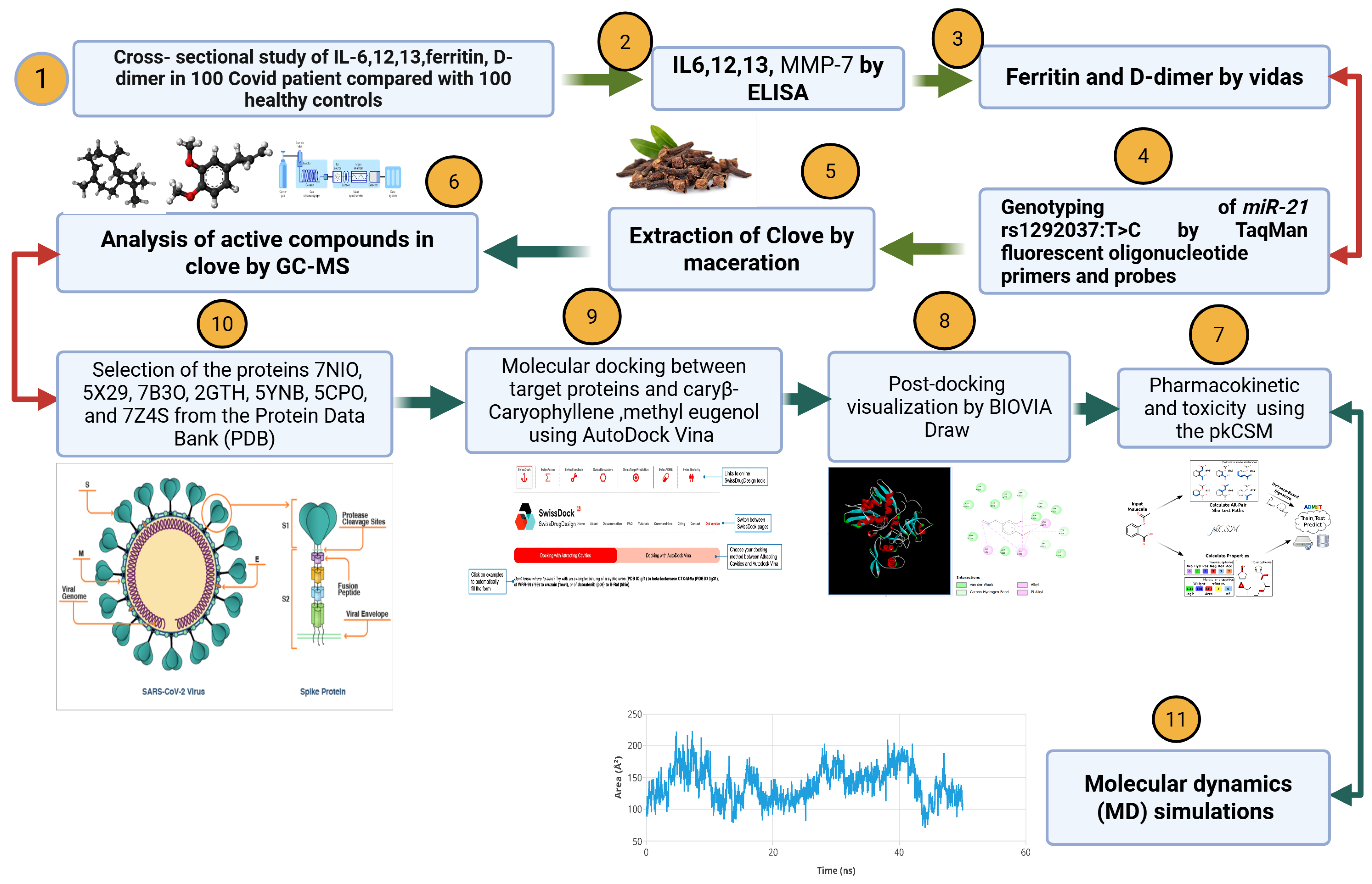
3.9. Molecular Docking Results of Eugenol Derivative and Caryophyllene Against SARS-CoV-2 Proteins
Table 12 and
Figure 6 present the results of the comparative molecular docking analysis of two phytocompounds—eugenol and caryophyllene—against seven critical SARS-CoV-2 proteins using AutoDock Vina. These proteins are involved in key stages of the viral life cycle, including RNA replication, protein maturation, and immune evasion.
The docking results indicate that eugenol generally exhibited stronger binding affinities than caryophyllene across most targets, particularly against the Envelope (5X29, −5.267 kcal/mol), Spike-RBD (7B3O, −4.979 kcal/mol), and NSP15 (2GTH, −5.317 kcal/mol) proteins. These values suggest potential for eugenol in interfering with viral entry and immune modulation.
In contrast, caryophyllene showed superior binding to NSP12 (RNA polymerase, 5CPO, −6.200 kcal/mol) and NSP16 (2′-O-methyltransferase, 5YNB, −6.000 kcal/mol), implying a possible inhibitory effect on viral genome replication and RNA capping processes. Caryophyllene also showed a moderately strong affinity for NSP5 (7Z4S, −5.500 kcal/mol), suggesting a role in inhibiting viral protease activity (
Figure 7).
