Predictive Value of Hepatitis B Core-Related Antigen for Multiple Recurrence Outcomes After Treatment Cessation in Chronic Hepatitis B: A Meta-Analysis Study
Abstract
1. Introduction
2. Method
2.1. Search Strategy
- (1)
- PubMed: Articles published before 1 April 2025 were retrieved using the search terms “HBcrAg” OR “Hepatitis B core-related Antigen.”
- (2)
- WOS: Literature indexed between 1 January 1900 and 1 April 2025 was identified using the keywords “hepatitis B core-related antigen” OR “HBcrAg.”
- (3)
- Wanfang: Journal articles, dissertations, and conference papers published from 1 January 1900 to 7 May 2025 were searched for with the terms “hepatitis B core-related antigen,” “HBcrAg,” or “hepatitis B core-related antigen (in Chinese)”.
- (4)
- CNKI: All literature from 1 January 1900 to 1 April 2025 was retrieved using the keywords “hepatitis B core-related antigen” OR “HBcrAg” OR “hepatitis B core-related antigen (in Chinese)”.
2.2. Quality Assessment
2.3. Data Analysis
3. Results
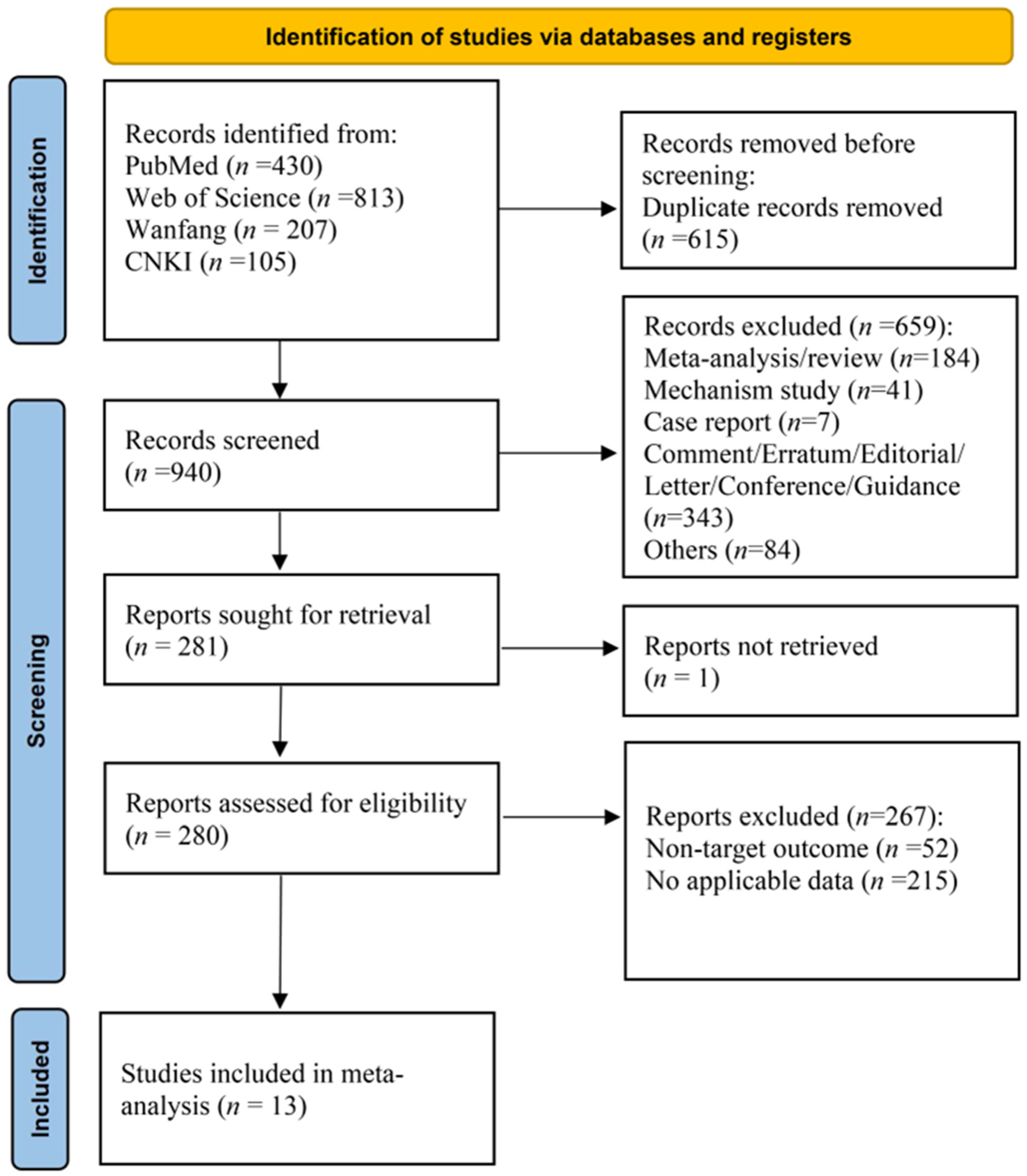
3.1. Study Selection and Characteristics
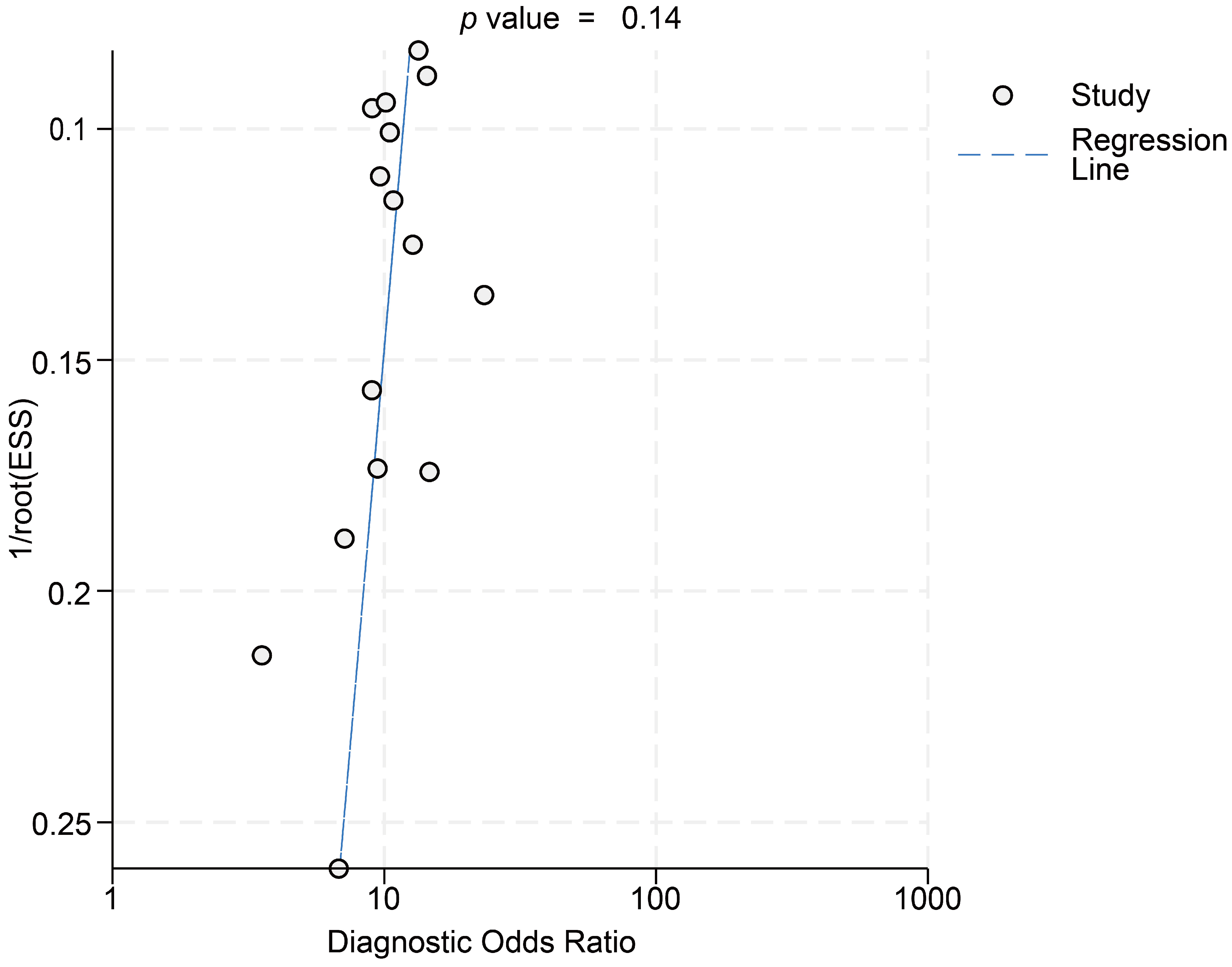
3.2. Risk of Bias Analysis
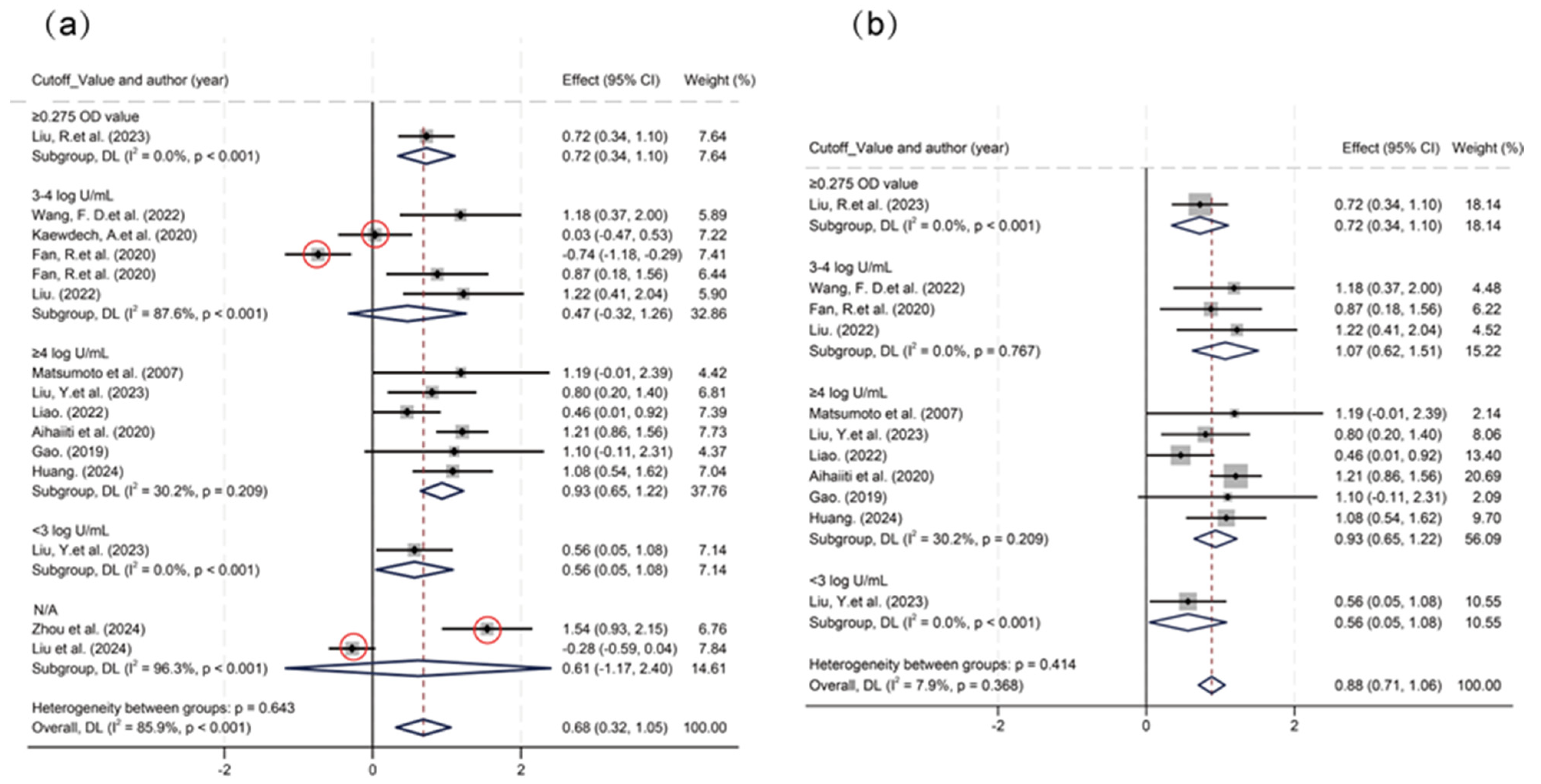
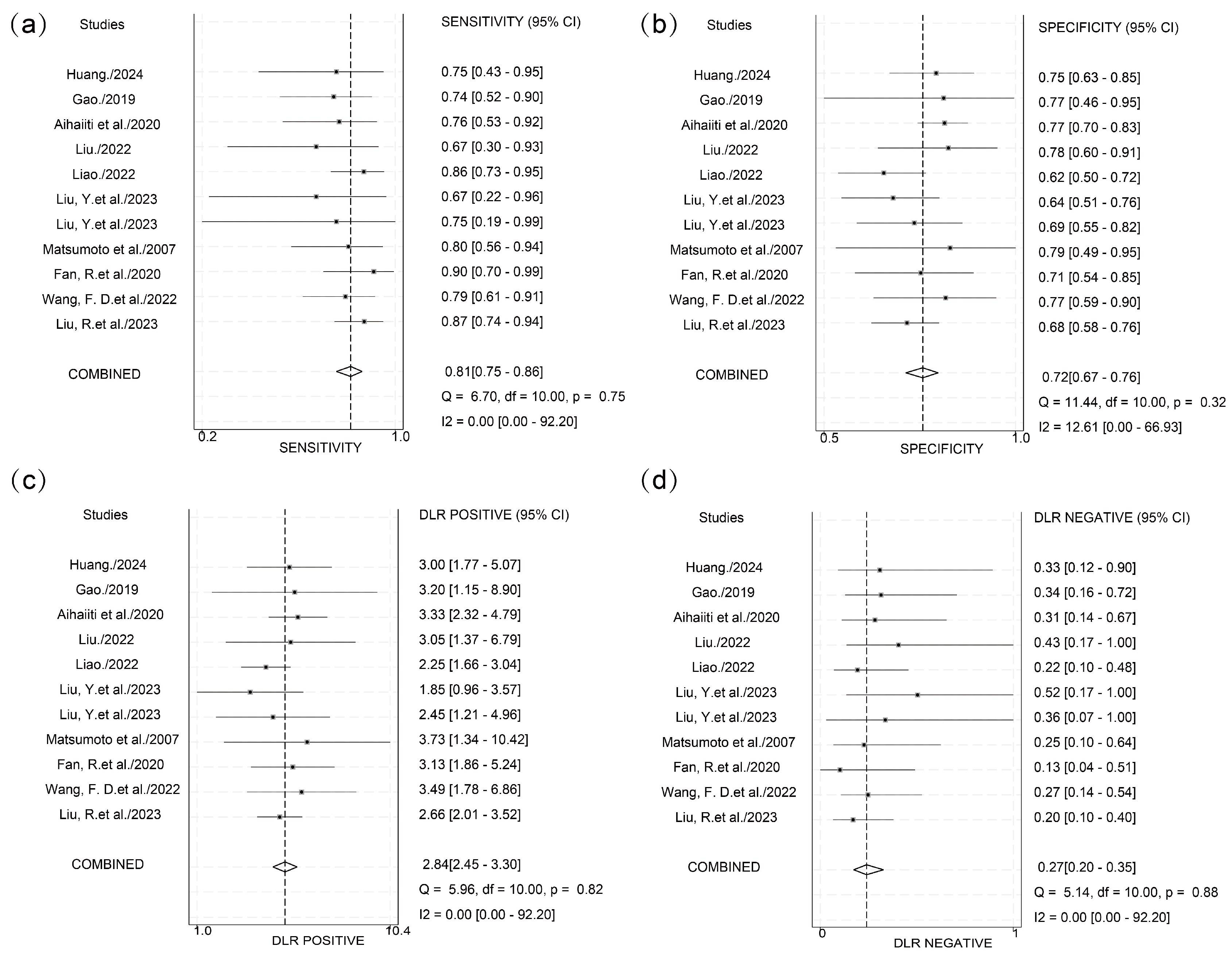
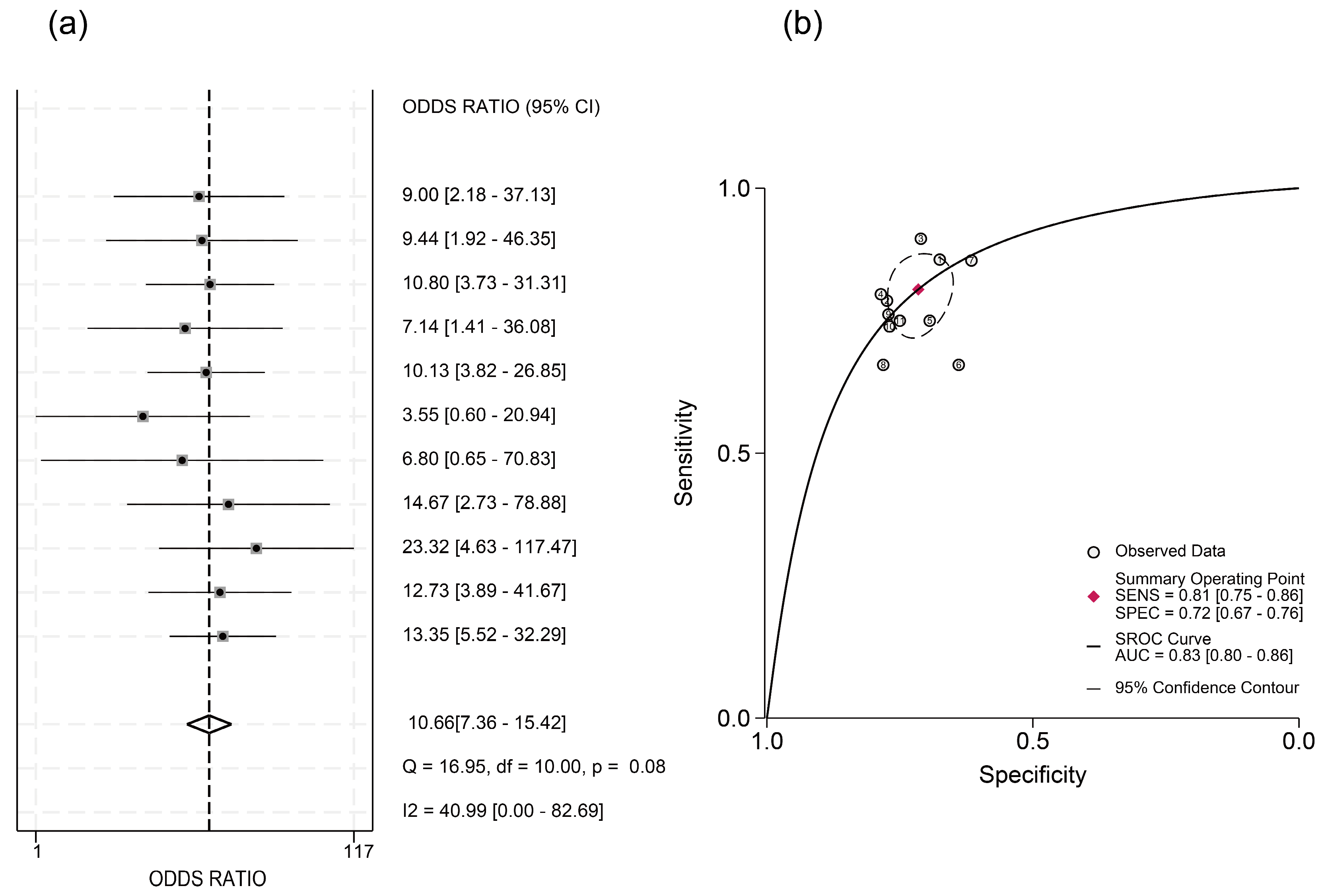
3.3. Predictive Efficacy of HBcrAg Across Various Recurrence Outcomes
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Polaris Observatory Collaborators. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: A modelling study. Lancet Gastroenterol. Hepatol. 2023, 8, 879–907. [Google Scholar] [CrossRef] [PubMed]
- The Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Kobayashi, M.; Suzuki, Y.; Saitoh, S.; et al. Ultrasensitive Assay for Hepatitis B Core-Related Antigen Predicts Hepatocellular Carcinoma Incidences During Entecavir. Hepatol. Commun. 2022, 6, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Kao, J.H. Development of hepatocellular carcinoma in treated and untreated patients with chronic hepatitis B virus infection. Clin. Mol. Hepatol. 2023, 29, 605–622. