Abstract
Porcine epidemic diarrhea (PED), a highly contagious enteric disease caused by the porcine epidemic diarrhea virus (PEDV), is characterized by vomiting, diarrhea, and dehydration, leading to high mortality in newborn piglets and significant economic losses in the swine industry. The shortage of effective PED vaccines emphasizes the need to explore potent natural compounds for therapeutic intervention. It has been shown that glycerol monolaurate (GML) effectively inhibits PEDV replication in vivo and in vitro. Further investigation is needed to assess whether complex medium-chain triglycerides (CMCTs), composed of glyceryl tricaprylate/caprate (GTCC) and GML, offer an efficient anti-PEDV activity. In this study, piglets were orally infected with PEDV and exhibited typical clinical signs, including diarrhea and vomiting, accompanied by intestinal inflammation, oxidative stress, and tissue damage. CMCTs were administered orally twice daily for one week. In vivo findings indicate that CMCT treatment alleviated clinical signs and prevented weight loss. It significantly increased serum immunoglobulins (IgG, IgM, and IgA) and intestinal mucosal sIgA and MUC-2 levels, while reducing pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, and IL-17) and increasing antiviral interferons (IFN-α and IFN-γ), anti-inflammatory cytokines (IL-4 and IL-10), and IL-22. Antioxidant enzyme activities (T-AOC, SOD, GSH-Px, and CAT) were elevated, whereas oxidative stress markers (iNOS, NO, and MDA) were decreased. Expression of intestinal tight junction proteins claudin-1 and ZO-1 was restored. Moreover, CD4+ and CD8+ T cell populations increased, and the functions of regulatory T cells (Tregs) were restored. Gut microbiota analysis showed increased beneficial genera (Streptococcus and Ligilactobacillus) and decreased pathogenic Escherichia-Shigella. These results demonstrate that CMCTs mitigate PEDV infection by enhancing anti-inflammation, antioxidation, and intestinal barrier function, as well as modulating gut microbiota composition. This study improves the understanding of the pathogenesis of PEDV and highlights CMCTs as a promising therapeutic candidate for PED.
1. Introduction
Porcine epidemic diarrhea (PED) is an acute, highly contagious enteric disease in swine caused by the porcine epidemic diarrhea virus (PEDV), a member of the genus Coronavirus within the family Coronaviridae. PEDV primarily infects the digestive tract of pigs, causing vomiting and diarrhea, and leading to particularly high mortality in piglets [1]. As the largest digestive and absorptive organ, the intestine is the body’s primary line of resistance to bacterial and viral invasion in pigs. The intestinal barrier functions in pigs are a complex system comprising four barriers: mechanical, biological, immune, and chemical [2,3]. Extensive viral replication in the intestinal epithelium results in significant damage, including marked villous atrophy, loss of enterocytes and goblet cells, destroyed tight junctions, and reduced mucin production. This damage facilitates the breach of the intestinal barrier by bacteria and toxins, which in turn induces the production of various inflammatory cytokines and leads to intestinal inflammation and oxidative stress [4,5]. Hence, it is crucial to preserve the intestinal barrier’s functions to resist PEDV infection.
Glyceryl tricaprylate/caprate (GTCC) is a triacylglycerol composed of caprylic (C8) and capric (C10) acids, two medium-chain fatty acids. As a specialized MCT, GTCC exhibits high stability, efficient absorption, and rapid energy metabolism, thereby promoting cellular repair and functional maintenance [6,7]. Glyceryl monolaurate (GML) is a monoglyceride derived from lauric acid, a medium-chain fatty acid (C12) commonly found in coconut oil and palm kernel oil. It possesses antimicrobial [8] and antiviral properties, significantly inhibiting enveloped viruses, including human immunodeficiency virus type 1 (HIV-1) [9] and African swine fever virus (ASFV) [10,11]. Beyond its antiviral effects, GML can modulate intestinal oxidative stress, counteract intestinal inflammation, and regulate the intestinal microbiota to maintain intestinal health, thereby reducing pathogenic microorganism infections in the intestinal tract [12,13,14,15].
Currently, a primary approach to controlling PEDV infection is vaccination. However, the immature immune systems of piglets may limit the efficacy of PED vaccines [16]. Consequently, this situation poses significant challenges for managing PED in piglets due to the lack of effective treatments. Therefore, this study aims to evaluate the protective potential of complex medium-chain triglycerides (CMCTs) against PEDV infection in piglets and elucidate their roles in regulating the intestinal microenvironment to enhance intestinal barrier functions.
2. Materials and Methods
2.1. Virus and Animals
The PEDV SC strain was supplied by the Animal Biotechnology Center of Sichuan Agricultural University. Twenty-five experimental piglets (Landrace × Yorkshire, one week old), all healthy and of similar body weight, were obtained from Chengdu Wangjiang Agriculture and Animal Husbandry Technology Co., Ltd. (Chengdu, China; a PEDV-free farm). These piglets were not vaccinated with a PED vaccine. The experimental milk replacer, used as the basal diet, was purchased from Tongwei Group Co., Ltd. (Chengdu, China) and confirmed to be free of anti-PEDV antibodies.
2.2. Drugs and Reagents
The CMCTs (consisting of GTCC and GML at a ratio of 2:1) were prepared by Nuacid Nutrition Co., Ltd. (Qingyuan, China). ELISA kits for IgG, IgM, IgA, and MUC-2, secretory IgA (sIgA), and IL-6, IL-1β, TNF-α, IL-4, IL-10, IFN-α, IFN-γ, IL-17, and IL-22 were purchased from Jiangsu Meimian Industrial Co., Ltd. (Yancheng, China). RIPA lysis buffer was obtained from Beyotime Biotechnology (Shanghai, China). The BCA Protein Assay Kit was purchased from Nanjing Vazyme Biotech Co., Ltd. (Nanjing, China). T-AOC, CAT, SOD, iNOS, GSH-Px, and MDA were supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). RNAiso Easy, PrimeScript™ RT reagent Kit (Perfect Real Time) and TB Green® Premix Ex Taq™ (Tli RNaseH Plus, Kusatsu, Japan) were supplied by Takara Biomedical Technology Co., Ltd. (Beijing, China). Immunofluorescence antibody was purchased from ABclonal Technology (Wuhan, China). All other chemicals and reagents were analytically pure and purchased from standard commercial suppliers.
