Screening of Active Compounds Against Porcine Epidemic Diarrhea Virus in Hypericum japonicum Thunb. ex Murray Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Line and Virus
2.3. Extraction of Herbal Extract and Chemical Compound
2.4. Cytotoxicity Assay
2.5. Inhibition of Virus Infection
2.6. qRT-PCR Analysis
2.7. Western Blot Analysis
2.8. TCID50 Analysis
2.9. UFLC-Q-TOF-MS/MS Analysis of T20 and T60
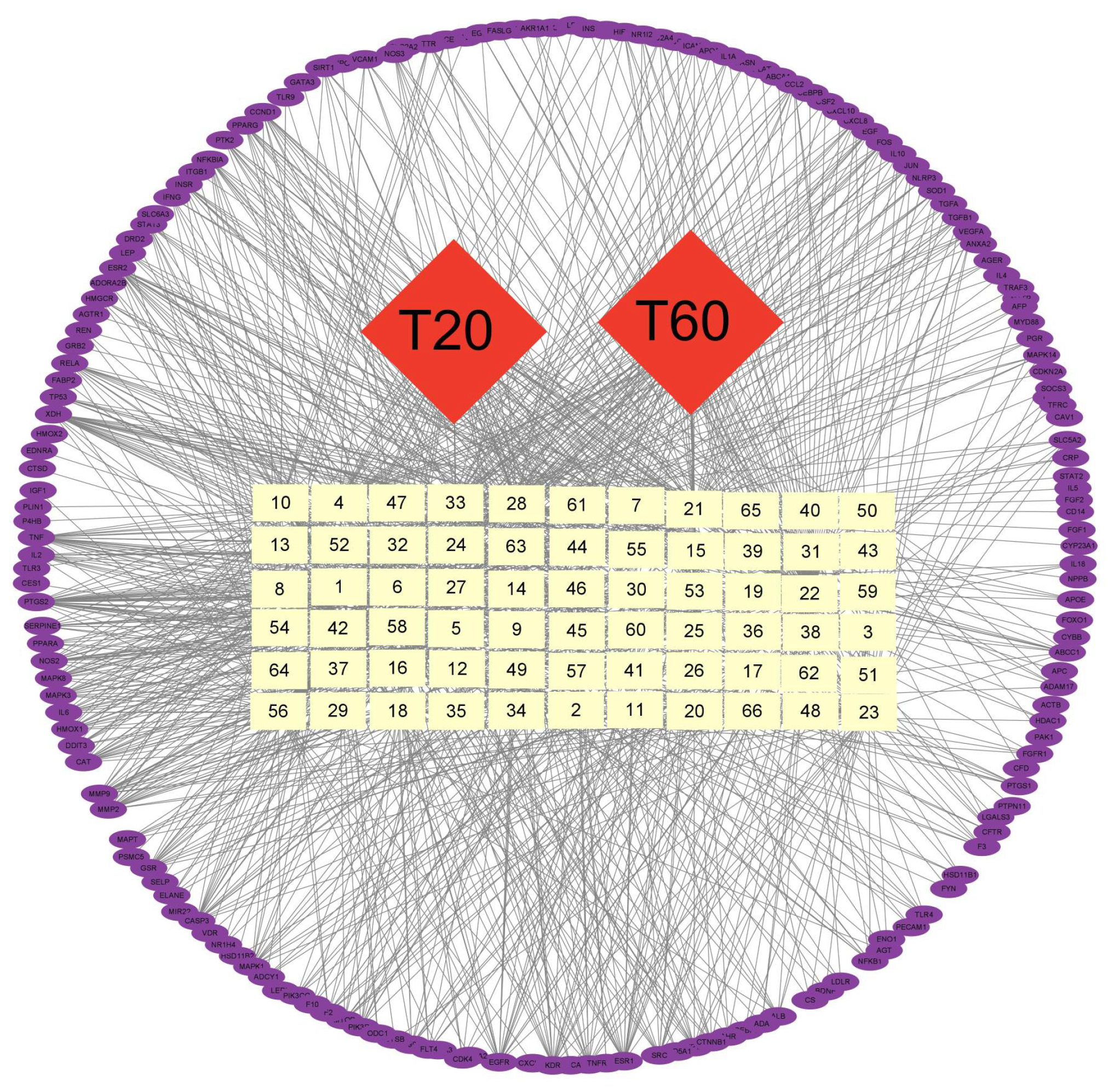
2.10. Network Pharmacological Analysis
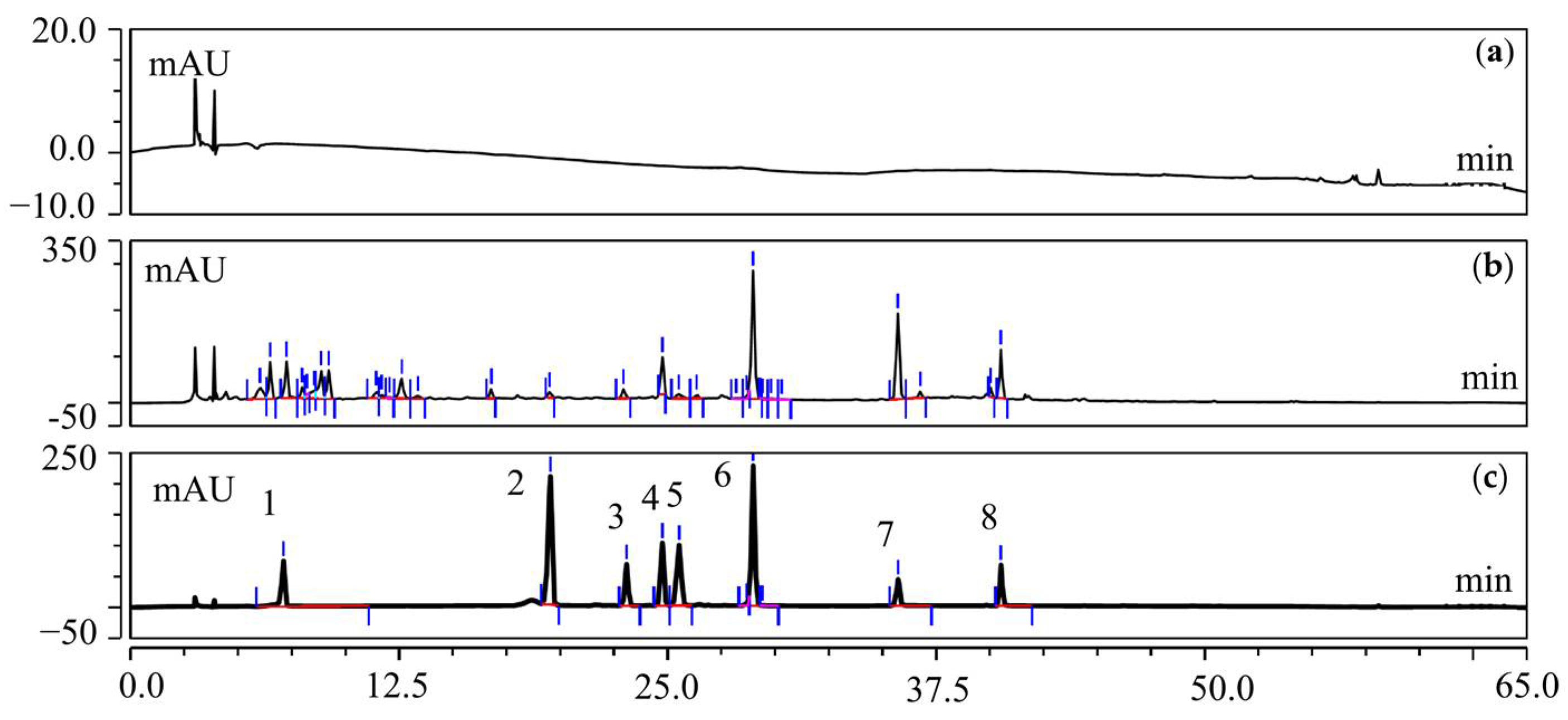
2.11. Determination of the Active Compounds and Contents of HJT Using HPLC
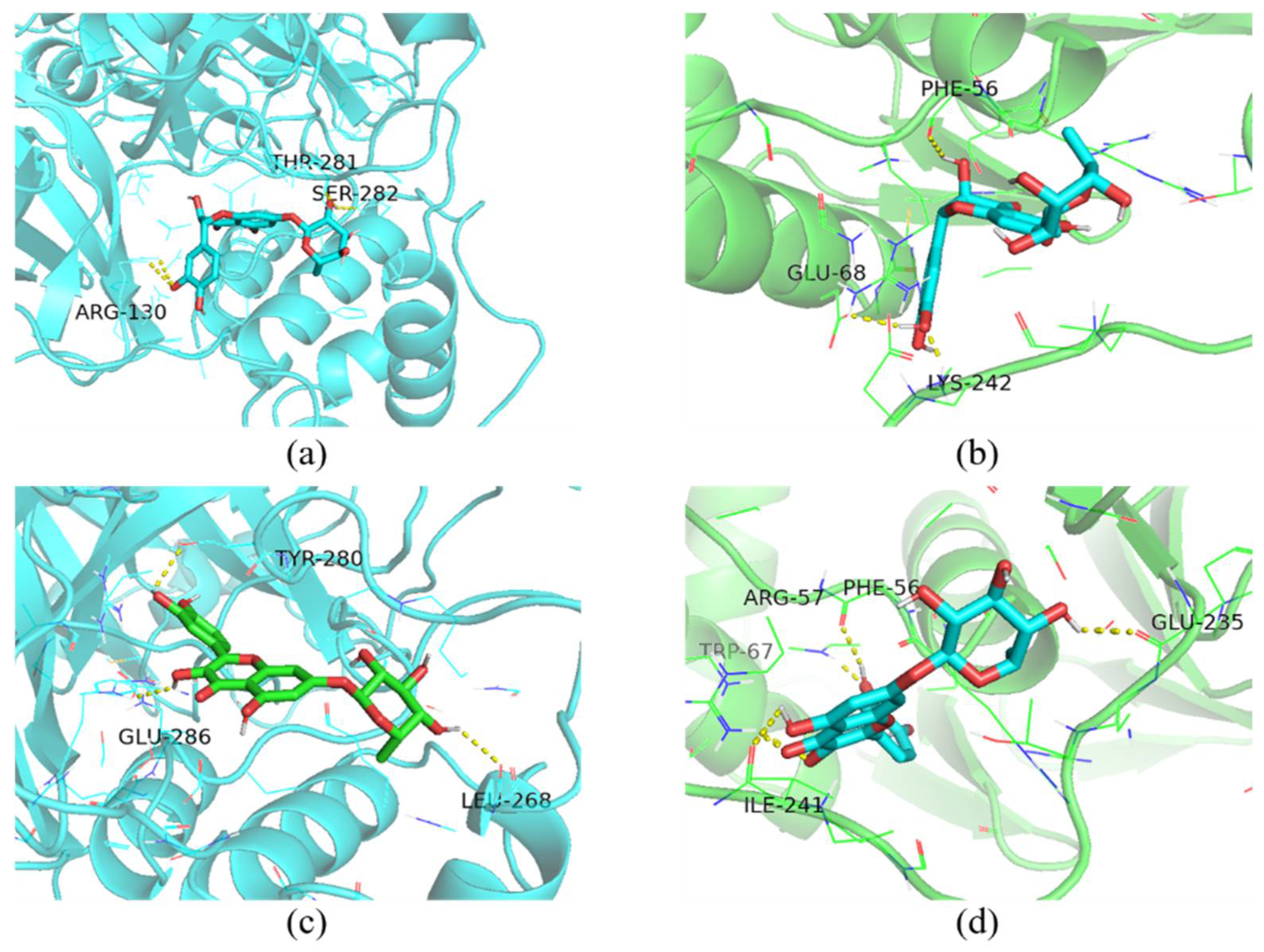
2.12. Molecular Docking
2.13. Statistical Analysis
3. Results
3.1. Evaluation of Cytotoxicity of HJT Extract
3.2. Inhibition of Viral Infections In Vitro by HJT Extracts
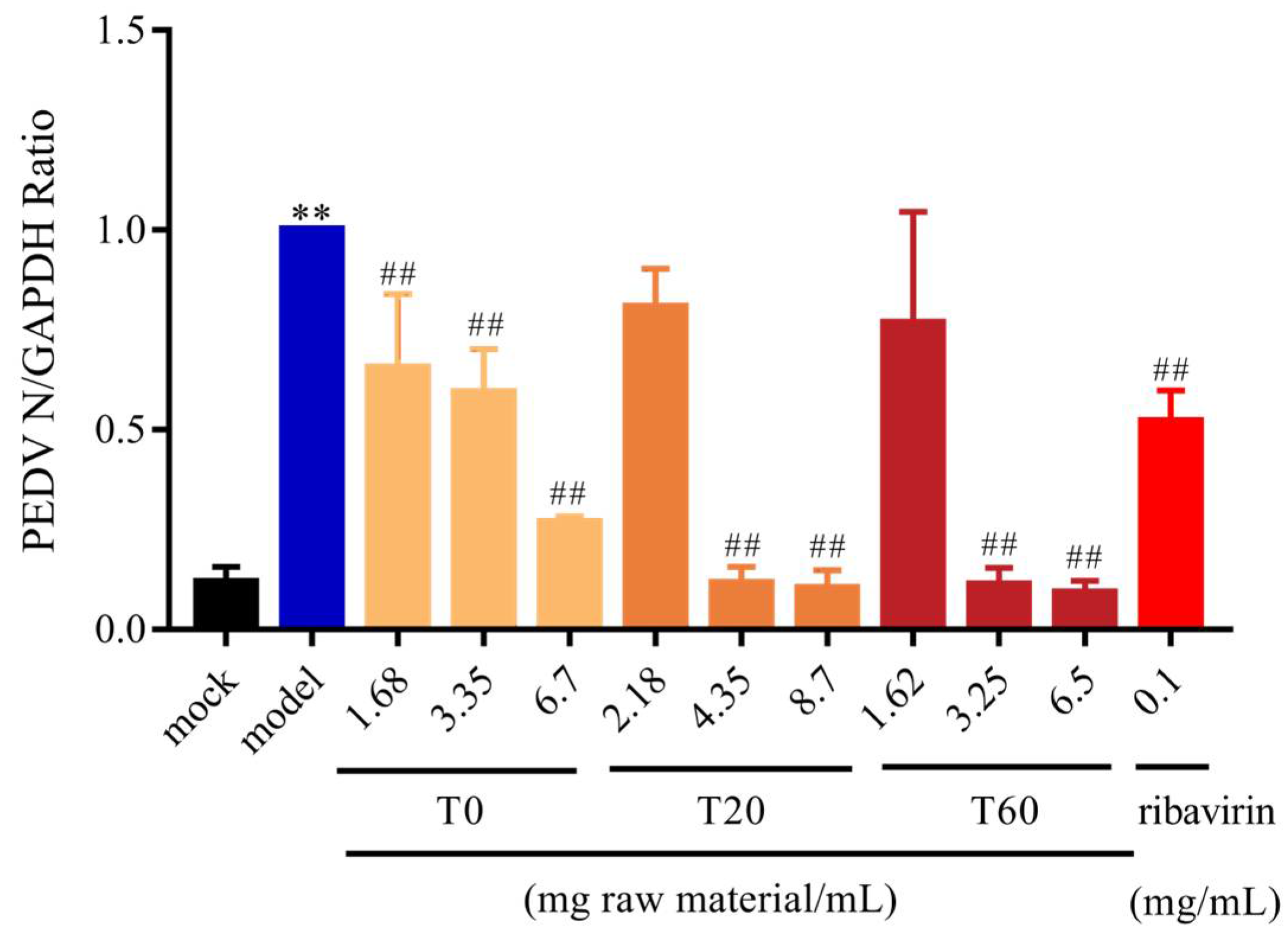
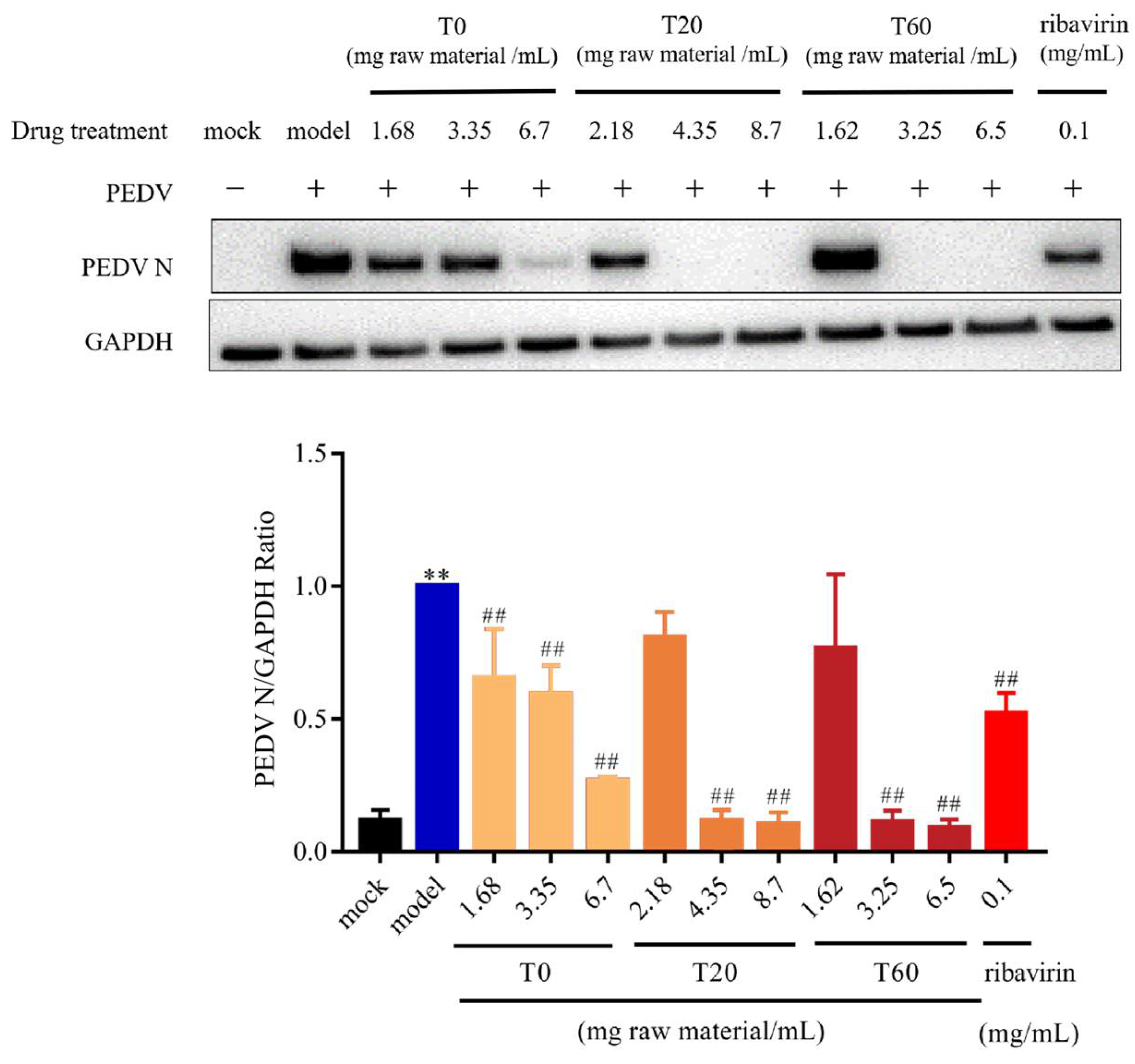
3.2.1. T0, T20, and T60 Inhibit PEDV N Protein Expression In Vitro
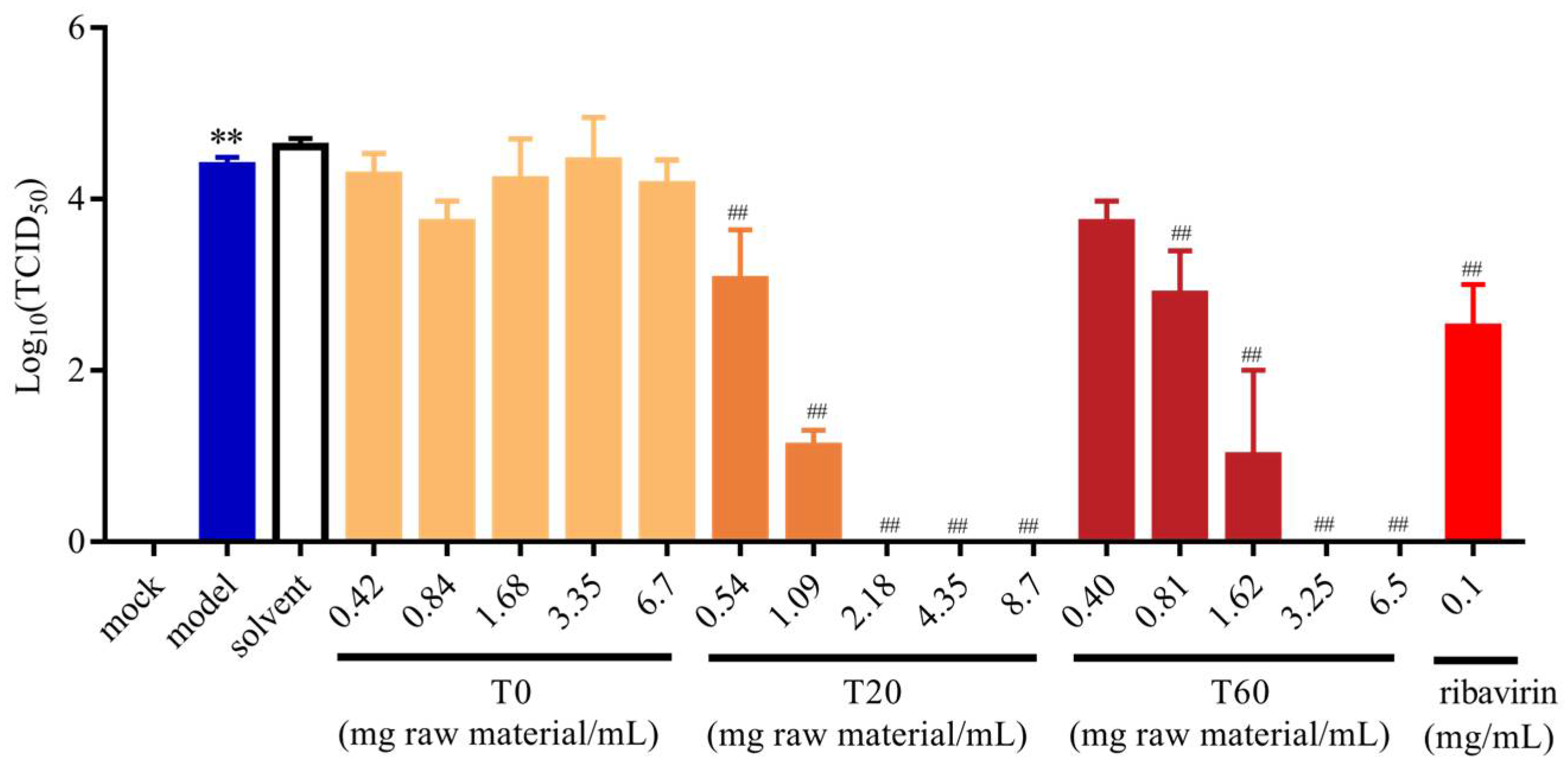
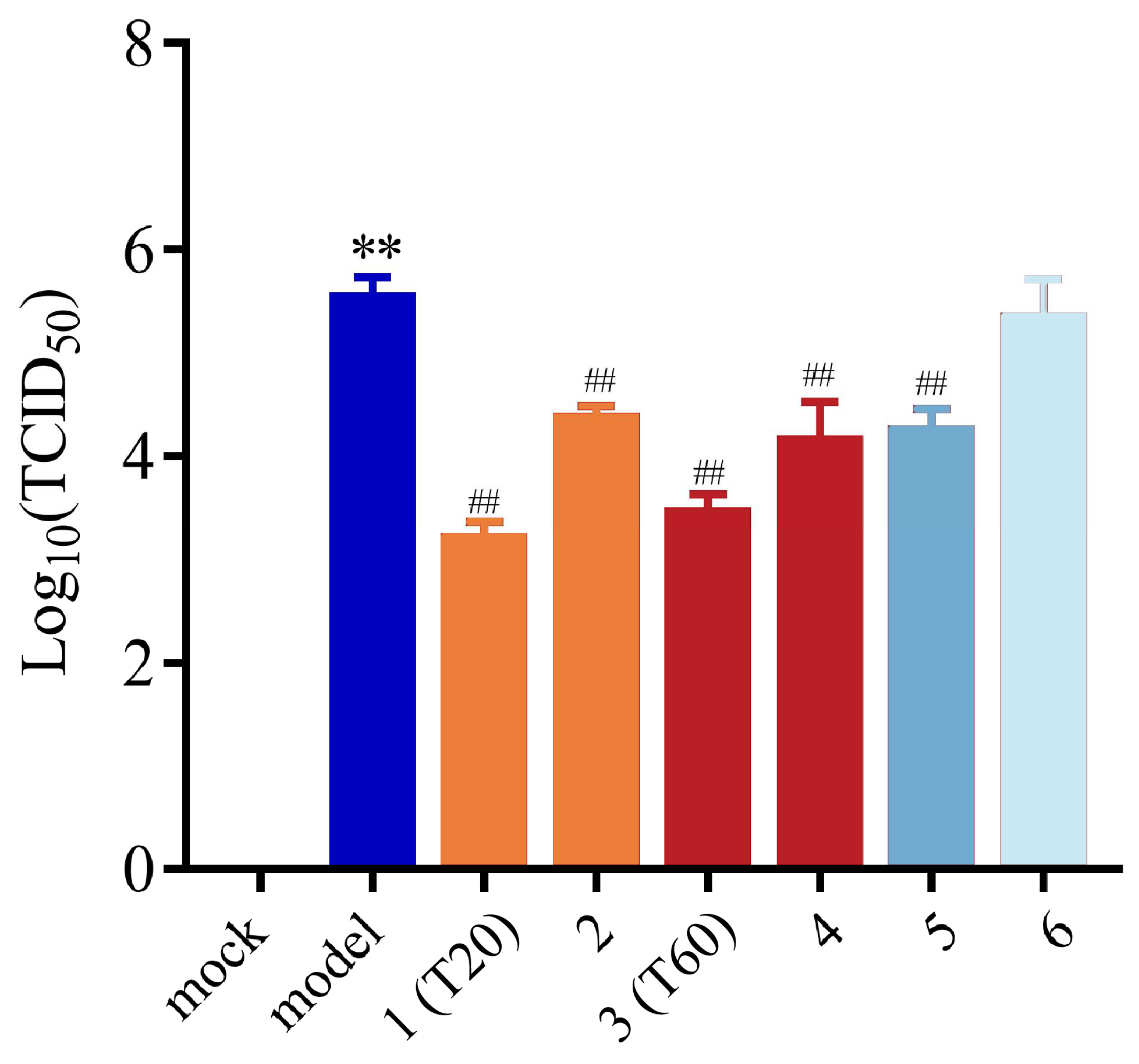
3.2.2. Effect of T0, T20, and T60 on PEDV Titers
3.3. Anti-PEDV Compounds in HJT Extracts
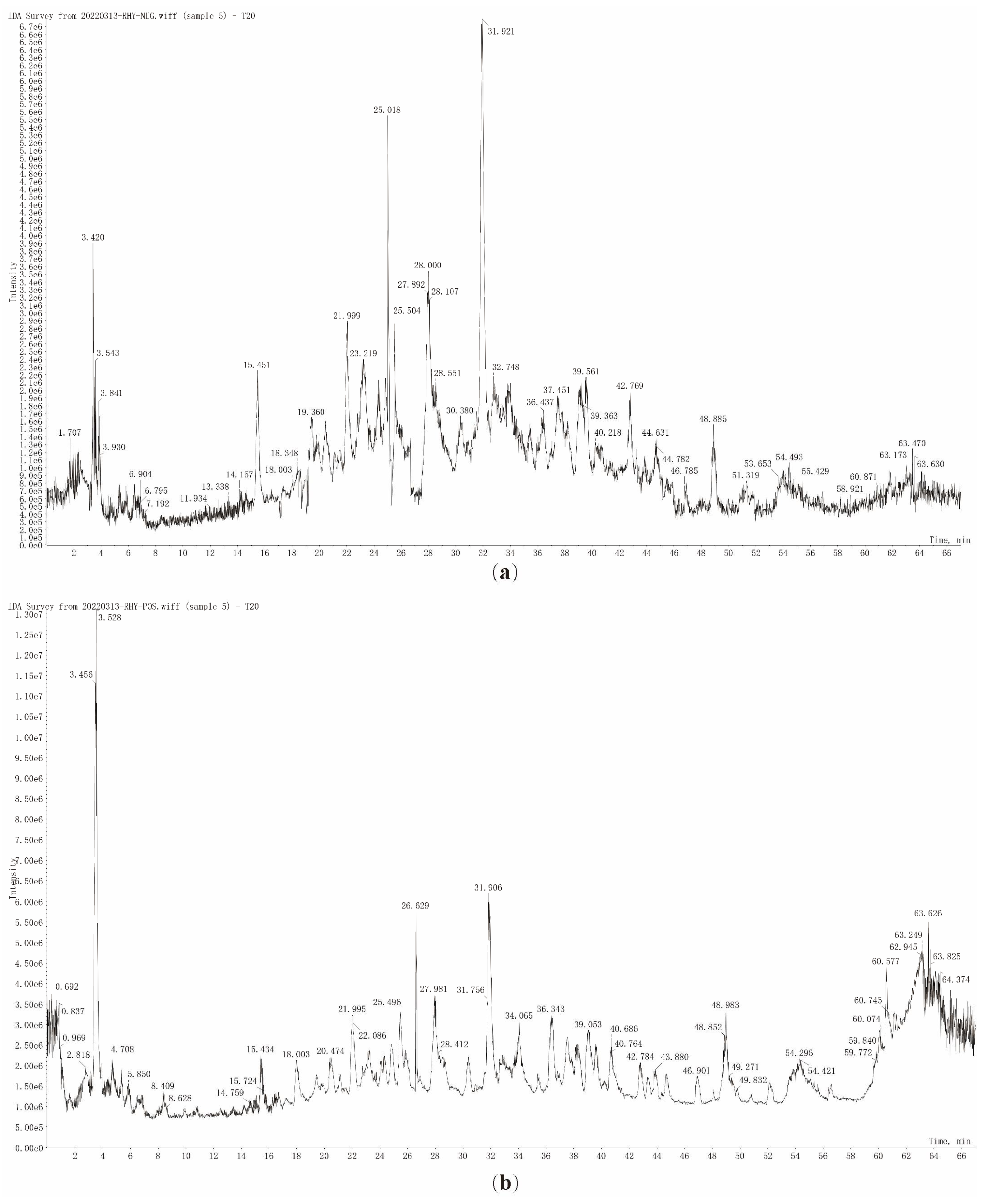
3.3.1. Characterization of the Chemical Compound Profiles of T20 and T60
3.3.2. Network Pharmacological Prediction of Anti-PEDV Compounds
3.3.3. Determination of Active Compounds
3.4. Analysis of Anti-PEDV Effects of HJT Active Compounds
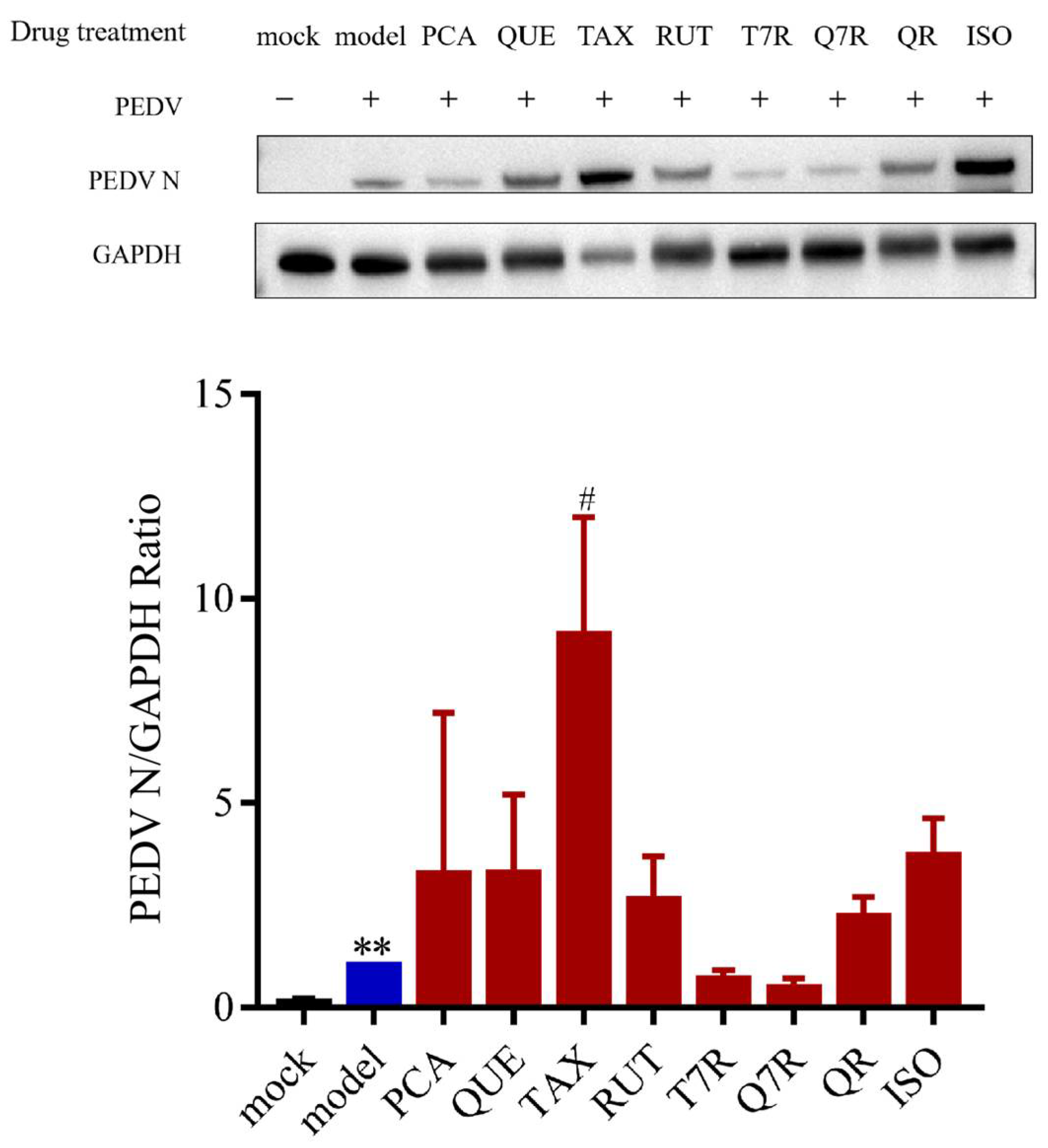
3.4.1. Anti-PEDV Effects of Individual Compounds
3.4.2. Anti-PEDV Effects of Mixed Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HJT | Hypericum japonicum Thunb. ex Murray |
| PEDV | Porcine epidemic diarrhea virus |
| PED | Porcine epidemic diarrhea |
| DMEM | Dulbecco’s modified Eagle medium |
| PBS | phosphate buffer solution |
| BSA | bovine serum albumin |
| CCK-8 | Cell counting Kit-8 |
| RIPA | Radio immunoprecipitation assay |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| PVDF | polyvinylidene fluoride |
| ECL | enhanced chemiluminescence |
| TBST | tris-buffered saline containing tween-20 |
| IPI-FX | immortal pig intestinal |
| UFLC-Q-TOF-MS/MS | ultra-fast liquid chromatography/quadrupole-time-of-flight tandem mass spectrometry system for analysis. |
| PLP-2 | papain-like protease 2 |
| 3CLpro | 3C-like protease |
| ARG | Arginine |
| SER | Serine |
| THR | Threonine |
| PHE | Phenylalanine |
| GLU | Glutamic acid |
| LYS | Lysine |
| LEU | Leucine |
| TYR | Tyrosine |
| TRP | Tryptophan |
| ILE | Isoleucine |
Appendix A
| No. | tR (min) | Molecular Function | Compounds | [M + H]+ | [M − H]− |
|---|---|---|---|---|---|
| 1 | 6.65 | C7H12O6 | Quinic acid | 193.0705 | 191.0562 |
| 2 | 22.03 | C13H20O3 | Vomifoliol | 225.1483 | \ |
| 3 | 23.17 | C16H18O9 | Chlorogenic acid | 355.1023 | 353.0870 |
| 4 | 23.18 | C9H6O3 | 7-hydroxycoumarine | 163.0388 | \ |
| 5 | 23.26 | C33H44O8 | 2,5-cyclohexadien-1-one, 2-[[3-[(2e)-3,7-dimethyl-2,6-octadien-1-yl]-2,4,6-trihydroxy-5-(2-methyl-1-oxopropyl)phenyl]methyl]-3,5-dihydroxy-4,4-dimethyl-6-(2-methyl-1-oxopropyl) | 569.3128 | \ |
| 6 | 23.34 | C21H22O11 | 7-o-rhamnoside of flagellin | 451.1228 | 449.1068 |
| 7 | 24.05 | C11H12O5 | Sinapic acid | 225.0755 | \ |
| 8 | 24.32 | C15H12O7 | Taxifolin | 305.0655 | 303.0505 |
| 9 | 24.42 | C28H36O8 | 3,5-dihydroxy-4,4-dimethyl-2-(1-oxoisobutyl)-6-[[5-(1-oxoisobutyl)-3-(3-methyl-2-butenyl)-2,4,6-trihydroxyphenyl]methyl]-2,5-cyclohexadiene-1-one | \ | 499.2356 |
| 10 | 24.44 | C15H14O6 | Catechin | 291.0862 | 289.0713 |
