Quantitative Measurement of Serum HBcrAg Can Be Used to Assess the Feasibility of Safe Discontinuation of Antiviral Therapy for Chronic Hepatitis B
Abstract
1. Introduction
2. Current Standards for Discontinuing Antiviral Therapy
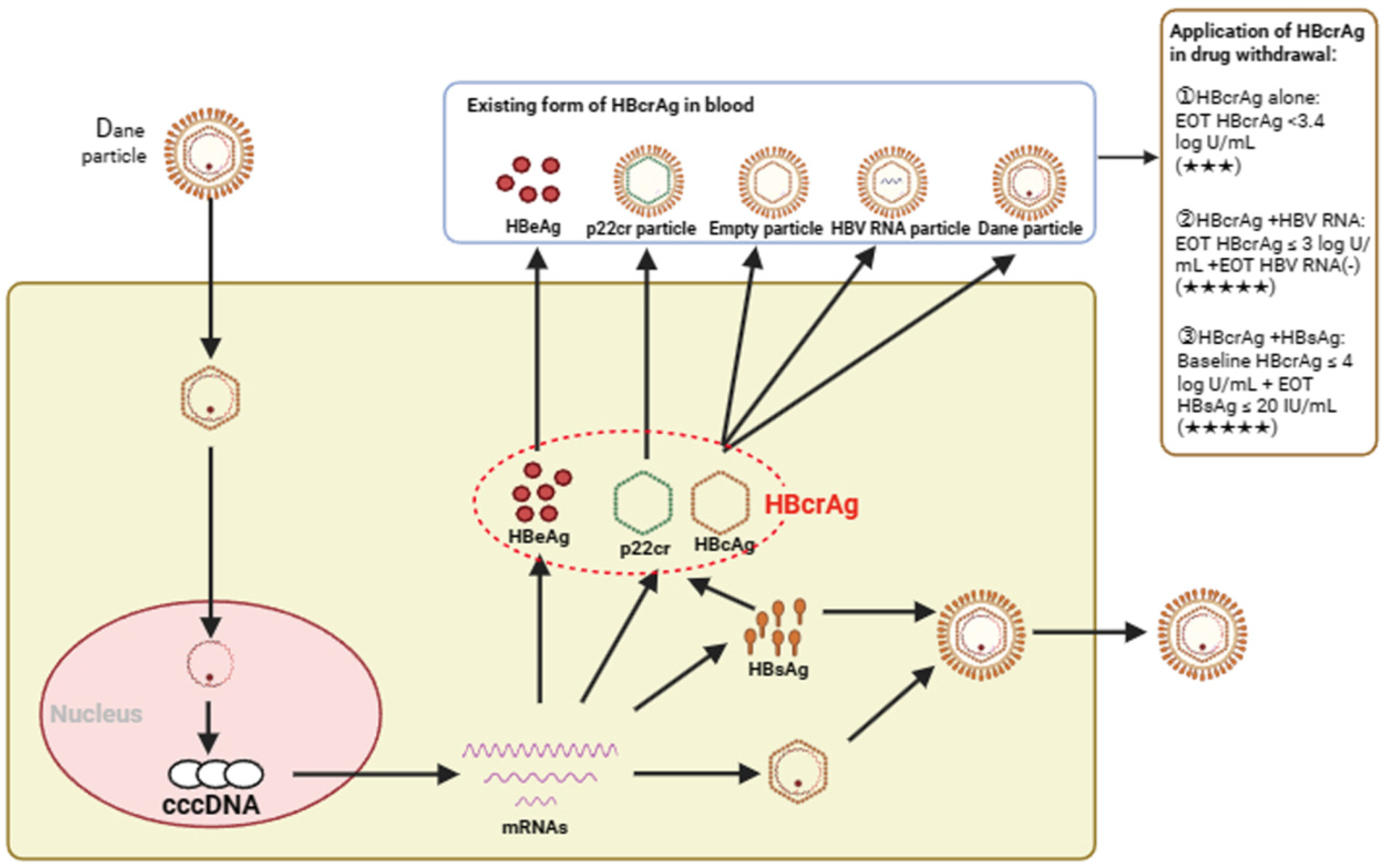
3. Serum HBcrAg Can Reflect the Transcription Level of Intrahepatic HBV cccDNA
4. Evidence of Serum HBcrAg in Predicting Discontinuing Antiviral Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef] [PubMed]
- The Polaris Observatory Collaborators. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: A modelling study. Lancet Gastroenterol. Hepatol. 2023, 8, 879–907. [CrossRef]
- Liu, Z.; Lin, C.; Mao, X.; Guo, C.; Suo, C.; Zhu, D.; Jiang, W.; Li, Y.; Fan, J.; Song, C.; et al. Changing prevalence of chronic hepatitis B virus infection in China between 1973 and 2021: A systematic literature review and meta-analysis of 3740 studies and 231 million people. Gut 2023, 72, 2354–2363. [Google Scholar] [CrossRef]
- Nassal, M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015, 64, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Chinese Society of Infectious Diseases, Chinese Medical Association. The expert consensus on clinical cure (functional cure) of chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi 2019, 27, 594–603. [Google Scholar] [CrossRef]
- Hall, S.A.L.; Vogrin, S.; Wawryk, O.; Burns, G.S.; Visvanathan, K.; Sundararajan, V.; Thompson, A. Discontinuation of nucleot(s)ide analogue therapy in HBeAg-negative chronic hepatitis B: A meta-analysis. Gut 2022, 71, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.A.; Lim, Y.S.; An, J.; Lee, D.; Shim, J.H.; Kim, K.M.; Lee, H.C.; Chung, Y.H.; Lee, Y.S.; Suh, D.J. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: Clinical outcomes and durability. Gut 2014, 63, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Wong, D.; Ip, P.; Kopaniszen, M.; Seto, W.K.; Fung, J.; Huang, F.Y.; Lee, B.; Cullaro, G.; Chong, C.K.; et al. Reduction of covalently closed circular DNA with long-term nucleos(t)ide analogue treatment in chronic hepatitis B. J. Hepatol. 2017, 66, 275–281. [Google Scholar] [CrossRef] [PubMed]
- EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [CrossRef]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Levrero, M.; Subic, M.; Villeret, F.; Zoulim, F. Perspectives and limitations for nucleo(t)side analogs in future HBV therapies. Curr. Opin. Virol. 2018, 30, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.S.Y.; Covert, E.; Wilson, E.; Kottilil, S. Chronic Hepatitis B Infection: A Review. JAMA 2018, 319, 1802–1813. [Google Scholar] [CrossRef]