Overall, while eugenol demonstrated consistent moderate binding across several viral targets, caryophyllene appeared to selectively bind more tightly to proteins involved in replication and post-transcriptional modification. These findings underscore the complementary potential of these two natural compounds as part of a multi-targeted antiviral strategy against SARS-CoV-2.
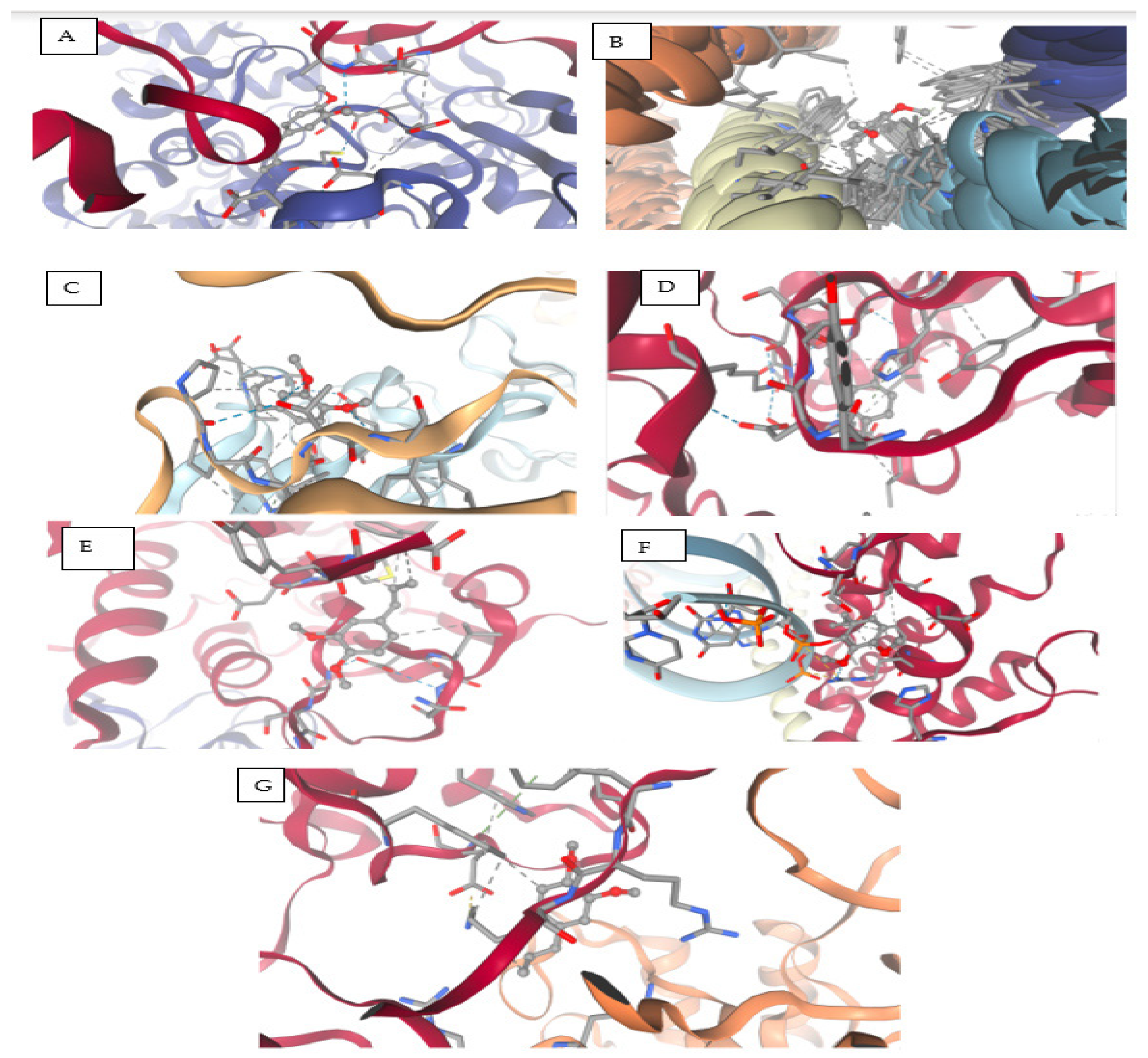
The compound (COC1=C(C=C(C=C1)CC=C)OC), a methoxy-substituted aromatic derivative, exhibits a diverse array of molecular interactions with critical amino acid residues across multiple SARS-CoV-2 protein structures, as revealed by molecular docking studies. The binding behavior includes hydrogen bonding, electrostatic contacts, π-π stacking, hydrophobic interactions, and van der Waals forces, contributing to ligand stabilization within active or allosteric sites of the viral targets, as shown in
Figure 8 and
Table 13.