| 11 | 25.5 | C26H34O8 | Saroaspidin c | 475.2291 | 473.2139 |
| 12 | 26.67 | C15H14O6 | Epicatechin | \ | 289.0709 |
| 13 | 26.73 | C14H10O4 | 5-hydroxy-1-methoxy-9h-xanthen-9-one | 243.0648 | \ |
| 14 | 26.74 | C15H12O6 | 5,5′-methylenedisalicylic acid | 289.0704 | \ |
| 15 | 27.36 | C27H30O15 | Kaempferol 7-o-rutinoside | 595.1644 | 593.1484 |
| 16 | 27.93 | C27H30O16 | Quercetin-7-o-rutinoside | 611.1595 | 609.1445 |
| 17 | 27.98 | C18H22O9 | 8-glucosyl-5,7-dihydroxy-2-isopropylchromone | 383.1330 | 381.1178 |
| 18 | 28.01 | C16H18O8 | 3-p-coumaroylquinic acid | \ | 337.0923 |
| 19 | 30.25 | C27H32O15 | Ellipticoside | 597.1800 | 595.1643 |
| 20 | 30.57 | C21H22O11 | Astilbin | \ | 449.1062 |
| 21 | 31.5 | C19H24O9 | 8-glucosyl-5,7-dihydroxy-2-(1-methylpropyl) chromone | 397.1488 | 395.1330 |
| 22 | 33.06 | C16H18O8 | 4-o-coumaroylquinic acid | \ | 337.0923 |
| 23 | 33.38 | C27H30O16 | Rutin | 611.1558 | 609.1442 |
| 24 | 34.87 | C10H10O4 | Isoferulic acid | \ | 193.0505 |
| 25 | 34.89 | C10H8O3 | 7-methoxycoumarin | 177.0544 | \ |
| 26 | 35.43 | C31H34O8 | 4-[[3-benzoyl-2,6-dihydroxy-4-(3-methylbut-2-enoxy)phenyl]methyl]-3,5-dihydroxy-6,6-dimethyl-2-(2-methylpropanoyl)cyclohexa-2,4-dien-1-one | \ | 533.2121 |
| 27 | 36.5 | C9H10O4 | Ethyl 3,4-dihydroxybenzoate | 183.0650 | 181.0505 |
| 28 | 36.93 | C27H30O15 | Quercetin 3,7-di-o-rhamnopyranoside | \ | 593.1486 |
| 29 | 37.33 | C21H20O12 | Hyperoside | 465.1025 | 463.0858 |
| 30 | 38.85 | C24H20O8 | Kielcorin | \ | 435.1069 |
| 31 | 38.95 | C15H12O7 | Taxifolin | \ | 303.0503 |
| 32 | 39.27 | C27H30O16 | Quercetin 3-o-rutinoside | 611.1594 | 609.1440 |
| 33 | 42.41 | C21H20O10 | Afzelin | 433.1119 | 431.0962 |
| 34 | 42.76 | C21H20O12 | Isoquercitrin | 465.1020 | 463.0859 |
| 35 | 43.71 | C12H16O4 | (1s,3s)-3,4-dihydro-8-methoxy-3,5-dimethyl-1h-2-benzopyran-1,6-diol | 225.1099 | 223.0956 |
| 36 | 46.87 | C21H20O11 | Quercitrin | 449.1067 | 447.0912 |
| 37 | 48.89 | C21H20O11 | Vincetoxicoside b | 449.1070 | 447.0913 |
| 38 | 53.85 | C21H18O13 | Quercetin-3-o-glucuronide | 479.0815 | \ |
| 39 | 56.88 | C15H10O7 | Quercetin | 303.0499 | 301.0351 |
| 40 | 57.52 | C13H8O6 | 1,3,5,6-tetrahydroxyxanthone | \ | 259.0246 |
| 41 | 60.25 | C13H8O5 | 1,3,5-trihydroxyxanthone | \ | 243.0296 |
| 42 | 63.41 | C28H42O5 | Hyperjaponol h | \ | 457.2943 |
| NO. | tR (min) | Molecular Formula | Compounds | [M + H]+ | [M − H]− |
|---|---|---|---|---|---|
| 1 | 6.61 | C7H12O6 | Quinic acid | 193.0703 | 191.0563 |
| 2 | 16.62 | C7H6O5 | Gallic acid | 171,0646 | 169.0144 |
| 3 | 23.14 | C7H6O4 | Protocatechuic acid | 155.0335 | 153.0195 |
| 4 | 23.2 | C16H18O9 | Chlorogenic acid | 355.1025 | 353.0876 |
| 5 | 23.2 | C7H6O3 | 4-hydroxybenzoic acid | 139.0386 | 137.0244 |
| 6 | 23.32 | C21H22O11 | Taxifolin 7-rhamnoside | 451.1233 | 449.1082 |
| 7 | 26.65 | C15H14O6 | L-epicatechin | 291.0864 | 289.0720 |
| 8 | 27.51 | C45H38O18 | Procyanidin c1 | 867.2130 | 865.1984 |
| 9 | 27.96 | C18H22O9 | 5,7-dihydroxy-2-isopropylchromone-8-beta-d-glucoside | 383.1335 | 381.1190 |
| 10 | 28.23 | C16H18O8 | 3-p-coumaroylquinic acid | \ | 337.0928 |
| 11 | 30.2 | C27H32O15 | Ellipticoside | 597.1809 | 595.1664 |
| 12 | 30.48 | C21H22O11 | Neoisoastilbin | 451.1233 | 449.1084 |
| 13 | 31.47 | C19H24O9 | 8-glucosyl-5,7-dihydroxy-2-(1-methylpropyl) chromone | 397.1495 | 395.1345 |
| 14 | 33.36 | C27H30O16 | Rutin | 611.1601 | 609.1455 |
| 15 | 34.86 | C10H10O4 | Isoferulic acid | \ | 193.0508 |