- Chevaliez, S.; Hézode, C.; Bahrami, S.; Grare, M.; Pawlotsky, J.M. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: Finite treatment duration unlikely. J. Hepatol. 2013, 58, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Zoutendijk, R.; Hansen, B.E.; van Vuuren, A.J.; Boucher, C.A.; Janssen, H.L. Serum HBsAg decline during long-term potent nucleos(t)ide analogue therapy for chronic hepatitis B and prediction of HBsAg loss. J. Infect. Dis. 2011, 204, 415–418. [Google Scholar] [CrossRef]
- Papatheodoridis, G.; Vlachogiannakos, I.; Cholongitas, E.; Wursthorn, K.; Thomadakis, C.; Touloumi, G.; Petersen, J. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology 2016, 63, 1481–1492. [Google Scholar] [CrossRef]
- Allweiss, L.; Dandri, M. The Role of cccDNA in HBV Maintenance. Viruses 2017, 9, 156. [Google Scholar] [CrossRef]
- Mani, H.; Kleiner, D.E. Liver biopsy findings in chronic hepatitis B. Hepatology 2009, 49, S61–S71. [Google Scholar] [CrossRef]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef]
- Liang, Y.; Jiang, J.; Su, M.; Liu, Z.; Guo, W.; Huang, X.; Xie, R.; Ge, S.; Hu, J.; Jiang, Z.; et al. Predictors of relapse in chronic hepatitis B after discontinuation of anti-viral therapy. Aliment. Pharmacol. Ther. 2011, 34, 344–352. [Google Scholar] [CrossRef]
- Tsuge, M.; Murakami, E.; Imamura, M.; Abe, H.; Miki, D.; Hiraga, N.; Takahashi, S.; Ochi, H.; Nelson Hayes, C.; Ginba, H.; et al. Serum HBV RNA and HBeAg are useful markers for the safe discontinuation of nucleotide analogue treatments in chronic hepatitis B patients. J. Gastroenterol. 2013, 48, 1188–1204. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, B.; Hou, J.; Jiang, D. Biomarkers for predicting nucleos(t)ide analogs discontinuation and hepatitis B virus recurrence after drug withdrawal in chronic hepatitis B patients. Hepatol. Res. 2022, 52, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Peng, J.; Xie, Q.; Tan, D.; Xu, M.; Niu, J.; Wang, H.; Ren, H.; Chen, X.; Wang, M.; et al. Combining Hepatitis B Virus RNA and Hepatitis B Core-Related Antigen: Guidance for Safely Stopping Nucleos(t)ide Analogues in Hepatitis B e Antigen-Positive Patients With Chronic Hepatitis B. J. Infect. Dis. 2020, 222, 611–618. [Google Scholar] [CrossRef]
- Inoue, T.; Watanabe, T.; Tanaka, Y. Hepatitis B core-related antigen: A novel and promising surrogate biomarker to guide anti-hepatitis B virus therapy. Clin. Mol. Hepatol. 2023, 29, 851–868. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Li, Z.; Hansen, B.E.; Yu, T.; Zhang, X.; Sun, J.; Hou, J.; Janssen, H.L.A.; Peng, J. Serum Level of Antibodies Against Hepatitis B Core Protein Is Associated With Clinical Relapse After Discontinuation of Nucleos(t)ide Analogue Therapy. Clin. Gastroenterol. Hepatol. 2019, 17, 182–191.e1. [Google Scholar] [CrossRef]
- Jaroszewicz, J.; Calle Serrano, B.; Wursthorn, K.; Deterding, K.; Schlue, J.; Raupach, R.; Flisiak, R.; Bock, C.T.; Manns, M.P.; Wedemeyer, H.; et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: A European perspective. J. Hepatol. 2010, 52, 514–522. [Google Scholar] [CrossRef]