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Armandi, A.; Rosso, C.; Ribaldone, D.G.; Pellicano, R.; Fagoonee, S. Hepatitis B Core-Related Antigen as Surrogate Biomarker of Intrahepatic Hepatitis B Virus Covalently-Closed-Circular DNA in Patients with Chronic Hepatitis B: A Meta-Analysis. Diagnostics 2021, 11, 187. [Google Scholar] [CrossRef]
- Nassal, M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015, 64, 1972–1984. [Google Scholar] [CrossRef]
- Mak, L.Y.; Wong, D.K.; Cheung, K.S.; Seto, W.K.; Lai, C.L.; Yuen, M.F. Review article: Hepatitis B core-related antigen (HBcrAg): An emerging marker for chronic hepatitis B virus infection. Aliment. Pharmacol. Ther. 2018, 47, 43–54. [Google Scholar] [CrossRef]
- Cao, Q.H.; Liu, H.; Yan, L.J.; Wang, H.C.; Ding, Z.N.; Mao, X.C.; Li, R.Z.; Pan, G.Q.; Zhang, X.; Tian, B.W.; et al. Role of hepatitis B core-related antigen in predicting the occurrence and recurrence of hepatocellular carcinoma in patients with chronic hepatitis B: A systemic review and meta-analysis. J. Gastroenterol. Hepatol. 2024, 39, 1464–1475. [Google Scholar] [CrossRef]
- Mukherjee, R.; Reddy, P.B.; Arava, J.; Rao, P.; Mitnala, S.; Gupta, R.; Reddy, D. Relationship between serum HBsAg level, HBV DNA level, and peripheral immune cells in patients with chronic hepatitis B virus infection. Hepat. Med. 2010, 2, 157–162. [Google Scholar] [CrossRef]
- Lim, S.G.; Teo, A.E.; Chan, E.S.; Phyo, W.W.; Chen, D.H.Y.; Hargreaves, C.A. Stopping Nucleos(t)ide Analogues in Chronic Hepatitis B Using HBsAg Thresholds: A Meta-Analysis and Meta-Regression. Clin. Gastroenterol. Hepatol. 2024, 22, 2403–2412. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, M.; Wu, S.; Jiang, W.; Feng, Y. Predictors of relapse after cessation of nucleos(t)ide analog treatment in HBeAg-negative chronic hepatitis B patients: A meta-analysis. Int. J. Infect. Dis. 2019, 86, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.A.L.; Vogrin, S.; Wawryk, O.; Burns, G.S.; Visvanathan, K.; Sundararajan, V.; Thompson, A. Discontinuation of nucleot(s)ide analogue therapy in HBeAg-negative chronic hepatitis B: A meta-analysis. Gut 2022, 71, 1629–1641. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Cote, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Ashrafian, H.; Skapinakis, P.; Arora, S.; Darzi, A.; Dimopoulos, K.; Athanasiou, T. Diagnostic accuracy meta-analysis: A review of the basic principles of interpretation and application. Int. J. Cardiol. 2010, 140, 138–144. [Google Scholar] [CrossRef]
- Chu, H.; Guo, H.; Zhou, Y. Bivariate random effects meta-analysis of diagnostic studies using generalized linear mixed models. Med. Decis. Mak. 2010, 30, 499–508. [Google Scholar] [CrossRef]
- Kaewdech, A.; Tangkijvanich, P.; Sripongpun, P.; Witeerungrot, T.; Jandee, S.; Tanaka, Y.; Piratvisuth, T. Hepatitis B surface antigen, core-related antigen and HBV RNA: Predicting clinical relapse after NA therapy discontinuation. Liver Int. 2020, 40, 2961–2971. [Google Scholar] [CrossRef]
- Liu, R.; Yang, L.; Jiang, T.; Lu, Y.; Zhang, L.; Shen, G.; Wu, S.; Chang, M.; Hao, H.; Hu, L.; et al. Hepatitis B core-related antigen serum levels may be a predictor of acute flare of chronic hepatitis B among pregnant women in the immune-tolerant phase of chronic HBV infection after short-course antiviral therapy. Virulence 2023, 14, 2186335. [Google Scholar] [CrossRef]
- Wang, F.D.; Zhou, J.; Li, L.Q.; Wang, M.L.; Tao, Y.C.; Wang, Y.H.; Zhang, D.M.; Chen, E.Q. Serum Pregenomic RNA Combined With Hepatitis B Core-Related Antigen Helps Predict the Risk of Virological Relapse After Discontinuation of Nucleos(t)ide Analogs in Patients With Chronic Hepatitis B. Front. Microbiol. 2022, 13, 901233. [Google Scholar] [CrossRef]
- Matsumoto, A.; Tanaka, E.; Minami, M.; Okanoue, T.; Yatsuhashi, H.; Nagaoka, S.; Suzuki, F.; Kobayashi, M.; Chayama, K.; Imamura, M.; et al. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol. Res. 2007, 37, 661–666. [Google Scholar] [CrossRef]
- Fan, R.; Peng, J.; Xie, Q.; Tan, D.; Xu, M.; Niu, J.; Wang, H.; Ren, H.; Chen, X.; Wang, M.; et al. Combining Hepatitis B Virus RNA and Hepatitis B Core-Related Antigen: Guidance for Safely Stopping Nucleos(t)ide Analogues in Hepatitis B e Antigen-Positive Patients With Chronic Hepatitis B. J. Infect. Dis. 2020, 222, 611–618. [Google Scholar] [CrossRef]