| 16 | 35.31 | C21H22O10 | Aromadendrin 7-o-rhamnoside | 435.1283 | 433.1136 |
| 17 | 36.11 | C21H20O12 | Hyperoside | 465.1024 | 463.0874 |
| 18 | 36.46 | C9H10O4 | Ethyl 3,4-dihydroxybenzoate | 183.0645 | 181.0513 |
| 19 | 36.88 | C27H30O15 | Quercetin 3,7-di-o-rhamnopyranoside | 595.1648 | 593.1501 |
| 20 | 37.11 | C14H10O6 | 1,3,7-trihydroxy-2-methoxyxanthone | 275.0548 | 273.0404 |
| 21 | 37.33 | C14H10O5 | 1,7-dihydroxy-4-methoxyxanthone | 259.0600 | 257.0457 |
| 22 | 37.38 | C9H8O3 | Trans-4-hydroxycinnamic acid | \ | 163.0400 |
| 23 | 38.49 | C24H20O9 | Cadesin a | \ | 451.1031 |
| 24 | 38.86 | C30H24O14 | Dehydropolyester catechins | \ | 607.1087 |
| 25 | 38.88 | C15H12O7 | Taxifolin | 305.0655 | 303.0511 |
| 26 | 39.04 | C32H36O8 | Hyperjaponicol a | 549.2421 | 547.2280 |
| 27 | 39.21 | C27H30O16 | Quercetin 3-o-rutinoside | 611.1602 | 609.1458 |
| 28 | 42.37 | C12H18O3 | Jasmonic acid | \ | 209.1182 |
| 29 | 42.45 | C19H18O10 | Lancerin | 407.0971 | 405.0822 |
| 30 | 42.7 | C21H20O12 | Isoquercitrin | 465.1025 | 463.0877 |
| 31 | 43.2 | C19H28O9 | 2-cyclohexen-1-one, 4-[(1e)-4-(β-d-glucopyranosyloxy)-3-oxo-1-buten-1-yl]-4-hydroxy-3,5,5-trimethyl-, (4s)- | 401.1223 | 399.1656 |
| 32 | 44.41 | C15H12O6 | 6,8-dihydroxy-1,2-dimethoxy-9h-xanthen-9-one | 289.0708 | 287.0562 |
| 33 | 46.79 | C21H20O11 | Quercitrin | 449.1076 | 447.0918 |
| 34 | 48.88 | C21H20O11 | 7-(α-l-rhamnopyranosyloxy)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-4h-1-benzopyran-4-one | 449.1074 | 447.0917 |
| 35 | 51.53 | C18H18O8 | Sampsone c | 363.1074 | 361.0916 |
| 36 | 53.53 | C13H8O6 | Norathyriol | 261.0393 | 259.0248 |
| 37 | 53.84 | C15H12O5 | Naringenin | 273.0756 | 271.0611 |
| 38 | 54.31 | C21H18O13 | Quercetin-3-o-glucuronide | 479.0816 | 477.0661 |
| 39 | 54.74 | C21H20O10 | Kaempferol-7-o-α-l-rhamnoside | 433.1129 | 431.0959 |
| 40 | 55.16 | C13H8O5 | 1,2,5-trihydroxyxanthone | 245.0445 | 243.0299 |
| 41 | 56.57 | C16H12O7 | Isorhamnetin | 317.0657 | 315.0507 |
| 42 | 56.77 | C15H10O7 | Quercetin | 303.0502 | 301.0351 |
| 43 | 57.52 | C13H8O6 | 1,3,5,6-tetrahydroxyxanthone | 261.0395 | 259.0246 |
| 44 | 60.06 | C12H16O4 | 3′,3′-dimethyl-6′-oxo-phlorisobutyrophenone | 225.1121 | 223.0977 |
| 45 | 61.62 | C15H10O6 | Kaempferol | 287.0552 | 285.0403 |
| 46 | 62.84 | C30H18O10 | 3,8′-biapigenin | 539.0967 | 537.0822 |
| NO. | Molecular Formula | Compounds | Cas | Categorization |
|---|---|---|---|---|
| 1 | C7H12O6 | Quinic acid | 77-95-2 | organic acid |
| 2 | C7H6O3 | 4-hydroxybenzoic acid | 99-96-7 | organic acid |
| 3 | C7H6O4 | Protocatechuic acid | 99-50-3 | organic acid |
| 4 | C7H6O5 | Gallic acid | 149-91-7 | organic acid |
| 5 | C7H6O4 | Ethyl 3,4-dihydroxybenzoate | 3943-89-3 | organic acid |
| 6 | C9H6O3 | 7-hydroxycoumarin | 93-35-6 | flavonoid |
| 7 | C9H8O3 | P-coumaric acid | 501-98-4 | organic acid |
| 8 | C10H10O4 | 3-hydroxy-4-methoxycinnamic acid | 537-73-5 | organic acid |
| 9 | C10H8O3 | 7-methoxycoumarin | 531-59-9 | else |
| 10 | C11H12O5 | 4-hydroxy-3,5-dimethoxycinnamic acid | 530-59-6 | organic acid |
| 11 | C12H16O4 | (1s,3s)-3,4-dihydro-8-methoxy-3,5-dimethyl-1h-2-benzopyran-1,6-diol | \ | else |
| 12 | C12H16O4 | 3′,3′-dimethyl-6′-oxo-phlorisobutyrophenone | \ | xanthone |
| 13 | C12H18O3 | Jasmonic acid | 6894-38-8 | organic acid |
| 14 | C13H20O3 | Vomifoliol | 23526-45-6 | flavonoid |
| 15 | C13H8O5 | 1,3,5-trihydroxyxanthone | 6732-85-0 | xanthone |
| 16 | C13H8O5 | 1,2,5-trihydroxyxanthone | 156640-23-2 | xanthone |