- Suslov, A.; Meier, M.A.; Ketterer, S.; Wang, X.; Wieland, S.; Heim, M.H. Transition to HBeAg-negative chronic hepatitis B virus infection is associated with reduced cccDNA transcriptional activity. J. Hepatol. 2021, 74, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.G.; Boyd, A.; Combe, E.; Testoni, B.; Zoulim, F. Covalently closed circular DNA: The ultimate therapeutic target for curing HBV infections. J. Hepatol. 2021, 75, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Testoni, B.; Scholtès, C.; Plissonnier, M.L.; Paturel, A.; Berby, F.; Facchetti, F.; Villeret, F.; Degasperi, E.; Scott, B.; Hamilton, A.; et al. Quantification of circulating HBV RNA expressed from intrahepatic cccDNA in untreated and NUC treated patients with chronic hepatitis B. Gut 2024, 73, 659–667. [Google Scholar] [CrossRef]
- Wang, X.; Chi, X.; Wu, R.; Xu, H.; Gao, X.; Yu, L.; Liu, L.; Zhang, M.; Tan, Y.; Niu, J.; et al. Serum HBV RNA correlated with intrahepatic cccDNA more strongly than other HBV markers during peg-interferon treatment. Virol. J. 2021, 18, 4. [Google Scholar] [CrossRef]
- Laras, A.; Papatheodoridi, M.; Panopoulou, E.; Papatheodoridis, G.V.; Hadziyannis, S.J.; Hadziyannis, E. Serum hepatitis B virus RNA detectability, composition and clinical significance in patients with ab initio hepatitis B e antigen negative chronic hepatitis B. Virol. J. 2022, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, B.; Valdes, J.D.; Sun, J.; Guo, H. Serum Hepatitis B Virus RNA: A New Potential Biomarker for Chronic Hepatitis B Virus Infection. Hepatology 2019, 69, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Abate, M.L.; Tandoi, F.; Ciancio, A.; Amoroso, A.; Salizzoni, M.; Saracco, G.M.; Rizzetto, M.; Romagnoli, R.; Smedile, A. Quantitation of HBV cccDNA in anti-HBc-positive liver donors by droplet digital PCR: A new tool to detect occult infection. J. Hepatol. 2018, 69, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.K.; Arita, J.; Akamatsu, N.; Maki, H.; Nishioka, Y.; Kawahara, T.; Miyata, A.; Kokudo, T.; Nagata, R.; Mihara, Y.; et al. The impact of the covalently closed circular DNA level on recurrence of hepatocellular carcinoma after initial hepatectomy: An analysis of patients with resolved hepatitis B virus infection. HPB 2022, 24, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Mak, L.Y.; Wong, D.K.; Cheung, K.S.; Seto, W.K.; Lai, C.L.; Yuen, M.F. Review article: Hepatitis B core-related antigen (HBcrAg): An emerging marker for chronic hepatitis B virus infection. Aliment. Pharmacol. Ther. 2018, 47, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.K.; Seto, W.K.; Cheung, K.S.; Chong, C.K.; Huang, F.Y.; Fung, J.; Lai, C.L.; Yuen, M.F. Hepatitis B virus core-related antigen as a surrogate marker for covalently closed circular DNA. Liver Int. 2017, 37, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Testoni, B.; Lebossé, F.; Scholtes, C.; Berby, F.; Miaglia, C.; Subic, M.; Loglio, A.; Facchetti, F.; Lampertico, P.; Levrero, M.; et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J. Hepatol. 