- Liu, Y.; Nuersulitan, R.; Zhang, C.; Huo, N.; Li, J.; Song, Y.; Zhu, J.; Liu, W.; Zhao, H. Steady Decline of HBV DNA Load under NAs in Lymphoma Patients and a Higher Level of qAnti-HBc Predict HBV Reactivation. J. Clin. Med. 2023, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yuan, Z.; Wu, D.; Yuan, W.; Chang, J.; Chen, Y.; Ning, Q.; Yan, W. HBV Antigen-Guided Switching Strategy From Nucleos(t)ide Analogue to Interferon: Avoid Virologic Breakthrough and Improve Functional Cure. J. Med. Virol. 2024, 96, e70021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, Y.; Ouyang, Y. Predictive value of HBV pgRNA combined with HBcrAg on the relapse of patients with chronic hepatitis B after drug withdrawal. Chin. J. Liver Dis. (Electron. Version) 2024, 16, 42–47. [Google Scholar]
- Liu Yaodan, L.M. Construction of a risk prediction model for postpartum hepatitis flare in HBV-infected pregnant women with mother-to-child transmission prevention. Prog. Obstet. Gynecol. 2024, 33, 196–200+204. [Google Scholar] [CrossRef]
- Liao, G. Effects of Hepatitis B Core-related Antigen and T cell Receptor Dynamic Changes on Relapse After Discontinuation of Nucleos(t)ide Analogues Treatment in Chronic Hepatitis B Patients. Ph.D. Thesis, Southern Medical University, Guangzhou, China, 2022. [Google Scholar] [CrossRef]
- Liu, Z. Clinical Study of Serum pgRNA, HBcrAg and HBsAg in Predicting the Efficacy of Pegylated Interferon in the Treatment of Chronic Hepatitis B. Master’s Thesis, Army Medical University, Shapingba, China, 2022. [Google Scholar] [CrossRef]
- Mikrenisha Aihaiti, K.K. Value of postoperative hepatitis B core-related antigen level to the prediction of hepatitis B virus relapse after liver transplantation. J. Chin. Pract. Diagn. Ther. 2020, 34, 1266–1269. [Google Scholar] [CrossRef]
- Gao, C. The Expression and Clinical Significance of Serum miRNA-122 After Cessation of Nucleos(t)ide Analogue Therapy for Chronic Hepatitis B. Master’s Thesis, Southern Medical University, Guangzhou, China, 2019. [Google Scholar] [CrossRef]