| 17 | C13H8O6 | 1,3,5,6-tetrahydroxyxanthone | 5084-31-1 | xanthone |
| 18 | C13H8O6 | Norathyriol | 3542-72-1 | xanthone |
| 19 | C14H10O4 | 3-methoxy-5-hydroxyxanthenone | \ | xanthone |
| 20 | C14H10O5 | 1,7-dihydroxy-4-methoxyxanthone | 87339-76-2 | xanthone |
| 21 | C14H10O6 | 1,3,7-trihydroxy-2-methoxyxanthone | 211948-69-5 | xanthone |
| 22 | C15H10O6 | Kaempferol | 520-18-3 | flavonoid |
| 23 | C15H10O7 | Quercetin | 117-39-5 | flavonoid |
| 24 | C15H12O5 | Naringenin | 480-41-1 | flavonoid |
| 25 | C15H12O6 | 5,5′-methylenedisalicylic acid | 122-25-8 | organic acid |
| 26 | C15H12O6 | 6,8-dihydroxy-1,2-dimethoxy-9h-xanthen-9-one | 25991-81-5 | xanthone |
| 27 | C15H12O7 | Epitaxanthin | 153666-25-2 | flavonoid |
| 28 | C15H12O7 | Taxifolin | 480-18-2 | flavonoid |
| 29 | C15H14O6 | Cianidanol | 154-23-4 | flavonoid |
| 30 | C15H14O6 | L-epicatechin | 490-46-0 | flavonoid |
| 31 | C16H12O7 | Isorhamnetin | 480-19-3 | flavonoid |
| 32 | C16H18O8 | 3-p-coumaroylquinic acid | 1899-30-5 | organic acid |
| 33 | C16H18O8 | 4-p-coumaroylquinic acid | 53539-37-0 | organic acid |
| 34 | C16H18O9 | Chlorogenic acid | 327-97-9 | organic acid |
| 35 | C18H18O8 | Sampsone c | \ | flavonoid |
| 36 | C18H22O9 | 8-glucosyl-5,7-dihydroxy-2-isopropylchromone | 188785-44-6 | flavonoid |
| 37 | C19H18O10 | 4-β-d-glucosyl-1,3,7-trihydroxyxanthenone | 81991-99-3 | xanthone |
| 38 | C19H24O9 | 8-glucosyl-5,7-dihydroxy-2-(1-methylpropyl) chromone | 188818-27-1 | xanthone |
| 39 | C19H28O9 | 2-cyclohexen-1-one,4-[(1e)-4-(β-d-glucopyranosyloxy)-3-oxo-1-buten-1-yl]-4-hydroxy-3,5,5-trimethyl-,(4s)- | 189344-54-5 | flavonoid |
| 40 | C21H18O13 | Quercetin-3-o-glucuronide | 22688-79-5 | flavonoid |
| 41 | C21H20O10 | Afzelin | 482-39-3 | flavonoid |
| 42 | C21H20O10 | Kaempferol 7-o-rhamnoside | 20196-89-8 | flavonoid |
| 43 | C21H20O11 | Quercitrin | 522-12-3 | flavonoid |
| 44 | C21H20O11 | 7-(α-l-rhamnopyranosyloxy)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-4h-1-benzopyran-4-one | 22007-72-3 | flavonoid |
| 45 | C21H20O12 | Hyperoside | 482-36-0 | flavonoid |
| 46 | C21H20O12 | Isoquercitrin | 482-35-9 | flavonoid |
| 47 | C21H22O10 | Aromadendrin 7-o-rhamnoside | 69135-41-7 | flavonoid |
| 48 | C21H22O11 | Taxifolin 7-rhamnoside | 137592-12-2 | flavonoid |
| 49 | C21H22O11 | 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-(3,4,5-trihydroxy-6-methyl-oxa n-2-yl)oxy-chroman-4-one | 54141-72-9 | flavonoid |
| 50 | C24H20O8 | Kielcorin | 64280-48-4 | xanthone |
| 51 | C24H20O9 | Cadesin a | 64280-46-2 | else |
| 52 | C26H34O8 | Saroaspidin c | 112663-70-4 | else |
| 53 | C27H30O15 | 4h-1-benzopyran-4-one, 7-[[6-o-(6-deoxy-α-l-mannopyranosyl)-β-d-glucopyranosyl]oxy]-3,5-dihydroxy-2-(4-hydroxyphenyl)- | 103102-81-4 | flavonoid |
| 54 | C27H30O15 | Quercetin 3,7-di-o-rhamnoside | 28638-13-3 | flavonoid |
| 55 | C27H30O16 | Quercetin-7-o-rutinoside | 147714-62-3 | flavonoid |
| 56 | C27H30O16 | Rutin | 153-18-4 | flavonoid |
| 57 | C27H30O16 | Quercetin-3-o-rutinose | 949926-49-2 | flavonoid |
| 58 | C27H32O15 | Ellipticoside | 128717-89-5 | flavonoid |
| 59 | C28H36O8 | 3,5-dihydroxy-4,4-dimethyl-2-(1-oxoisobutyl)-6-[[5-(1-oxoisobutyl)-3-(3-methyl-2-butenyl)-2,4,6-trihydroxyphenyl]methyl]-2,5-cyclohexadiene-1-one | 19809-78-0 | flavonoid |
| 60 | C28H42O5 | Hyperjaponol h | \ | else |
| 61 | C30H18O10 | Amentoflavone | 101140-06-1 | flavonoid |
| 62 | C30H24O14 | Dehydropolyester catechins | \ | flavonoid |
