Respiratory Syncytial Virus Induces B Cell Activating Factor (BAFF) in Airway Epithelium: A Potential Avenue for Mucosal Vaccine Development
Abstract
1. Introduction
2. Material and Methods
2.1. RSV Challenge and IFN-β Treatment of BEAS-2B Airway Epithelial Cells
2.2. Inhibition of IFN-β Follwing RSV Challenge
2.3. Assessment Survival of B Cells Using MTT Assay
2.4. Identification of BAFF Forms in BEAS-2B Cells Post-RSV Challenge
2.5. Investigation of How RSV Challenge in BEAS-2B Cells Promotes BAFF Cleavage
2.6. BAFF Protein Quantification and Immunofluorescence Staining
2.7. Statistical Analysis
3. Results
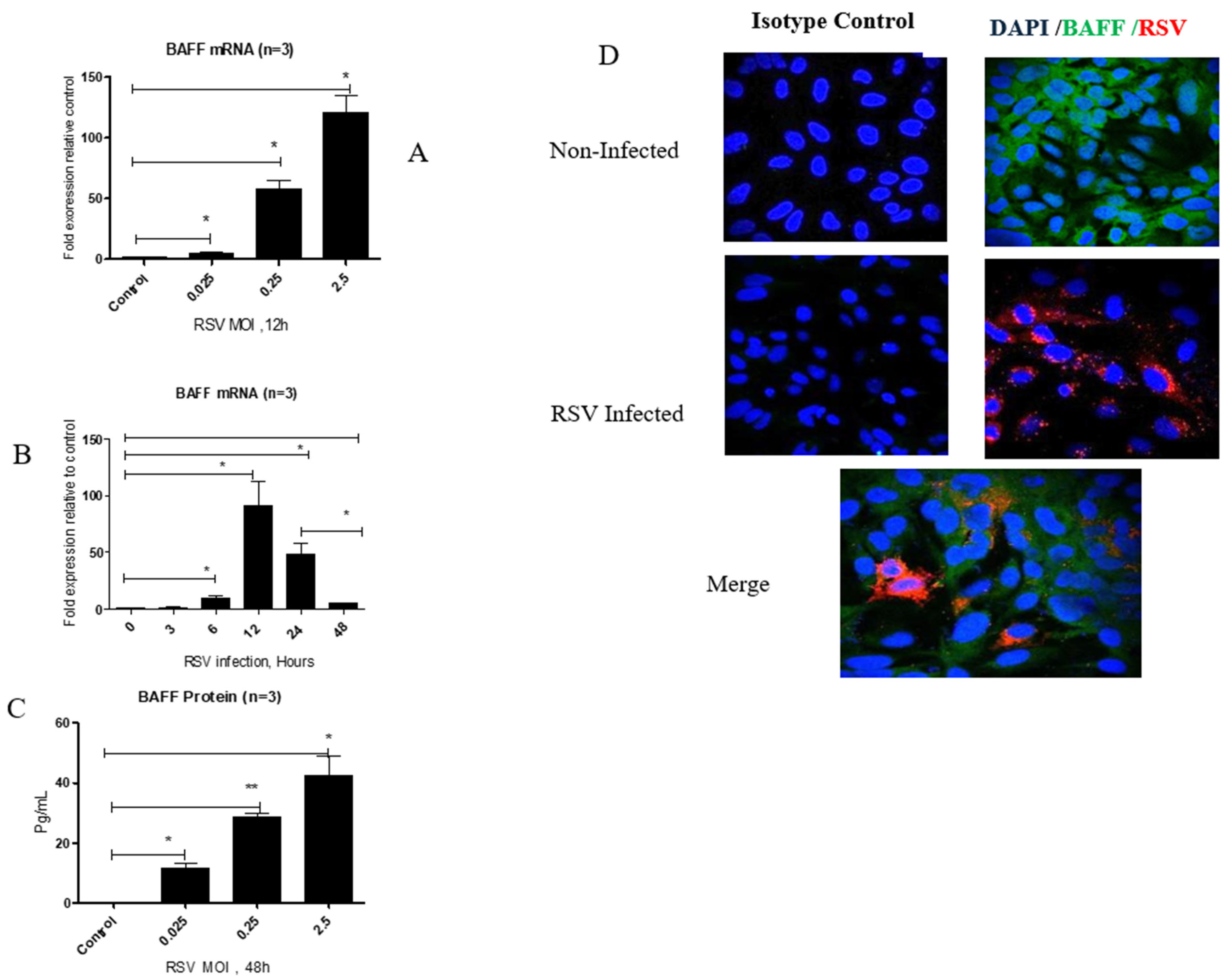
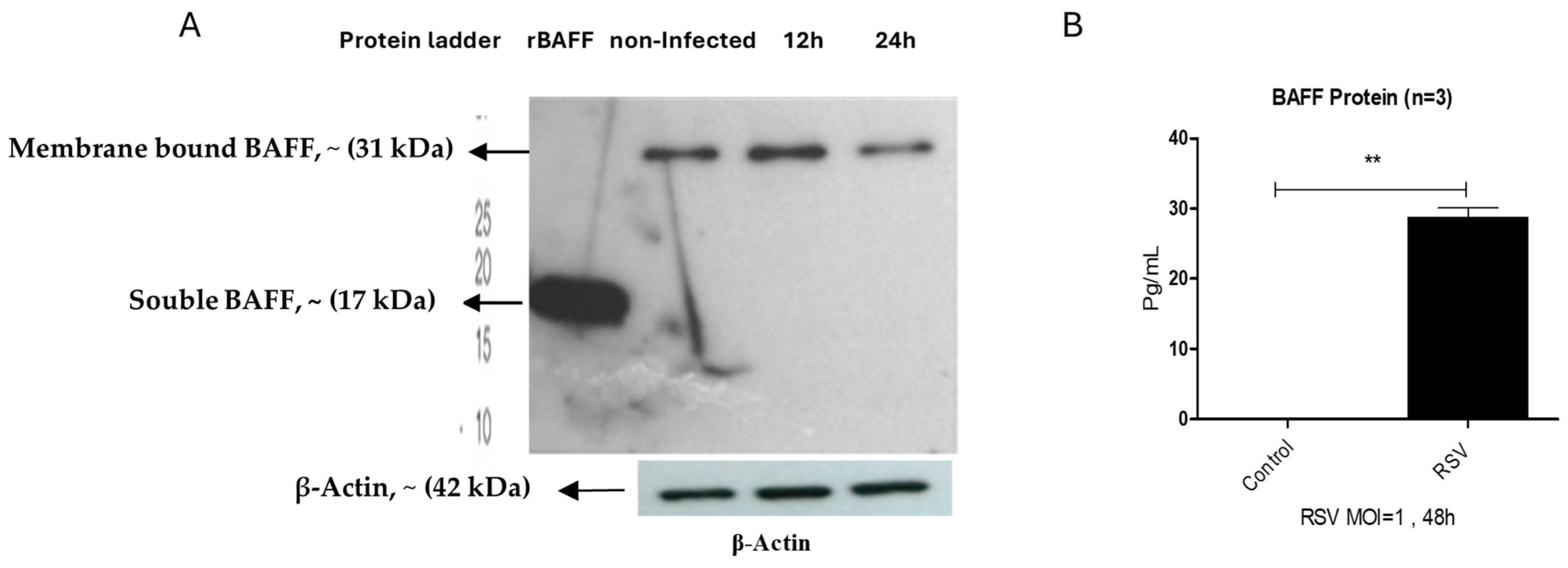
3.1. RSV Infection Enhances BAFF Gene and Protein Expression in BEAS-2B Cells in a Time- and Dose-Dependent Manner
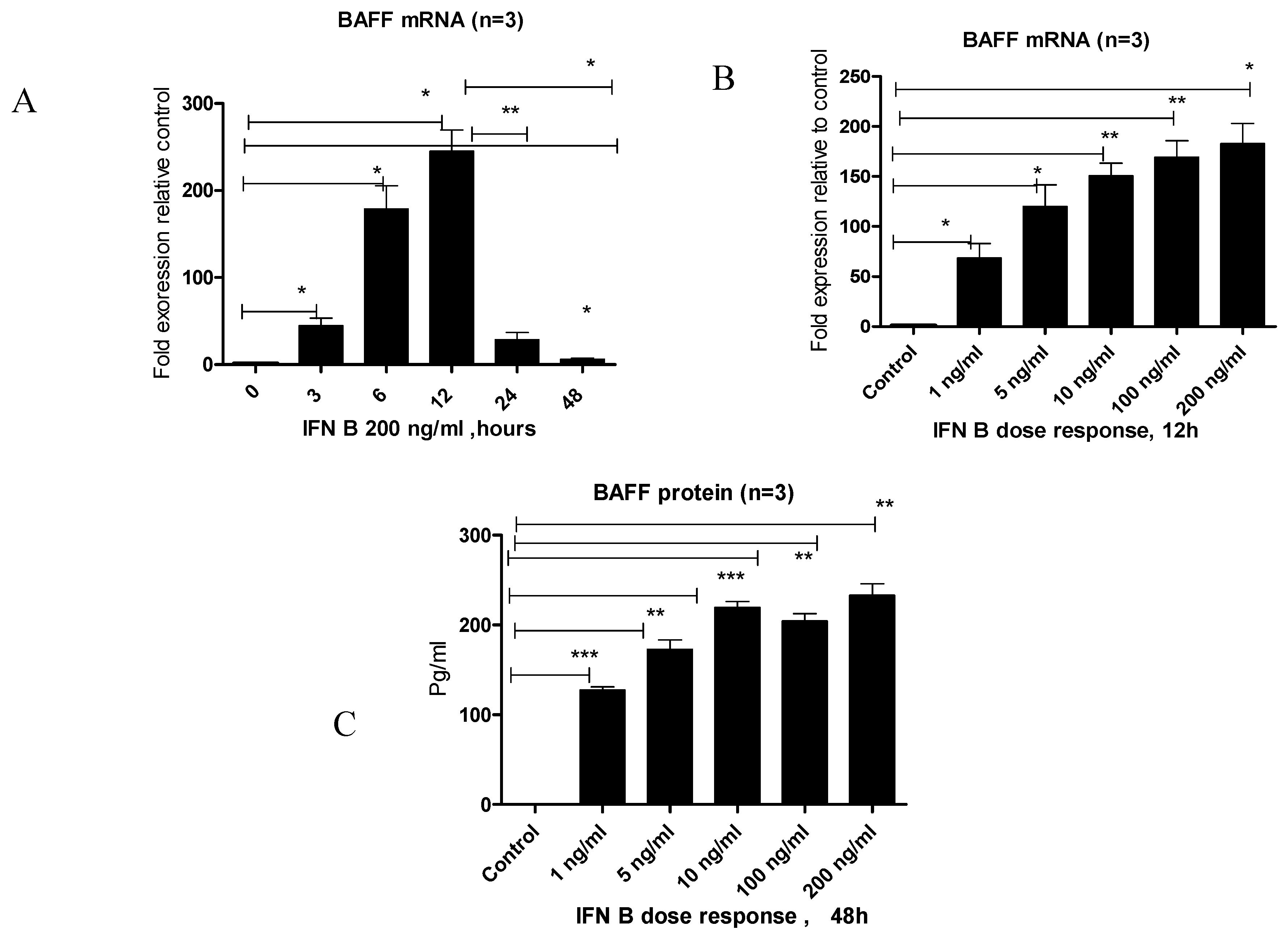
3.2. IFN-β Treatment Induces BAFF Gene and Protein Expression in BEAS-2B Cells
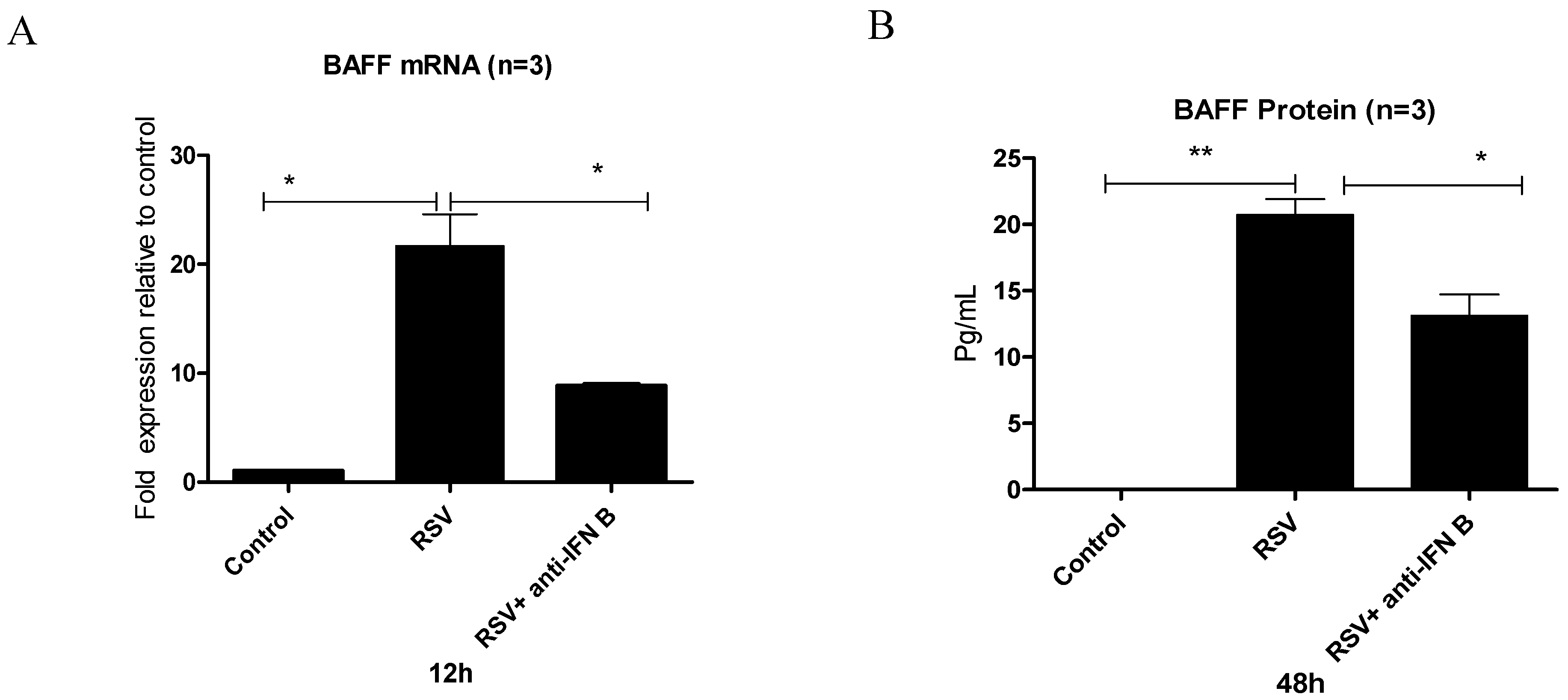
3.3. Does RSV Infection Induce BAFF Through an IFN-β-Mediated Mechanism?