2019, 70, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Q.; Feng, S.; Wang, M.L.; Liang, L.B.; Zhou, L.Y.; Du, L.Y.; Yan, L.B.; Tao, C.M.; Tang, H. Serum hepatitis B core-related antigen is a satisfactory surrogate marker of intrahepatic covalently closed circular DNA in chronic hepatitis B. Sci. Rep. 2017, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Q.; Wang, M.L.; Tao, Y.C.; Wu, D.B.; Liao, J.; He, M.; Tang, H. Serum HBcrAg is better than HBV RNA and HBsAg in reflecting intrahepatic covalently closed circular DNA. J. Viral Hepat. 2019, 26, 586–595. [Google Scholar] [CrossRef]
- Wong, D.K.; Inoue, T.; Mak, L.Y.; Hui, R.W.; Fung, J.; Cheung, K.S.; Seto, W.K.; Tanaka, Y.; Yuen, M.F. A longitudinal study to detect hepatitis B surface and core-related antigens in chronic hepatitis B patients with hepatitis B surface antigen seroclearance using highly sensitive assays. J. Clin. Virol. 2023, 160, 105375. [Google Scholar] [CrossRef]
- Kimura, T.; Rokuhara, A.; Sakamoto, Y.; Yagi, S.; Tanaka, E.; Kiyosawa, K.; Maki, N. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J. Clin. Microbiol. 2002, 40, 439–445. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Wang, J.H.; Hung, C.H.; Lu, S.N.; Hu, T.H.; Chen, C.H. Combining end-of-treatment HBsAg and baseline hepatitis B core-related antigen reduce HBV relapse rate after tenofovir cessation. Hepatol. Int. 2021, 15, 301–309. [Google Scholar] [CrossRef]
- Adraneda, C.; Tan, Y.C.; Yeo, E.J.; Kew, G.S.; Khakpoor, A.; Lim, S.G. A critique and systematic review of the clinical utility of hepatitis B core-related antigen. J. Hepatol. 2023, 78, 731–741. [Google Scholar] [CrossRef]
- Hong, X.; Luckenbaugh, L.; Mendenhall, M.; Walsh, R.; Cabuang, L.; Soppe, S.; Revill, P.A.; Burdette, D.; Feierbach, B.; Delaney, W.; et al. Characterization of Hepatitis B Precore/Core-Related Antigens. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- Inoue, T.; Kusumoto, S.; Iio, E.; Ogawa, S.; Suzuki, T.; Yagi, S.; Kaneko, A.; Matsuura, K.; Aoyagi, K.; Tanaka, Y. Clinical efficacy of a novel, high-sensitivity HBcrAg assay in the management of chronic hepatitis B and HBV reactivation. J. Hepatol. 2021, 75, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Carey, I.; Gersch, J.; Wang, B.; Moigboi, C.; Kuhns, M.; Cloherty, G.; Dusheiko, G.; Agarwal, K. Pregenomic HBV RNA and Hepatitis B Core-Related Antigen Predict Outcomes in Hepatitis B e Antigen-Negative Chronic Hepatitis B Patients Suppressed on Nucleos(T)ide Analogue Therapy. Hepatology 2020, 72, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.F.; Seto, W.K.; Wong, D.; Cheung, K.S.; Fung, J.; Mak, L.Y.; Yuen, J.; Chong, C.K.; Lai, C.L.; Yuen, M.F. Seven-Year Treatment Outcome of Entecavir in a Real-World Cohort: Effects on Clinical Parameters, HBsAg and HBcrAg Levels. Clin. Transl. Gastroenterol. 