| 63 | C31H34O8 | 4-[[3-benzoyl-2,6-dihydroxy-4-(3-methylbut-2-enoxy)phenyl]methyl]-3,5-dihydroxy-6,6-dimethyl-2-(2-methylpropanoyl)cyclohexa-2,4-dien-1-one | 96624-40-7 | flavonoid |
| 64 | C32H36O8 | Hyperjaponicol a | \ | flavonoid |
| 65 | C33H44O8 | 2,5-cyclohexadien-1-one, 2-[[3-[(2e)-3,7-dimethyl-2,6-octadien-1-yl]-2,4,6-trihydroxy-5-(2-methyl-1-oxopropyl)phenyl]methyl]-3,5-dihydroxy-4,4-dimethyl-6-(2-methyl-1-oxopropyl)- | 105214-57-1 | flavonoid |
| 66 | C45H38O18 | Procyanidin c1 | 37064-30-5 | flavonoid |


References
- Shen, Z.; Ye, G.; Deng, F.; Wang, G.; Cui, M.; Fang, L.; Xiao, S.; Fu, Z.F.; Peng, G. Structural Basis for the Inhibition of Host Gene Expression by Porcine Epidemic Diarrhea Virus Nsp1. J. Virol. 2018, 92, e01896-17. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Kong, N.; Zhang, Y.; Song, Y.; Qin, W.; Yang, X.; Ye, C.; Ye, M.; Tong, W.; Liu, C.; et al. N Protein of PEDV Plays Chess Game with Host Proteins by Selective Autophagy. Autophagy 2023, 19, 2338–2352. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine Epidemic Diarrhea Virus (PEDV): An Update on Etiology, Transmission, Pathogenesis, and Prevention and Control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Zhou, J.; Wang, X.; Ma, L.; Li, J.; Yang, L.; Yuan, H.; Pang, D.; Ouyang, H. Porcine Epidemic Diarrhea Virus: An Updated Overview of Virus Epidemiology, Virulence Variation Patterns and Virus–Host Interactions. Viruses 2022, 14, 2434. [Google Scholar] [CrossRef]
- Lei, J.; Miao, Y.; Bi, W.; Xiang, C.; Li, W.; Zhang, R.; Li, Q.; Yang, Z. Porcine Epidemic Diarrhea Virus: Etiology, Epidemiology, Antigenicity, and Control Strategies in China. Animals 2024, 14, 294. [Google Scholar] [CrossRef]
- Li, M.; Pan, Y.; Xi, Y.; Wang, M.; Zeng, Q. Insights and Progress on Epidemic Characteristics, Genotyping, and Preventive Measures of PEDV in China: A Review. Microb. Pathog. 2023, 181, 106185. [Google Scholar] [CrossRef]
- Lee, C. Porcine Epidemic Diarrhea Virus: An Emerging and Re-Emerging Epizootic Swine Virus. Virol. J. 2015, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- August-GSDMR-Report-8-6-2024.Pdf. Available online: https://www.swinehealth.org/global-disease-surveillance-reports/ (accessed on 16 December 2024).
- Kikuti, M.; Drebes, D.; Robbins, R.; Dufresne, L.; Sanhueza, J.M.; Corzo, C.A. Growing Pig Incidence Rate, Control and Prevention of Porcine Epidemic Diarrhea Virus in a Large Pig Production System in the United States. Porc. Health Manag. 2022, 8, 23. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.-X.; Li, C.; Wang, H.; Wang, H.; Meng, X.-Z.; Ma, J.; Ni, H.-B.; Zhang, X.; Qi, Y.; et al. Epidemiology of Porcine Epidemic Diarrhea Virus among Chinese Pig Populations: A Meta-Analysis. Microb. Pathog. 2019, 129, 43–49. [Google Scholar] [CrossRef]
- Liu, L.-S.; Liu, M.-H.; He, J.-Y. Hypericum Japonicum Thunb. Ex Murray: Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics of an Important Herbal Medicine. Molecules 2014, 19, 10733–10754. [Google Scholar] [CrossRef]
- Du, H.; Zhang, S.; Song, M.; Wang, Y.; Zeng, L.; Chen, Y.; Xiong, W.; Yang, J.; Yao, F.; Wu, Y.; et al. Assessment of a Flavone-Polysaccharide Based Prescription for Treating Duck Virus Hepatitis. PLoS ONE 2016, 11, e0146046. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, M.; Yu, M.; Zheng, C.; Wei, S. Study on the Chemical Constituents and Anti-HBV Activity of Hypericum Japonica. MATEC Web Conf. 2016, 60, 03002. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Zhu, H.; Liu, J.; Li, H.; Li, X.-N.; Sun, W.; Zeng, J.; Xue, Y.; Zhang, Y. Filicinic Acid Based Meroterpenoids with Anti-Epstein–Barr Virus Activities from Hypericum Japonicum. Org. Lett. 2016, 18, 2272–2275. [Google Scholar] [CrossRef]