- Wong, D.K.; Seto, W.K.; Cheung, K.S.; Chong, C.K.; Huang, F.Y.; Fung, J.; Lai, C.L.; Yuen, M.F. Hepatitis B virus core-related antigen as a surrogate marker for covalently closed circular DNA. Liver Int. 2017, 37, 995–1001. [Google Scholar] [CrossRef]
- Tseng, T.C.; Chiang, C.; Liu, C.J.; Hong, C.M.; Su, T.H.; Yang, H.C.; Yang, W.T.; Liu, C.H.; Chen, P.J.; Kao, J.H. Low Hepatitis B Core-Related Antigen Levels Correlate Higher Spontaneous Seroclearance of Hepatitis B Surface Antigen in Chronic Hepatitis B Patients With High Hepatitis B Surface Antigen Levels. Gastroenterology 2023, 164, 669–679.e6. [Google Scholar] [CrossRef]
- Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. Japan Society of Hepatology Guidelines for the Management of Hepatitis B Virus Infection: 2019 update. Hepatol. Res. 2020, 50, 892–923. [Google Scholar] [CrossRef]
- Brakenhoff, S.M.; de Knegt, R.J.; van Campenhout, M.J.H.; van der Eijk, A.A.; Brouwer, W.P.; van Bommel, F.; Boonstra, A.; Hansen, B.E.; Berg, T.; Janssen, H.L.A.; et al. End-of-treatment HBsAg, HBcrAg and HBV RNA predict the risk of off-treatment ALT flares in chronic hepatitis B patients. J. Microbiol. Immunol. Infect. 2023, 56, 31–39. [Google Scholar] [CrossRef]
- Xie, Y.; Li, M.; Ou, X.; Zheng, S.; Gao, Y.; Xu, X.; Yang, Y.; Ma, A.; Li, J.; Huang, Y.; et al. HBeAg-positive patients with HBsAg < 100 IU/mL and negative HBV RNA have lower risk of virological relapse after nucleos(t)ide analogues cessation. J. Gastroenterol. 2021, 56, 856–867. [Google Scholar] [CrossRef]
| Author and Year | Country | Study Type | Sample Year | No. of Patients (F/M) | Baseline HBeAg Status (+/Total Patients) | Baseline HBsAg (Log10 U/mL) | Baseline HBcrAg (Log10 U/mL) | Average Age (Years) | Detection Time/Prediction Time/Outcome | Cut-off Value (Log10 U/mL) | AUC (95% CI) [Sen/Sep] | Method of Measurement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kaewdech, Apichat et al. (2020) [16] | Thailand | A prospective, observational study | February 2018–August 2019 | 33/59 | 20/92 | 2.96 (2.40–3.43) | 3.20 (<3.00–3.90) | 55.0 (50.0–63.0) | * EOT/EOT 48 w/Relapse | 3 | 0.773 (0.677–0.869) [90.3%/50.8%] | CLEIA |