2017, 8, e125. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, X.; Wang, Z.; Gao, Y.; Deng, J.; Liu, X.; Zhuang, H. Correlation of HBcrAg with Intrahepatic Hepatitis B Virus Total DNA and Covalently Closed Circular DNA in HBeAg-Positive Chronic Hepatitis B Patients. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Kimura, T.; Ohno, N.; Terada, N.; Rokuhara, A.; Matsumoto, A.; Yagi, S.; Tanaka, E.; Kiyosawa, K.; Ohno, S.; Maki, N. Hepatitis B virus DNA-negative dane particles lack core protein but contain a 22-kDa precore protein without C-terminal arginine-rich domain. J. Biol. Chem. 2005, 280, 21713–21719. [Google Scholar] [CrossRef]
- Wong, D.K.; Tanaka, Y.; Lai, C.L.; Mizokami, M.; Fung, J.; Yuen, M.F. Hepatitis B virus core-related antigens as markers for monitoring chronic hepatitis B infection. J. Clin. Microbiol. 2007, 45, 3942–3947. [Google Scholar] [CrossRef]
- Wang, M.L.; Deng, R.; Chen, E.Q.; Tao, C.M.; Liao, J.; Zhou, T.Y.; Wang, J.; Tang, H. Performance of serum HBcrAg in chronic hepatitis B patients with 8-year nucleos(t)ide analogs therapy. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Inoue, T.; Tanaka, Y. Hepatitis B Core-Related Antigen and New Therapies for Hepatitis B. Microorganisms 2021, 9, 2083. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Tanaka, E.; Minami, M.; Okanoue, T.; Yatsuhashi, H.; Nagaoka, S.; Suzuki, F.; Kobayashi, M.; Chayama, K.; Imamura, M.; et al. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol. Res. 2007, 37, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, N.; Tanaka, Y.; Orito, E.; Ito, K.; Ohno, T.; Hirashima, N.; Hasegawa, I.; Sugauchi, F.; Ueda, R.; Mizokami, M. Measurement of hepatitis B virus core-related antigen as predicting factor for relapse after cessation of lamivudine therapy for chronic hepatitis B virus infection. Hepatol. Res. 2006, 36, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.S.; Park, J.Y.; Chon, Y.E.; Kim, H.S.; Kang, W.; Kim, B.K.; Kim, S.U.; Kim, Y.d.; Han, K.H.; Ahn, S.H. Clinical outcomes and predictors for relapse after cessation of oral antiviral treatment in chronic hepatitis B patients. J. Gastroenterol. 2016, 51, 830–839. [Google Scholar] [CrossRef]
- Jaroenlapnopparat, A.; Chayanupatkul, M.; Tangkijvanich, P. Novel viral markers and the prediction of off-treatment relapse in chronic hepatitis B patients: A systematic review. J. Gastroenterol. Hepatol. 2021, 36, 2349–2362. [Google Scholar] [CrossRef]