- Hu, L.; Xue, Y.; Zhang, J.; Zhu, H.; Chen, C.; Li, X.-N.; Liu, J.; Wang, Z.; Zhang, Y.; Zhang, Y. (±)-Japonicols A–D, Acylphloroglucinol-Based Meroterpenoid Enantiomers with Anti-KSHV Activities from Hypericum Japonicum. J. Nat. Prod. 2016, 79, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Su, W.; Zhang, X.; Wang, Y.; Li, T.; Li, J.; Zeng, X.; Li, P. Hypericum Japonicum Extract Inhibited Porcine Epidemic Diarrhea Virus in Vitro and in Vivo. Front. Pharmacol. 2023, 14, 1112610. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, X.; Qu, Q.; Lin, Y.; Chen, R.; Zhu, Y.; Lv, W.; Guo, S. Lizhong Decoction Inhibits Porcine Epidemic Diarrhea Virus in Vitro and in Vivo. J. Ethnopharmacol. 2024, 333, 118428. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty per Cent Endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. UFLC-Q-TOF-MS/MS-Based Screening and Identification of Flavonoids and Derived Metabolites in Human Urine after Oral Administration of Exocarpium Citri Grandis Extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, S.; Huang, Y.; Zhang, S.; Wang, H.; Bao, W. The Aqueous Leaf Extract of M. Oleifera Inhibits PEDV Replication through Suppressing Oxidative Stress-Mediated Apoptosis. Animals 2022, 12, 458. [Google Scholar] [CrossRef]
- Maikhunthod, B.; Chaipayang, S.; Jittmittraphap, A.; Thippornchai, N.; Boonchuen, P.; Tittabutr, P.; Eumkeb, G.; Sabuakham, S.; Rungrotmongkol, T.; Mahalapbutr, P.; et al. Exploring the Therapeutic Potential of Thai Medicinal Plants: In Vitro Screening and in Silico Docking of Phytoconstituents for Novel Anti-SARS-CoV-2 Agents. BMC Complement. Med. Ther. 2024, 24, 274. [Google Scholar] [CrossRef]
- Graci, J.D.; Cameron, C.E. Mechanisms of Action of Ribavirin against Distinct Viruses. Rev. Med. Virol. 2006, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Menis Candela, F.; Soria, E.A.; Moliva, M.V.; Suárez Perrone, A.; Reinoso, E.B.; Giordano, W.; Sabini, M.C. Anti-DENV-2 Activity of Ethanolic Extracts from Arachis Hypogaea L.: Peanut Skin as a Relevant Resource of Bioactive Compounds against Dengue Virus. Plants 2024, 13, 2881. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Xian, Y.; Jiang, F. Anti-Virus Activity and Mechanisms of Natural Polysaccharides from Medicinal Herbs. Carbohydr. Res. 2024, 542, 109205. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.-T.; Yao, X.; Lu, W.-H.; Zhang, Y.-Q.; Malhi, K.K.; Li, H.-X.; Li, J.-L. Matrine Exhibits Antiviral Activities against PEDV by Directly Targeting Spike Protein of the Virus and Inducing Apoptosis via the MAPK Signaling Pathway. Int. J. Biol. Macromol. 2024, 270, 132408. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, X.; Yin, D.; Yin, L.; Shen, X.; Xu, F.; Dai, Y.; Pan, X. In Silico and in Vitro Evaluation of Antiviral Activity of Wogonin against Main Protease of Porcine Epidemic Diarrhea Virus. Front. Cell. Infect. Microbiol. 2023, 13, 1123650. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Bai, J.; Liu, X.; Wang, M.; Wang, X.; Jiang, P. Tomatidine Inhibits Porcine Epidemic Diarrhea Virus Replication by Targeting 3CL Protease. Vet. Res. 2020, 51, 136. [Google Scholar] [CrossRef]
- Gong, M.; Xia, X.; Chen, D.; Ren, Y.; Liu, Y.; Xiang, H.; Li, X.; Zhi, Y.; Mo, Y. Antiviral Activity of Chrysin and Naringenin against Porcine Epidemic Diarrhea Virus Infection. Front. Vet. Sci. 2023, 10, 1278997. [Google Scholar] [CrossRef]