| Liu, Ruyu et al. (2023) [17] | China | A prospective study | January 2019–April 2022 | 172/0 | 172/172 | >3 | <3 | 26.49 ± 3.77 | Postpartum 12-week (12 w) EOT/Postpartum 12 w/Hepatitis B acute flare | 0.275 (OD value) | 0.84 (0.78–0.91) [86.5%/67.5%] | ELISA |
| Wang, Fa-Da et al. (2022) [18] | China | A retrospective study | June 2020–January 2021 | 32/32 | 36/64 | HBeAg(+): 3.41 (2.97–2.47) HBeAg(−): 3.05 (2.77–3.42) | HBeAg(+): 3.34 ± 0.10 HBeAg(−): 3.08 ± 0.09 | HBeAg(+): 47.28 ± 6.86 HBeAg(−): 47.39 ± 6.45 | EOT/Within 1 year after treatment cessation/Relapse | 3.3 | 0.817 [78%/77%] | CLEIA |
| Matsumoto, Akihiro et al. (2007) [19] | Japan | A retrospective study | [N/A] | 14/20 | 16/34 | positive | 6.3 (5.0–7.7) | 47 (35–59) | EOT/Within 1 Year After Treatment Cessation/Acute Hepatitis B Flare | 4.1–4.6 | 0.764 [80%/80%] | CLEIA |
| Fan, Rong et al. (2020) [20] | China | A prospective study | [N/A] | 35/92 | 127/127 | 3.3 (2.9–3.7) | 4.3 (4.0–4.7) | 30 (25–35) | EOT/Within 4 Years After EOT/Relapse | 4 | 0.621 [N/A] | CLEIA |
| 13/46 | 59/59 | 2.6 (1.4–3.0) | 3.9 (3.3–4.5) | 36 (30–43) | EOT/Within 4 Years After EOT/Relapse | 4 | 0.798 [N/A] | CLEIA | ||||
| Liu, Yiqi et al. (2023) [21] | China | A retrospective study | June 2010–December 2021 | 23/30 | 53 | Positive | 4.27 ± 1.99 | 53.2 ± 12.0 | Pre-chemotherapy/48 Weeks Post-chemotherapy/HBV Reactivation | 4.668 | 0.689 [N/A] | CLEIA |
| 29/38 | 67 | Negative | 2.67 ± 0.54 | 62.5 ± 11.5 | Pre-chemotherapy/48 Weeks Post-chemotherapy/HBV Reactivation | 2.54 | 0.605 [N/A] | CLEIA | ||||
| Huang, Da et al. (2024) [22] | China | RCT | N/A | 14/66 | 33/80 | 2.797 ± 0.425 | N/A | 35.4 ± 9.8 | NAs EOT/During Interferon Conversion Therapy/HBV Reactivation | 5 | 0.755 [N/A] | CLEIA |
| Baseline HBcrAg Prediction/During Interferon Transition Therapy/HBV Reactivation | N/A | 0.713 [N/A] | ||||||||||
