Medicinal Mushrooms, Probiotics and Combination of Natural Compounds in the Management of HPV: A Comparative Look at Viral Clearance and Lesion Resolution
Abstract
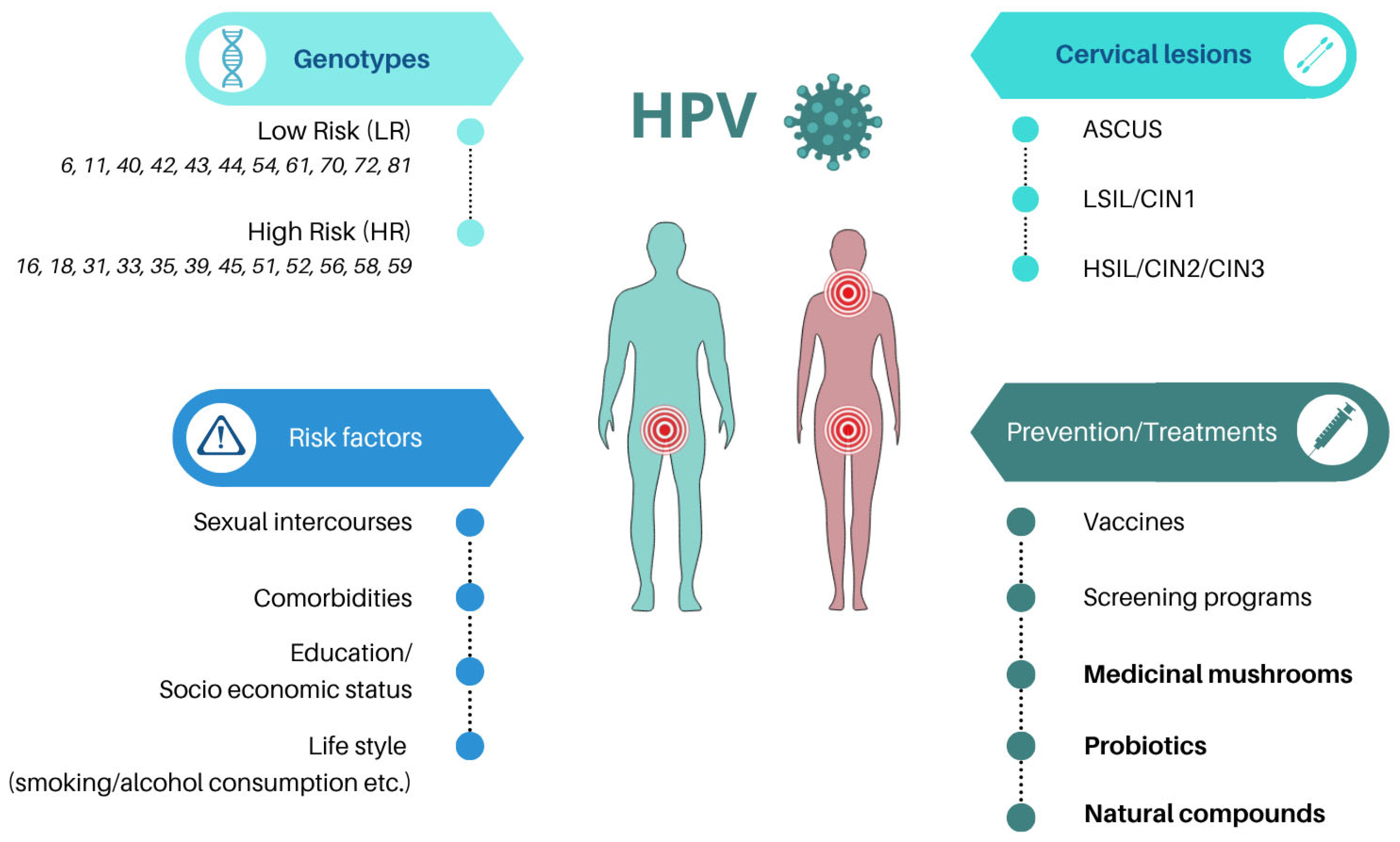
1. Introduction
2. Materials and Methods
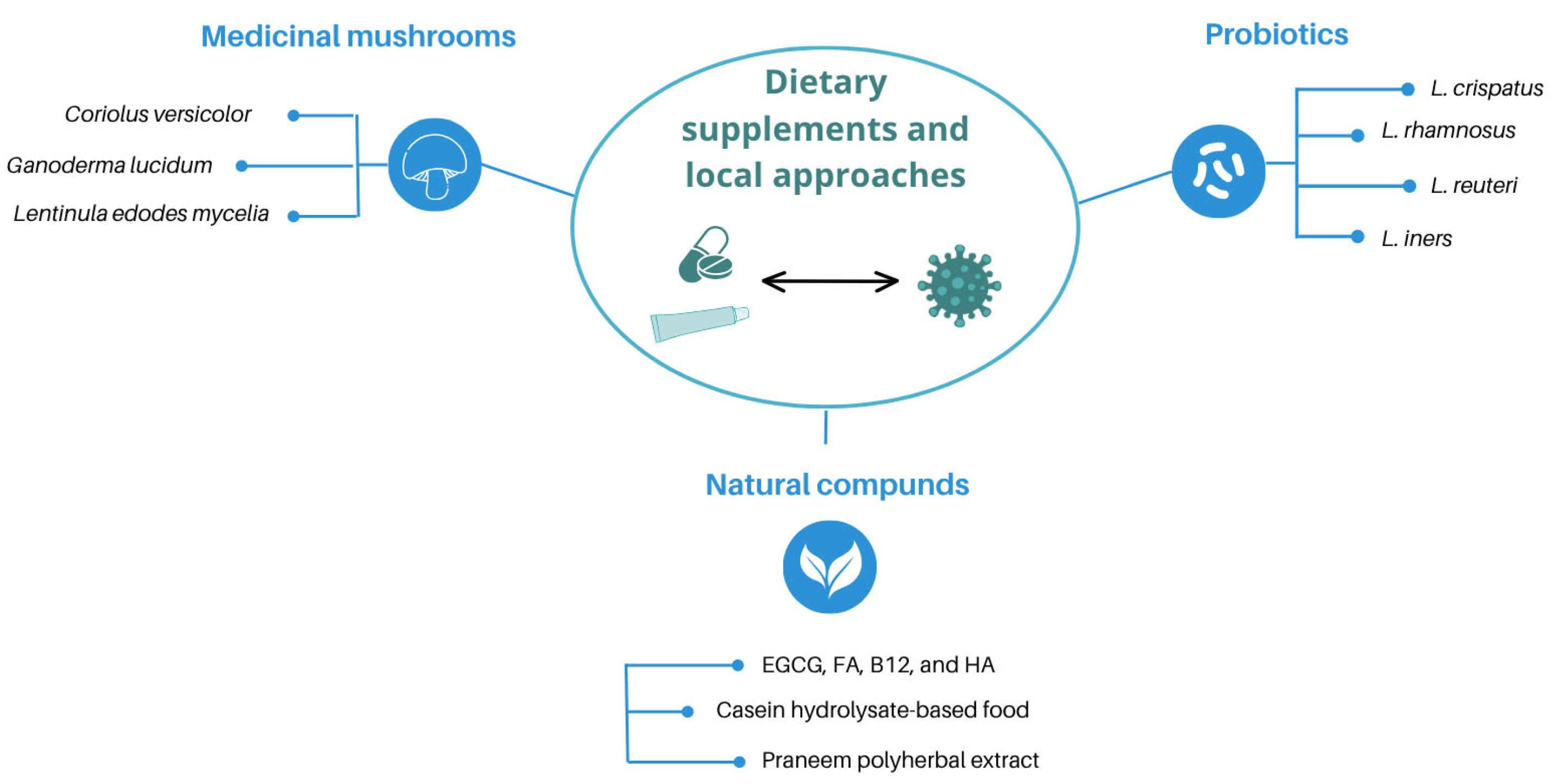
3. Dietary-Based and Local Approaches in the Management of HPV Infection
3.1. Medicinal Mushrooms
3.2. Probiotics
3.3. Natural Compounds
| Dietary Supplementation Treatment | Outcomes | Dosage | Reported Limitations | Duration of Treatment | Sample Size | Study Design | HPV Genotypes | |
|---|---|---|---|---|---|---|---|---|
| HPV Clearance (%) | Lesion Resolution (%) | |||||||
| Medicinal mushrooms | ||||||||
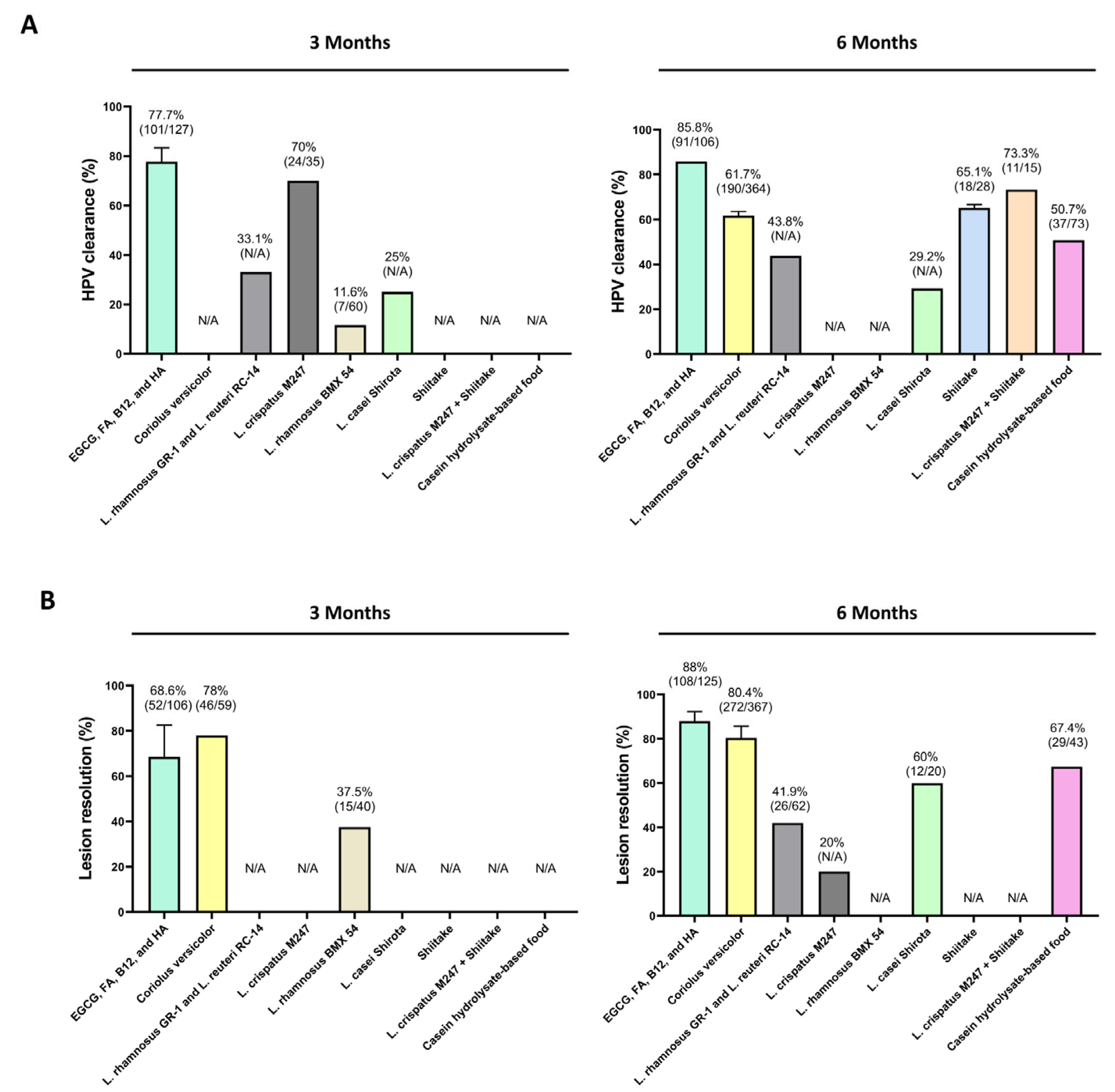
| Coriolus versicolor + Ganoderma lucidum [35] | 88% (n = 36/41) | N/A | Two capsules of 200 mg/day | Limited sample size | 2 months | 61 (41 treated; 20 controls) | Randomized, controlled clinical trial | HR (HPV 16, 18) genotypes |
| Coriolus versicolor [36] | 67% (n = 65/97) | 77.3% (n = 75/97) | Vaginal gel application (1 cannula/day for 21 days; repeated for 3 months) | Bias derived from non-experimental study design | 6 months | 183 (97 treated; 86 controls) | Longitudinal retrospective observational, controlled trial | HR genotypes (no additional specification) |
| Coriolus versicolor [37] | N/A | 78% (n = 46/59) | Vaginal gel application (1 cannula/day for 21 days; repeated for 3 months) | Lack of biopsies, unblinded assessments, and missing data on cofactors like smoking | 3 months | 91 (59 treated; 32 controls) | Open-label, randomized, controlled trial | HR (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) genotypes |
| 59.6% (n = 31/52) | 84.9% (n = 45/53) | 6 months | ||||||
| Coriolus versicolor [38] | 61.5% (n = 16/26) | 92.3% (n = 24/26) | Vaginal gel application (1 cannula/day for 21 days; repeated for 3 months) | Limited sample size (especially in HR-HPV 16-18-31 strains) | 6 months | 38 (26 treated; 12 controls) | Open-label, randomized, controlled trial | LR (not specified) and HR (6, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) genotypes |
| Coriolus versicolor [39] | 58.7% (n = 78/189) | 67% (n = 128/191) | Vaginal gel application (1 cannula/day for 21 days; repeated for 3 months) | Absence of a control arm, high number of drop-out patients, lack of info on HPV-related cofactors, lack of analysis of regression and clearance for specific HPV genotypes | 6 months | 192 (all treated) | Multicentric real-world study | LR (not specified) and HR (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) genotypes |
| 71.6% (n = 54/190) | 77.1% (n = 148/192) | 12 months | ||||||
| Lentinula edodes mycelia (Shiitake) | 66.7% (n = 4/6) | N/A | 3 g/day | N/A | 3–6 months | 10 reported (6 analyzed) | Pilot uncontrolled study | HR genotypes (not specified) |
| Trial 1 and 2 [43] | 44% (n = 4/9) | N/A | 1 g/day | N/A | >8 months | 10 reported (9 analyzed) | ||
| Lentinula edodes mycelia (Shiitake) [44] | 63.6% (n = 14/22) | N/A | 3 g/day | Limited sample size, single-arm analysis | 6 months | 34 (22 treated; 12 controls) | Randomized, double-blind, placebo-controlled trial | HR genotypes (not specified) |
| Probiotics | ||||||||
| L. crispatus [54] | N/A | 20% | L. crispatus M247 (2 × 1010 CFU) for 12 months | No statistical differences between groups for HPV clearance; lack of specific microbiota community analysis | 6 months | 160 (80 treated; 80 conrols) | Randomized, untreated controlled retrospective trial | HR (HPV 16, 18, 66, 68, 58, 45, 53, 51, 52, 35); LR (HPV 6, 11, 40) |
| 15.3% | 61.5% | 12 months follow-up | ||||||
| L. crispatus M247 [53] | 70% (n = 24/35) | N/A | N/A | Open, non-controlled study | 3 months | 35 (all treated) | Open-label, non-controlled clinical study | HPV+ (Not specified) |