2.3. Piglet Infection Model and Treatments
Prior to the experiment, rigorous disinfection measures were implemented at the experimental site, including overnight formaldehyde fumigation and subsequent spraying with a broad-spectrum disinfectant. After acquiring the piglets, continuous attention was given to maintaining their hygiene, and they were fed a milk replacer optimized for growth. A 3-day observation period was conducted to ensure the health of the piglets before starting the experiment. Twenty-five one-week-old piglets were randomly assigned to five groups: Control (unchallenged, untreated), Model (PEDV-challenged, untreated), CMCTs high/medium/low (PEDV-challenged, treated with high, medium, or low doses of CMCTs, respectively). Piglets in the Model and CMCTs groups were orally inoculated with 5 mL of PEDV stock at 105 TCID50/mL per piglet, while those in the Control group received an equivalent volume of sterile saline. The PEDV dose (105 TCID50/mL, 5 mL) was chosen based on previous studies to induce infection [17]. Immediately after the PEDV challenge, piglets in the high (2.0 g/kg), medium (1.0 g/kg), and low (0.5 g/kg) dose CMCT groups received oral administration of the treatments twice daily for 7 consecutive days. The CMCTs were suspended in sterile normal saline and thoroughly mixed to ensure uniform dispersion and accurate dosing. The piglets in the model group received an equal volume of sterile normal saline following the same schedule.
2.4. Clinical Observation and Sample Collection
The control and infected groups were housed in separate rooms under strict biosecurity protocols. A unidirectional workflow was implemented, beginning with the control room and proceeding to the infected room to minimize the risk of cross-contamination. Protective clothing and footwear were changed between rooms, and hands and equipment were disinfected prior to each entry. Clinical signs, including diarrhea, dehydration, and vomiting, were monitored daily, and body weights were recorded every other day throughout the 7-day observation period. At 7 days post-infection and treatment, all piglets were euthanized for sample collection. For animals that developed severe clinical signs prior to the scheduled endpoint, tissue and serum samples were collected immediately upon reaching a moribund state to prevent sample loss and maintain a consistent sample size across groups. Euthanasia was performed under inhalation anesthesia using isoflurane, with the concentration gradually increased to 4–5% in 100% oxygen until deep anesthesia was achieved. Exsanguination via the anterior vena cava was then carried out to complete euthanasia. Blood, intestinal mucosa, tissues, and intestinal contents were collected; some samples were fixed in 4% paraformaldehyde, while others were snap-frozen and stored at −80 °C for subsequent analyses.
2.5. Histological Analysis
The intestinal tissues were fixed with 4% paraformaldehyde. Subsequently, the fixed tissues were sectioned, dehydrated, embedded in paraffin, and stained with hematoxylin and eosin (H&E). A light microscope was utilized to observe histopathological changes and capture images.
2.6. Serum Immunoglobulin Quantification
After natural coagulation, blood was centrifuged (1000× g, 4 °C, 10 min) in a refrigerated centrifuge to separate the serum. Immunoglobulin (IgG, IgM, and IgA) concentrations in the serum were assessed using commercial ELISA kits.
2.7. Quantification of MUC-2 and sIgA in Intestinal Mucosa
Porcine jejunal mucosa was carefully collected, transferred to sterilized centrifuge tubes, and homogenized in PBS (1:5). The homogenate was then centrifuged at 8000× g at 4 °C for 15 min to obtain the supernatant, which was used to assess the protein expression of mucins (MUC-2) and secretory IgA (sIgA) in the intestinal mucosa.
2.8. Jejunal Cytokine Expression Analysis
PEDV primarily infects the small intestine of pigs, with a particular predilection for the jejunum and ileum regions, resulting in severe atrophic enteritis [18]. To examine the protective effect of CMCTs on the porcine small intestine, we used homogenized jejunal tissues (PBS, 1:5), which were then centrifuged at 8000× g at 4 °C for 15 min. The supernatant was utilized to measure cytokine concentrations (IL-6, IL-1β, TNF-α, IL-4, IL-10, IFN-α, IFN-γ, IL-17, and IL-22) in the jejunal tissues by ELISA kits.
2.9. Jejunal Oxidative Stress Analysis
Jejunal tissues were homogenized in RIPA lysis buffer to extract total proteins. The homogenate was then centrifuged at 12,000× g and 4 °C for 15 min. Protein concentrations were measured using a bicinchoninic acid (BCA) assay kit according to the manufacturer’s instructions. The concentrations of oxidative stress markers, including T-AOC, CAT, SOD, iNOS, GSH-Px, and MDA, were measured using commercial kits. These assessments were conducted to evaluate the effect of CMCTs on oxidative stress in PEDV-infected intestinal tissues.
2.10. Indirect Immunofluorescence Analysis
The intestinal tissues were fixed in 4% paraformaldehyde and then embedded in paraffin. Immunofluorescence staining was used to assess the protein expression of claudin-1 and ZO-1, as well as the colonization of T cells and their subsets, identified by T cell surface markers CD127, CD4, and CD8. After sectioning, the tissues underwent deparaffinization, rehydration, and blocking. Primary antibodies targeting the proteins were incubated overnight at 4 °C. Fluorescently labeled secondary antibodies were then applied and incubated. To visualize cell nuclei, DAPI was used as a counterstain. The images were captured using a Leica microscope and analyzed with the ImageJ software (v1.8.0).