This ligand engages in hydrogen bonding and hydrophobic interactions with Ala553, supporting mixed-polarity contact. Thr552 lies in close proximity, participating in potential polar contacts, while Glu551 facilitates electrostatic stabilization, likely via a salt bridge or hydrogen bond. Cys556 and Asp578/580 further enhance binding through van der Waals and ionic interactions. Notably, Leu581 creates a hydrophobic microenvironment, reinforcing non-polar affinity.
Aromatic interactions are predominant here, with Phe25–26 forming π-π stacking contacts, indicative of strong binding to aromatic regions of the protein. A hydrophobic patch formed by Ala, Leu, and Val (residues 19–29) stabilizes the ligand via van der Waals and alkyl interactions.
Key anchoring is established through hydrogen bonding and π–alkyl interactions with Phe99, Leu45, Ala60, and Pro95, suggesting stable aromatic and alkyl engagement. Additional contributions from Ser, Asp, Trp, Glu, and Thr residues form weak van der Waals contacts that may fine-tune the ligand’s spatial orientation within the binding site.
Moderate C–H and π–alkyl interactions occur with His304 and Ser301, while multiple residues including Tyr, Asp, Leu, Lys, Ile, Arg, and Val form a network of van der Waals contacts, reinforcing the fit through hydrophobic and spatial complementarity. Ala89 and Leu279 further stabilize the complex through alkyl interactions.
Significant electrostatic and hydrogen bonding is observed with Asp99, 130, and 133 via π–anion interactions, C–H bonds, and van der Waals forces. A diverse set of residues (Asn101, Gly71/73, Met131, and Tyr132) contribute to polar and van der Waals contacts, indicating a broad interaction surface. Leu100 and Phe149 provide hydrophobic anchoring through π–alkyl interactions.
Strong stabilizing effects are noted with Arg844 and His448, mediated through hydrogen bonds and π–alkyl interactions. However, steric clashes may arise with U:P32–33 and A:P31, as indicated by unfavorable bumps. Several additional residues (Asp, Pro, Gln, Ile, and Ser) contribute to non-specific van der Waals stabilization.
Weak polar interactions such as carbon–hydrogen bonds are observed with Arg4 and Leu282, while Lys5, Trp207, and Phe291 establish π–alkyl interactions, securing the ligand in an aromatic-rich environment. Glu288 and Phe3 form additional van der Waals contacts, suggesting a supplementary role in complex stabilization.