| L. rhamnosus BMX 54 [57] | 11.6% (n = 7/60) | 37.5% (n = 15/40) | Vaginal tablet containing 104 CFU L. rhamnosus (once every 3 days for initial 20 days + once a week until 3/6 months) | Lack of controls | ≤3 months | 117 (60 short treatment; 57 long treatment) | Randomized controlled clinical study | HR (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) |
| 31.2% (n = 18/58) | 79.4% (n = 31/39) | Follow-up median 14 months (range 9–30 months) | ||||||
| L. rhamnosus GR-1 and L. reuteri RC-14 [59] | 58.1% (n = 36/62) | One caps/day (≥109 CFU/g of each strain) | Low statistical power (55.8%); loss to follow-up; no significant impact on HPV clearance rates | 3–12 months (total clearance) | 121 (62 treated; 59 controls) | Randomized clinical trial | HR genotypes (Not specified) | |
| 33.1% | ns | 3 months | ||||||
| 43.8% | 41.9% (n = 26/62) | 6 months | ||||||
| 47.9% | N/A | 9 months | ||||||
| 56.2% | N/A | 12 months | ||||||
| L. casei Shirota [55] | 25% | N/A | Daily probiotic drink during the study period (6 months) | Heterogeneity among participants (e.g., age, duration of infection. Lack of randomization; absence of statistical power | 3 months | 51 (24 treated; 27 controls) | Prospective, controlled (untreated) pilot study | HR genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 58, 59, 66, 68, 73, and 82) |
| 29.2% | 60% (n = 12/20) | 6 months | ||||||
| Probiotics + Mushroom | ||||||||
| L. crispatus M247 + Lentinula edodes mycelia (Shiitake) [60] | 73.3% (n = 11/15) | N/A (regression to chronic cervicitis in 13% of cases; n = 2/15) | 6 caps (500 mg each)/day + L. crispatus M247 (2 × 1010 CFU) | Sample size; lack of randomized groups | 6 months | 34 (15 treated; 19 controls) | Prospective observational study | LR (HPV-6, -11, -40, -42, -43, -44, -54, -61, -70, -72, -81); HR (HPV-16, -18, -26, -31, -33, -35, -39, -45, -51, -52, -53, -56, -58, -59, -66, -68, -73, -82) genotypes |
| Natural extracts | ||||||||
| Praneem (Azadirachta indica + Sapindus muckerossi + Mentha citrate + Quinine Hydrochloride) | 60% (n = 6/10) 80% (n = 8/10) | N/A N/A | 500 mg/day 500 mg/day | N/A | 1 month 2 months | 20 (10 treated; 10 controls) | Non-randomized, open-label, placebo-controlled pilot study | HR (HPV 16) genotype |
| [62] | ||||||||
| Casein hydrolysate-based food [63] | 50.7% (n = 37/73) | 67.4% (n = 29/43) | 6 g/day | Lack of demographic and anamnestic data; lack of randomization; info for tolerability only for 20 patients | 6 months | 118 (73 treated; 45 controls) | Prospective, non-interventional observational study with untreated controls | HR (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) genotypes |
| EGCG, FA, B12, and HA [68] | N/A | 100% (n = 1/1) | 2 tabs/day | Need for longer monitoring | 8 months | 1 treated | Case report | HR (HPV 16) genotype |
| EGCG, FA, B12, and HA [70] | 85% (n = 17/20) | 85% (n = 17/20) | 1 tab/day | Sample size; lack of randomization; lack of placebo | 3 months | 41 (20 treated; 21 controls) | Open-label, controlled (no treatment) clinical trial | HR (HPV 16, 18, 33, 45 and 52) genotypes |
| EGCG, FA, B12, and HA [69] | 100% (n = 5/5) | 100% (n = 5/5) | 1 or 2 tab/day | Sample size; lack of randomization and control group | 3–6 months | 5 (all treated) | Case reports | HR (HPV 16, 18, 31, 51, 45, 52) genotypes |
| EGCG, FA, B12, and HA [71] | 81.4% (n = 70/86) | 40.7 (n = 35/86) | 1 tab/day | Lack of randomization; no genotyping or viral load quantification | 3 months | 163 (86 treated; 77 controls) | Open-label, controlled clinical trial | HR (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) genotypes |
| 83.7 (n = 72/86) | 1 tab/day | 6 months | ||||||