2.11. 16S rRNA Sequencing of Gut Microbiota
The jejunal contents were collected and transported to Novogene Co., Ltd. (Tokyo, Japan) in dry ice. Total DNA was extracted using a DNA extraction kit following the manufacturer’s protocols. The concentration, purity, and quality of the extracted DNA were then evaluated. The primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′) were utilized to amplify the V3-V4 domain of the microbial 16S rRNA gene via PCR. The amplified products were sequenced using the Illumina MiSeq platform. Data processing was then performed using the R programming language, involving analysis of intergroup microbial abundance, diversity, clustering, and other pertinent information.
2.12. Statistical Analysis
All data were presented as means ± SD and analyzed using SPSS v.22.0 (IBM Corp., Armonk, NY, USA). One-way ANOVA was employed to compare groups, followed by Tukey’s post hoc test. For 16S rRNA sequencing, differential microbiota were identified using the Wilcoxon rank-sum test (for two groups) or the Kruskal–Wallis H test with Bonferroni correction (for multiple groups) with a 95% confidence interval. Statistical significance was indicated by * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
3. Results
3.1. CMCTs Can Ameliorate Clinical Signs
Piglets infected with PEDV displayed vomiting and watery diarrhea. The model group experienced severe dehydration, emaciation, contamination with vomit and excreta, and death (Table 1). In contrast, the control group showed normal growth. Piglets receiving low-dose treatment exhibited clinical signs similar to the model group but gradually recovered. In the medium-to-high dose treatment group, vomiting was absent, and transient diarrhea was quickly resolved, indicating the effectiveness of the treatment. Additionally, the piglets tolerated CMCTs well, showing no signs of toxicity.

Table 1.
Statistics of clinical signs in each group after PEDV infection.
3.2. CMCTs Prevent Weight Loss in Infected Piglets
After infection, piglets in the model group rapidly developed dehydration, appetite reduction, and persistent weight loss without recovery. In contrast, control group piglets maintained a normal appetite and gained weight. The medium and high dose treatment groups exhibited improved appetite and regained physical strength, initiating weight gain by the fourth day post-treatment (Figure 1). The results indicated that CMCTs effectively slowed weight loss in piglets and promoted the restoration of their growth performance.
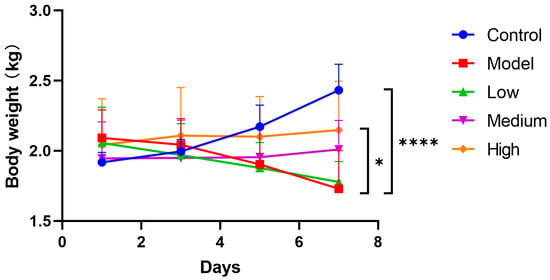
Figure 1.
Effects of CMCTs on piglet body weight after PEDV infection. After the challenge, the treatment groups received low (0.5 g/kg), medium (1.0 g/kg), and high (2.0 g/kg) doses of CMCTs orally twice daily for 7 days, with body weight measured every other day. Data were presented as means ± SD (n = 5). Statistical analyses were performed using two-way ANOVA followed by Dunnett’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared with the model group.
3.3. CMCTs Effectively Suppressed PEDV-Induced Inflammation and Small Intestine Damage
Compared to the control group, the model group exhibited pronounced histopathological alterations in the jejunum and ileum, including severe mucosal thinning (yellow arrows), villous atrophy (red arrows), and epithelial exfoliation (blue arrows). Necrotic cellular debris was also evident within the intestinal lumen (purple arrows). Post-treatment, these lesions in the jejunum and ileum were markedly ameliorated, with a dose-dependent restoration of villous architecture observed (green arrows) (Figure 2).
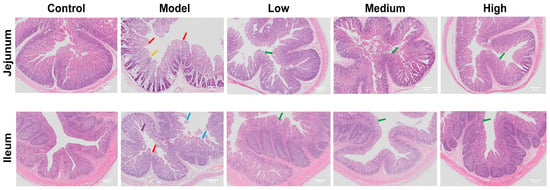
Figure 2.
The effects of CMCTs on piglet jejunum and ileum. Histological analysis of H&E staining images. Scale bar, 50 μm. Magnification, 50×. Yellow arrows: mucosal thinning; red arrows: villous atrophy; blue arrows: epithelial exfoliation; purple arrows: necrotic cellular debris; green arrows: restored villous structure.
3.4. CMCTs Enhance Serum Immunoglobulin Levels in Infected Piglets
The levels of serum immunoglobulins reflect the general humoral immune status of the host. PEDV infection in piglets led to a significant reduction in total IgG, IgM, and IgA concentrations. However, administration of CMCTs significantly increased IgM levels in a dose-dependent manner (Figure 3A), suggesting a potential role for CMCTs in promoting the early activation of humoral immunity. In addition, CMCTs significantly elevated total IgG (Figure 3B) and IgA (Figure 3C) levels at medium and high doses. These findings indicate that CMCTs may help maintain or restore humoral immune balance during PEDV infection.
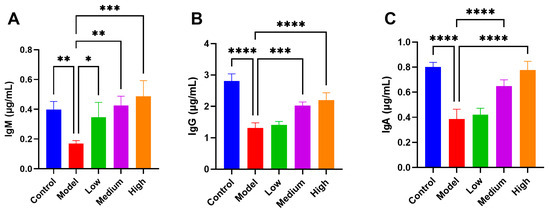
Figure 3.
Effects of CMCTs on piglet serum immunoglobulin levels. Concentrations of three blood immunoglobulins, IgM (A), IgG (B), and IgA (C) in the serum. Data were presented as means ± SD (n = 5). Statistical significance was determined using one-way ANOVA, followed by Dunnett’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared with the model group.