- He, C.; Zhang, R.; Yang, L.; Xiang, B. Andrographolide Inhibits Porcine Epidemic Diarrhea Virus by Inhibiting the JAK2-STAT3 Pathway and Promoting Apoptosis. Vet. Microbiol. 2024, 298, 110235. [Google Scholar] [CrossRef]
- Sun, P.; Wang, M.; Li, J.; Qiu, Y.; Li, H.; Lv, M.; Bo, Z.; Shen, H.; Li, L. Inhibitory Effect of Buddlejasaponin IVb on Porcine Epidemic Diarrhea Virus in Vivo and in Vitro. Vet. Microbiol. 2022, 272, 109516. [Google Scholar] [CrossRef]
- Song, Z.; Deng, C.; Chen, Q.; Zhao, S.; Li, P.; Wu, T.; Hou, Y.; Yi, D. Protective Effects and Mechanisms of Ellagic Acid on Intestinal Injury in Piglets Infected with Porcine Epidemic Diarrhea Virus. Front. Immunol. 2024, 15, 1323866. [Google Scholar] [CrossRef]
- Stefanelli, I.; Corona, A.; Cerchia, C.; Cassese, E.; Improta, S.; Costanzi, E.; Pelliccia, S.; Morasso, S.; Esposito, F.; Paulis, A.; et al. Broad-Spectrum Coronavirus 3C-like Protease Peptidomimetic Inhibitors Effectively Block SARS-CoV-2 Replication in Cells: Design, Synthesis, Biological Evaluation, and X-Ray Structure Determination. Eur. J. Med. Chem. 2023, 253, 115311. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Xu, W.; Yang, Y.; Li, D.; Shi, W.; Li, X.; Chen, N.; Lv, Q.; Shi, Y.; Xu, J.; et al. Diverse Strategies Utilized by Coronaviruses to Evade Antiviral Responses and Suppress Pyroptosis. Int. J. Biol. Macromol. 2025, 296, 139743. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Kim, W.-I.; Kim, J.-M. Targeting the PEDV 3CL Protease for Identification of Small Molecule Inhibitors: An Insight from Virtual Screening, ADMET Prediction, Molecular Dynamics, Free Energy Landscape, and Binding Energy Calculations. J. Biol. Eng. 2023, 17, 29. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kim, J.-H.; Lee, C.-H.; Ahn, Y.-J.; Song, J.-H.; Baek, S.-H.; Kwon, D.-H. Antiviral Activity of Quercetin 7-Rhamnoside against Porcine Epidemic Diarrhea Virus. Antiviral Res. 2008, 81, 77. [Google Scholar] [CrossRef]
- Song, J.H.; Shim, J.K.; Choi, H.J. Quercetin 7-Rhamnoside Reduces Porcine Epidemic Diarrhea Virus Replication via Independent Pathway of Viral Induced Reactive Oxygen Species. Virol. J. 2011, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ocelotl, M.R.; Rosas-Murrieta, N.H.; Moreno, D.A.; Vallejo-Ruiz, V.; Reyes-Leyva, J.; Domínguez, F.; Santos-López, G. Taraxacum Officinale and Urtica Dioica Extracts Inhibit Dengue Virus Serotype 2 Replication in Vitro. BMC Complement. Altern. Med. 2018, 18, 95. [Google Scholar] [CrossRef]
- Schadich, E.; Kaczorová, D.; Béres, T.; Džubák, P.; Hajdúch, M.; Tarkowski, P.; Ćavar Zeljković, S. Secondary Metabolite Profiles and anti-SARS-CoV-2 Activity of Ethanolic Extracts from Nine Genotypes of Cannabis sativa L. Arch. Pharm. 2025, 358, e2400607. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-Derived Immunomodulators: An Insight on Their Preclinical Evaluation and Clinical Trials. Front. Plant Sci. 2015, 6, 655. [Google Scholar] [CrossRef]
- Lin, T.-L.; Lu, C.-C.; Lai, W.-F.; Wu, T.-S.; Lu, J.-J.; Chen, Y.-M.; Tzeng, C.-M.; Liu, H.-T.; Wei, H.; Lai, H.-C. Role of Gut Microbiota in Identification of Novel TCM-Derived Active Metabolites. Protein Cell 2020, 12, 394. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Zhou, F.; Xiao, S.; Zhong, L.; Hu, S.; Zhou, Z.; Li, L.; Tan, Y. Protocatechuic Acid Alleviates Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Mice via the Regulation of Intestinal Flora and Ferroptosis. Molecules 2023, 28, 3775. [Google Scholar] [CrossRef]