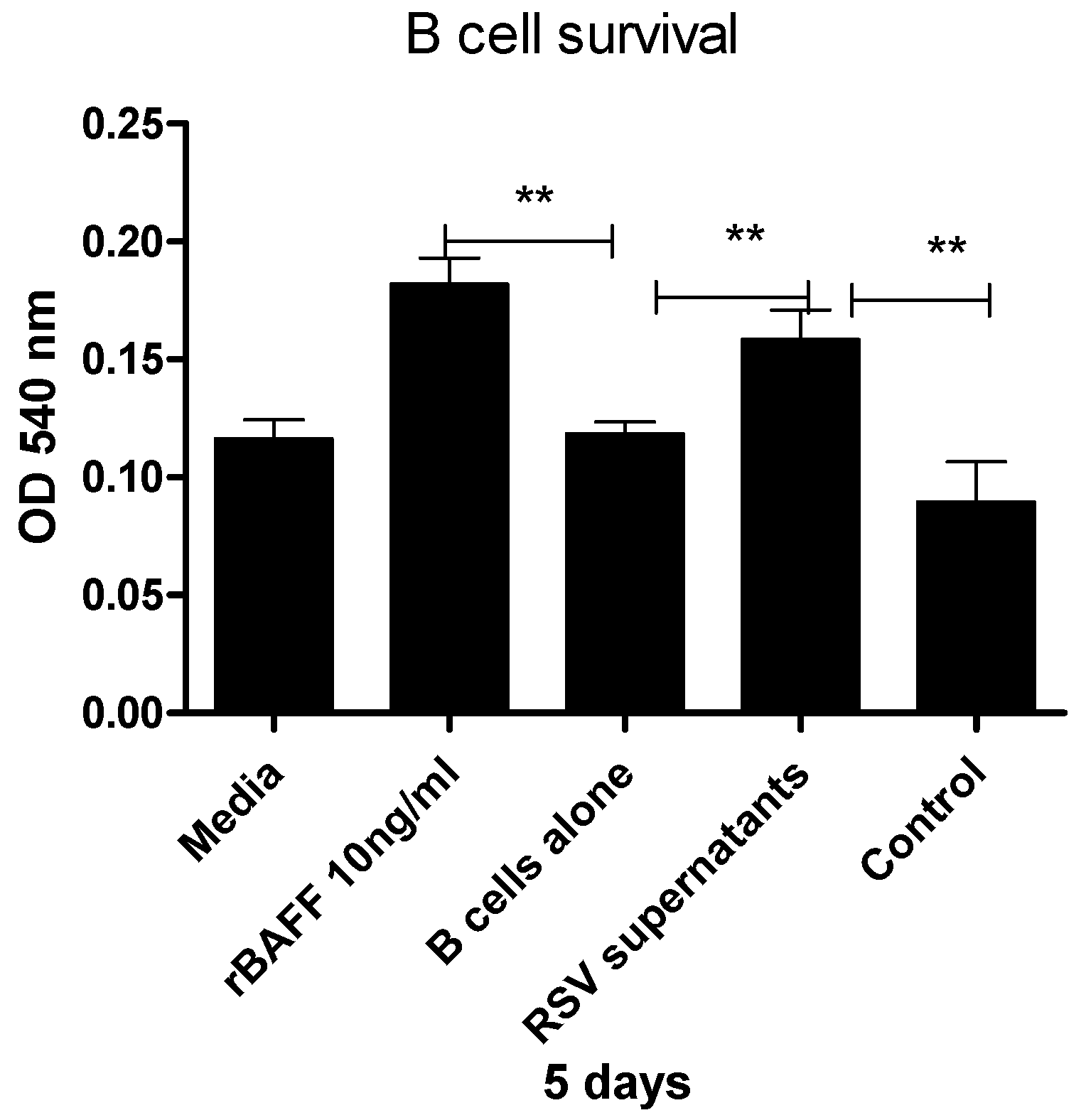
3.4. Epithelial Cell-Derived BAFF Following RSV Infection Enhances B Cell Survival
3.5. BAFF Isoform Expression in BEAS-2B Cells Post-RSV Challenge
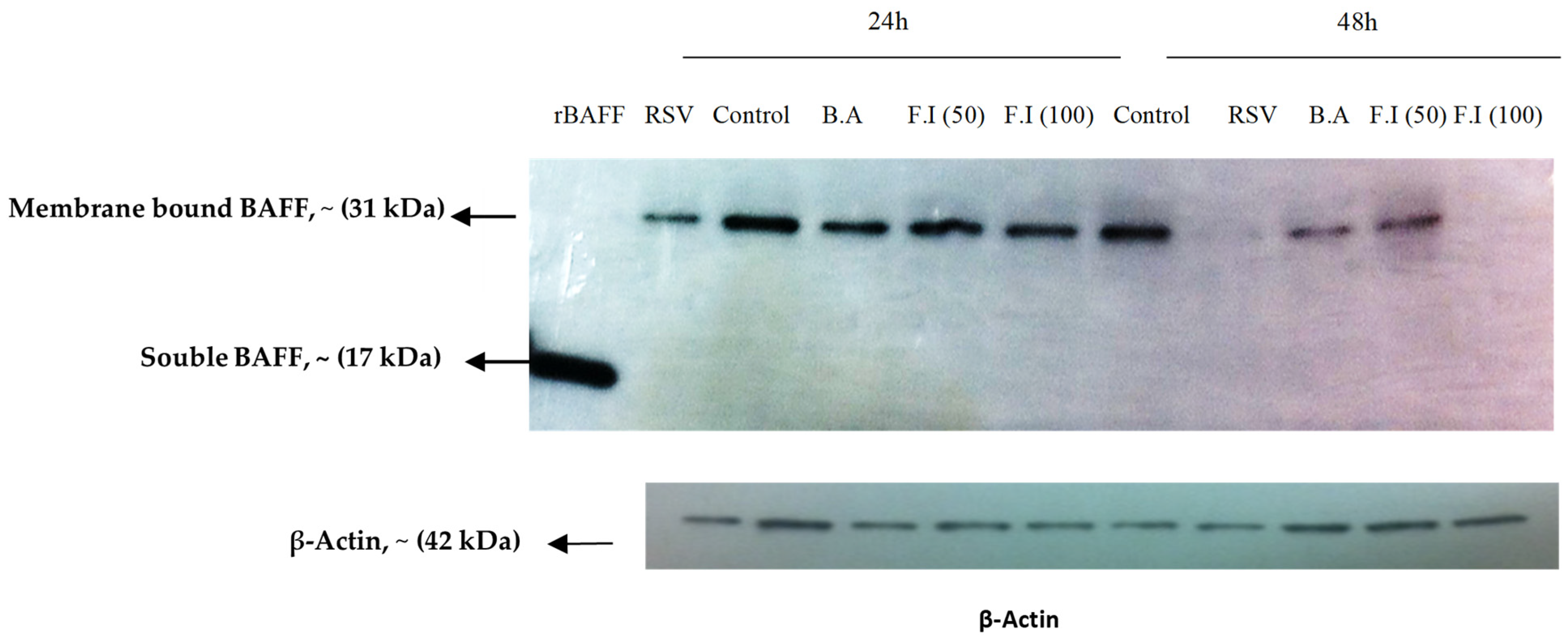
3.6. Characterization of BAFF Cleavage Pathways
4. Discussion
5. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alturaiki, W. Exploring the development of a promising mucosal adjuvant vaccine for human respiratory syncytial virus (RSV) infection. J. King Saud Univ. Sci. 2024, 36, 103289. [Google Scholar] [CrossRef]
- Alturaiki, W. Considerations for novel COVID-19 mucosal vaccine development. Vaccines 2022, 10, 1173. [Google Scholar] [CrossRef]
- Xiao, L.; Yu, W.; Shen, L.; Yan, W.; Qi, J.; Hu, T. Mucosal SARS-CoV-2 nanoparticle vaccine based on mucosal adjuvants and its immune effectiveness by intranasal administration. ACS Appl. Mater. Interfaces 2023, 15, 35895–35905. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Poo, H.; Han, D.P.; Hong, S.-P.; Kim, K.; Cho, M.W.; Kim, E.; Sung, M.-H.; Kim, C.