3.5. CMCTs Augment the Expression of Intestinal Mucosal MUCs and sIgA in Infected Piglets
In the pigs infected with PEDV, the expression of intestinal mucosal secretory immunoglobulin A (sIgA) and mucins (MUC-2) reduced considerably (Figure 4). sIgA is vital for intestinal immune defense and maintaining intestinal health. Studies indicate that sIgA, secreted by intestinal mucosal epithelial cells, efficiently neutralizes viruses at invasion sites [19]. MUC-2 plays a significant role in preserving the integrity of the intestinal mucosa, shielding the intestine from pathogen invasion [20]. After treatment, sIgA (Figure 4A) and MUC-2 (Figure 4B) showed dose-dependent upregulation under CMCTs induction, significantly strengthening the intestinal barrier’s resistance.
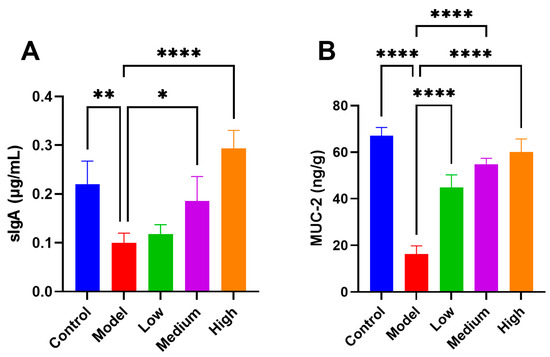
Figure 4.
The CMCTs exhibit the capacity to augment the expression of sIgA and MUC-2 in the jejunal mucosa of piglets. Concentrations of sIgA (A) and MUC-2 (B) in jejunal mucosa; data were presented as means ± SD (n = 5). Statistical significance was determined using one-way ANOVA, followed by Dunnett’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared with the model group.
3.6. CMCTs Modulate Inflammatory Cytokine and Interferon Expression in Jejunal Tissue
After PEDV infection, various pro-inflammatory cytokines, including IL-1β (Figure 5A), IL-6 (Figure 5B), TNF-α (Figure 5C), and IL-17 (Figure 5D), are simultaneously released, resulting in intestinal tissue damage. Treatment with CMCTs in infected piglets significantly decreased the levels of IL-1β, IL-6, TNF-α, and IL-17 in intestinal tissues across all three dosage conditions. These findings indicate that CMCTs effectively mitigate the inflammatory response in infected intestinal tissues, facilitating tissue repair.
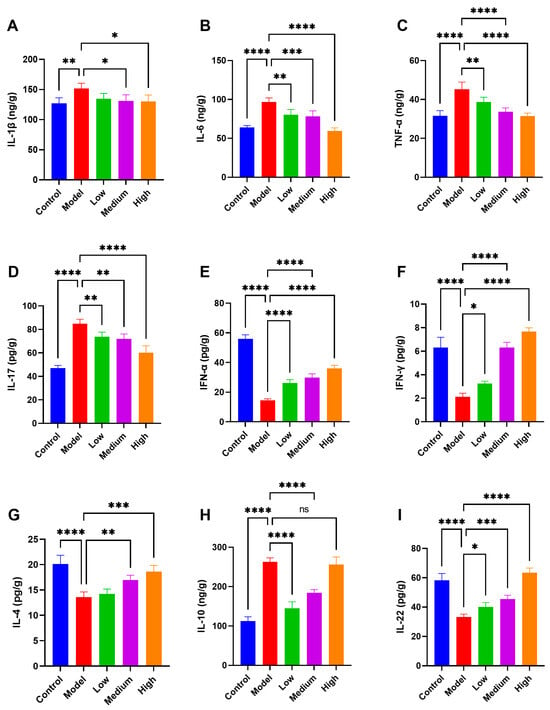
Figure 5.
The CMCTs enhance jejunal tissue’s antiviral, anti-inflammatory, and repair factor expression while reducing inflammation. Concentrations of four representative pro-inflammatory cytokines, IL-1β (A), IL-6 (B), TNF-α (C) and IL-17 (D); IFN-α (E), and IFN-γ (F); anti-inflammatory cytokines IL-4 (G) and IL-10 (H); the intestinal mucosal repair factor IL-22 (I) in jejunal tissue. Data were presented as means ± SD (n = 5). Statistical significance was determined using one-way ANOVA, followed by Dunnett’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared with the model group.
PEDV infection significantly decreases the expression of antiviral interferons IFN-α (Figure 5E) and IFN-γ (Figure 5F) in piglet intestinal tissues. CMCTs upregulate these factors in a dose-dependent manner, enhancing piglets’ intestinal resistance to PEDV infection. Moreover, at medium to high doses, the expression levels of anti-inflammatory cytokines IL-4 (Figure 5G), IL-10 (Figure 5H), and the intestinal mucosal repair factor IL-22 (Figure 5I) are significantly increased, bolstering the intestine’s capacity to modulate inflammation and repair tissue damage. CMCTs enhance the expression levels of intestinal antiviral factors, anti-inflammatory factors, and tissue repair factors, thereby strengthening the intestinal defense against infection, reducing inflammation, and promoting tissue repair.
3.7. CMCTs Reduced PEDV-Induced Oxidative Stress in Jejunal Tissue
Oxidative stress, characterized by an imbalance in oxidative-reduction processes in the body, is a significant contributor to various diseases. Viral infections can induce oxidative stress in cells [21]. The intestine is particularly susceptible to oxidative damage from free radicals [22]. In this research, we found a substantial reduction in antioxidant enzyme levels in the intestines of the PEDV-infected pigs, including total antioxidant capacity (T-AOC), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px), as well as glutathione (GSH) (Figure 6A–D). Additionally, catalase (CAT) levels were markedly reduced (Figure 6E), while inducible nitric oxide synthase (iNOS), nitric oxide (NO), and the oxidative stress metabolite malondialdehyde (MDA) levels were significantly elevated (Figure 6F–H), indicating pronounced oxidative stress in the intestines following infection. After CMCT therapy, antioxidant enzyme levels significantly increased while oxidative metabolites decreased, indicating enhanced endogenous antioxidant capacity. This therapy notably improved oxidative stress in the intestines of infected pigs, especially at medium to high dosages.