| EGCG, FA, B12, and HA [72] | 66.7% (n = 14/21) | 80% (n = 4/5) | 1 tab/day | Lack of control; lack of immune biomarker analysis | 3 months | 106 (all treated) | Single arm, open-label clinical study | HR (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) genotypes |
| 85.8% (n = 91/106) | 92.3% (n = 36/39) | 1 tab/day | 6 months | |||||
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASCUS | Atypical Squamous Cells of Undetermined Significance |
| B12 | Vitamin B12 |
| CIN1 | Cervical Intraepithelial Neoplasia Grade 1 |
| CIN2/CIN3 | Cervical Intraepithelial Neoplasia Grade 2/Grade 3 |
| E6/E7 | Oncogenic Proteins E6 and E7 |
| EGCG | Epigallocatechin Gallate |
| FA | Folic Acid |
| FDA | Food and Drug Administration |
| HA | Hyaluronic Acid |
| HIV | Human Immunodeficiency Virus |
| HPV | Human Papillomavirus |
| HR | High-Risk |
| HSIL | High-Grade Squamous Intraepithelial Lesion |
| IFN-γ | Interferon-gamma |
| IL | Interleukin (e.g., IL12) |
| LEEP | Loop Electrosurgical Excision Procedure |
| LMICs | Low- and Middle-Income Countries |
| LR | Low-Risk |
| LSIL | Low-Grade Squamous Intraepithelial Lesion |
| N/A | Not applicable |
| p53 | Tumor Suppressor Protein p53 |
| pRb | Retinoblastoma Protein |
| PSP | Polysaccharopeptides |
| TNF-α | Tumor necrosis factor alpha |
References
- Bartosik, M.; Moranova, L.; Izadi, N.; Strmiskova, J.; Sebuyoya, R.; Holcakova, J.; Hrstka, R. Advanced Technologies towards Improved HPV Diagnostics. J. Med. Virol. 2024, 96, e29409. [Google Scholar] [CrossRef] [PubMed]
- Fashedemi, O.; Ozoemena, O.C.; Peteni, S.; Haruna, A.B.; Shai, L.J.; Chen, A.; Rawson, F.; Cruickshank, M.E.; Grant, D.; Ola, O.; et al. Advances in Human Papillomavirus Detection for Cervical Cancer Screening and Diagnosis: Challenges of Conventional Methods and Opportunities for Emergent Tools. Anal. Methods 2024, 17, 1428–1450. [Google Scholar] [CrossRef] [PubMed]
- Alemany, L.; Felsher, M.; Giuliano, A.R.; Waterboer, T.; Mirghani, H.; Mehanna, H.; Roberts, C.; Chen, Y.T.; Lara, N.; Lynam, M.; et al. Oral Human Papillomavirus (HPV) Prevalence and Genotyping among Healthy Adult Populations in the United States and Europe: Results from the PROGRESS (PRevalence of Oral hpv infection, a Global aSSessment) Study. eClinicalMedicine 2025, 79, 103018. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, A.; Assefa, N.; Gure, T.; Seyoum, B.; Mulu, A.; Mihret, A. Prevalence and Genotype Distribution of High-Risk Human Papillomavirus Infection Among Sub-Saharan African Women: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 890880. [Google Scholar] [CrossRef]
- Krasniqi, E.; Barba, M.; Venuti, A.; Pizzuti, L.; Cappuzzo, F.; Landi, L.; Carpano, S.; Marchetti, P.; Villa, A.; Vizza, E.; et al. Circulating HPV DNA in the Management of Oropharyngeal and Cervical Cancers: Current Knowledge and Future Perspectives. J. Clin. Med. 2021, 10, 1525. [Google Scholar] [CrossRef]
- Quinlan, J.D. Human Papillomavirus: Screening, Testing, and Prevention. Am. Fam. Physician 2021, 104, 152–159. [Google Scholar]
- Yang, D.; Zhang, J.; Cui, X.; Ma, J.; Wang, C.; Piao, H. Risk Factors Associated With Human Papillomavirus Infection, Cervical Cancer, and Precancerous Lesions in Large-Scale Population Screening. Front. Microbiol. 2022, 13, 914516. [Google Scholar] [CrossRef]
- Mathur, S.; Conway, D.I.; Worlledge-Andrew, H.; Macpherson, L.M.D.; Ross, A.J. Assessment and Prevention of Behavioural and Social Risk Factors Associated with Oral Cancer: Protocol for a Systematic Review of Clinical Guidelines and Systematic Reviews to Inform Primary Care Dental Professionals. Syst. Rev. 2015, 4, 184. [Google Scholar] [CrossRef]