- Tseng, T.N.; Jeng, W.J.; Hu, T.H.; Wang, J.H.; Hung, C.H.; Lu, S.N.; Chen, C.H. Combined baseline HBcrAg and end-of-treatment HBsAg predict HBV relapse after entecavir or tenofovir cessation. J. Antimicrob. Chemother. 2023, 78, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.Y.; Wang, J.H.; Hung, C.H.; Lu, S.N.; Hu, T.H.; Chen, C.H. The role of hepatitis B virus core-related antigen in predicting hepatitis B virus relapse after cessation of entecavir in hepatitis B e antigen-negative patients. J. Viral Hepat. 2021, 28, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.K.; Liu, K.S.; Mak, L.Y.; Cloherty, G.; Wong, D.K.; Gersch, J.; Lam, Y.F.; Cheung, K.S.; Chow, N.; Ko, K.L.; et al. Role of serum HBV RNA and hepatitis B surface antigen levels in identifying Asian patients with chronic hepatitis B suitable for entecavir cessation. Gut 2021, 70, 775–783. [Google Scholar] [CrossRef]
- Tseng, T.N.; Hu, T.H.; Wang, J.H.; Kuo, Y.H.; Hung, C.H.; Lu, S.N.; Jeng, W.J.; Chen, C.H. Incidence and Factors Associated With HBV Relapse After Cessation of Entecavir or Tenofovir in Patients With HBsAg Below 100 IU/mL. Clin. Gastroenterol. Hepatol. 2020, 18, 2803–2812.e2. [Google Scholar] [CrossRef]
- Sonneveld, M.J.; Chiu, S.M.; Park, J.Y.; Brakenhoff, S.M.; Kaewdech, A.; Seto, W.K.; Tanaka, Y.; Carey, I.; Papatheodoridi, M.; van Bömmel, F.; et al. Probability of HBsAg loss after nucleo(s)tide analogue withdrawal depends on HBV genotype and viral antigen levels. J. Hepatol. 2022, 76, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Japan Society of Hepatology Guidelines for the Management of Hepatitis B Virus Infection: 2019 update. Hepatol. Res. 2020, 50, 892–923. [CrossRef] [PubMed]
- Kaewdech, A.; Tangkijvanich, P.; Sripongpun, P.; Witeerungrot, T.; Jandee, S.; Tanaka, Y.; Piratvisuth, T. Hepatitis B surface antigen, core-related antigen and HBV RNA: Predicting clinical relapse after NA therapy discontinuation. Liver Int. 2020, 40, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
- Zoulim, F.; Testoni, B.; Lebossé, F. Kinetics of intrahepatic covalently closed circular DNA and serum hepatitis B surface antigen during antiviral therapy for chronic hepatitis B: Lessons from experimental and clinical studies. Clin. Gastroenterol. Hepatol. 2013, 11, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridi, M.; Papatheodoridis, G. Can we stop nucleoside analogues before HBsAg loss? J. Viral Hepat. 2019, 26, 936–941. [Google Scholar] [CrossRef]
- van Bömmel, F.; Stein, K.; Heyne, R.; Petersen, J.; Buggisch, P.; Berg, C.; Zeuzem, S.; Stallmach, A.; Sprinzl, M.; Schott, E.; et al. A multicenter randomized-controlled trial of nucleos(t)ide analogue cessation in HBeAg-negative chronic hepatitis B. J. Hepatol. 2023, 78, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Peng, C.Y.; Kuo, Y.H.; Hu, T.H.; Hung, C.H.; Wang, J.H.; Lu, S.N. Earlier and Higher Rate of Hepatitis B Virus Relapse After Discontinuing Tenofovir Versus Entecavir in Hepatitis B e Antigen-Positive Patients. J. Infect. Dis. 2022, 225, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Azarkar, Z.; Ziaee, M.; Ebrahimzadeh, A.; Sharifzadeh, G.; Javanmard, D. Epidemiology, risk factors, and molecular characterization of occult hepatitis B infection among anti-hepatitis B core antigen alone subjects. J. Med. Virol. 2019, 91, 615–622. [Google Scholar] [CrossRef]