Molecular Docking Analysis of Caryophyllene with Various Protein Targets (
Figure 5 and
Table 14)
A comprehensive molecular docking study was conducted to assess the binding interactions of caryophyllene, a non-polar sesquiterpene, with diverse protein targets implicated in viral replication, microbial resistance, and quorum sensing.
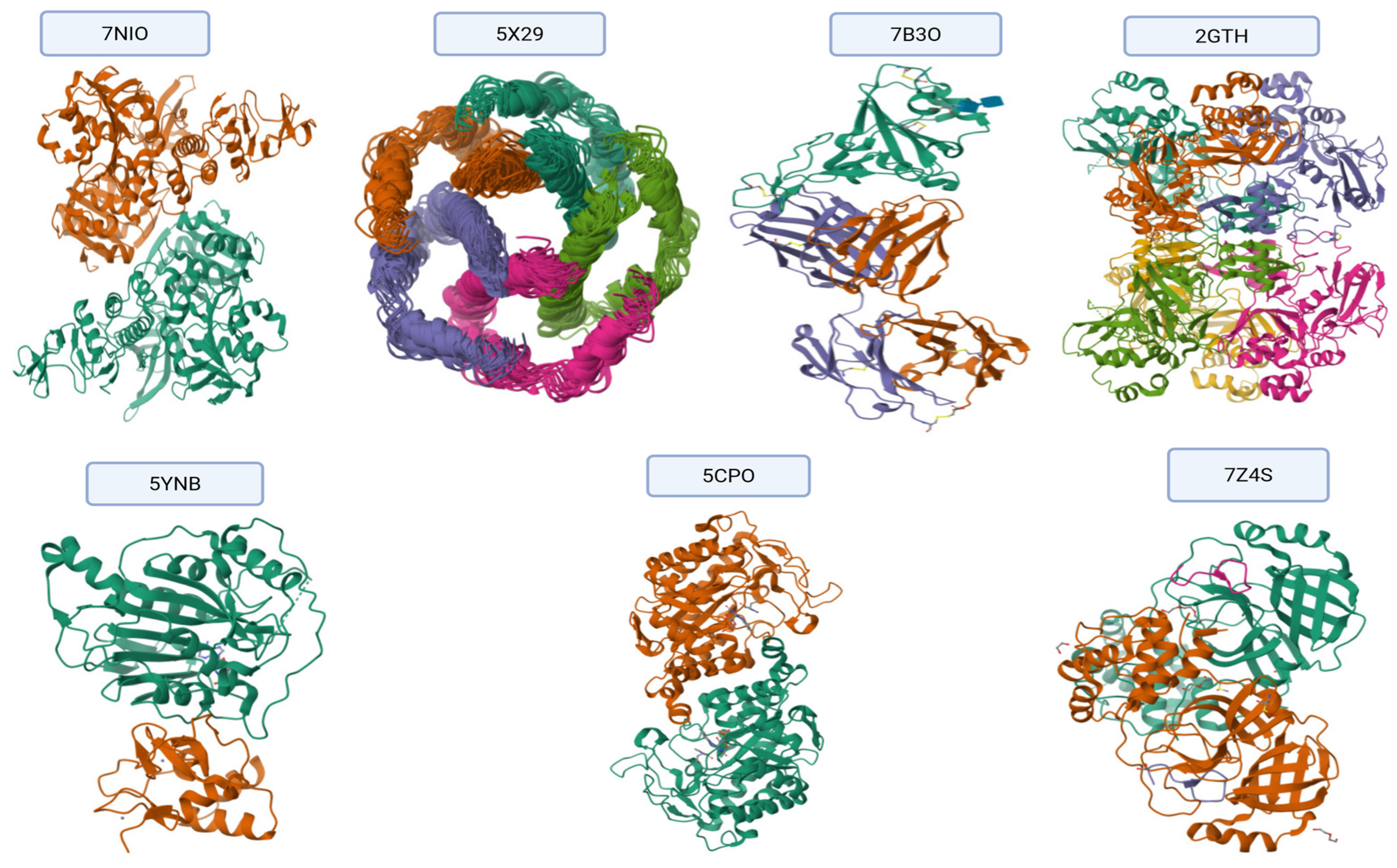
Figure 9A displays the docking results of caryophyllene with the main SARS-CoV-2 protease (PDB ID: 7NIO). The interaction diagram reveals hydrophobic
Pi–alkyl contacts with LEU405, ARG560, HIS554, and PRO408, highlighted in pink. Additionally,
van der Waals interactions are observed with ASP534, LEU412, and LEU417 (green shading). The absence of polar or hydrogen bonds reflects the non-polar nature of caryophyllene, indicating that hydrophobic and steric factors largely drive binding stabilization in this protease.
Figure 9B illustrates the interaction between caryophyllene and the quorum sensing receptor protein (PDB ID: 5X29). The ligand engages in
Pi–alkyl interactions with PHE28 and LEU19, suggesting strong hydrophobic anchoring. A single
van der Waals contact with SER16 contributes to ligand stabilization. The pocket’s predominantly hydrophobic environment supports caryophyllene’s fit and potential to interfere with receptor activity.
Figure 9C shows caryophyllene docked into the bacterial resistance-associated protein 7B3O. Here, the ligand forms an
alkyl interaction with LEU455, alongside multiple
van der Waals contacts with LYS417, TYR453, and ARG403. These findings support a non-polar binding mechanism, consistent with caryophyllene’s hydrophobic nature, and suggest possible interference with protein function.
Figure 9D presents the interaction of caryophyllene with the eukaryotic protein 2GTP. The ligand displays
alkyl interactions with LEU227, VAL160, and ALA89, supported by
van der Waals contacts involving ASP300, GLU68, GLY158, TYR88, ASP155, and ASP87. This combination of non-polar and polar residues highlights a moderate binding mode, largely governed by hydrophobic interactions.
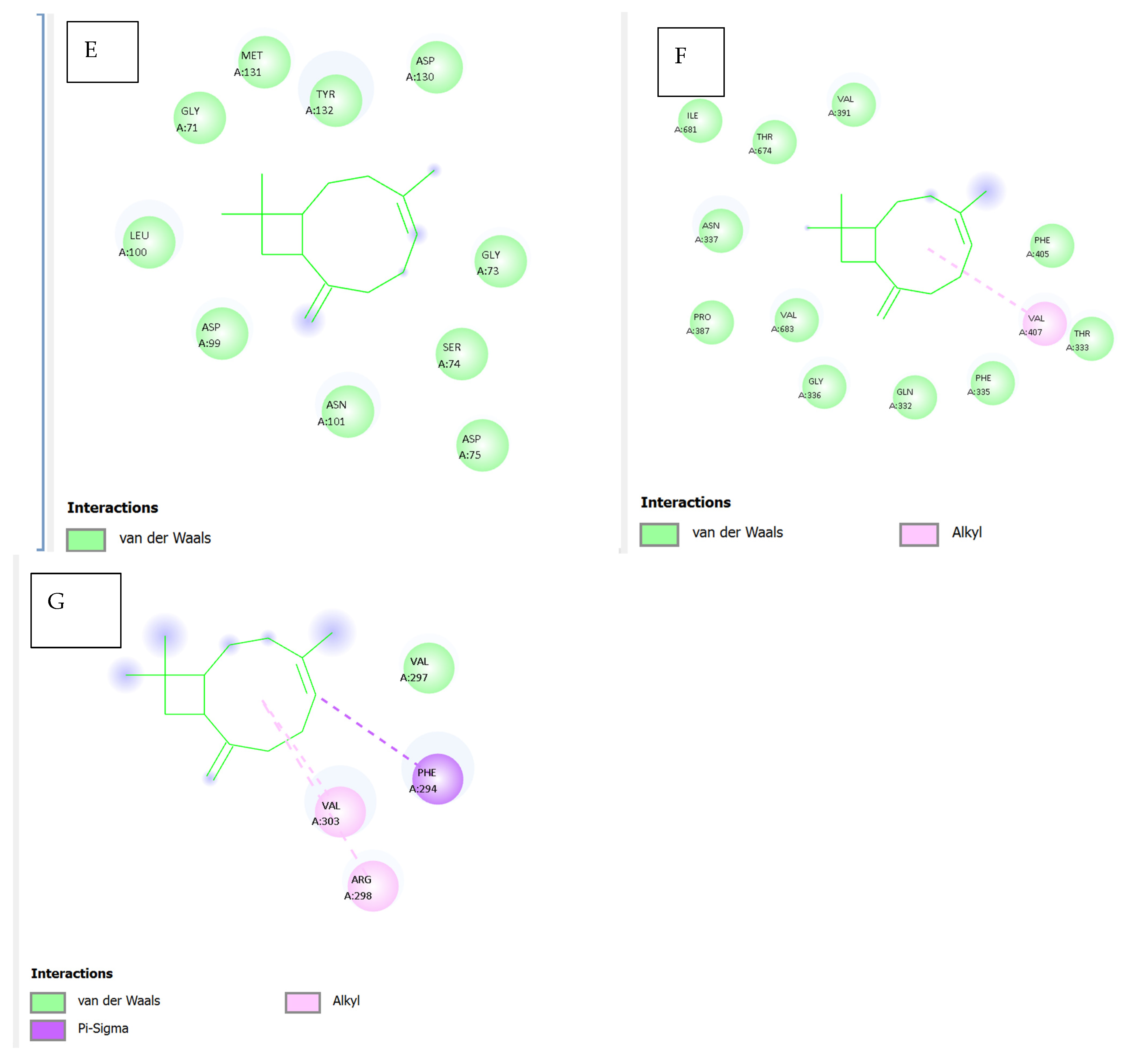
Figure 9E illustrates the docking pose of caryophyllene within the bacterial protein 5YNB. All observed contacts are