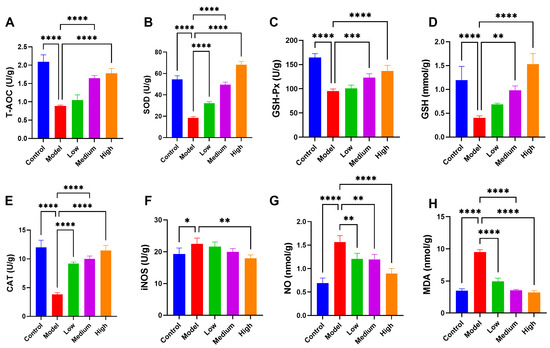
Figure 6.
The CMCTs mitigated oxidative stress in the jejunum. Concentrations of T-AOC (A), SOD (B), GSH-Px (C), GSH (D), CAT (E), iNOS (F), NO (G), and MDA (H) in jejunal tissue from each group. Data were presented as means ± SD (n = 5). Statistical significance was determined using one-way ANOVA, followed by Dunnett’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared with the model group.
3.8. CMCTs Improved the Mechanical Barrier Functions of the Intestine
Tight junction proteins are fundamental to the mechanical barrier, ensuring the integrity of paracellular permeability and effectively blocking the invasion of pathogens, metabolites, and other toxic substances [23]. The study observed a decrease in the fluorescence expression levels of intestinal tight junction proteins claudin-1 (Figure 7A) and ZO-1 (Figure 7B) in the PEDV-infected pigs. After CMCT administration, the fluorescence expression levels of claudin-1 and ZO-1 significantly increased, indicating that CMCTs confer protective effects on intestinal barrier functions.
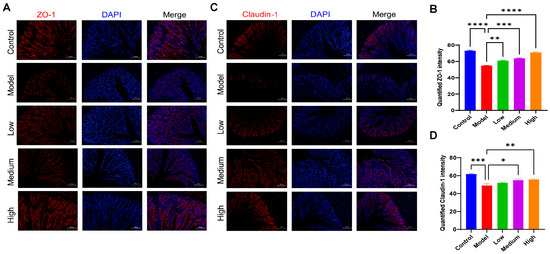
Figure 7.
The CMCTs promote the expression of tight junction proteins in the jejunal tissue of piglets. Representative fluorescence microscopy images show ZO-1 (A,B) and claudin-1 (C,D) in jejunal tissue. claudin-1 and ZO-1 are shown in red, and nuclei are blue. Scale bar, 100 μm, magnification, 200×. Quantification of fluorescence signals was performed using Image J. Statistical significance was determined using one-way ANOVA, followed by Dunnett’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared with the model group.
3.9. CMCTs Improved the Intestinal Immune Barrier Functions
Immune cells are essential components of the intestinal immune defense system [24]. CD4 and CD8 markers identify T helper and cytotoxic T cells, respectively, which are crucial for immune activation and cytotoxicity. CD127 (IL-7 receptor), a marker for regulatory T cells (Tregs), is generally expressed at low levels in Tregs. The elevated expression of CD127 is associated with impaired or suppressed Treg functions [25].
The activation of these immune cells is pivotal for mounting an effective antiviral defense within the intestinal tract. In the piglets infected with PEDV, fluorescence signals for CD4+ and CD8+ T cells (red fluorescence) in the intestinal tract are significantly diminished. Treatment leads to a notable enhancement of these signals (Figure 8A–D). Conversely, CD127 expression on Tregs is significantly elevated in piglets after PEDV infection, with levels markedly reduced following treatment (Figure 8E,F). These findings indicate that PEDV infection results in a substantial reduction of CD4+ and CD8+ T cells and impaired Treg functions, suggesting an immune tolerant or suppressive state in the intestinal immune environment. In contrast, CMCTs effectively modulate immune cell populations in the intestinal tract, enhancing immune barrier functions, promoting viral clearance, and reducing inflammation.
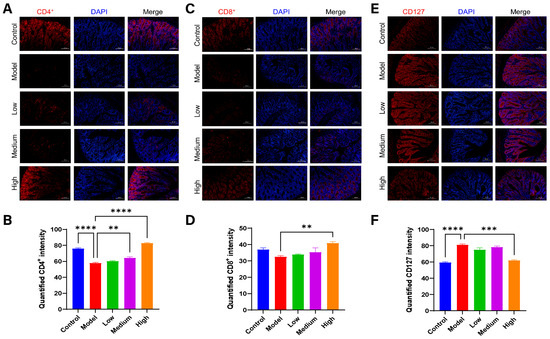
Figure 8.
The CMCTs exert a positive regulatory effect on immune cells in the jejunal tissue of piglets. Representative fluorescence microscopy images show CD4+ (A,B), CD8+ (C,D), and CD127 (E,F) in jejunal tissue. CD4+, CD8+, and CD127 are shown in red, and nuclei are blue. Scale bar, 100 μm, magnification, 200×. Quantification of fluorescence signals was performed using Image J. Statistical significance was determined using one-way ANOVA, followed by Dunnett’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared with the model group.
3.10. CMCTs Regulated the Composition of Gut Microbiota
Using 16S rRNA gene sequencing, we observed how CMCTs affected the gut microbiota composition of the PEDV-infected pigs. PEDV infection alters intestinal microbiota composition and diversity through inflammation, oxidative stress, and immune dysregulation, which exacerbates intestinal damage and hinders recovery. Results indicate shifts in microbiota diversity (Figure 9A) and relative species abundance in the jejunal contents of the model group post-infection. Specifically, the abundance of Firmicutes decreases, while Proteobacteria increases at the phylum level (Figure 9B). At the genus level, the abundance of Escherichia-Shigella sharply increases, while the abundance of Streptococcus and Ligilactobacillus decreases (Figure 9C). After treatment, the microbiota composition in treated piglets significantly differs from the model group and more closely resembles that of healthy piglets.