- Keller, K.; Ramos-Cartagena, J.M.; Guiot, H.M.; Muñoz, C.; Rodríguez, Y.; Colón-López, V.; Deshmukh, A.A.; Tirado-Gómez, M.; Ortiz, A.P. Association of Smoking with Anal High-Risk HPV Infection and Histologically Confirmed Anal High-Grade Squamous Intraepithelial Lesions among a Clinic-Based Population in Puerto Rico. Cancer Treat. Res. Commun. 2022, 30, 100503. [Google Scholar] [CrossRef]
- Madathil, S.; Rousseau, M.C.; Durán, D.; Alli, B.Y.; Joseph, L.; Nicolau, B. Life Course Tobacco Smoking and Risk of HPV-Negative Squamous Cell Carcinomas of Oral Cavity in Two Countries. Front. Oral Health 2022, 3, 844230. [Google Scholar] [CrossRef]
- Best, S.R.; Niparko, K.J.; Pai, S.I. Biology of Human Papillomavirus Infection and Immune Therapy for HPV-Related Head and Neck Cancers. Otolaryngol. Clin. N. Am. 2012, 45, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.; Kelsall, G.; Tellier, P.; Voyer, H.; Abrahamowicz, M.; Ferenczy, A.; Coutlée, F.; Franco, E.L. The Natural History of Type-Specific Human Papillomavirus Infections in Female University Students. Cancer Epidemiol. Biomark. Prev. 2003, 12, 485–490. [Google Scholar]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic Human Papillomavirus Infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.B.; Shew, M.L.; Qadadri, B.; Neptune, N.; Vargas, M.; Tu, W.; Juliar, B.E.; Breen, T.E.; Fortenberry, J.D. A Longitudinal Study of Genital Human Papillomavirus Infection in a Cohort of Closely Followed Adolescent Women. J. Infect. Dis. 2005, 191, 182–192. [Google Scholar] [CrossRef]
- Franco, E.L.; Villa, L.L.; Sobrinho, J.P.; Prado, J.M.; Rousseau, M.C.; Désy, M.; Rohan, T.E. Epidemiology of Acquisition and Clearance of Cervical Human Papillomavirus Infection in Women from a High-Risk Area for Cervical Cancer. J. Infect. Dis. 1999, 180, 1415–1423. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Harris, R.; Sedjo, R.L.; Baldwin, S.; Roe, D.; Papenfuss, M.R.; Abrahamsen, M.; Inserra, P.; Olvera, S.; Hatch, K. Incidence, Prevalence, and Clearance of Type-Specific Human Papillomavirus Infections: The Young Women’s Health Study. J. Infect. Dis. 2002, 186, 462–469. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Lee, J.H.; Fulp, W.; Villa, L.L.; Lazcano, E.; Papenfuss, M.R.; Abrahamsen, M.; Salmeron, J.; Anic, G.M.; Rollison, D.E.; et al. Incidence and Clearance of Genital Human Papillomavirus Infection in Men (HIM): A Cohort Study. Lancet 2011, 377, 932–940. [Google Scholar] [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2020, 10, 3116. [Google Scholar] [CrossRef]
- Syrjänen, S. Oral Manifestations of Human Papillomavirus Infections. Eur. J. Oral Sci. 2018, 126, 49–66. [Google Scholar] [CrossRef]
- Gholamzad, A.; Khakpour, N.; Hashemi, M.; Gholamzad, M. Prevalence of High and Low Risk HPV Genotypes among Vaccinated and Non-Vaccinated People in Tehran. Virol. J. 2024, 21, 9. [Google Scholar] [CrossRef]
- Mao, C.; Koutsky, L.A.; Ault, K.A.; Wheeler, C.M.; Brown, D.R.; Wiley, D.J.; Alvarez, F.B.; Bautista, O.M.; Jansen, K.U.; Barr, E. Efficacy of Human Papillomavirus-16 Vaccine to Prevent Cervical Intraepithelial Neoplasia: A Randomized Controlled Trial. Obstet. Gynecol. 2006, 107, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, K.S.; Cubie, H.A.; Whitley, M.W.; Gilkison, G.; Arends, M.J.; Graham, C.; McGoogan, E. Persistent High Risk HPV Infection Associated with Development of Cervical Neoplasia in a Prospective Population Study. J. Clin. Pathol. 2005, 58, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Lindsay, L.; Pimenta, J.M.; Poole, C.; Jenkins, D.; Smith, J.S. Persistent Human Papillomavirus Infection and Cervical Neoplasia: A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2008, 168, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Rositch, A.F.; Koshiol, J.; Hudgens, M.G.; Razzaghi, H.; Backes, D.M.; Pimenta, J.M.; Franco, E.L.; Poole, C.; Smith, J.S. Patterns of Persistent Genital Human Papillomavirus Infection among Women Worldwide: A Literature Review and Meta-Analysis. Int. J. Cancer 2013, 133, 1271–1285. [Google Scholar] [CrossRef]
