; Barrett, T.; Suo, L.; Zhao, W.; Liu, Y.; Liu, C.; Li, J. Development of one-step real-time RT-PCR assay for detection and quantitation of peste des petits ruminants virus. J. Virol. Methods 2008, 148, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Couacy-Hymann, E.; Roger, F.; Hurard, C.; Guillou, J.P.; Libeau, G.; Diallo, A. Rapid and sensitive detection of peste des petits ruminants virus by a polymerase chain reaction assay. J. Virol. Methods 2002, 100, 17–25. [Google Scholar] [CrossRef]
- Zhao, H.; Njeumi, F.; Parida, S.; Benfield, C.T.O. Progress towards eradication of peste des petits ruminants through vaccination. Viruses 2021, 13, 59. [Google Scholar] [CrossRef]
- Elzein, E.M.; Housawi, F.M.; Bashareek, Y.; Gameel, A.A.; AI-Afaleq, A.I.; Anderson, E. Severe PPR infection in gazelles kept under semi-free range conditions. J. Vet. Med. B Infect. Dis. Vet. Public Health 2004, 51, 68–71. [Google Scholar] [CrossRef]
- Gur, S.; Albayrak, H. Seroprevalance of peste des petits ruminants (PPR) in goitered gazelle (Gazella subgutturosa subgutturosa) in Turkey. J. Wildl. Dis. 2010, 46, 673–677. [Google Scholar] [CrossRef]
- Li, J.M.; Li, L.; Wu, X.D.; Liu, F.X.; Zou, Y.L.; Wang, Q.H.; Liu, C.; Bao, J.; Wang, W.; Ma, W.; et al. Diagnosis of Peste des Petits Ruminants in Wild and Domestic Animals in Xinjiang, China, 2013–2016. Transbound. Emerg. Dis. 2017, 64, e43–e47. [Google Scholar] [CrossRef]
- Mahapatra, M.; Neto, M.M.; Khunti, A.; Njeumi, F.; Parida, S. Development and evaluation of a nested PCR for Improved Diagnosis and Genetic Analysis of Peste Des Petits ruminants Virus (PPRV) for future use in nascent PPR eradication Programme. Animals 2021, 11, 3170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Zhang, Z.; Mei, L.; Wang, J.; Wu, S.; Lin, X. Development of recombinase polymerase amplification assays for the rapid detection of peste des petits ruminants virus. J. Virol. Methods 2018, 254, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Hou, G.; Cao, P.; Ding, Y.; Zhao, C.; Wang, F.; Liu, Y.; Fan, Y.; Liu, S. Establishment and validation of a real-time fluorescent PCR freeze-dried type assay for 11 sheep and goats pathogens. Vet. J. 2024, 308, 106255. [Google Scholar] [CrossRef]
- Rojas, J.M.; Moreno, H.; Valcarcel, F.; Pena, L.; Sevilla, N.; Martin, V. Vaccination with recombinant adenoviruses expressing the peste des petits ruminants virus F or H proteins overcomes viral immunosuppression and induces protective immunity against PPRV challenge in sheep. PLoS ONE 2014, 9, e101226. [Google Scholar] [CrossRef]
- Lelisa, K.; Chibssa, T.R.; Desissa, F.; Emiyu, K.; Muluneh, A.; Lobago, D.S.; Gebreweld, D.S.; Debebe, K.; Mohammed, A.A. Evaluation of diagnostic performance of H-based blocking ELISA for specific detection of peste des petits ruminants in domestic sheep, goats, cattle and camels. BMC Microbiol. 2022, 22, 254. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V.; Bokade, P.P.; Kumar, K.V.; SowjanyaKumari, S.; Nagalingam, M.; Hemadri, D.; Shome, B.R. Comparative diagnostic efficacy of Avidin-Biotin recombinant nucleoprotein competitive ELISA for serosurveillance and monitoring of peste des petits ruminants in sheep and goats. J. Immunol. Methods 2023, 512, 113409. [Google Scholar] [CrossRef]
- Mantip, S.; Sigismeau, A.; Shamaki, D.; Woma, T.Y.; Kwiatek, O.; Libeau, G.; Farougou, S.; Bataille, A. Molecular epidemiology of peste des petits ruminants virus in Nigeria: An update. Transbound. Emerg. Dis. 2022, 69, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Kinimi, E.; Mahapatra, M.; Kgotlele, T.; Makange, M.R.; Tennakoon, C.; Njeumi, F.; Odongo, S.; Muyldermans, S.; Kock, R.; Parida, S.; et al. Complete genome sequencing of field isolates of Peste des petits ruminants virus from Tanzania revealed a high nucleotide identity with lineage III PPR viruses. Animals 2021, 11, 2976. [Google Scholar] [CrossRef] [PubMed]
- Tshilenge, G.M.; Walandila, J.S.; Kikukama, D.B.; Masumu, J.; Balowa, L.K.; Cattolo, G.; Bushu, E.; Tshipambe, S.M.; Dundon, W.G. Peste des petits ruminants viruses of lineages II and III identified in the Democratic Republic of the Congo. Vet. Microbiol. 2019, 239, 108493. [Google Scholar] [CrossRef]
- Rume, V.N.; Dundon, W.G.; Belay, G.; Baziki, J.; Diakite, A.; Paul, A.; Tessema, Y.D.; Nwankpa, N.; Gizaw, D.; Cattoli, G.; et al. Molecular epidemiological update of Peste des Petits Ruminants virus (PPRV) in Ethiopia. Vet. Microbiol. 2019, 235, 229–233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).