- Fu, M.X.; Simmonds, P.; Andersson, M.; Harvala, H. Biomarkers of transfusion transmitted occult hepatitis B virus infection: Where are we and what next? Rev. Med. Virol. 2024, 34, e2525. [Google Scholar] [CrossRef]
- Ye, X.; Zhao, Y.; Li, R.; Li, T.; Zheng, X.; Xiong, W.; Zeng, J.; Xu, M.; Chen, L. High Frequency Occult Hepatitis B Virus Infection Detected in Non-Resolved Donations Suggests the Requirement of Anti-HBc Test in Blood Donors in Southern China. Front. Immunol. 2021, 12, 699217. [Google Scholar] [CrossRef]
- Tang, X.; Yang, L.; Zhang, P.; Wang, C.; Luo, S.; Liu, B.; Fu, Y.; Candotti, D.; Allain, J.P.; Zhang, L.; et al. Occult Hepatitis B Virus Infection and Liver Fibrosis in Chinese Patients. J. Infect. Dis. 2023, 228, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Wang, F.; Li, T.; Xu, X.; Sun, Y.; Nan, Y.; Wang, G.; Hou, J.; Duan, Z.; Wei, L.; et al. Guidelines for the Prevention and Treatment of Chronic Hepatitis B (version 2022). J. Clin. Transl. Hepatol. 2023, 11, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.K.; Wong, D.K.; Chan, T.S.; Hwang, Y.Y.; Fung, J.; Liu, K.S.; Gill, H.; Lam, Y.F.; Cheung, K.S.; Lie, A.K.; et al. Association of Hepatitis B Core-Related Antigen With Hepatitis B Virus Reactivation in Occult Viral Carriers Undergoing High-Risk Immunosuppressive Therapy. Am. J. Gastroenterol. 2016, 111, 1788–1795. [Google Scholar] [CrossRef]
- Seto, W.K.; Wong, D.K.; Fung, J.; Huang, F.Y.; Liu, K.S.; Lai, C.L.; Yuen, M.F. Linearized hepatitis B surface antigen and hepatitis B core-related antigen in the natural history of chronic hepatitis B. Clin. Microbiol. Infect. 2014, 20, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Lou, B.; Lv, F.; Zhao, D.; Chen, H.; Ye, X.; Chen, Y. HBcrAg, pg RNA and HBsAg dynamically supervise the seroconversion of HBsAg with anti-viral therapy: “Loss of HBsAg” maybe not a good end-point of anti-viral therapy. Clin. Chim. Acta 2020, 501, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Wong, D.K.; Sablon, E.; Tse, E.; Ng, I.O.; Yuan, H.J.; Siu, C.W.; Sander, T.J.; Bourne, E.J.; Hall, J.G.; et al. HBsAg seroclearance in chronic hepatitis B in the Chinese: Virological, histological, and clinical aspects. Hepatology 2004, 39, 1694–1701. [Google Scholar] [CrossRef]
- Huang, D.; Wu, D.; Wang, P.; Wang, Y.; Yuan, W.; Hu, D.; Hu, J.; Wang, Y.; Tao, R.; Xiao, F.; et al. End-of-treatment HBcrAg and HBsAb levels identify durable functional cure after Peg-IFN-based therapy in patients with CHB. J. Hepatol. 2022, 77, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.J.; Hirode, G.; Chen, C.H.; Su, T.H.; Seto, W.K.; Van Hees, S.; Papatheodoridi, M.; Lens, S.; Wong, G.L.H.; Brakenhoff, S.M.; et al. Differential Relapse Patterns After Discontinuation of Entecavir vs Tenofovir Disoproxil Fumarate in Chronic Hepatitis B. Clin. Gastroenterol. Hepatol. 