| Zhou, Fang et al. (2024) [23] | China | A prospective study | June 2020–June 2021 | Relapse group: 16/22 Non-relapse group: 36/34 | Not mentioned/ 108 | 2.82 ± 0.08 | 6.075 ± 1.52 | 42.83 ± 9.63 | EOT/1 Year Later/Post-Treatment Relapse | N/A | 0.783 (0.650–0.884) [68.42%/82.68%] | CLEIA |
| Liu, Yaodan et al. (2024) [24] | China | A prospective observational study | January 2020–October 2022 | 198/0 | 198/198 | Postpartum Hepatitis Non-flare Group: 3.66 (3.20–4.01) Postpartum Hepatitis Flare Group: 4.05 (3.70–4.24) | Postpartum Hepatitis Non-flare Group: 6.98 (6.77–7.18) Postpartum Hepatitis Flare Group: 7.35 (7.31–7.52) | Postpartum Hepatitis Non-flare Group: 30.89 ± 6.13 Postpartum Hepatitis Flare Group: 29.88 ± 4.55 | Antiviral Treatment Initiation/48 Weeks Postpartum/ alt flare | N/A | 0.713 [N/A] | ELISA |
| Liao, Guichan. (2022) [25] | China | A prospective study | November 2011–December 2018 | 27/95 | 122/122 | 2.55 ± 1.11 | 3.80 ± 0.83 | 34 (29–40) | EOT/5 Years Post-EOT/Relapse | 4.0 | 0.71 (0.62–0.81) [87.1%/61.5%] | CLEIA |
| Liu, Zhongwei. (2022) [26] | China | A prospective study | [N/A] | [N/A] | Not mentioned/ 41 | 2.07 (1.315–2.808) | 3.58 ± 0.75 | 42.09 ± 8.13 | EOT/During Follow-up/HBsAg Recurrence | 3.8 | 0.793 [66.67%/78.12%] | CLEIA |
| Aihaiti et al. (2020) [27] | China | A prospective study | August 2014–June 2017 | 30/166 | Not mentioned/ 196 | N/A | 4.9 (2–7.5) | 55 (40–74) | Post-LT Day 3/3 Years Post-Transplant/HBV Recurrence | 4.5 | 0.818 (0.72–0.917) [76.19%/77.14%] | CLEIA |
| Gao, Chang. (2019) [28] | China | A prospective study | January 2003–July 2011 | 3/33 | 25/36 | 2.78 (2.02–3.32) | 3.85 (3.0–4.4) | 35.5 (29.25–41) | EOT 12-week/EOT 48-week/Relapse | 4.1 | 0.719 (0.536–0.902) [73.7%/76.9%] | CLEIA |
| Author (Year) | Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analysis and Reporting | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| Kaewdech, Apichat et al. (2020) [16] | Low | Low | Low | Low | Low | Low | Low |
| Liu, Ruyu et al. (2023) [17] | Low | Low | Low | Low | Low | Low | Low |