- Shulzhenko, N.; Lyng, H.; Sanson, G.F.; Morgun, A. Ménage à Trois: An Evolutionary Interplay between Human Papillomavirus, a Tumor, and a Woman. Trends Microbiol. 2014, 22, 345–353. [Google Scholar] [CrossRef]
- Tainio, K.; Athanasiou, A.; Tikkinen, K.A.O.; Aaltonen, R.; Cárdenas, J.; Hernándes; Glazer-Livson, S.; Jakobsson, M.; Joronen, K.; Kiviharju, M.; et al. Clinical Course of Untreated Cervical Intraepithelial Neoplasia Grade 2 under Active Surveillance: Systematic Review and Meta-Analysis. BMJ 2018, 360, k499. [Google Scholar] [CrossRef]
- Huber, J.; Mueller, A.; Sailer, M.; Regidor, P.A. Human Papillomavirus Persistence or Clearance after Infection in Reproductive Age. What Is the Status? Review of the Literature and New Data of a Vaginal Gel Containing Silicate Dioxide, Citric Acid, and Selenite. Women’s Health 2021, 17, 17455065211020702. [Google Scholar] [CrossRef]
- Palefsky, J. CHAPTER 5 HPV Infection and HPV-Associated Neoplasia in Immunocompromised Women. Int. J. Gynecol. Obstet. 2006, 94, 56–64. [Google Scholar] [CrossRef]
- Kilic, D.; Guler, T.; Atigan, A.; Avsaroglu, E.; Karakaya, Y.A.; Kaleli, I.; Kaleli, B. Predictors of Human Papillomavirus (HPV) Persistence after Treatment of High Grade Cervical Lesions; Does Cervical Cytology Have Any Prognostic Value in Primary HPV Screening? Ann. Diagn. Pathol. 2020, 49, 151626. [Google Scholar] [CrossRef]
- Abate, A.; Munshea, A.; Nibret, E.; Alemayehu, D.H.; Alemu, A.; Abdissa, A.; Mihret, A.; Abebe, M.; Mulu, A. Persistence and Clearance Rates of Human Papillomaviruses in a Cohort of Women Treated or Not Treated for Cervical Dysplasia in Northwest Ethiopia. Sci. Rep. 2025, 15, 8218. [Google Scholar] [CrossRef]
- Laganà, A.S.; Chiantera, V.; Gerli, S.; Proietti, S.; Lepore, E.; Unfer, V.; Carugno, J.; Favilli, A. Preventing Persistence of HPV Infection with Natural Molecules. Pathogens 2023, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Gardella, B.; Gritti, A.; Soleymaninejadian, E.; Pasquali, M.F.; Riemma, G.; La Verde, M.; Schettino, M.T.; Fortunato, N.; Torella, M.; Dominoni, M. New Perspectives in Therapeutic Vaccines for HPV: A Critical Review. Medicina 2022, 58, 860. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front. Immunol. 2022, 12, 805695. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.H.; Rashedi, I.; Keating, A. Immunomodulatory Properties of Coriolus Versicolor: The Role of Polysaccharopeptide. Front. Immunol. 2017, 8, 1087. [Google Scholar] [CrossRef]
- Donatini, B. Control of Oral Human Papillomavirus (HPV) by Medicinal Mushrooms, Trametes Versicolor and Ganoderma Lucidum: A Preliminary Clinical Trial. Int. J. Med. Mushrooms 2014, 16, 497–498. [Google Scholar] [CrossRef]
- Criscuolo, A.A.; Sesti, F.; Piccione, E.; Mancino, P.; Belloni, E.; Gullo, C.; Ciotti, M. Therapeutic Efficacy of a Coriolus Versicolor-Based Vaginal Gel in Women with Cervical Uterine High-Risk HPV Infection: A Retrospective Observational Study. Adv. Ther. 2021, 38, 1202–1211. [Google Scholar] [CrossRef]
- Serrano, L.; López, A.C.; González, S.P.; Palacios, S.; Dexeus, D.; Centeno-Mediavilla, C.; Coronado, P.; De La Fuente, J.; López, J.A.; Vanrell, C.; et al. Efficacy of a Coriolus Versicolor-Based Vaginal Gel in Women with Human Papillomavirus-Dependent Cervical Lesions: The PALOMA Study. J. Low. Genit. Tract. Dis. 2021, 25, 130–136. [Google Scholar] [CrossRef]