van der Waals in nature, involving residues such as GLY71, GLY73, ASP75, ASN101, LEU100, and TYR132. The absence of stronger interactions (e.g., hydrogen bonds or π interactions) suggests a lower binding affinity and a relatively passive accommodation within the binding cavity.
Figure 9F shows the interaction with the 5CPO protein. Caryophyllene forms
alkyl interactions with VAL407 and PHE405, alongside
van der Waals contacts involving ILE681, VAL391, GLN332, ASN337, and PRO387, among others. The diversity of surrounding residues indicates a flexible and largely hydrophobic binding site, suitable for the bulky, cyclic nature of the ligand.
Figure 9G highlights caryophyllene’s interactions with the 7Z4S protein. Notable contacts include
van der Waals and
alkyl interactions with VAL297, VAL303, and PHE294, in addition to a potential
pi–sigma interaction involving PHE294. These hydrophobic and aromatic interactions may support stable binding within the protein’s functional domain, although experimental studies are needed to confirm this.
Table 14.
Summary of caryophyllene–protein interactions.
Table 14.
Summary of caryophyllene–protein interactions.
| Figure | Protein (PDB ID) | Key Interactions | Notable Residues | Binding Nature |
|---|
| 9A | 7NIO (main protease) | Pi–alkyl, van der Waals | LEU405, ARG560, ASP534 | Hydrophobic |
| 9B | 5X29 (QS receptor) | Pi–alkyl, van der Waals | PHE28, LEU19, SER16 | Hydrophobic |
| 9C | 7B3O (resistance) | Alkyl, van der Waals | LEU455, TYR453, ARG403 | Hydrophobic |
| 9D | 2GTP | Alkyl, van der Waals | LEU227, VAL160, ASP300 | Mixed hydrophobic |
| 9E | 5YNB | Van der Waals only | GLY71, ASP130, TYR132 | Weak/non-specific |
| 9F | 5CPO | Alkyl, van der Waals | VAL407, PHE405, ILE681 | Hydrophobic |
| 9G | 7Z4S | Alkyl, van der Waals, π-σ | VAL297, PHE294, ARG298 | Aromatic–hydrophobic |
This analysis underscores that caryophyllene preferentially binds to hydrophobic and semi-polar regions of diverse proteins through non-covalent interactions, predominantly alkyl and van der Waals forces. Its lack of hydrogen bonding is consistent with its lipophilic terpenoid structure, suggesting that its biological activity may derive from steric interference with active or regulatory sites in proteins.
The comparative ADMET profile presented in
Table 15 offers a comprehensive evaluation of methyl
eugenol and β-
caryophyllene using predictive in silico models, emphasizing key pharmacokinetic and toxicological parameters essential for drug development and safety assessment.