-J. Mucosal immunization with surface-displayed severe acute respiratory syndrome coronavirus spike protein on Lactobacillus casei induces neutralizing antibodies in mice. J. Virol. 2006, 80, 4079–4087. [Google Scholar] [CrossRef]
- Rossi, A.; Michelini, Z.; Leone, P.; Borghi, M.; Blasi, M.; Bona, R.; Spada, M.; Grasso, F.; Gugliotta, A.; Klotman, M.E.; et al. Optimization of mucosal responses after intramuscular immunization with integrase defective lentiviral vector. PLoS ONE 2014, 9, e107377. [Google Scholar] [CrossRef] [PubMed]
- Vajdy, M.; Singh, M.; Ugozzoli, M.; Briones, M.; Soenawan, E.; Cuadra, L.; Kazzaz, J.; Ruggiero, P.; Peppoloni, S.; Norelli, F.; et al. Enhanced mucosal and systemic immune responses to Helicobacter pylori antigens through mucosal priming followed by systemic boosting immunizations. Immunology 2003, 110, 86–94. [Google Scholar] [CrossRef]
- Wang, B.-Z.; Xu, R.; Quan, F.-S.; Kang, S.-M.; Wang, L.; Compans, R.W.; Fouchier, R.A.M. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS ONE 2010, 5, e13972. [Google Scholar] [CrossRef]