- Gil-Antuñano, S.P.; Serrano Cogollor, L.; López Díaz, A.C.; González Rodríguez, S.P.; Dexeus Carter, D.; Centeno Mediavilla, C.; Coronado Martín, P.; de la Fuente Valero, J.; López Fernández, J.A.; Vanrell Barbat, C.; et al. Efficacy of a Coriolus Versicolor-Based Vaginal Gel in Human Papillomavirus-Positive Women Older Than 40 Years: A Sub-Analysis of PALOMA Study. J. Pers. Med. 2022, 12, 1559. [Google Scholar] [CrossRef]
- Cortés Bordoy, J.; de Santiago García, J.; Agenjo González, M.; Dexeus Carter, D.; Fiol Ruiz, G.; García Ferreiro, C.; González Rodríguez, S.P.; Gurrea Soteras, M.; Martínez Lamela, E.; Palacios Gil-Antuñano, S.; et al. Effect of a Multi-Ingredient Coriolus-Versicolor-Based Vaginal Gel in Women with HPV–Dependent Cervical Lesions: The Papilobs Real-Life Prospective Study. Cancers 2023, 15, 3863. [Google Scholar] [CrossRef]
- Kajiyama, S.; Nagatake, T.; Ishikawa, S.; Hosomi, K.; Shimada, Y.; Matsui, Y.; Kunisawa, J. Lentinula Edodes Mycelia Extract Regulates the Function of Antigen-Presenting Cells to Activate Immune Cells and Prevent Tumor-Induced Deterioration of Immune Function. BMC Complement. Med. Ther. 2023, 23, 281. [Google Scholar] [CrossRef]
- Kuroki, T.; Lee, S.; Hirohama, M.; Taku, T.; Kumakura, M.; Haruyama, T.; Nagata, K.; Kawaguchi, A. Inhibition of Influenza Virus Infection by Lentinus Edodes Mycelia Extract through Its Direct Action and Immunopotentiating Activity. Front. Microbiol. 2018, 9, 1164. [Google Scholar] [CrossRef] [PubMed]
- Bugajewski, M.; Angerhoefer, N.; Pączek, L.; Kaleta, B. Lentinula Edodes as a Source of Bioactive Compounds with Therapeutical Potential in Intestinal Inflammation and Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 3320. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Mathew, L.; Gaikwad, A.; Rech, B.; Burney, M.N.; Faro, J.P.; Lucci, J.A.; Bai, Y.; Olsen, R.J.; Byrd, T.T. From Bench to Bedside: Evaluation of AHCC Supplementation to Modulate the Host Immunity to Clear High-Risk Human Papillomavirus Infections. Front. Oncol. 2019, 9, 173. [Google Scholar] [CrossRef]
- Smith, J.A.; Gaikwad, A.A.; Mathew, L.; Rech, B.; Faro, J.P.; Lucci, J.A.; Bai, Y.; Olsen, R.J.; Byrd, T.T. AHCC® Supplementation to Support Immune Function to Clear Persistent Human Papillomavirus Infections. Front. Oncol. 2022, 12, 881902. [Google Scholar] [CrossRef]
- Alizhan, D.; Ukybassova, T.; Bapayeva, G.; Aimagambetova, G.; Kongrtay, K.; Kamzayeva, N.; Terzic, M. Cervicovaginal Microbiome: Physiology, Age-Related Changes, and Protective Role Against Human Papillomavirus Infection. J. Clin. Med. 2025, 14, 1521. [Google Scholar] [CrossRef]
- Lin, F.-C.; Young, H.A. Interferons: Success in Anti-Viral Immunotherapy. Cytokine Growth Factor. Rev. 2014, 25, 369–376. [Google Scholar] [CrossRef]
- Zeng, M.; Li, X.; Jiao, X.; Cai, X.; Yao, F.; Xu, S.; Huang, X.; Zhang, Q.; Chen, J. Roles of Vaginal Flora in Human Papillomavirus Infection, Virus Persistence and Clearance. Front. Cell Infect. Microbiol. 2023, 12, 1036869. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef]
- Brusselaers, N.; Shrestha, S.; van de Wijgert, J.; Verstraelen, H. Vaginal Dysbiosis and the Risk of Human Papillomavirus and Cervical Cancer: Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2019, 221, 9–18.e8. [Google Scholar] [CrossRef]
- Papamentzelopoulou, M.; Pitiriga, V.C. Unlocking the Interactions Between the Whole-Body Microbiome and HPV Infection: A Literature Review. Pathogens 2025, 14, 293. [Google Scholar] [CrossRef]