In terms of absorption, eugenol displays a markedly higher aqueous solubility than caryophyllene (log S = −2.671 vs. −5.555), which favors its dissolution and potential oral bioavailability. Both compounds show comparable Caco-2 permeability and high human intestinal absorption, suggesting effective gastrointestinal uptake. Despite their favorable absorption profiles, both exhibit relatively low skin permeability, with eugenol being slightly less permeable dermally. Notably, neither molecule is a substrate or inhibitor of P-glycoproteins, indicating a reduced risk of efflux-related drug interactions and enhanced systemic retention.
Regarding distribution, caryophyllene shows a higher volume of distribution (log VDss = 0.652) compared to eugenol (0.265), suggesting more extensive tissue penetration. Both compounds exhibit moderate plasma protein binding, ensuring an adequate unbound drug fraction. Additionally, caryophyllene demonstrates a greater capacity to permeate the blood–brain barrier, though both show limited CNS permeability, which may restrict their central effects.
Under metabolic profiling, neither compound is a substrate or inhibitor of major cytochrome P450 enzymes except for CYP1A2, where eugenol acts as an inhibitor. This interaction implies a potential for metabolic interference when co-administered with substrates of this enzyme. Otherwise, their metabolic stability appears favorable, with low risk of enzymatic inhibition-based drug interactions.
In the context of excretion, caryophyllene exhibits higher predicted total clearance, suggesting faster systemic elimination. Neither compound is likely to be excreted via renal OCT2-mediated pathways.
Concerning toxicity, a notable distinction arises in AMES test predictions: eugenol may possess mutagenic potential, whereas caryophyllene is predicted to be non-mutagenic. Eugenol, however, is tolerated at a higher dose in humans and demonstrates lower acute and chronic toxicity in rodent models. Both compounds show potential for skin sensitization. Environmentally, eugenol exhibits higher minnow toxicity, while caryophyllene is more toxic to T. pyriformis, indicating species-specific ecological risks.
In summary, eugenol and caryophyllene share several favorable ADMET traits, such as high absorption and low hepatic toxicity, but differ in solubility, metabolic enzyme interaction, and certain toxicity endpoints. These differences could influence their suitability in pharmaceutical or nutraceutical applications, depending on the target profile and safety requirements.
A suite of molecular dynamics (MD) simulations was conducted to evaluate the dynamic stability, structural adaptability, and interaction profiles of methyl eugenol in complex with seven critical SARS-CoV-2 target proteins: 7NIO (nucleocapsid phosphoprotein), 5X29 (Envelope Protein), 7B3O (RNA-dependent RNA polymerase), 2GTH (main protease), 5YNB (RNA-binding domain), 5CPO (papain-like protease), and 7Z4S (spike receptor-binding domain). The simulations were carried out over a 50 ns timescale under explicit solvent conditions, with systematic evaluation of key structural descriptors, including root mean square deviation (RMSD), root mean square fluctuation (RMSF), radius of gyration (Rg), molecular and polar surface area (MSA/PSA), solvent-accessible surface area (SASA), radial distribution function (RDF), and ligand–protein contact profiles as shown in
Supplementary S1,
Figure 10, and
Table 16.
Across all protein complexes, RMSD values stabilized within the first 5–6 ns, with most systems maintaining deviations below 2.5 Å. This included the 7Z4S and 7NIO complexes, which exhibited particularly low RMSD plateaus around 1.8–2.1 Å, underscoring rapid convergence and minimal global rearrangement. These results reflect strong structural resilience upon ligand binding, with methyl eugenol being effectively accommodated in shallow grooves, hydrophobic pockets, or active site clefts without inducing deleterious conformational changes. The 7B3O and 5CPO systems similarly stabilized early, with average RMSD values suggesting tight ligand anchoring and thermodynamic compatibility.
The RMSF analyses revealed that loop regions and termini consistently displayed the highest fluctuations (>2.0 Å), while structured α-helices and β-strands remained notably rigid (≤1.2 Å). Binding-site residues in all complexes showed suppressed mobility, particularly in the 7Z4S RBD, where ACE2-interacting loops exhibited reduced flexibility, hinting at potential inhibitory effects through rigidification. In the catalytic proteins (5CPO, 2GTH), ligand-induced dampening of flexibility in enzymatically active residues supports the hypothesis of functional interference. The 5YNB complex, involving the RNA-binding domain, displayed ligand-induced stabilization within its β-sheet core, aligning with the notion of conformational anchoring.
All protein systems maintained compact tertiary structures throughout the simulations, with Rg fluctuations confined within ±0.3 Å of their initial values. No evidence of large-scale unfolding or domain collapse was observed, even in structurally sensitive systems like 2GTH and 5CPO. This compactness confirms the biophysical compatibility of methyl eugenol with diverse viral protein architectures and further supports its non-denaturing interaction profile.