| Wang, Fa-Da et al. (2022) [18] | Low | Low | Low | Low | Low | Low | Low |
| Matsumoto, Akihiro et al. (2007) [19] | Moderate | Low | Low | Low | Low | Low | Moderate |
| Fan, Rong et al. (2020) [20] | Low | Moderate | Low | Low | Low | Low | Moderate |
| Liu, Yiqi et al. (2023) [21] | Moderate | Low | Low | Low | Low | Low | Moderate |
| Huang, Da et al. (2024) [22] | Low | Low | Low | Low | Low | Low | Low |
| Zhou, Fang et al. (2024) [23] | Low | Low | Moderate | Low | Low | Low | Moderate |
| Liu, Yaodan et al. (2024) [24] | Low | Moderate | High | Low | Low | Low | High |
| Liao, Guichan. (2022) [25] | Low | Low | Low | Low | Low | Low | Low |
| Liu, Zhongwei. (2022) [26] | Moderate | Low | Low | Low | Low | Low | Moderate |
| Aihaiti et al. (2020) [27] | Low | Low | Low | Low | Low | Low | Low |
| Gao, Chang. (2019) [28] | Moderate | Low | Low | Low | Low | Low | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Cheng, M.; Duan, Y.; Kang, M.; Jiang, N.; Yan, W.; Yin, J. Predictive Value of Hepatitis B Core-Related Antigen for Multiple Recurrence Outcomes After Treatment Cessation in Chronic Hepatitis B: A Meta-Analysis Study. Viruses 2025, 17, 929. https://doi.org/10.3390/v17070929
Yu G, Cheng M, Duan Y, Kang M, Jiang N, Yan W, Yin J. Predictive Value of Hepatitis B Core-Related Antigen for Multiple Recurrence Outcomes After Treatment Cessation in Chronic Hepatitis B: A Meta-Analysis Study. Viruses. 2025; 17(7):929. https://doi.org/10.3390/v17070929
Chicago/Turabian StyleYu, Guoyang, Meiqi Cheng, Yuxin Duan, Minrong Kang, Ning Jiang, Wei Yan, and Jianhua Yin. 2025. "Predictive Value of Hepatitis B Core-Related Antigen for Multiple Recurrence Outcomes After Treatment Cessation in Chronic Hepatitis B: A Meta-Analysis Study" Viruses 17, no. 7: 929. https://doi.org/10.3390/v17070929
APA StyleYu, G., Cheng, M., Duan, Y., Kang, M., Jiang, N., Yan, W., & Yin, J. (2025). Predictive Value of Hepatitis B Core-Related Antigen for Multiple Recurrence Outcomes After Treatment Cessation in Chronic Hepatitis B: A Meta-Analysis Study. Viruses, 17(7), 929. https://doi.org/10.3390/v17070929