- Tertilt, C.; Joh, J.; Krause, A.; Chou, P.; Schneeweiss, K.; Crystal, R.G.; Worgall, S. Expression of B-cell activating factor enhances protective immunity of a vaccine against Pseudomonas aeruginosa. Infect. Immun. 2009, 77, 3044–3055. [Google Scholar] [CrossRef]
- Bornacelly, A.; Mercado, D.; Acevedo, N.; Caraballo, L. The strength of the antibody response to the nematode Ascaris lumbricoides inversely correlates with levels of B-Cell Activating Factor (BAFF). BMC Immunol. 2014, 15, 22. [Google Scholar] [CrossRef]
- Nascimento, M.; Huot-Marchand, S.; Gombault, A.; Panek, C.; Bourinet, M.; Fanny, M.; Savigny, F.; Schneider, P.; Le Bert, M.; Ryffel, B.; et al. B-cell activating factor secreted by neutrophils is a critical player in lung inflammation to cigarette smoke exposure. Front. Immunol. 2020, 11, 1622. [Google Scholar] [CrossRef]
- Morissette, M.C.; Gao, Y.; Shen, P.; Thayaparan, D.; Bérubé, J.; Paré, P.D.; Brandsma, C.; Hao, K.; Bossé, Y.; Ettinger, R.; et al. Role of BAFF in pulmonary autoantibody responses induced by chronic cigarette smoke exposure in mice. Physiol. Rep. 2016, 4, e13057. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, L. CsBAFF, a teleost B cell activating factor, promotes pathogen-induced innate immunity and vaccine-induced adaptive immunity. PLoS ONE 2015, 10, e0136015. [Google Scholar] [CrossRef]
- Zhang, K.; Roy, N.K.; Vicioso, Y.; Woo, J.; Beck, R.; de Lima, M.; Caimi, P.; Feinberg, D.; Parameswaran, R. BAFF receptor antibody for mantle cell lymphoma therapy. Oncoimmunology 2021, 10, 1893501. [Google Scholar] [CrossRef]
- Schneider, P.; MacKay, F.; Steiner, V.; Hofmann, K.; Bodmer, J.-L.; Holler, N.; Ambrose, C.; Lawton, P.; Bixler, S.; Acha-Orbea, H.; et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 1999, 189, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.S.; Fonceca, A.M.; Howarth, D.; Correia, J.B.; Slupsky, J.R.; Trinick, R.E.; Al Turaiki, W.; Smyth, R.L.; Flanagan, B.F. Respiratory syncytial virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax 2013, 68, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Truong-Tran, A.Q.; Scott, A.L.; Matsumoto, K.; Schleimer, R.P. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J. Immunol. 2006, 177, 7164–7172. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Xie, J.; Xu, Y. Cigarette smoke inhibits BAFF expression and mucosal immunoglobulin A responses in the lung during influenza virus infection. Respir. Res. 2015, 16, 37. [Google Scholar] [CrossRef]
- Ittah, M.; Miceli-Richard, C.; Lebon, P.; Pallier, C.; Lepajolec, C.; Mariette, X. Induction of B cell-activating factor by viral infection is a general phenomenon, but the types of viruses and mechanisms depend on cell type. J. Innate Immun. 2011, 3, 200–207. [Google Scholar] [CrossRef]
- Neill, D.R.; Saint, G.L.; Bricio-Moreno, L.; Fothergill, J.L.; Southern, K.W.; Winstanley, C.; Christmas, S.E.; Slupsky, J.R.; McNamara, P.S.; Kadioglu, A.; et al. The B lymphocyte differentiation factor (BAFF) is expressed in the airways of children with CF and in lungs of mice infected with Pseudomonas aeruginosa. PLoS ONE 2014, 9, e95892. [Google Scholar] [CrossRef]