The MSA and PSA trends were consistent across simulations, with moderate increases during early equilibration phases, reflecting solvent reorganization and mild residue rearrangements. PSA elevations (typically ~300 Å2) were most notable in proteins with solvent-facing polar grooves (e.g., 5X29, 7B3O), suggesting polar residue reorientation around the ligand. In the 7Z4S system, a slight reduction in MSA indicated hydrophobic cavity occupation by methyl eugenol, leading to reduced surface entropy and enthalpic stabilization.
RDF profiles across all complexes exhibited sharp, high-density peaks between 2.2 and 4.8 Å, confirming persistent and spatially localized interactions. Notably, 5CPO and 7Z4S showed strong RDF peaks corresponding to active-site residues and receptor-binding motifs, respectively, indicating sustained contact zones critical for function modulation. The narrowness and symmetry of RDF peaks in all systems confirmed single, well-defined binding poses, with no evidence of alternative orientations or transient dissociation events.
The SASA trajectories across complexes revealed minor oscillations consistent with protein breathing motions. Importantly, no spikes indicative of partial unfolding were observed. Slight SASA reductions in the vicinity of the ligand (e.g., 5CPO and 7Z4S) reflected hydrophobic shielding and potential reductions in conformational entropy—features correlated with enhanced binding affinity and reduced solvation energy penalties.
Methyl eugenol exhibited diverse, yet consistent interaction profiles, dominated by hydrophobic contacts, hydrogen bonds, and π-π or π–cation interactions. In catalytic proteins such as 5CPO and 2GTH, key residues like Cys111 and His272 engaged in stabilizing interactions with the ligand, suggesting active-site targeting. In the 7Z4S RBD, aromatic residues (Tyr453, Tyr505) formed π-π stacking interactions, while Gln493 and Asn487 provided hydrogen bonds—highlighting a pharmacophoric alignment potentially disruptive to ACE2 engagement. The interaction persistence across simulations supports the ligand’s high residence time and specificity.
Finally, methyl eugenol demonstrates a robust and adaptable binding profile across several critical SARS-CoV-2 proteins, maintaining structural stability and inducing local conformational reinforcement at functional domains. Its capacity to stably occupy enzymatic clefts, receptor interfaces, and RNA-binding grooves, while preserving protein fold and compactness, highlights its promise as a phytochemical scaffold for antiviral intervention. These molecular dynamics simulations offer compelling evidence for its further exploration through in vitro validation, SAR optimization, and structure-based drug design initiatives targeting viral replication and host-cell entry.
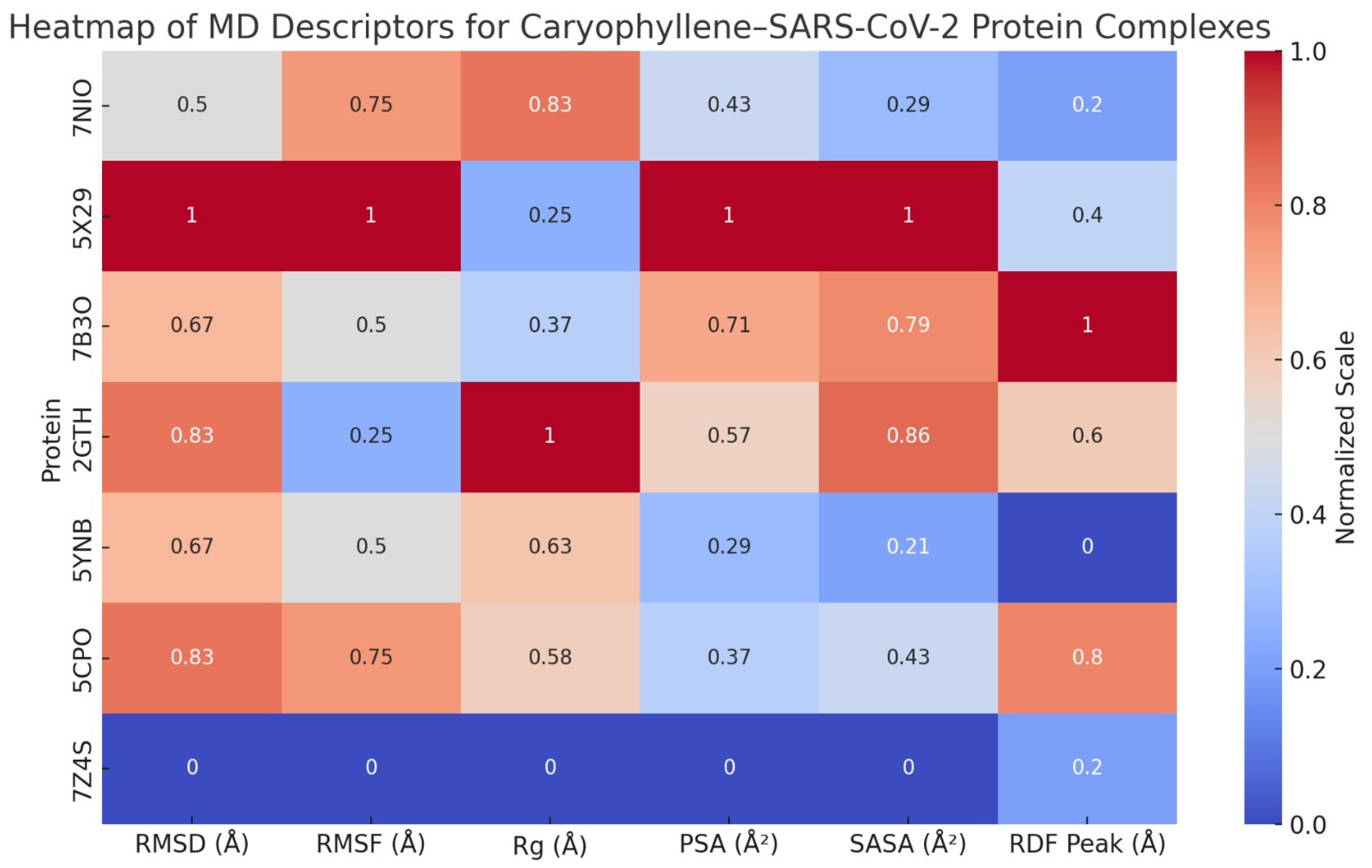
A series of molecular dynamics (MD) simulations were conducted to assess the dynamic behavior and structural stability of caryophyllene when bound to seven critical SARS-CoV-2 proteins: nucleocapsid phosphoprotein (7NIO), Envelope Protein (5X29), RNA-dependent RNA polymerase (7B3O), main protease (2GTH), RNA-binding domain (5YNB), papain-like protease (5CPO), and the spike receptor-binding domain (7Z4S). Each protein–ligand complex was simulated over a 50 ns timescale, and key structural descriptors were evaluated to understand the conformational response, interaction patterns, and overall compatibility of the non-polar sesquiterpene ligand, as shown in