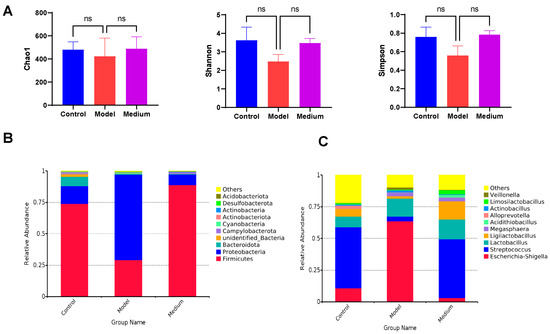
Figure 9.
CMCTs regulated the composition of intestinal microbiota. (A) Difference in alpha diversity, demonstrated by Chao1, Shannon, and Simpson indexes. Differences in relative microbial abundance between the two groups at the phylum level (B) and the genus level (C). Statistical significance was determined using one-way ANOVA, followed by Dunnett’s post hoc test. ns, not significant; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared with the model group.
4. Discussion
PEDV infects pigs of all ages, with a 100% mortality rate in infected suckling piglets. Analysis of fecal and intestinal tissue samples from diarrheal pigs identified PEDV as the most prevalent virus in recent years in China, with a prevalence rate of 61.8%. Additionally, PEDV strains circulating in China exhibit higher recombination frequency and faster evolutionary rate than those from other regions [26]. This finding may clarify the limited efficacy of current PED vaccines, highlighting the necessity for the swine industry to investigate effective therapeutic options to safeguard pigs against PEDV infection. In this study, we found that CMCTs alleviated clinical signs and significantly increased the levels of total IgM, IgG, and IgA, which may indicate an overall enhancement of systemic immune status. IgM is typically the first antibody produced during acute infection and is indicative of early immune activation [27], while IgG and IgA are generally associated with long-term systemic and mucosal immunity, respectively [28]. A limitation of this study is that only total immunoglobulin levels were measured, without assessing anti-PEDV antibodies. To more accurately evaluate the immunomodulatory effects of CMCTs, future studies employing PEDV-specific ELISAs or virus neutralization assays are warranted to determine whether CMCTs elicit virus-specific humoral immune responses.
PEDV infection in neonatal pigs induces severe atrophic enteritis, primarily affecting the jejunum and ileum, and triggers a significant release of inflammatory factors [1], oxidative stress, and disruption of intestinal barrier functions. While moderate inflammation is essential for clearing infections, excessive activation of inflammatory cells and the subsequent release of numerous inflammatory mediators can impede tissue repair [29]. Research findings indicate that CMCTs effectively reduce histopathological lesions in the jejunum and ileum, decrease the expression of pro-inflammatory factors, and increase the expression of anti-inflammatory factors, thereby mitigating intestinal damage caused by excessive inflammation. Additionally, CMCTs enhance intestinal antioxidant capacity and elevate the expression of the intestinal mucosal repair factor IL-22, promoting intestinal damage repair.
The induction of IFN-α and IFN-γ plays a pivotal role in the host’s innate immune defense against viral pathogens. Previous studies have demonstrated that PEDV infection suppresses type I and II IFN induction in vivo [30,31]. Consistent with these findings, our results show that CMCTs significantly enhance IFN-α and IFN-γ expression by intestinal immune cells, promoting viral clearance by intestinal epithelial cells.
The intestinal barrier can be divided into a mechanical barrier, biological barrier, chemical barrier, and immune barrier [32]. claudin-1, occludin, and ZO-1 are essential components of the structural and functional units of tight junctions (TJs) [33], and play an important role in intestinal mechanical barrier function [34]. Our results showed that CMCTs enhance the expression of claudin-1 and ZO-1 in intestinal tissue. Additionally, CMCTs upregulate MUC-2 and sIgA levels and modulate immune cell populations, strengthening both the intestinal chemical barrier and mucosal immunity, thereby enhancing resistance to viral infection and mitigating virus-induced intestinal damage.
The intestinal microbiota is a vital component of the gut’s biological barrier, playing a key role in maintaining homeostasis and defending against pathogenic infections [35]. In this study, PEDV infection significantly disrupted the ecological balance of the gut microbiota in piglets, as evidenced by decreased microbial diversity, a reduction in the relative abundance of Firmicutes, and an increase in Proteobacteria. These alterations are consistent with previous reports describing gut dysbiosis following PEDV infection [36,37]. Growing evidence suggests that the intestinal microbiota and its metabolites actively contribute to resistance against PEDV infection through multiple mechanisms, including modulation of mucosal immunity [38], and reinforcement of the intestinal barrier [39,40,41]. Our results further revealed a marked increase in pathogenic genera such as Escherichia-Shigella, alongside a reduction in beneficial genera including Streptococcus and Ligilactobacillus. The overgrowth of Escherichia-Shigella has been linked to intestinal inflammation and barrier dysfunction [42], while Ligilactobacillus species are known to support gut health and immune modulation [43], and have also been implicated in the maintenance of overall animal health [44]. These findings suggest that maintaining a balanced microbial community may be critical for mitigating PEDV-induced intestinal damage and promoting recovery. Treatment with CMCTs significantly modulated the gut microbiota composition, shifting it toward a profile more closely resembling that of healthy piglets. This modulation is likely to promote gastrointestinal health by supporting a balanced and stable microbial ecosystem. However, the specific mechanisms by which CMCTs regulate individual bacterial genera remain unclear and require further investigation.