- Alturaiki, W.; McFarlane, A.J.; Rose, K.; Corkhill, R.; McNamara, P.S.; Schwarze, J.; Flanagan, B.F. Expression of the B cell differentiation factor BAFF and chemokine CXCL13 in a murine model of respiratory syncytial virus infection. Cytokine 2018, 110, 267–271. [Google Scholar] [CrossRef]
- Reed, J.L.; Welliver, T.P.; Sims, G.P.; McKinney, L.; Velozo, L.; Avendano, L.; Hintz, K.; Luma, J.; Coyle, A.J.; Welliver, S.R.C. Innate immune signals modulate antiviral and polyreactive antibody responses during severe respiratory syncytial virus infection. J. Infect. Dis. 2009, 199, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Clark, E.S.; Termini, J.M.; Boucher, J.; Kanagavelu, S.; LeBranche, C.C.; Abraham, S.; Montefiori, D.C.; Khan, W.N.; Stone, G.W.; et al. DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers. J. Virol. 2015, 89, 4158–4169. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.G.; Ng, C.-H.; Woehl, B.; Sutherland, A.P.R.; Huo, J.; Xu, S.; Mackay, F.; Lam, K.-P. BAFF costimulation of Toll-like receptor-activatedB-1 cells. Eur. J. Immunol. 2006, 36, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alturaiki, W.; Flanagan, B. Respiratory Syncytial Virus Induces B Cell Activating Factor (BAFF) in Airway Epithelium: A Potential Avenue for Mucosal Vaccine Development. Viruses 2025, 17, 946. https://doi.org/10.3390/v17070946
Alturaiki W, Flanagan B. Respiratory Syncytial Virus Induces B Cell Activating Factor (BAFF) in Airway Epithelium: A Potential Avenue for Mucosal Vaccine Development. Viruses. 2025; 17(7):946. https://doi.org/10.3390/v17070946
Chicago/Turabian StyleAlturaiki, Wael, and Brian Flanagan. 2025. "Respiratory Syncytial Virus Induces B Cell Activating Factor (BAFF) in Airway Epithelium: A Potential Avenue for Mucosal Vaccine Development" Viruses 17, no. 7: 946. https://doi.org/10.3390/v17070946
APA StyleAlturaiki, W., & Flanagan, B. (2025). Respiratory Syncytial Virus Induces B Cell Activating Factor (BAFF) in Airway Epithelium: A Potential Avenue for Mucosal Vaccine Development. Viruses, 17(7), 946. https://doi.org/10.3390/v17070946