Supplementary S1,
Figure 11, and
Table 17.
Across all complexes, RMSD values stabilized within the first 4–6 ns, with average deviations ranging from ~1.8 Å (7Z4S) to ~2.4 Å (5X29), indicating high structural fidelity upon ligand binding. No global unfolding or large-scale structural rearrangements were observed. The radius of gyration (Rg) profiles remained within a narrow ±0.3 Å band, further underscoring the preservation of protein compactness. Similarly, SASA trends demonstrated minimal deviation, suggesting that ligand binding did not provoke abnormal solvent exposure or collapse of tertiary structures.
RMSF analyses showed generally low residue-level fluctuations (<1.5 Å) across the α-helical, β-sheet, and catalytic cores. Notably, loop regions in RNA-binding or receptor-interacting domains displayed slightly elevated mobility, yet this remained within expected dynamic limits. In many cases (e.g., 7B3O, 2GTH, 5YNB), ligand presence actually dampened flexibility near active or binding sites, suggesting a stabilizing influence on functionally relevant residues.
Molecular surface area (MSA) increased modestly in most systems due to accommodation of the ligand’s hydrophobic volume. However, polar surface area (PSA) remained largely stable, indicating that critical polar interactions and solvation interfaces were conserved. Radial distribution function (RDF) analyses consistently revealed sharp peaks between 4.3 and 4.8 Å, reflecting organized hydration shells that supported ligand stability via water-mediated van der Waals and dispersive forces.
Despite lacking polar functional groups, caryophyllene consistently engaged in van der Waals, alkyl–alkyl, and π–alkyl interactions across all protein targets. Key residues involved included Leu50 and Val73 in 7NIO, Leu28 and Phe56 in 5X29, Ile548 and Phe793 in 7B3O, Met49 and His163 in 2GTH, Phe55 and Ile89 in 5YNB, Leu162 and Val266 in 5CPO, and Tyr489 and Leu455 in 7Z4S. These hydrophobic contacts formed stable clusters that anchored the ligand within shallow grooves, clefts, or surface pockets, without disrupting polar interaction networks or essential catalytic motifs.
The simulation data suggest that caryophyllene maintains protein structural integrity while inducing localized rigidification in flexible or catalytically important regions. In spike RBD (7Z4S), reduced mobility in the receptor-binding loop implies potential interference with ACE2 docking. In proteases (2GTH, 5CPO), occupancy near the catalytic triad hints at possible non-competitive inhibition. For RNA-interacting proteins (7NIO, 5YNB), the ligand may modulate RNA accessibility without obstructing functional core motifs.
Finally, the molecular dynamics simulations across seven SARS-CoV-2 proteins demonstrated that caryophyllene binding is structurally non-disruptive and thermodynamically favorable. The ligand’s hydrophobic nature enabled stable anchoring through non-polar contacts, while the preservation of compactness, surface integrity, and functional domain architecture suggests strong compatibility. These findings advocate for the potential of caryophyllene as a scaffold for further antiviral drug development, particularly in targeting protein surfaces involved in viral assembly, RNA binding, or host interaction.