Based on the above discussion, CMCTs demonstrate a pronounced protective effect against PEDV-induced lesions. The mechanisms primarily involve anti-inflammatory, antioxidant, and intestinal barrier regulation functions, all contributing to enhancing the endogenous antiviral capacity, promoting viral clearance, and assisting the organism in improving recovery capabilities, thus exerting therapeutic effects against PEDV. Nevertheless, despite these promising findings, certain limitations of the study should be acknowledged. The relatively small sample size, limited observation period, and specific aspects of the experimental design may restrict the generalizability and robustness of the conclusions. Consequently, further research involving larger cohorts and extended clinical evaluations is necessary to comprehensively validate the protective efficacy of CMCTs and to support their translation into practical applications. A limitation of the present study is the absence of direct viral load measurements, which restricts definitive demonstration of CMCTs’ antiviral effects in this model. Although previous research has documented the antiviral activity of CMCTs, and our results indicate improvements in clinical and immunological parameters, future investigations incorporating quantitative viral assays, such as RT-qPCR, are warranted to strengthen mechanistic insights. Moreover, the precise antiviral mechanisms of CMCTs remain to be elucidated. Future studies focusing on the molecular and immunological pathways involved will be essential to provide a stronger theoretical foundation for the development and clinical use of CMCT-based interventions.
Author Contributions
T.H.: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing—original draft. Y.L. and S.G.: Formal analysis, Investigation. X.Z., H.C., and Y.H.: Resources. H.T. and Z.X.: Investigation, Methodology, Resources. C.F.: Supervision, Project administration, Methodology, Validation, Visualization, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Ethics and Welfare Committee of Sichuan Agricultural University, Sichuan, China (approval No. 20220078, dated 1 March 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are available in the China National Centre for Bioinformation Repository (GSA: CRA018805) and are publicly accessible at https://ngdc.cncb.ac.cn/gsub/submit/gsa/subCRA030312/finishedOverview (accessed on 9 April 2025).
Acknowledgments
The authors would like to thank the personnel of these teams for their kind assistance.
Conflicts of Interest
Authors Xiaonan Zhao, Huangzuo Cheng and Youjun Hu were employed by the Innovation Center of Guangdong Nuacid Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef]
- Oswald, I.P. Role of intestinal epithelial cells in the innate immune defence of the pig intestine. Vet. Res. 2006, 37, 359–368. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhou, J.; Zhao, Y.; Zhu, X.; Zhu, M.; Peng, Q.; Li, J.; Chang, X.; Shi, D.; Yin, J.; et al. Identification of cell types and transcriptome landscapes of porcine epidemic diarrhea virus-infected porcine small intestine using single-cell RNA sequencing. J. Immunol. 2023, 210, 271–282. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, Z.; Zhang, S.; Wang, H.; Wu, S.; Bao, W. Analysis of intestinal mucosa integrity and GLP-2 gene functions upon porcine epidemic diarrhea virus infection in pigs. Animals 2021, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Nimbkar, S.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Medium chain triglycerides (MCT): State-of-the-art on chemistry, synthesis, health benefits and applications in food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 843–867. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Tang, T.K.; Chan, E.S.; Phuah, E.T.; Lai, O.M.; Tan, C.P.; Wang, Y.; Ab Karim, N.A.; Mat Dian, N.H.; Tan, J.S. Medium chain triglyceride and medium-and long chain triglyceride: Metabolism, production, health impacts and its applications—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4169–4185. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Peterson, M.L. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS ONE 2012, 7, e40350. [Google Scholar] [CrossRef]
- Welch, J.L.; Xiang, J.; Okeoma, C.M.; Schlievert, P.M.; Stapleton, J.T. Glycerol monolaurate, an analogue to a factor secreted by Lactobacillus, is vrucidal against enveloped viruses, including HIV-1. mBio 2020, 11, e00686-20. [Google Scholar] [CrossRef]
- Jackman, J.A.; Arabyan, E.; Zakaryan, H.; Elrod, C.C. Glycerol monolaurate inhibits wild-type african swine fever virus infection in porcine macrophages. Pathogens 2023, 12, 1193. [Google Scholar] [CrossRef]
- Jackman, J.A.; Hakobyan, A.; Zakaryan, H.; Elrod, C.C. Inhibition of African swine fever virus in liquid and feed by medium-chain fatty acids and glycerol monolaurate. J. Anim. Sci. Biotechnol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- He, K.J.; Dong, J.H.; Ouyang, X.M.; Huo, Y.N.; Cheng, X.S.; Lin, Y.; Li, Y.; Gong, G.; Liu, J.; Ren, J.L.; et al. Glycerol monolaurate ameliorates DSS-induced acute colitis by inhibiting infiltration of Th17, neutrophils, macrophages and altering the gut microbiota. Front. Nutr. 2022, 9, 911315. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, Z.; Weng, M.; Shen, Y.; Lai, W.; Hao, T.; Yao, C.; Bu, X.; Du, J.; Li, Y.; et al. Glycerol monolaurate improved intestinal barrier, antioxidant capacity, inflammatory response and microbiota dysbiosis in large yellow croaker (Larimichthys crocea) fed with high soybean oil diets. Fish Shellfish Immunol. 2023, 141, 109031. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Zhang, Y.; Li, X.; Jiang, X.; Ding, H. Effects of Dietary Supplementation with Glycerol Monolaurate (GML) or the Combination of GML and Tributyrin on Growth Performance and Rumen Microbiome of Weaned Lambs. Animals 2022, 12, 1309. [Google Scholar] [CrossRef]