- So, B.R.; Kim, S.; Jang, S.H.; Kim, M.J.; Lee, J.J.; Kim, S.R.; Jung, S.K. Dietary Protocatechuic Acid Redistributes Tight Junction Proteins by Targeting Rho-Associated Protein Kinase to Improve Intestinal Barrier Function. Food Funct. 2023, 14, 4777–4791. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Montoya, C.A.; Mace, L.; Rettedal, E.A.; Fraser, K.; McNabb, W.C.; Moughan, P.J.; Roy, N.C. Biotransformation of Rutin in In Vitro Porcine Ileal and Colonic Fermentation Models. J. Agric. Food Chem. 2023, 71, 12487–12496. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Shi, D.-Z.; Wang, T.-Y.; Zheng, S.-Q.; Liu, L.-J.; Sun, Z.-X.; Wang, R.-F.; Ding, Y. Transformation of Trollioside and Isoquercetin by Human Intestinal Flora in Vitro. Chin. J. Nat. Med. 2016, 14, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, Pharmacokinetics, Pharmacological Activities, and Toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.; Li, Y.; Xie, X.; Huang, S.; Pan, S.; Zou, Y.; Pan, Z.; Wang, Q.; Chen, J.; et al. Quercetin Ameliorates Ulcerative Colitis by Activating Aryl Hydrocarbon Receptor to Improve Intestinal Barrier Integrity. Phytother. Res. 2024, 38, 253–264. [Google Scholar] [CrossRef]
- Liu, G.; Fang, Y.; Zhang, Y.; Zhu, M. Dihydroquercetin Improves the Proliferation of Porcine Intestinal Epithelial Cells via the Wnt/β-Catenin Pathway. Biochem. Biophys. Res. Commun. 2024, 734, 150460. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| PEDV N | GAAAATCCTGACAGGCATAAGCA | TTGCCGCTGTTGTCAGACTT |
| GAPDH | CCTTCCGTGTCCCTACTGC CAAC | GACGCCTGCTTCAC CACCTTCT |
| Extraction Fraction | Content (mg/g Raw Material) | |||||||
|---|---|---|---|---|---|---|---|---|
| Protocatechuic Acid | Rutin | Taxifolin 7-Rhamnoside | Isoquercitrin | Taxifolin | Quercitrin | Quercetin 7-Rhamnoside | Quercetin | |
| T20 | 0.009 | 0.275 | 0.694 | 0.589 | 0.121 | 0.560 | 0.420 | 0.046 |
| T60 | 0.008 | 0.076 | 0.478 | 1.140 | 0.132 | 1.978 | 0.673 | 0.247 |
| Ligand | Protein | Binding Energy (Kcal/mol) | Amino Acid Residues Contributing to Interactions |
|---|---|---|---|
| taxifolin-7-O-rhamnoside | 3CLpro | −9.5 | ARG-130, THR-281, SER-282 |
| PLP-2 | −8.0 | PHE-56, GLU-68, LYS-242 | |
| quercetin-7-rhamnoside | 3CLpro | −10.4 | LEU-268, TYR-280, GLU-286 |
| PLP-2 | −8.2 | PHE-56, ARG-57, TRP-67, GLU-235, ILE-241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, H.; Liu, S.; Wu, H.; Wang, W.; Wang, W.; Su, W.; Li, P. Screening of Active Compounds Against Porcine Epidemic Diarrhea Virus in Hypericum japonicum Thunb. ex Murray Extracts. Viruses 2025, 17, 900. https://doi.org/10.3390/v17070900
Rao H, Liu S, Wu H, Wang W, Wang W, Su W, Li P. Screening of Active Compounds Against Porcine Epidemic Diarrhea Virus in Hypericum japonicum Thunb. ex Murray Extracts. Viruses. 2025; 17(7):900. https://doi.org/10.3390/v17070900
Chicago/Turabian StyleRao, Hongyu, Siqi Liu, Hao Wu, Wenlong Wang, Weiyue Wang, Weiwei Su, and Peibo Li. 2025. "Screening of Active Compounds Against Porcine Epidemic Diarrhea Virus in Hypericum japonicum Thunb. ex Murray Extracts" Viruses 17, no. 7: 900. https://doi.org/10.3390/v17070900
APA StyleRao, H., Liu, S., Wu, H., Wang, W., Wang, W., Su, W., & Li, P. (2025). Screening of Active Compounds Against Porcine Epidemic Diarrhea Virus in Hypericum japonicum Thunb. ex Murray Extracts. Viruses, 17(7), 900. https://doi.org/10.3390/v17070900