2023, 21, 1513–1522.e4. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.T.; Hu, T.H.; Hung, C.H.; Wang, J.H.; Lu, S.N.; Tsai, K.L.; Chen, C.H. Hepatitis B virus relapse rates in chronic hepatitis B patients who discontinue either entecavir or tenofovir. Aliment. Pharmacol. Ther. 2019, 49, 218–228. [Google Scholar] [CrossRef]
- Sonneveld, M.J.; Chiu, S.M.; Park, J.Y.; Brakenhoff, S.M.; Kaewdech, A.; Seto, W.K.; Tanaka, Y.; Carey, I.; Papatheodoridi, M.; Colombatto, P.; et al. HBV DNA and HBsAg Levels at 24 Weeks Off-Treatment Predict Clinical Relapse and HBsAg Loss in HBeAg-Negative Patients Who Discontinued Antiviral Therapy. Gastroenterology 2024, 166, 168–177.e8. [Google Scholar] [CrossRef]
| Biomarkers | No. of Subjects | Drugs | Drug Withdrawal Time | VR(%) | CR(%) | Cut-Off Value | Ref. |
|---|---|---|---|---|---|---|---|
| HBcrAg | 34 | LAM | 12 months | - | 0 | EOT HBcrAg < 4.5 log U/mL | [53] |
| 22 | LAM | 28 months | - | 0 | EOT HBcrAg < 3.4 log U/mL | [54] | |
| 68 | LAM, ETV | 12 months | 0 | - | EOT HBcrAg ≤ 3.7 log U/mL | [55] | |
| HBcrAg + HBV RNA | 127 | LDT | 48 months | - | 0 | EOT HBcrAg < 4 log U/mL + EOT HBV RNA(-) | [23] |
| 92 | LAM, LDT, ADV, ETV, TDF | 12 months | - | 0 | EOT HBcrAg ≤ 3 log U/mL + EOT HBV RNA(-) | [63] | |
| HBcrAg + HBsAg | 31 | ETV, TDF | 60 months | 6.5 | 0 | Baseline HBcrAg ≤ 4 log U/mL + EOT HBsAg ≤ 20 IU/mL | [57] |
| 84 | ETV | 60 months | 27.9 | 18 | Baseline HBcrAg ≤ 4 log U/mL + EOT HBsAg < 150 IU/mL | [58] | |
| 53 | TDF | 36 months | 20.3 | 10.3 | Baseline HBcrAg < 4.7 log U/mL + EOT HBsAg < 100 IU/mL | [42] | |
| 36 | ETV, TDF | 60 months | 5.9 | 2.8 | Baseline HBcrAg < 4log U/mL + EOT HBsAg < 40 IU/mL | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-H.; Tang, H.; Chen, E.-Q. Quantitative Measurement of Serum HBcrAg Can Be Used to Assess the Feasibility of Safe Discontinuation of Antiviral Therapy for Chronic Hepatitis B. Viruses 2024, 16, 529. https://doi.org/10.3390/v16040529
Wang Y-H, Tang H, Chen E-Q. Quantitative Measurement of Serum HBcrAg Can Be Used to Assess the Feasibility of Safe Discontinuation of Antiviral Therapy for Chronic Hepatitis B. Viruses. 2024; 16(4):529. https://doi.org/10.3390/v16040529
Chicago/Turabian StyleWang, Yong-Hong, Hong Tang, and En-Qiang Chen. 2024. "Quantitative Measurement of Serum HBcrAg Can Be Used to Assess the Feasibility of Safe Discontinuation of Antiviral Therapy for Chronic Hepatitis B" Viruses 16, no. 4: 529. https://doi.org/10.3390/v16040529
APA StyleWang, Y.-H., Tang, H., & Chen, E.-Q. (2024). Quantitative Measurement of Serum HBcrAg Can Be Used to Assess the Feasibility of Safe Discontinuation of Antiviral Therapy for Chronic Hepatitis B. Viruses, 16(4), 529. https://doi.org/10.3390/v16040529