- Zheng, C.; Xiao, G.; Yan, X.; Qiu, T.; Liu, S.; Ou, J.; Cen, M.; Gong, L.; Shi, J.; Zhang, H. Complex of lauric acid monoglyceride and cinnamaldehyde ameliorated subclinical necrotic enteritis in yellow-feathered broilers by regulating gut morphology, barrier, inflammation and serum biochemistry. Animals 2023, 13, 516. [Google Scholar] [CrossRef]
- Wang, D.; Fang, L.; Xiao, S. Porcine epidemic diarrhea in China. Virus Res. 2016, 226, 7–13. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, L.; Zhao, X.; Liu, J.; Cheng, H.; Zhang, L.; Tang, H.; Sun, X.; Hu, Y.; Xu, Z. Effects of oral of administration of monoglycide laurate on virus load and inflammation in PEDV infected porcine. Front. Vet. Sci. 2022, 9, 980381. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and pathogen interaction with the immune system. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Zhu, L.; Ma, S.; Luo, Y.; Liang, H.; Liu, Q.; Chen, J.; Guli, S.; Chen, X. Orchestration of MUC2—The key regulatory target of gut barrier and homeostasis: A review. Int. J. Biol. Macromol. 2023, 236, 123862. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Bartosch, B.; Isaguliants, M.G. Oxidative stress in infection and consequent disease. Oxid. Med. Cell. Longev. 2017, 2017, 3496043. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight junction proteins in gastrointestinal and liver disease. Gut 2019, 68, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; Fazekas de St Groth, B.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, C.; Peng, O.; Ashraf, U.; Xu, Q.; Gong, L.; Fan, B.; Zhang, Y.; Xu, Z.; Xue, C.; et al. Global Dynamics of Porcine Enteric Coronavirus PEDV Epidemiology, Evolution, and Transmission. Mol. Biol. Evol. 2023, 40, msad052. [Google Scholar] [CrossRef]
- Wang, H.; Coligan, J.E.; Morse, H.C., 3rd. Emerging Functions of Natural IgM and Its Fc Receptor FCMR in Immune Homeostasis. Front. Immunol. 2016, 7, 99. [Google Scholar] [CrossRef]
- Chen, M.; Qin, R.; Jiang, M.; Yang, Z.; Wen, W.; Li, J. Clinical applications of detecting IgG, IgM or IgA antibody for the diagnosis of COVID-19: A meta-analysis and systematic review. Int. J. Infect. Dis. 2021, 104, 415–422. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Chan, F.K. Inflammasome, Inflammation, and Tissue Homeostasis. Trends Mol. Med. 2018, 24, 304–318. [Google Scholar] [CrossRef]
- Yin, L.; Liu, X.; Hu, D.; Luo, Y.; Zhang, G.; Liu, P. Swine Enteric Coronaviruses (PEDV, TGEV, and PDCoV) Induce Divergent Interferon-Stimulated Gene Responses and Antigen Presentation in Porcine Intestinal Enteroids. Front. Immunol. 2021, 12, 826882. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, K.; Yoo, D. Suppression of type I interferon production by porcine epidemic diarrhea virus and degradation of CREB-binding protein by nsp1. Virology 2016, 489, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Che, Q.; Luo, T.; Shi, J.; He, Y.; Xu, D.L. Mechanisms by Which Traditional Chinese Medicines Influence the Intestinal Flora and Intestinal Barrier. Front. Cell. Infect. Microbiol. 2022, 12, 863779. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Q.; Wu, M.; Zhang, Y.; Li, Z.; Li, H.; Yu, C.; Zhang, X.; Zhao, D.; Wang, L.; et al. Lactobacillus rhamnosus GG powder supplementation alleviates intestinal injury in piglets challenged by porcine epidemic diarrhea virus. Front. Cell. Infect. Microbiol. 2024, 14, 1371916. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Z.; Wang, S.Y.; Wang, H.; Cui, D.A.; Yang, Y.J.; Liu, X.W.; Kong, X.J.; Li, J.Y. Differences in the intestinal microbiota between uninfected piglets and piglets infected with porcine epidemic diarrhea virus. PLoS ONE 2018, 13, e0192992. [Google Scholar] [CrossRef]
- Yang, S.; Liu, G.; Savelkoul, H.F.J.; Jansen, C.A.; Li, B. Mini-review: Microbiota have potential to prevent PEDV infection by improved intestinal barrier. Front. Immunol. 2023, 14, 1230937. [Google Scholar] [CrossRef]
- Xing, J.H.; Niu, T.M.; Zou, B.S.; Yang, G.L.; Shi, C.W.; Yan, Q.S.; Sun, M.J.; Yu, T.; Zhang, S.M.; Feng, X.Z.; et al. Gut microbiota-derived LCA mediates the protective effect of PEDV infection in piglets. Microbiome 2024, 12, 20. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Cui, T.; Bao, H.; Gao, M.; Cheng, M.; Sun, Y.; Lu, Y.; Guan, J.; Zhang, D.; et al. AhR ligands from LGG metabolites promote piglet intestinal ILC3 activation and IL-22 secretion to inhibit PEDV infection. J. Virol. 2024, 98, e0103924. [Google Scholar] [CrossRef]
- Xing, J.; Niu, T.; Yu, T.; Zou, B.; Shi, C.; Wang, Y.; Fan, S.; Li, M.; Bao, M.; Sun, Y.; et al. Faecalibacterium prausnitzii-derived outer membrane vesicles reprogram gut microbiota metabolism to alleviate porcine epidemic diarrhea virus infection. Microbiome 2025, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Cai, R.; Wang, Q.; Shi, L.; Li, C.; Yan, H. Dynamic change of gut microbiota during porcine epidemic diarrhea virus infection in suckling piglets. Front. Microbiol. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, X.; Wang, G.; Xia, Y.; Xiong, Z.; Ai, L. Understanding Ligilactobacillus salivarius from probiotic properties to omics technology: A review. Foods 2024, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Liu, Z.; Chen, J.; Jiang, J.; Zhao, M.; Gong, D. Ligilactobacillus Salivarius improve body growth and anti-oxidation capacity of broiler chickens via regulation of the microbiota-gut-brain axis. BMC Microbiol. 2023, 23, 395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).