- Reid, G.; Abrahamsson, T.; Bailey, M.; Bindels, L.B.; Bubnov, R.; Ganguli, K.; Martoni, C.; O’Neill, C.; Savignac, H.M.; Stanton, C.; et al. How Do Probiotics and Prebiotics Function at Distant Sites? Benef. Microbes 2017, 8, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Q.; Zhao, J.; Gong, L.; Zhang, Y.; Wang, X.; Yuan, Z. Altered Diversity and Composition of the Gut Microbiome in Patients with Cervical Cancer. AMB Express 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Criscuolo, A.A.; Dei Giudici, A.; Senatori, R.; Sesti, F.; Ciotti, M.; Piccione, E. Oral Administration of Lactobacillus Crispatus M247 to Papillomavirus-Infected Women: Results of a Preliminary, Uncontrolled, Open Trial. Minerva Obstet. Gynecol. 2021, 73, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus Crispatus M247 Oral Administration: Is It Really an Effective Strategy in the Management of Papillomavirus-Infected Women? Infect. Agent. Cancer 2022, 17, 53. [Google Scholar] [CrossRef]
- Verhoeven, V.; Renard, N.; Makar, A.; Van Royen, P.; Bogers, J.P.; Lardon, F.; Peeters, M.; Baay, M. Probiotics Enhance the Clearance of Human Papillomavirus-Related Cervical Lesions: A Prospective Controlled Pilot Study. Eur. J. Cancer Prev. 2013, 22, 46–51. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a Better Understanding of Lactobacillus Rhamnosus GG—Host Interactions. Microb. Cell Fact. 2014, 13, S7. [Google Scholar] [CrossRef]
- Palma, E.; Recine, N.; Domenici, L.; Giorgini, M.; Pierangeli, A.; Panici, P.B. Long-Term Lactobacillus Rhamnosus BMX 54 Application to Restore a Balanced Vaginal Ecosystem: A Promising Solution against HPV-Infection. BMC Infect. Dis. 2018, 18, 13. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus Reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Ou, Y.C.; Fu, H.C.; Tseng, C.W.; Wu, C.H.; Tsai, C.C.; Lin, H. The Influence of Probiotics on Genital High-Risk Human Papilloma Virus Clearance and Quality of Cervical Smear: A Randomized Placebo-Controlled Trial. BMC Womens Health 2019, 19, 103. [Google Scholar] [CrossRef]
- Salimbeni, V.; Martinelli, C.; Porto, L.; Le Donne, M.; Alibrandi, A.; Di Pierro, F.; Cau, F.; Granese, R.; Iannone, V.; Marchetta, L.; et al. A Prospective Observational Study to Evaluate Impact of Oral Supplementation with AHCC and Lactobacillus Crispatus M247 on HPV Clearance and Low-Grade Squamous Intraepithelial Lesion Regression. Ann. Res. Oncol. 2024, 4, 3–18. [Google Scholar] [CrossRef]
- Mitra, A.; Gultekin, M.; Burney Ellis, L.; Bizzarri, N.; Bowden, S.; Taumberger, N.; Bracic, T.; Vieira-Baptista, P.; Sehouli, J.; Kyrgiou, M. Genital Tract Microbiota Composition Profiles and Use of Prebiotics and Probiotics in Gynaecological Cancer Prevention: Review of the Current Evidence, the European Society of Gynaecological Oncology Prevention Committee Statement. Lancet Microbe 2024, 5, e291–e300. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Bharti, A.C.; Hussain, S.; Mahata, S.; Hedau, S.; Kailash, U.; Kashyap, V.; Bhambhani, S.; Roy, M.; Batra, S.; et al. Elimination of High-Risk Human Papillomavirus Type HPV16 Infection by “Praneem” Polyherbal Tablet in Women with Early Cervical Intraepithelial Lesions. J. Cancer Res. Clin. Oncol. 2009, 135, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Pingarron, C.; Duque, A.; López, A.; Ferragud, J. A Prospective, Non-Interventional Observational Study to Assess the Efficacy, Safety, and Tolerability of the Casein Hydrolysate-Based Food Supplement in High-Risk Human Papillomavirus-Positive Women. Cureus 2025, 17, e80201. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Cong, Q.; Wu, D.; Chen, Y.; Qiu, L.H.; Hong, Z.B.; Yang, Y.B.; Xu, L.; Wang, L.F.; Huang, L.X.; et al. A Prospective Multicentre Controlled Study of Gaoweikang (Chinese Multiherb Extract-Based Tincture) Used in High-Risk HPV Infections. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8985–8992. [Google Scholar] [CrossRef]
- Kiyoshima, C.; Kimura, I.; Ishida, K.; Hirano, T.; Ishida, T.; Shigekawa, K.; Yoshikawa, K.; Yotsumoto, F. Effectiveness of the Traditional Japanese Herbal Medicine, Yokuinin (Kampo), in the Treatment of Cervical Precancerous Lesions. Cureus 2025, 17, e77114. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Lu, J.L.; Liang, Y.R.; Li, Q.S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef]
- Frega, A.; Gentili, C.; Proietti, S.; Lepore, E.; Unfer, V.; Fuso, A. Epigallocatechin Gallate, Folic Acid, Vitamin B12, and Hyaluronic Acid Significantly Increase Apoptosis and P53 Expression in HeLa Cells. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5240–5245. [Google Scholar] [CrossRef]
- Grandi, G.; Botticelli, L.; Fraia, P.D.; Babalini, C.; Masini, M.; Unfer, V. The Association of Four Natural Molecules—EGCG, Folic Acid, Vitamin B12, and HA—To Counteract HPV Cervical Lesions: A Case Report. J. Pers. Med. 2023, 13, 567. [Google Scholar] [CrossRef]
- Calcagno, M.; Incocciati, B.; Di Fraia, L.; Unfer, V. Counteracting HPV Cervical and Anal Infection through Dietary Supplementation of EGCG, Folic Acid, Vitamin B12 and Hyaluronic Acid: Clinical Case Reports. J. Clin. Med. 2024, 13, 3597. [Google Scholar] [CrossRef]
- Aragona, C.; Bezerra Espinola, M.S.; Bilotta, G.; Porcaro, G.; Calcagno, M. Evaluating the Efficacy of Pervistop®, a New Combination Based on EGCG, Folic Acid, Vitamin B12 and Hyaluronic Acid on Patients with Human Papilloma Virus (HPV) Persistent Infections and Cervical Lesions: A Pilot Study. J. Clin. Med. 2023, 12, 2171. [Google Scholar] [CrossRef]
- Tinelli, A.; Gustapane, S.; Licchelli, M.; Coluccia, A.C.; Panese, G.; Proietti, S.; Gambioli, R. Treatment with Epigallocatechin Gallate, Folic Acid, Vitamin B12, and Hyaluronic Acid Decreases HPV Positivity in Women Attending Regional Screening in Puglia. Microorganisms 2024, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, G.; Pavone-Cossut, M.R.; Moretti, S.; Bilotta, G.; Aragona, C.; Unfer, V. Oral Treatment with EGCG, Folic Acid, Vitamin B12, and Hyaluronic Acid Improves HPV Clearance and Counteracts Its Persistence: A Clinical Study. Int. J. Mol. Sci. 2025, 26, 5251. [Google Scholar] [CrossRef] [PubMed]
- Rokos, T.; Pribulova, T.; Kozubik, E.; Biringer, K.; Holubekova, V.; Kudela, E. Exploring the Bioactive Mycocompounds (Fungal Compounds) of Selected Medicinal Mushrooms and Their Potentials against HPV Infection and Associated Cancer in Humans. Life 2023, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Senba, M.; Mori, N. Mechanisms of Virus Immune Evasion Lead to Development from Chronic Inflammation to Cancer Formation Associated with Human Papillomavirus Infection. Oncol. Rev. 2012, 6, e17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcaro, G.; Calcagno, M.; Tinelli, A. Medicinal Mushrooms, Probiotics and Combination of Natural Compounds in the Management of HPV: A Comparative Look at Viral Clearance and Lesion Resolution. Viruses 2025, 17, 942. https://doi.org/10.3390/v17070942
Porcaro G, Calcagno M, Tinelli A. Medicinal Mushrooms, Probiotics and Combination of Natural Compounds in the Management of HPV: A Comparative Look at Viral Clearance and Lesion Resolution. Viruses. 2025; 17(7):942. https://doi.org/10.3390/v17070942
Chicago/Turabian StylePorcaro, Giuseppina, Marco Calcagno, and Andrea Tinelli. 2025. "Medicinal Mushrooms, Probiotics and Combination of Natural Compounds in the Management of HPV: A Comparative Look at Viral Clearance and Lesion Resolution" Viruses 17, no. 7: 942. https://doi.org/10.3390/v17070942
APA StylePorcaro, G., Calcagno, M., & Tinelli, A. (2025). Medicinal Mushrooms, Probiotics and Combination of Natural Compounds in the Management of HPV: A Comparative Look at Viral Clearance and Lesion Resolution. Viruses, 17(7), 942. https://doi.org/10.3390/v17070942







